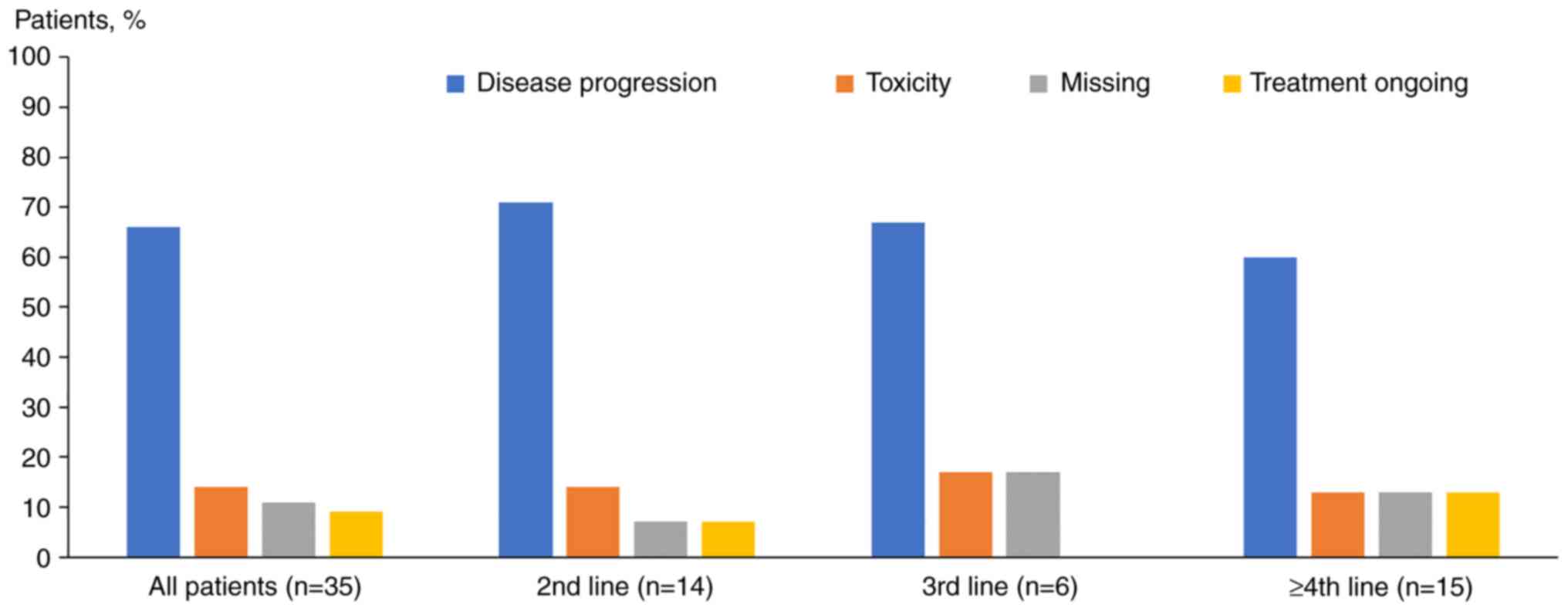
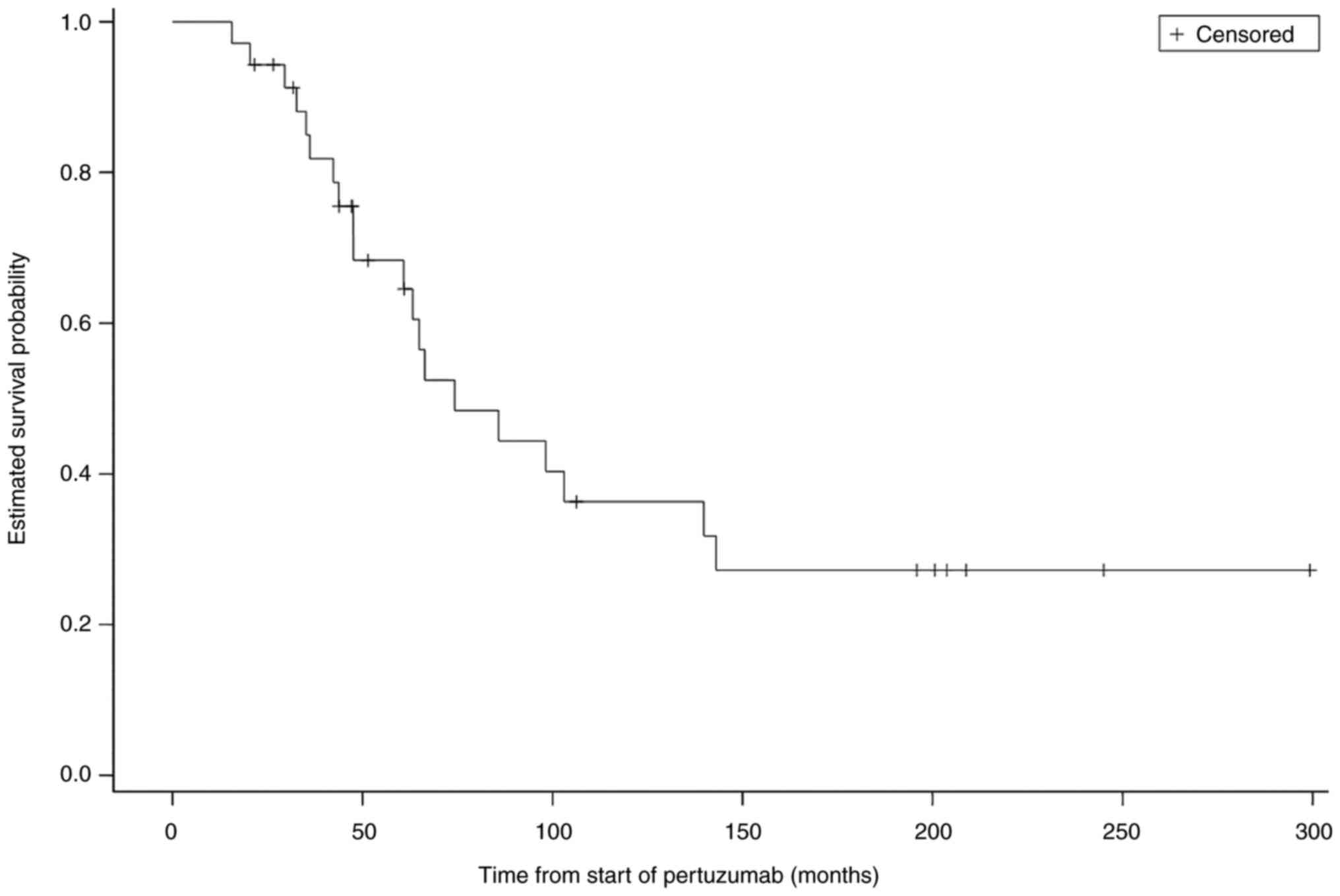
|
1
|
World Health Organization, Breast cancer,
2020. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/.
|
|
2
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel members.
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Andrulis IL, Bull SB, Blackstein ME,
Sutherland D, Mak C, Sidlofsky S, Pritzker KP, Hartwick RW, Hanna
W, Lickley L, et al: neu/erbB-2 amplification identifies a
poor-prognosis group of women with node-negative breast cancer.
Toronto breast cancer study group. J Clin Oncol. 16:1340–1349.
1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Adams CW, Allison DE, Flagella K, Presta
L, Clarke J, Dybdal N, McKeever K and Sliwkowski MX: Humanization
of a recombinant monoclonal antibody to produce a therapeutic HER
dimerization inhibitor, pertuzumab. Cancer Immunol Immunother.
55:717–727. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Scheuer W, Friess T, Burtscher H,
Bossenmaier B, Endl J and Hasmann M: Strongly enhanced antitumor
activity of trastuzumab and pertuzumab combination treatment on
HER2-positive human xenograft tumor models. Cancer Res.
69:9330–9336. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baselga J, Cortés J, Kim SB, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast
cancer. N Engl J Med. 366:109–119. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Swain SM, Baselga J, Kim SB, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Heeson S, et al: Pertuzumab, trastuzumab, and docetaxel in
HER2-positive metastatic breast cancer. N Engl J Med. 372:724–734.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bachelot T, Ciruelos E, Schneeweiss A,
Puglisi F, Peretz-Yablonski T, Bondarenko I, Paluch-Shimon S,
Wardley A, Merot JL, du Toit Y, et al: Preliminary safety and
efficacy of first-line pertuzumab combined with trastuzumab and
taxane therapy for HER2-positive locally recurrent or metastatic
breast cancer (PERUSE). Ann Oncol. 30:766–773. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Perez EA, López-Vega JM, Petit T, Zamagni
C, Easton V, Kamber J, Restuccia E and Andersson M: Safety and
efficacy of vinorelbine in combination with pertuzumab and
trastuzumab for first-line treatment of patients with HER2-positive
locally advanced or metastatic breast cancer: VELVET Cohort 1 final
results. Breast Cancer Res. 18(126)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Andersson M, López-Vega JM, Petit T,
Zamagni C, Easton V, Kamber J, Restuccia E and Perez EA: Efficacy
and safety of pertuzumab and trastuzumab administered in a single
infusion bag, followed by vinorelbine: VELVET Cohort 2 final
results. Oncologist. 22:1160–1168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A and
Kaufman B: Lapatinib plus capecitabine for HER2-positive advanced
breast cancer. N Engl J Med. 355:2733–2743. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
National Comprehensive Cancer Network,
NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, 2021.
https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
|
|
15
|
Rimawi MF and Osborne CK: Revisiting the
use of HER2-targeted therapies in the adjuvant treatment of early
breast cancer. J Clin Oncol. 33:1236–1238. 2015.
|
|
16
|
Biserni GB, Engstrøm MJ and Bofin AM: HER2
gene copy number and breast cancer-specific survival.
Histopathology. 69:871–879. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gradishar WJ, Anderson BO, Abraham J, Aft
R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD,
et al: Breast cancer, version 3.2020, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 18:452–478.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Abemaciclib in combination with fulvestrant in women
with HR+/HER2-advanced breast cancer who had progressed while
receiving endocrine therapy. J Clin Oncol. 35:2875–2884.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive breast
cancer. N Engl J Med. 382:610–621. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Barok M, Tanner M, Köninki K and Isola J:
Trastuzumab-DM1 causes tumour growth inhibition by mitotic
catastrophe in trastuzumab-resistant breast cancer cells in vivo.
Breast Cancer Res. 13(R46)2011.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Murthy RK, Loi S, Okines A, Paplomata E,
Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, et
al: Tucatinib, trastuzumab, and capecitabine for HER2-positive
metastatic breast cancer. N Engl J Med. 382:597–609.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Waddell T, Kotsori A, Constantinidou A,
Yousaf N, Ashley S, Parton M, Allen M, Starling N, Papadopoulos P,
O'Brien M, et al: Trastuzumab beyond progression in HER2-positive
advanced breast cancer: The Royal Marsden experience. Br J Cancer.
104:1675–1679. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Baselga J, Gelmon KA, Verma S, Wardley A,
Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA, et al:
Phase II trial of pertuzumab and trastuzumab in patients with human
epidermal growth factor receptor 2-positive metastatic breast
cancer that progressed during prior trastuzumab therapy. J Clin
Oncol. 28:1138–1144. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Urruticoechea A, Rizwanullah M, Im SA,
Ruiz ACS, Láng I, Tomasello G, Douthwaite H, Badovinac Crnjevic T,
Heeson S, Eng-Wong J and Muñoz M: Randomized phase III trial of
trastuzumab plus capecitabine with or without pertuzumab in
patients with human epidermal growth factor receptor 2-positive
metastatic breast cancer who experienced disease progression during
or after trastuzumab-based therapy. J Clin Oncol. 35:3030–3038.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gianni L, Pienkowski T, Im YH, Roman L,
Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J,
Im SA, et al: Efficacy and safety of neoadjuvant pertuzumab and
trastuzumab in women with locally advanced, inflammatory, or early
HER2-positive breast cancer (NeoSphere): A randomised multicentre,
open-label, phase 2 trial. Lancet Oncol. 13:25–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Amiri-Kordestani L, Wedam S, Zhang L, Tang
S, Tilley A, Ibrahim A, Justice R, Pazdur R and Cortazar P: First
FDA approval of neoadjuvant therapy for breast cancer: Pertuzumab
for the treatment of patients with HER2-positive breast cancer.
Clin Cancer Res. 20:5359–5364. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
ClinicalTrials.gov: Phase III trial of pertuzumab and
trastuzumab plus endocrine therapy versus chemotherapy plus
trastuzumab and pertuzumab in patients with hormone
receptor-positive, HER2-positive primary breast cancer (Detect
V/CHEVENDO), 2015. https://clinicaltrials.gov/ct2/show/NCT02344472.
|
|
28
|
Kute T, Lackey KA and Sutton LM: Targeted
therapy in HER2-positive metastatic breast cancer: A review of the
literature. J Adv Pract Oncol. 9:401–414. 2018.
|
|
29
|
Huober J, Fasching PA, Barsoum M,
Petruzelka L, Wallwiener M, Thomssen C and Untch M: Higher efficacy
of the combination of trastuzumab and pertuzumab in the neoadjuvant
setting compared with the adjuvant setting in patients with
HER2-positive breast cancer. J Clin Oncol. 33:983–990. 2015.
|