Introduction
Nasopharyngeal carcinoma (NPC) is common in
Southeast Asia and is less common in Europe and the United States.
The incidence of NPC in endemic areas can reach 10-50 per 100,000
individuals (1). With the use of
intensity-modulated radiation therapy (IMRT), the 5-year local
recurrence-free survival rate and overall survival rate are
approximately 90 and 80%, respectively (2,3).
However, the extension of survival time leads to the occurrence of
late adverse effects (AEs) of radiotherapy, which are associated
with poor quality of life in patients with NPC. The most common AEs
in patients with NPC treated with radiotherapy include hearing
loss, tinnitus, subcutaneous fibrosis, and xerostomia (4). Radiation-related nasopharyngeal
necrosis (RRNN) is a rare AE of radiotherapy, with an incidence of
1-2% in patients with primary NPC (5,6).
However, in patients with recurrent NPC treated with reirradiation,
the incidence of RRNN is 30-40% (7,8).
Predominant symptoms caused by RRNN include foul nasal odor,
persistent headache, and nasal hemorrhage. Most patients with RRNN
succumb, as a result of massive nasopharyngeal bleeding due to
internal carotid artery rupture (9).
There are currently no effective treatments for RRNN
due to a lack of knowledge surrounding its pathophysiology.
Conservative management includes nasal irrigation, systemic or
topical antibiotics, intravenous nutritional supplements,
hyperbaric oxygen, and debridement guided by nasal endoscopy;
however, outcomes with these treatments remain suboptimal (9). Endoscopic or open surgery have also
been suggested to remove necrotic tissue, followed by flap
covering; however, these approaches are associated with high costs
and can only be performed by few skilled surgeons in China
(10-12).
As such, there is a need to identify effective, low cost therapies
that can effectively treat RRNN in patients with NPC.
Endostar, as a recombinant form of endostatin,
exerts antitumor effects via inhibition of the VEGF pathway,
similar to bevacizumab (13-16).
Endostar, in combination with chemotherapy, was approved by the
China Food and Drug Administration as first-line therapy for stage
III-IV non-small cell lung cancer (NSCLC) (16). In addition to its anti-angiogenic
effect, Endostar was also demonstrated to reduce radiation damage
to normal tissues. Guan et al revealed that Endostar
decreased the occruence of RRNN in patients with local recurrent
NPC who were treated with radiotherapy (17). Xing et al also successfully
treated patients with radiotherapy-induced brain necrosis, with
Endostar (18). In addition, a
pre-clinical study revealed that Endostar could reduce
radiation-induced fibrosis (19).
Based on these studies, two patients with RRNN were
treated with Endostar to determine its effectiveness. To the best
of our knowledge, this is the first study of an anti-angiogenic
approach for the treatment of RRNN.
Case report
The present study was approved (approval no.
PYRC2023090) by the Ethics Committee of Panyu Central Hospital
(Guangzhou, China). The patients agreed to participate in the
present study and submitted written informed consent.
The first patient (case 1), was a 50-year-old man
who in June 2019 presented to the Outpatient Department of Panyu
Central Hospital (Guangzhou, China), with a headache and was
diagnosed with undifferentiated non-keratinizing carcinoma of the
nasopharynx [World Health Organization (WHO) type III], tumor stage
III (T3N1M0) according to the eighth edition of the American Joint
Committee on Cancer staging system (20,21).
From June 26, 2019, the patient received two cycles of induction
chemotherapy with docetaxel plus cisplatin, followed by concurrent
chemoradiotherapy with IMRT, administered according to the
following dosing schedule: Gross tumor volume (GTV), high risk area
around primary tumor (CTV1), low risk area around tumor and
bilateral cervical lymph node drainage area (CTV2), left positive
lymph node and right positive lymph node were dosed with 70.4, 60,
54, 66 and 64 Gy, respectively. The fractionation number was 32. A
complete response was revealed using MRI and a nasopharyngoscope on
September 10, 2019. On July 3, 2020, the patient again presented to
the hospital due to a refractory, severe headache. A
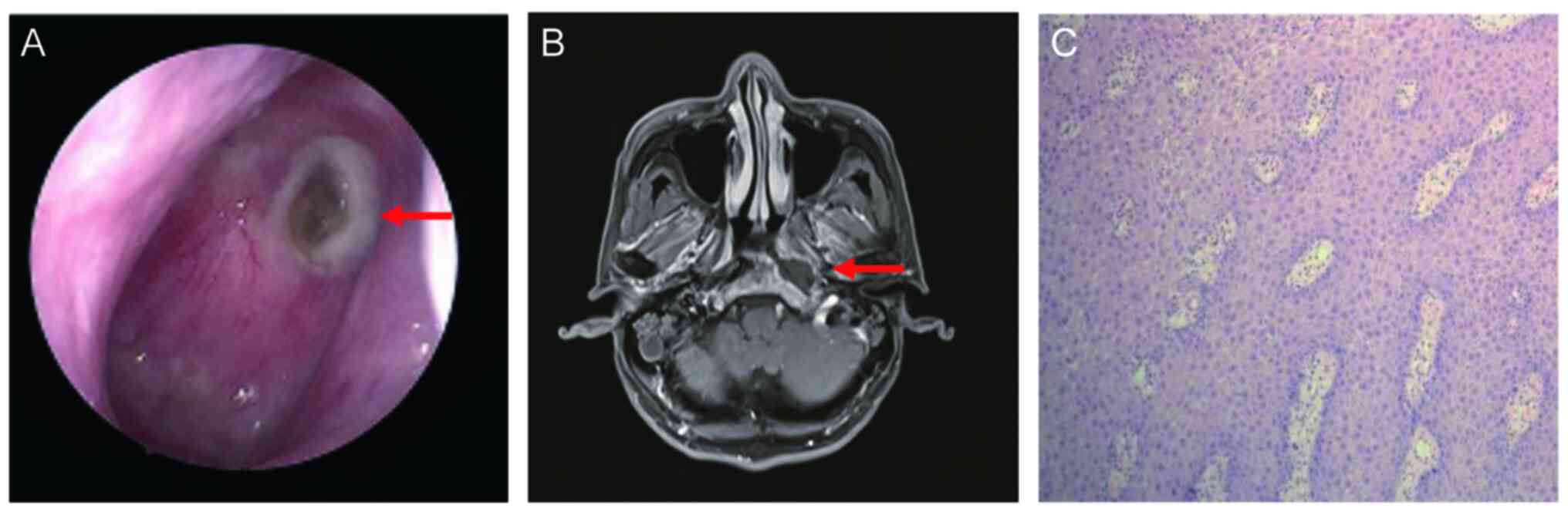
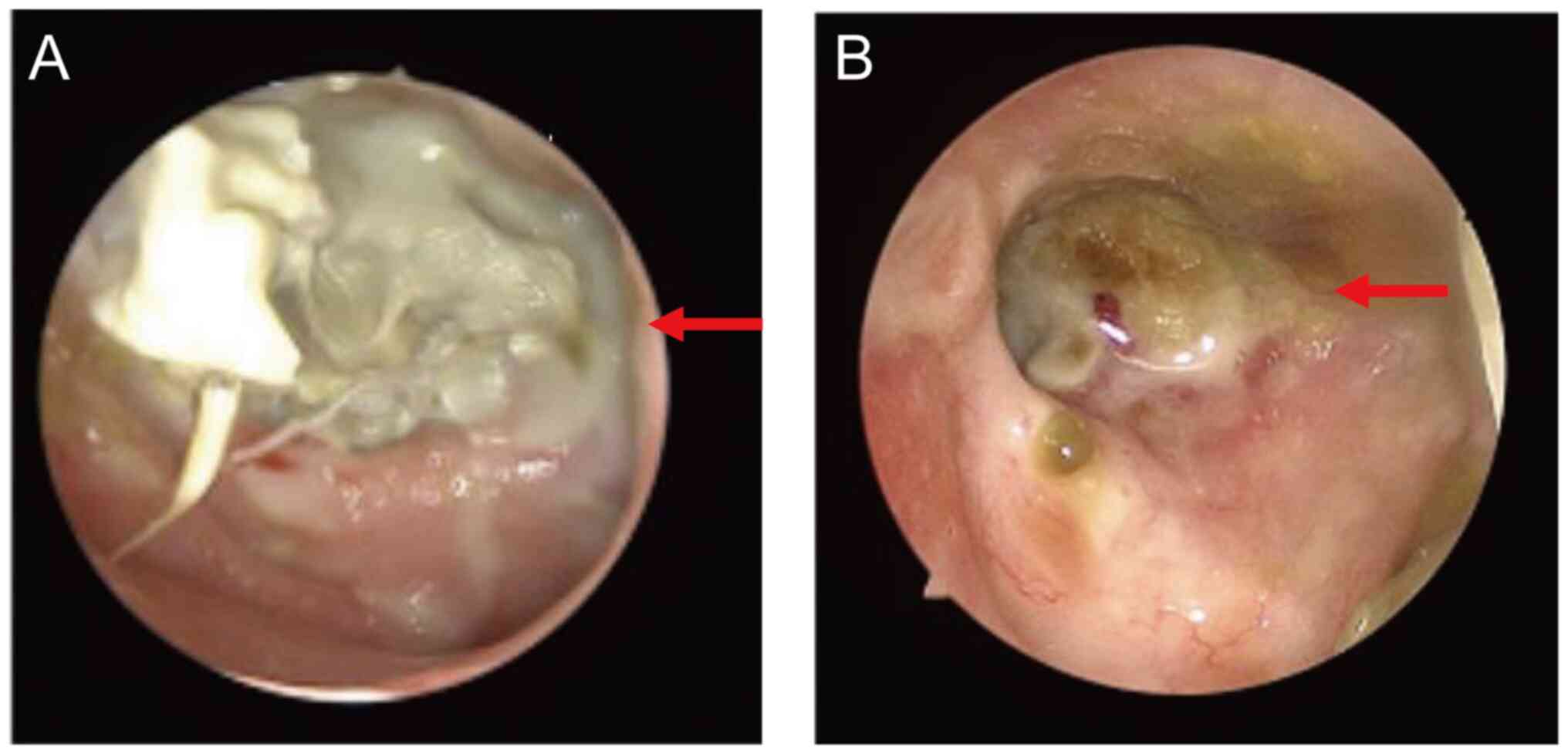
nasopharyngoscope revealed a yellowish necrotic tissue on the left
parietal-posterior wall of the nasopharynx, surrounded by purulent
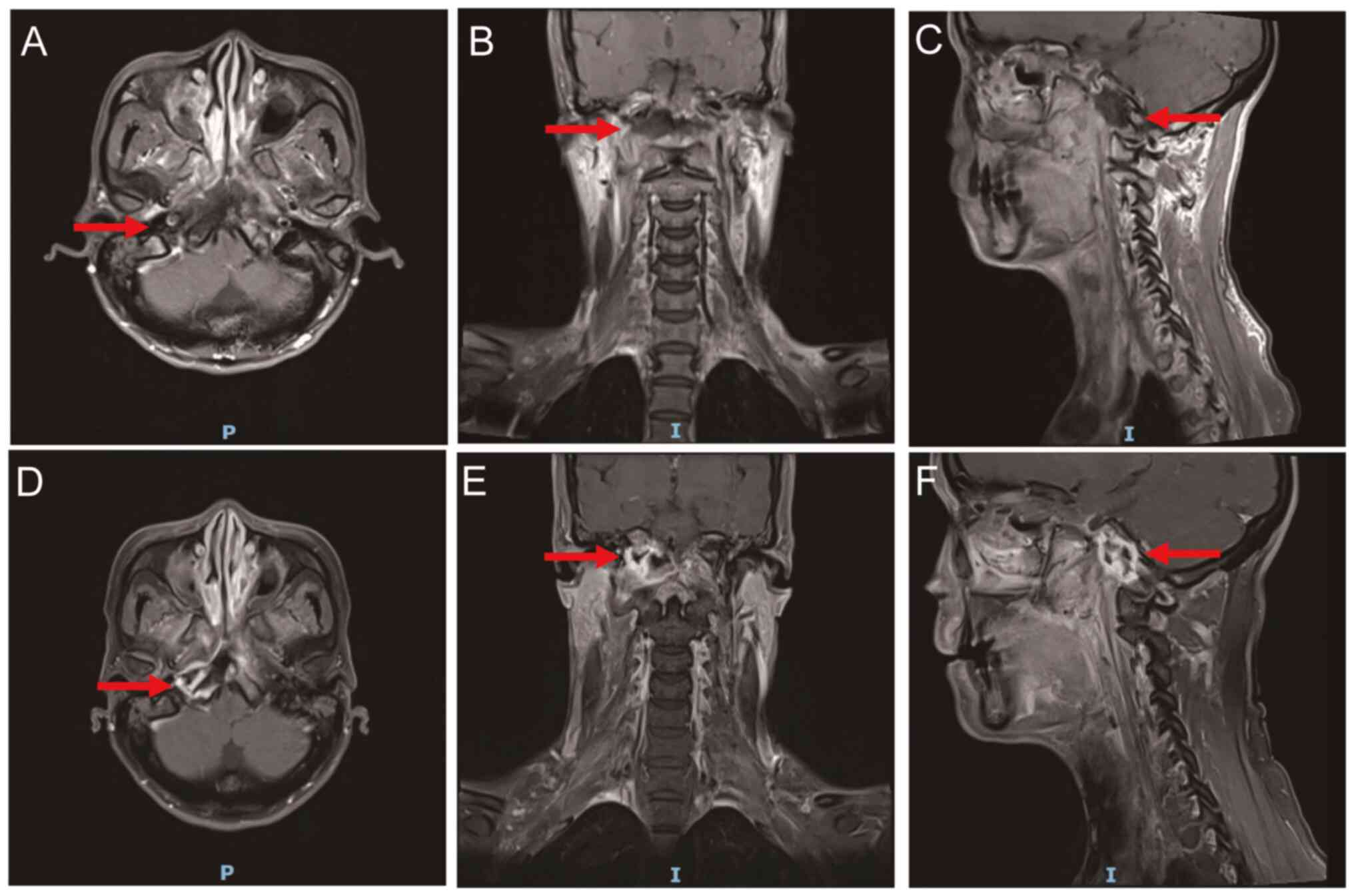
secretions (Fig. 1A). MRI revealed
necrotic lesions on the left side of the nasopharynx (Fig. 1B). No tumor metastases were
detected by contrast-enhanced CT of the chest and abdomen. The
patient was negative for Epstein-Barr virus. Repetitive
histological examination (10% formalin-fixed for 12 h at room
temperature; embedded in paraffin; section thickness, 3 µm;
staining, hematoxylin and eosin stain used for 15 min at room
temperature; and visualized using a light microscope with a
magnification of x100) of nasopharyngeal specimens showed no
recurrent tumor (Fig. 1C).
Initially, conservative treatments including nasal irrigations,
systemic antibiotics and intravenous nutrition were used to treat
the patient. However, the headache of the patient worsened one week
later. The decision was made to use Endostar following approval
(approval no. PYRC2023090) by the Ethics Committee of Panyu Central
Hospital (Guangzhou, China). The headache of the patient was
significantly relieved after one course of Endostar (15 mg per day,
from day 1 to day 7, every three weeks), and oxycodone was not
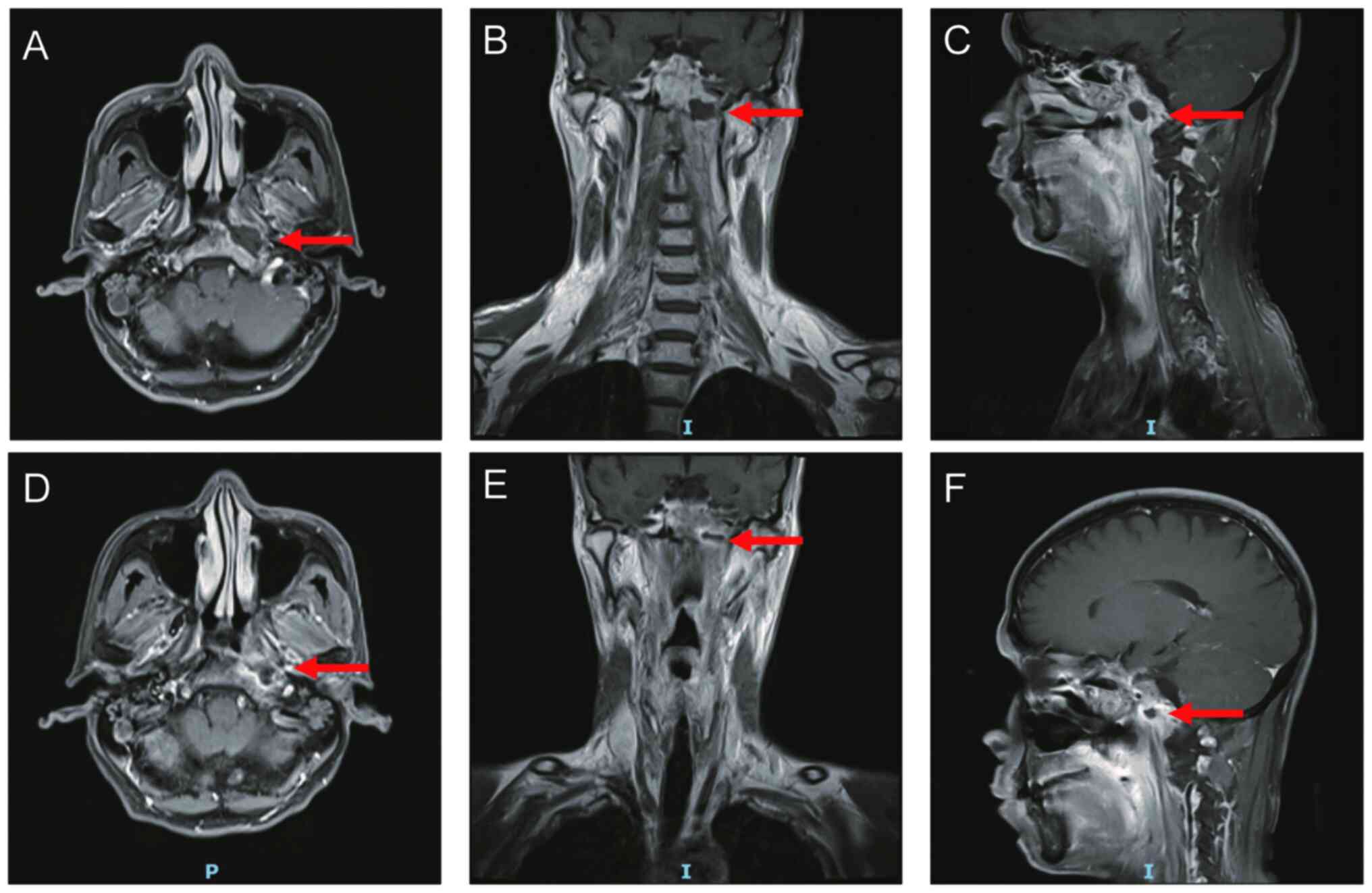
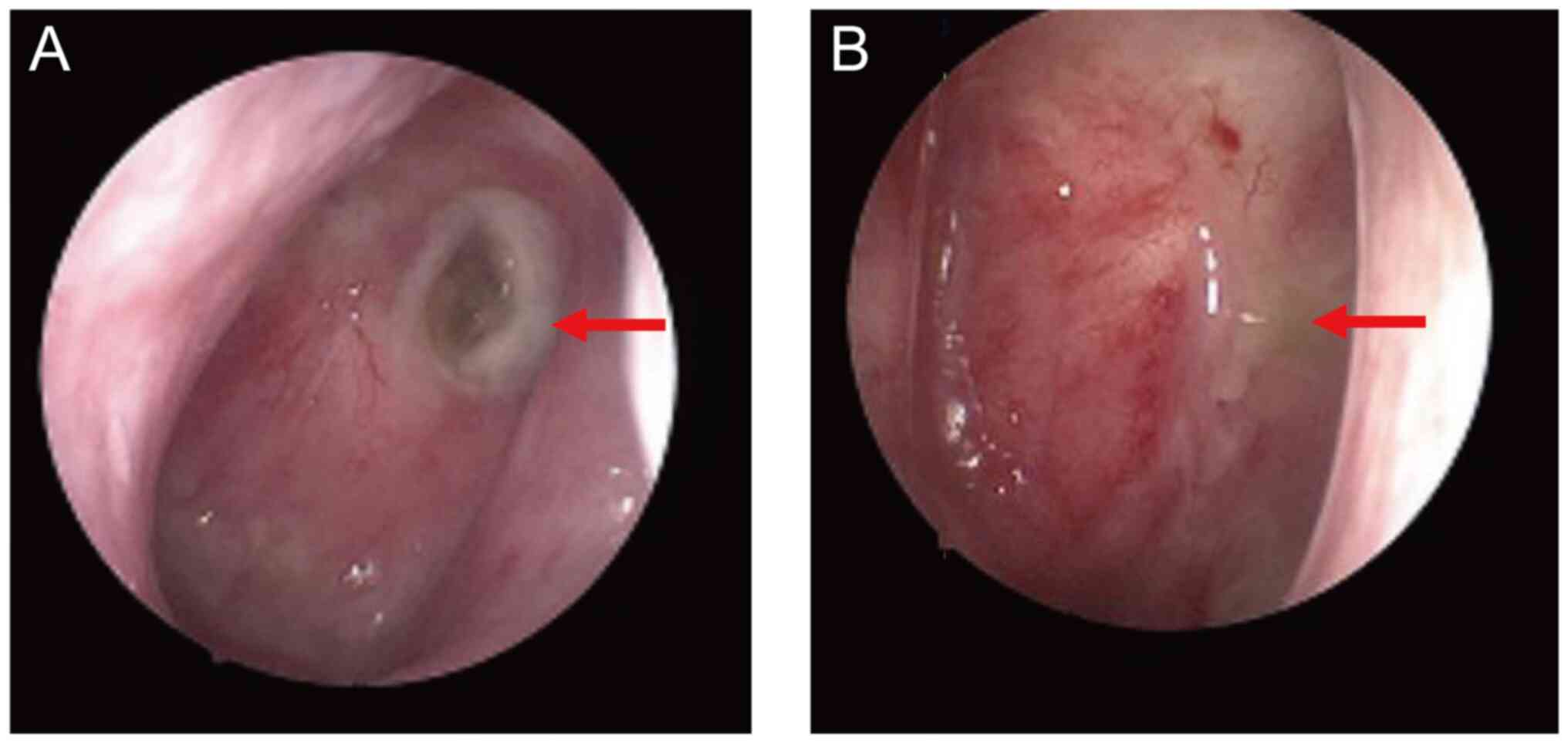
required for pain relief after four courses of treatment. MRI and a
nasopharyngoscope revealed that the necrotic lesion had disappeared
after two cycles of Endostar (Figs.
2 and 3). Due to financial
constraints, the patient declined to return for examination and
treatment after four cycles of Endostar. However, on August 20,
2022, the patient was revealed to be doing well without any
discomfort such as headache, nasal odor, or nasal hemorrhage, upon
examination at the Outpatient Department.
The second patient (case 2), was a 50-year-old woman
who in November 2019 was diagnosed with undifferentiated
non-keratinizing carcinoma of the nasopharynx (WHO type III), tumor
stage IVA (T4N1M0) at Panyu Central Hospital (Guangzhou, China)
according to the eighth edition of the American Joint Committee on
Cancer staging system. The patient received three cycles of
chemotherapy with gemcitabine, cisplatin, and sintilimab, followed
by concurrent chemoradiotherapy as part of a randomized controlled
trial for patients with local advanced nasopharyngeal carcinoma.
IMRT was administered at 70, 60, 54, 66 and 64 Gy for the GTV,
CTV1, CTV2, left positive lymph node and right positive lymph node,
respectively. The fractionation number was 33. A total of six
additional cycles of immunotherapy with sintilimab were
administered after chemoradiotherapy and the last dose of
sintilimab was administered on September 9, 2020. The patient was
admitted to the Department of Oncology, Panyu Central Hospital
(Guangzhou, China) on September 23, 2020 due to foul nasal odor and
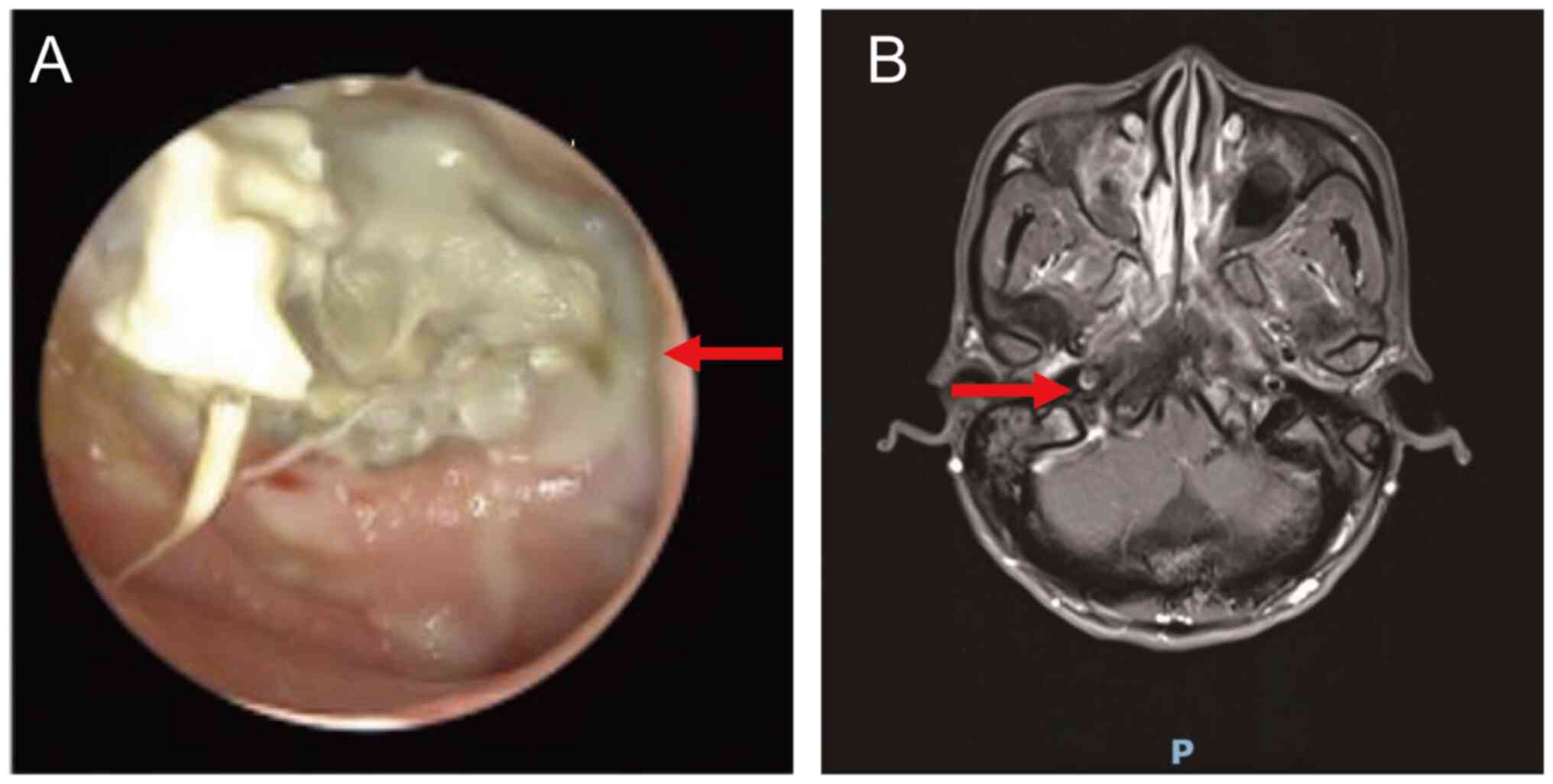
hearing loss. A nasopharyngoscope revealed a large amount of
necrotic tissue and bone on the posterior wall of the nasopharynx
(Fig. 4A). An endotoscope revealed
bilateral middle ear effusion. MRI revealed osteonecrosis of the
skull base and necrosis of the posterior wall of the nasopharyngx
(Fig. 4B). No distant metastases
were identified by CT scan of the chest and abdomen. The patient
was negative for Epstein-Barr virus. Due to insufficient exposure
of the nasopharynx with the use of the nasopharyngoscope, as well
as severe necrosis and adhesion, a forceps biopsy was not permitted
as there was a possibility of massive nasopharyngeal hemorrhage.
The patient was eventually diagnosed with RRNN based on the results
of MRI and the nasopharyngoscope and was treated with Endostar
following approval (approval no. PYRC2023090) by the Ethics
Committee of Panyu Central Hospital (Guangzhou, China). The patient
received bilateral middle ear tube drainage and seven cycles of
Endostar (15 mg per day, from day 1 to day 7, every three weeks)
between September 30, 2020 and February 23, 2021. The symptoms of
the patient were relieved after two courses of Endostar. The
nasopharyngoscope and MRI showed that the nasopharyngeal necrotic
lesions had disappeared and the nasopharyngx was filled with new
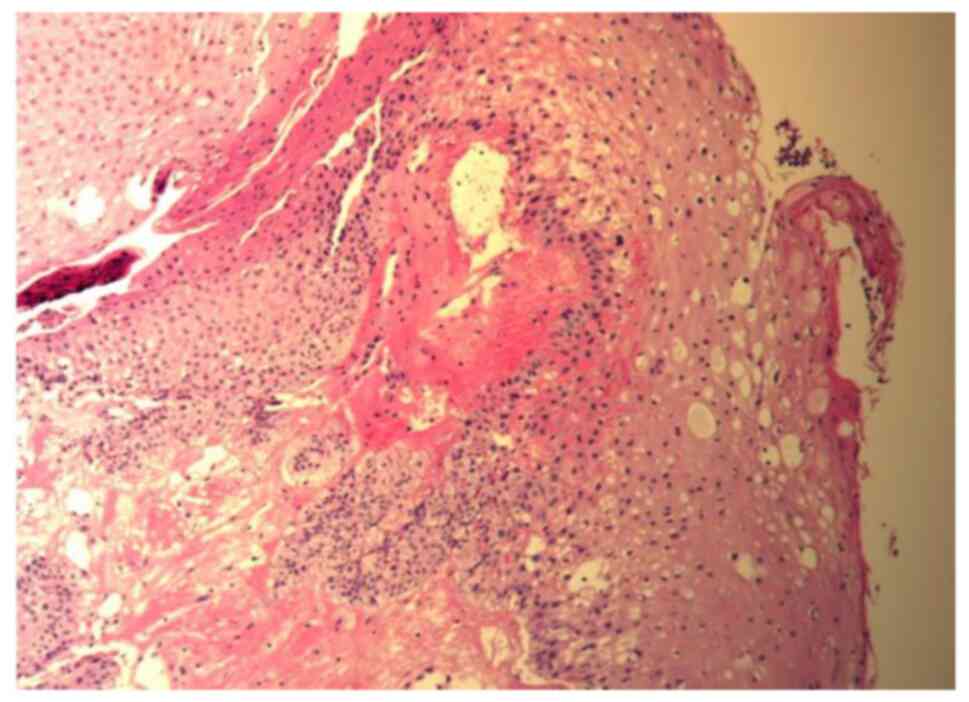
tissue (Figs. 5 and 6). Pathological biopsy (10%
formalin-fixed for 12 h at room temperature; embedded in paraffin;
section thickness, 3 µm; staining, hematoxylin and eosin stain used
for 15 min at room temperature; and visualized using a light
microscope with a magnification of x100) of the posterior wall of
the nasopharynx revealed that there was no tumor recurrence
(Fig. 7).
Discussion
RRNN is defined as necrosis of the nasopharyngeal
mucosa, adjacent muscles, or the skull base following exposure to
high doses of radiation in patients with NPC (22). Reirradiation is the most important
risk factor for the development of RRNN. Other risk factors include
tumor stage, nutritional status during radiotherapy, and anemia
(5). Patients with RRNN often
present with headache, foul nasal odor, and recurrent epistaxis,
which may occur alone or together (6,9,23).
RRNN can significantly reduce the quality of life of the patient
and is associated with mortality. The reported 2-year overall
survival (OS) in patients with RRNN is 51.6% (11). In patients in whom necrosis or
ulcers have eroded the skull base bone, survival rates are even
worse (9). The diagnosis of RRNN
can be achieved via a nasopharyngoscope and MRI of the nasopharynx;
however, the gold standard of diagnosis is a pathological biopsy
(11).
RRNN is challenging for physicians to treat. The
mainstays of treatment include conservative and surgical
management. The conservative approach consists of daily rinsing of
the nasopharynx with 2% aqueous hydrogen peroxide solution or
saline, hyperbaric oxygen, systemic antibiotics, intravenous
nutrition and surgical debridement, which is associated with a cure
rate of only 13.4% (9). In
addition, a combination of pentoxifylline, tocopherol, and
clodronate, or PENTOCLO, was revealed to be effective for treating
refractory osteoradionecrosis, which is a serious complication of
RRNN (24,25). However, a study by Huang et
al (12) revealed that only
9.5% of patients with mucosal defects caused by RRNN could be
effectively treated with PENTOCLO, which is lower than the 59% cure
rate reported by Robard et al (25). Surgical management, including
complete resection of necrotic nasopharyngeal tissue under
endoscopy, and maxillary swing or mandibulotomy with free vastus
lateralis flap for reconstruction, may be effective methods for the
treatment of RRNN (11,12). However, surgery is a complex and
risky procedure that can only be performed by specific experts.
Additionally, only 32% of patients with osteoradionecrosis were
eligible for surgery in a study by Chen et al (11).
In the present study, the successful treatment of
two patients with RRNN using the anti-angiogenic drug, Endostar was
reported. As of October 8, 2022, neither patient reported headache,
nasal odor, nasal bleeding or other symptoms and both patients did
not require analgesic drugs.
The main mechanism underlying the development of
RRNN is radiation-induced fibrosis (RIF) (26). This process can be divided into
three phases; phase I involves the injury of endothelial cells by
radiation leading to the generation of chemotactic cytokines that
can trigger acute, non-specific inflammation. This inflammation is
characterized by increased vascular permeability, local edema
formation and local ischaemia. This process is very similar to the
changes observed in the vasculature of the blood-spinal cord
barrier following radiotherapy, which may be due to upregulation of
VEGF expression induced by radiotherapy. Phase II is characterized
by the activation of fibroblasts by cytokines that seep into tissue
from the micro vasculature and become myofibroblasts, leading to
the formation of RIF tissue in place of normal tissue. Among these
cytokines, transforming growth factor β1 (TGF-β1) plays a
predominant role. Finally, phase III involves the death of
myofibroblasts in RIF tissue, which leads the tissue to become
poorly vascularized, paucicellular, and unable to heal once it is
subjected to trauma and is therefore prone to necrosis (26,27).
Endostatin was shown to exert antitumor effects by
inhibiting tumor angiogenesis in xenografted mouse models (28-31).
Endostar is a recombinant form of endostatin, which was approved by
the China Food and Drug Administration in combination with
vinorelbine and cisplatin, for the treatment of NSCLC in
2005(16). However, a phase II
study showed that Endostar alone did not cause tumor regression in
highly vascular neuroendocrine tumors (32). These results indicate that the
antitumor activity of Endostar is more complex than previously
considered. Another study in a xenografted mouse model of human NPC
demonstrated that Endostar can restore normal vasculature. The
tumor vasculature had fewer sprouts and branches in the Endostar
group compared with controls. In addition, Endostar significantly
increased the pericyte coverage, and the percentage of basement
membrane in the blood vessels. By contrast, Endostar decreased the
permeability of the tumor vasculature and improved hypoxia in tumor
tissue compared with controls in a xenografted mouse model of human
NPC (P<0.05). These effects are attributable to the
antiangiogenic activity of Endostar (33). Wang et al demonstrated that
Endostar could significantly increase the blood volume and blood
flow velocity in the tumors of patients with NPC (34). Thus, Endostar may treat RRNN by
downregulating the VEGF pathway, leading to decreased vascular
permeability in irradiated tissue. Additionally, Endostar may also
downregulate TGF-β1 to treat RRNN (19).
Guan et al demonstrated that treatment with
Endostar decreased the incidence of RRNN in patients with recurrent
NPC who received radiotherapy (17). In the present study, the effect of
Endostar in the treatment of RRNN was investigated. In case 1,
T1-enhanced imaging showed that the signal intensity of the skull
base bone significantly decreased after treatment. This may be due
to reduced vascular permeability following treatment with Endostar.
In both cases, new tissue was observed in the nasopharynx, which
presented with a high signal in T1-enhanced images. Pathological
biopsy from the posterior wall of the nasopharynx in case 2
revealed that the tissue was full of squamous cells, which is in
contrast to RIF tissue decribed in a previous study (26).
Endostar is generally dosed at 7.5
mg/m2/day for 14 consecutive days (13,16).
However, clinical trials have evaluated different doses, including
15 mg/day for 14 days or 45 mg/day on days 1, 3, and 4 (35,36).
In the present study, a treatment regimen of 15 mg daily for 7 days
was evaluated. Total therapeutic dosage was determined based on the
response of each patient to treatment. After each cycle of
treatment, the patient was evaluated for symptoms, and underwent a
nasopharyngoscope examination and MRI every two cycles. If the
following criteria were met, treatment was discontinued: Resolution
or significant improvement of symptoms; signs of ulcer repair with
the use of the nasopharyngoscope; no infectious secretions in the
nasopharynx; resolution of necrotic lesions observed with MRI.
However, given the limited clinical experience of the authors,
further studies are required to explore the optimal total
dosage.
Previous clinical research has reported several AEs
associated with Endostar including arrhythmia, prolonged or
shortened Q-T interval, hematological adverse reactions such as
anemia and prolonged thrombin time, and digestive adverse reactions
such as transaminase elevation (37). In the present study, no
Endostar-related AEs were observed. Morevover, previous
pharmacokinetic studies have shown that the maximum total dose of
Endostar in humans can reach 240 mg/m2/day for 168 days
(38), which demonstrates the
favorable safety profile of Endostar.
In conclusion, in the present study it was revealed
that two patients with RRNN were cured with Endostar, suggesting
that it may be a promising treatment for this intractable disease.
However, large-scale, prospective, controlled trials are required
to confirm the effectiveness of Endostar for the treatment of
RRNN.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the General Medical
and Health Projects of Science and Technology Bureau of Panyu
District (grant no. 2022-Z04-048 to JT), the Hubei Provincial
Natural Science Foundation (grant no. 2020CFB397 to BCW), and the
Independent Innovation Foundation of Wuhan Union Hospital (grant
no. 2019-109 to BCW).
Availability of data and materials
The data generated in the present study are included
in the figures of this article.
Authors' contributions
JT, XWL and YW contributed to the design of the
study. YHL, ZS and XLC contributed to the conception of the study.
JT, XWL, GRZ, YHL, ZS, and XLC performed the extraction of images.
JT, BCW, XWS, YH and XWL performed the analysis of the images. JT
and BCW wrote the manuscript. GRZ, YW and JT edited the manuscript.
JT and BCW confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
PYRC2023090) by the Ethics Committee of Panyu Central Hospital
(Guangzhou, China). The patients agreed to participate in the
present study and submitted written informed consent.
Patient consent for publication
The patients provided consent for their information
to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luo J, Chia KS, Chia SE, Reilly M, Tan CS
and Ye W: Secular trends of nasopharyngeal carcinoma incidence in
Singapore, Hong Kong and Los Angeles Chinese populations,
1973-1997. Eur J Epidemiol. 22:513–521. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xu M, Zang J, Luo S, Wang J and Li X:
Long-term survival outcomes and adverse effects of nasopharyngeal
carcinoma patients treated with IMRT in a non-endemic region: A
population-based retrospective study. BMJ Open.
11(e045417)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang MX, Li J, Shen GP, Zou X, Xu JJ,
Jiang R, You R, Hua YJ, Sun Y, Ma J, et al: Intensity-modulated
radiotherapy prolongs the survival of patients with nasopharyngeal
carcinoma compared with conventional two-dimensional radiotherapy:
A 10-year experience with a large cohort and long follow-up. Eur J
Cancer. 51:2587–2595. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zeng L, Tian YM, Sun XM, Chen CY, Han F,
Xiao WW, Deng XW and Lu TX: Late toxicities after
intensity-modulated radiotherapy for nasopharyngeal carcinoma:
Patient and treatment-related risk factors. Br J Cancer. 110:49–54.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li XY, Sun XS, Liu SL, Chen QY, Guo SS,
Liu LT, Yan JJ, Xie HJ, Tang QN, Liang YJ, et al: The development
of a nomogram to predict post-radiation necrosis in nasopharyngeal
carcinoma patients: A large-scale cohort study. Cancer Manag Res.
11:6253–6263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han P, Wang X, Liang F, Liu Y, Qiu X, Xu
Y, Chen R, Yu S and Huang X: Osteoradionecrosis of the Skull base
in nasopharyngeal carcinoma: Incidence and risk factors. Int J
Radiat Oncol Biol Phys. 102:552–555. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kong F, Zhou J, Du C, He X, Kong L, Hu C
and Ying H: Long-term survival and late complications of
intensity-modulated radiotherapy for recurrent nasopharyngeal
carcinoma. BMC Cancer. 18(1139)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Han F, Zhao C, Huang SM, Lu LX, Huang Y,
Deng XW, Mai WY, The BS, Butler EB and Lu TX: Long-term outcomes
and prognostic factors of re-irradiation for locally recurrent
nasopharyngeal carcinoma using intensity-modulated radiotherapy.
Clin Oncol (R Coll Radiol). 24:569–576. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen MY, Mai HQ, Sun R, Guo X, Zhao C,
Hong MH and Hua YJ: Clinical findings and imaging features of 67
nasopharyngeal carcinoma patients with postradiation nasopharyngeal
necrosis. Chin J Cancer. 32:533–538. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang XM, Zheng YQ, Zhang XM, Mai HQ, Zeng
L, Liu X, Liu W, Zou H and Xu G: Diagnosis and management of skull
base osteoradionecrosis after radiotherapy for nasopharyngeal
carcinoma. Laryngoscope. 116:1626–1631. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang Q, Zou X, You R, Liu YP, Han Y, Zhang
YN, Guo L, Mai HQ, Xie CM, Li L, et al: Proposal for a new risk
classification system for nasopharyngeal carcinoma patients with
post-radiation nasopharyngeal necrosis. Oral Oncol. 67:83–88.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang WB, Wong STS and Chan JYW: Role of
surgery in the treatment of osteoradionecrosis and its
complications after radiotherapy for nasopharyngeal carcinoma. Head
Neck. 40:369–376. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen J, Yao Q, Huang M, Wang B, Zhang J,
Wang T, Ming Y, Zhou X, Jia Q, Huan Y, et al: A randomized Phase
III trial of neoadjuvant recombinant human endostatin, docetaxel
and epirubicin as first-line therapy for patients with breast
cancer (CBCRT01). Int J Cancer. 142:2130–2138. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bao Y, Peng F, Zhou QC, Yu ZH, Li JC,
Cheng ZB, Chen L, Hu X, Chen YY, Wang J, et al: Phase II trial of
recombinant human endostatin in combination with concurrent
chemoradiotherapy in patients with stage III non-small-cell lung
cancer. Radiother Oncol. 114:161–166. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Han BH, Xiu QY, Wang HM, Shen J, Gu AQ,
Luo Y, Bai CX, Guo SL, Liu WC, Zhuang ZX, et al: A multicenter,
randomized, double-blind, placebo-controlled safety study to
evaluate the clinical effects and quality of life of
paclitaxel-carboplatin (PC) alone or combined with endostar for
advanced non-small cell lung cancer (NSCLC). Zhonghua Zhong Liu Za
Zhi. 33:854–859. 2011.PubMed/NCBI(In Chinese).
|
|
16
|
Wang J, Sun Y, Liu Y, Yu Q, Zhang Y, Li K,
Zhu Y, Zhou Q, Hou M, Guan Z, et al: (Results of randomized,
multicenter, double-blind phase III trial of rh-endostatin (YH-16)
in treatment of advanced non-small cell lung cancer patients).
Zhongguo Fei Ai Za Zhi. 8:283–290. 2005.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
17
|
Guan Y, Li A, Xiao W, Liu S, Chen B, Lu T,
Zhao C and Han F: The efficacy and safety of Endostar combined with
chemoradiotherapy for patients with advanced, locally recurrent
nasopharyngeal carcinoma. Oncotarget. 6:33926–33934.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xing S, Fan Z, Shi L, Yang Z and Bai Y:
Successful treatment of brain radiation necrosis resulting from
triple-negative breast cancer with Endostar and short-term
hyperbaric oxygen therapy: A case report. Onco Targets Ther.
12:2729–2735. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang K, Yang S, Zhu Y, Mo A, Zhang D and
Liu L: Protection against acute radiation-induced lung injury: A
novel role for the anti-angiogenic agent Endostar. Mol Med Rep.
6:309–315. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He T, Yan RN, Chen HY, Zeng YY, Xiang ZZ,
Liu F, Shao BF, Ma JC, Wang XR and Liu L: Comparing the 7th and 8th
editions of UICC/AJCC staging system for nasopharyngeal carcinoma
in the IMRT era. BMC Cancer. 21(327)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pan JJ, Ng WT, Zong JF, Chan LL,
O'Sullivan B, Lin SJ, Sze HC, Chen YB, Choi HC, Guo QJ, et al:
Proposal for the 8th edition of the AJCC/UICC staging system for
nasopharyngeal cancer in the era of intensity-modulated
radiotherapy. Cancer. 122:546–558. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hua YJ, Chen MY, Qian CN, Hong MH, Zhao C,
Guo L, Guo X and Cao KJ: Postradiation nasopharyngeal necrosis in
the patients with nasopharyngeal carcinoma. Head Neck. 31:807–812.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu J, Ning X, Sun X, Lu H, Gu Y and Wang
D: Endoscopic sequestrectomy for skull base osteoradionecrosis in
nasopharyngeal carcinoma patients: A 10-year experience. Int J Clin
Oncol. 24:248–255. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Delanian S, Chatel C, Porcher R, Depondt J
and Lefaix JL: Complete restoration of refractory mandibular
osteoradionecrosis by prolonged treatment with a
pentoxifylline-tocopherol-clodronate combination (PENTOCLO): A
phase II trial. Int J Radiat Oncol Biol Phys. 80:832–839.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Robard L, Louis MY, Blanchard D, Babin E
and Delanian S: Medical treatment of osteoradionecrosis of the
mandible by PENTOCLO: Preliminary results. Eur Ann Otorhinolaryngol
Head Neck Dis. 131:333–338. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lyons A and Ghazali N: Osteoradionecrosis
of the jaws: Current understanding of its pathophysiology and
treatment. Br J Oral Maxillofac Surg. 46:653–660. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nordal RA, Nagy A, Pintilie M and Wong CS:
Hypoxia and hypoxia-inducible factor-1 target genes in central
nervous system radiation injury: A role for vascular endothelial
growth factor. Clin Cancer Res. 10:3342–3353. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Perletti G, Concari P, Giardini R, Marras
E, Piccinini F, Folkman J and Chen L: Antitumor activity of
endostatin against carcinogen-induced rat primary mammary tumors.
Cancer Res. 60:1793–1796. 2000.PubMed/NCBI
|
|
30
|
Bergers G, Javaherian K, Lo KM, Folkman J
and Hanahan D: Effects of angiogenesis inhibitors on multistage
carcinogenesis in mice. Science. 284:808–812. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Boehm T, Folkman J, Browder T and O'Reilly
MS: Antiangiogenic therapy of experimental cancer does not induce
acquired drug resistance. Nature. 390:404–407. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Kulke MH, Bergsland EK, Ryan DP, Enzinger
PC, Lynch TJ, Zhu AX, Meyerhardt JA, Heymach JV, Fogler WE, Sidor
C, et al: Phase II study of recombinant human endostatin in
patients with advanced neuroendocrine tumors. J Clin Oncol.
24:3555–3561. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Peng F, Xu Z, Wang J, Chen Y, Li Q, Zuo Y,
Chen J, Hu X, Zhou Q, Wang Y, et al: Recombinant human endostatin
normalizes tumor vasculature and enhances radiation response in
xenografted human nasopharyngeal carcinoma models. PLoS One.
7(e34646)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Y, Xuan Z, Zheng X, Han R, Zhou L,
Liu H and Liu Y: Efficacy of endostar combined with transcatheter
arterial chemoembolization and analysis of vascular endothelial
factor and C-reactive protein levels in patients with advanced
hepatocellular carcinoma under contrast enhanced ultrasound. J
BUON. 25:407–414. 2020.PubMed/NCBI
|
|
35
|
Cheng YJ, Meng CT, Ying HY, Zhou JF, Yan
XY, Gao X, Zhou N and Bai CM: Effect of Endostar combined with
chemotherapy in advanced well-differentiated pancreatic
neuroendocrine tumors. Medicine (Baltimore).
97(e12750)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou ZT, Zhou FX, Wei Q, Zou LY, Qin BF
and Peng XS: Phase II study of cisplatin/etoposide and endostar for
extensive-stage small-cell lung cancer. Cancer Chemother Pharmacol.
68:1027–1032. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang X, Shi Y, Jia Y, Zhao W, Zhang L, Bai
G, Ren Y, Chen YZ and Tong Z: Tolerance and pharmacokinetics of
recombinant human endostatin administered as single-dose or
multiple-dose infusions in patients with advanced solid tumors: A
phase I clinical trial. Technol Cancer Res Treat.
20(15330338211064434)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Eder JP Jr, Supko JG, Clark JW, Puchalski
TA, Garcia-Carbonero R, Ryan DP, Shulman LN, Proper J, Kirvan M,
Rattner B, et al: Phase I clinical trial of recombinant human
endostatin administered as a short intravenous infusion repeated
daily. J Clin Oncol. 20:3772–3784. 2002.PubMed/NCBI View Article : Google Scholar
|