Introduction
The main treatment options for breast cancer include
surgery, chemotherapy, radiotherapy, and endocrine and targeted
therapies. Different patients may select one or more treatment
methods based on their condition. Post-mastectomy radiotherapy
(PMRT) is a critical and validated treatment modality for patients
with breast cancer who have at least four positive nodes (1,2);
however, the efficacy of PMRT in patients with one to three
positive lymph nodes remains unclear (3). Several randomized clinical trials
(4-6)
and the Early Breast Cancer Trialists' Collaborative Group (EBCTCG)
(7) have outlined clear benefits
for patients with one to three positive nodes (N1) undergoing PMRT.
Furthermore, the American Society of Clinical Oncology has updated
its recommendation of PMRT to the strong level for patients with
tumors sized ≤5 cm (T1-2) and with one to three involved lymph
nodes (8). However, these research
studies (4-6)
predominantly recruited patients in the 1970s and 1980s when
systemic therapies differed from the modern adjuvant treatment, and
they also did not take into account high-risk factors, including
age, estrogen receptor (ER)/progesterone receptor (PR), human
epidermal growth factor receptor-2 (HER-2) and Ki67. A
retrospective study from the MD Anderson Cancer Center indicated
that the locoregional recurrence rate (LRR) for patients with T1-2
breast cancer with one to three positive lymph nodes (T1-2N1) was
highly dependent on the era of treatment (9). Thus, certain controversies remain
regarding the use of PMRT for patients with one to three positive
lymph nodes (10).
It is well recognized that the immediate and
long-term side effects of PMRT, including radiation-induced cardiac
disease, arm lymphedema, secondary cancer and further complications
with reconstruction, are important (5). Therefore, the present retrospective
study aimed to examine which patients may be able to avoid the use
of PMRT and thus its related side effects.
Materials and methods
Patients
A retrospective consecutive analysis was conducted
on patients with breast cancer who were treated between January
2011 and June 2020 at the Second Affiliated Hospital, Medical
School of Xi'an Jiaotong University (Xi'an, China). Patient
information was only collected after June 2020 and a total of 728
patients were included in the present study. The inclusion criteria
were as follows: i) Patients with clinical T1-2N1M0 stage; ii)
patients who had undergone radical mastectomy; iii) patients who
had undergone chemotherapy, endocrine and targeted therapy
according to the National Comprehensive Cancer Network guidelines
(11); iv) patients for which
ER/PR, HER-2 and Ki67 had been detected; and v) patients who had
completed a follow-up study period. Notably, male patients were
excluded. The clinicopathological data of the patients were
collected from the electronic medical records of the university.
Among these, 438 patients received PMRT.
Definition of molecular markers
Immunohistochemical staining was used to detect the
proportion of ER/PR-, HER-2- and Ki-67-positive tumor cells. The
results of immunohistochemical analysis were obtained from the
medical records. The percentage score was defined as the percentage
of positive tumor cells in the total number of malignant cells
evaluated. According to the experience of various pathologists, as
well as the current national and international recommendations
(12,13), the following definitions were used:
i) ER/PR were categorized as negative (<1%) and positive (≥1%)
according to the percentage of tumor cell nuclear staining; ii) a
negative HER-2 status was defined when HER-2 expression was
negative or ‘+’ as detected by immunohistochemical staining, and a
positive HER-2 status was defined when its expression was positive
‘+++’; its expression was further determined by in situ
fluorescence hybridization when status was ‘++’ (14); iii) Ki67 expression was divided
into low (<14%) or high (≥14%) labeling indexes (15).
Follow-up study and study
endpoints
Follow-up data were obtained via medical records,
and making telephone calls every 3 months for the first 2 years,
every 6 months for years 3-5 and annually after 5 years.
Therapeutic evaluation indicators included LRR, distant metastasis
(DM) and overall survival (OS). LRR was defined as recurrent breast
cancer in the ipsilateral chest wall, skin, axilla, internal
mammary or supraclavicular lymph nodes. DM included all sites of
recurrence, with the exception of locoregional recurrence, and
contralateral breast cancer. OS was determined as the time from
surgery until the date of mortality (from any cause) or was
censored at the date of last follow-up. The follow-up deadline was
December 2020.
Statistical analysis
The baseline characteristics of the patients were
examined using the χ2 test or Fisher's exact test for
categorical variables assuming equal variance. LRR, DM and OS were
assessed using Kaplan-Meier survival curves; group differences were
compared using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference. All statistical
calculations were conducted using SPSS Statistics 26.0 (IBM Corp.)
The figures in the study were generated using SPSS Statistics 26.0
and GraphPad Prism 9 (Dotmatics).
Results
Baseline characteristics
Among the 728 patients with a T1-2N1 status
following radical mastectomy, 438 patients (60.2%) received PMRT
and 290 patients (39.8%) did not. All patients were considered to
have negative surgical margins in the database following radical
mastectomy, and received irradiation of the chest wall and regional
lymph nodes.
The characteristics of the patients and tumors in
the PMRT and non-PMRT subgroups are presented in Table I. The age of the patients ranged
between 24 and 79 years, with a mean age at diagnosis of 59 years;
29 patients (4.0%) were ≤35 years at the time of diagnosis. In
total, 26.5% of the patients had a positive HER-2 status, 70.5% had
a positive ER/PR expression and 80.6% had a Ki67 expression status
of ≥14%.
 | Table IBaseline characteristics of the study
population (n=728). |
Table I
Baseline characteristics of the study
population (n=728).
| Characteristic | Non-PMRT subgroup, n
(%) | PMRT subgroup, n
(%) | χ2 | P-value |
|---|
| Age, years | | | 6.22 | 0.01 |
|
≤35 | 18 (6.21) | 11 (2.51) | | |
|
>35 | 272 (93.79) | 427 (97.49) | | |
| ER/PR | | | 0.19 | 0.66 |
|
Negative | 83 (28.62) | 132 (30.14) | | |
|
Positive | 207 (71.38) | 306 (69.86) | | |
| HER-2 | | | 1.94 | 0.16 |
|
Negative | 205 (70.69) | 330 (75.34) | | |
|
Positive | 85 (29.31) | 108 (24.66) | | |
| Ki67 | | | 0.17 | 0.68 |
|
<14% | 54 (18.62) | 87 (19.86) | | |
|
≥14% | 236 (81.38) | 351 (80.14) | | |
| T stage | | | 11.16 | <0.01 |
|
T1 | 87(30) | 185 (42.24) | | |
|
T2 | 203(70) | 253 (57.76) | | |
| Positive lymph nodes,
n | | | 4.60 | 0.10 |
|
1 | 155 (53.45) | 204 (46.58) | | |
|
2 | 94 (32.41) | 149 (34.02) | | |
|
3 | 41 (14.14) | 85 (19.40) | | |
No benefit of PMRT in patients aged
≤35 years or those with a positive HER-2 status
The median follow-up time was 45 months (range,
6-108 months). At the cut-off date for this analysis, 66 patients
(9.1%) had experienced local recurrence (3.0% in the PMRT group and
18.3% in the non-PMRT group); 103 patients (14.1%) had experienced
DM (11.9 and 17.6%, respectively), and 97 patients (13.3%) had
succumbed (10.5 and 17.6%, respectively).
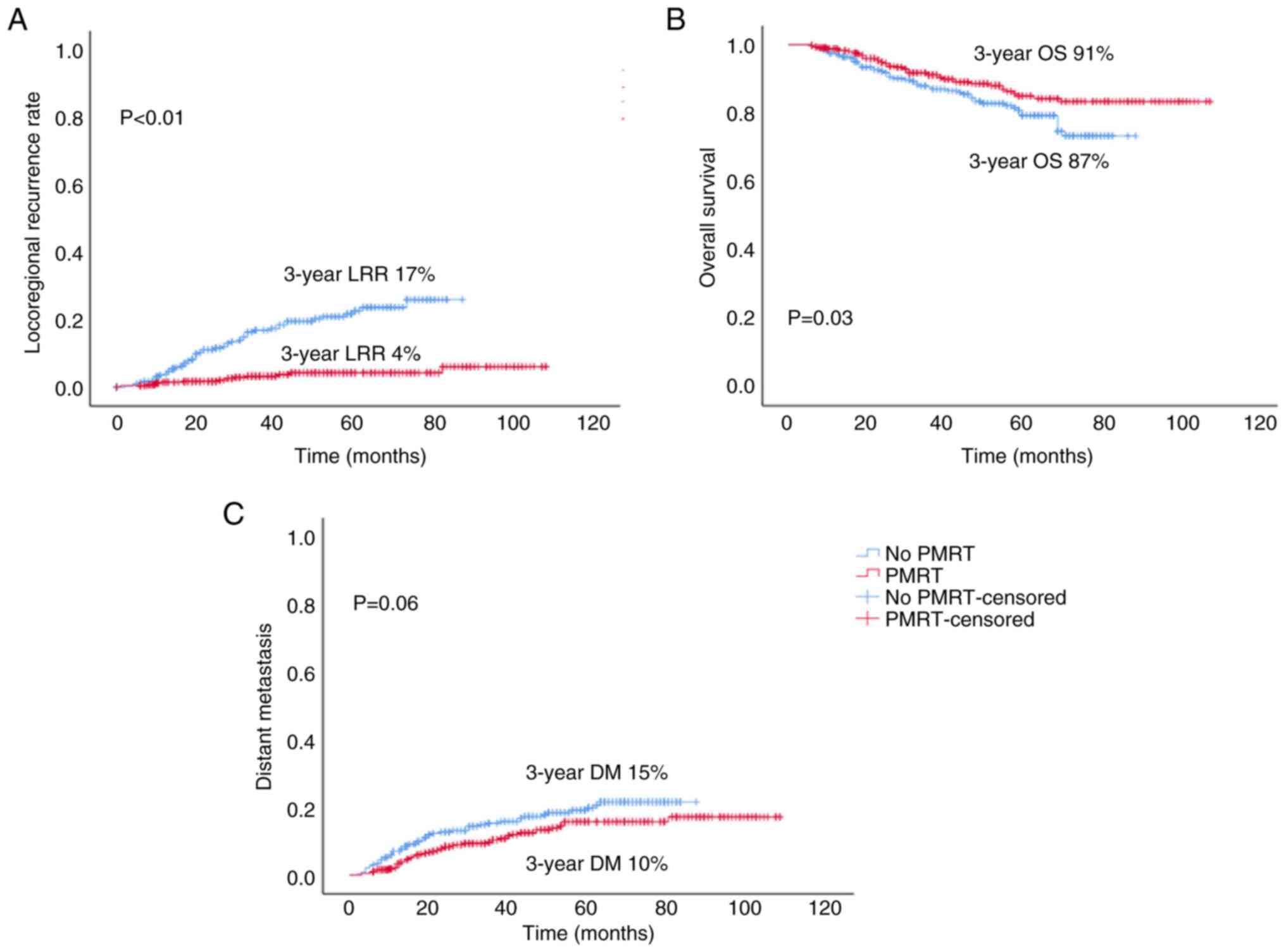
PMRT significantly decreased the LRR [hazard ratio
(HR)=5.602, 95% confidence interval (CI)=3.139-9.998, P<0.01;
3-year LRR: 4 vs. 17%] and improved OS (HR=0.651, 95%
CI=0.437-0.971, P=0.03; 3-year OS: 91 vs. 87%); however, it had no
significant effect on the DM rate (HR=0.691, 95% CI=0.468-1.019,
P=0.06; 3-year DM: 10 vs. 15%), compared with the non-PMRT group
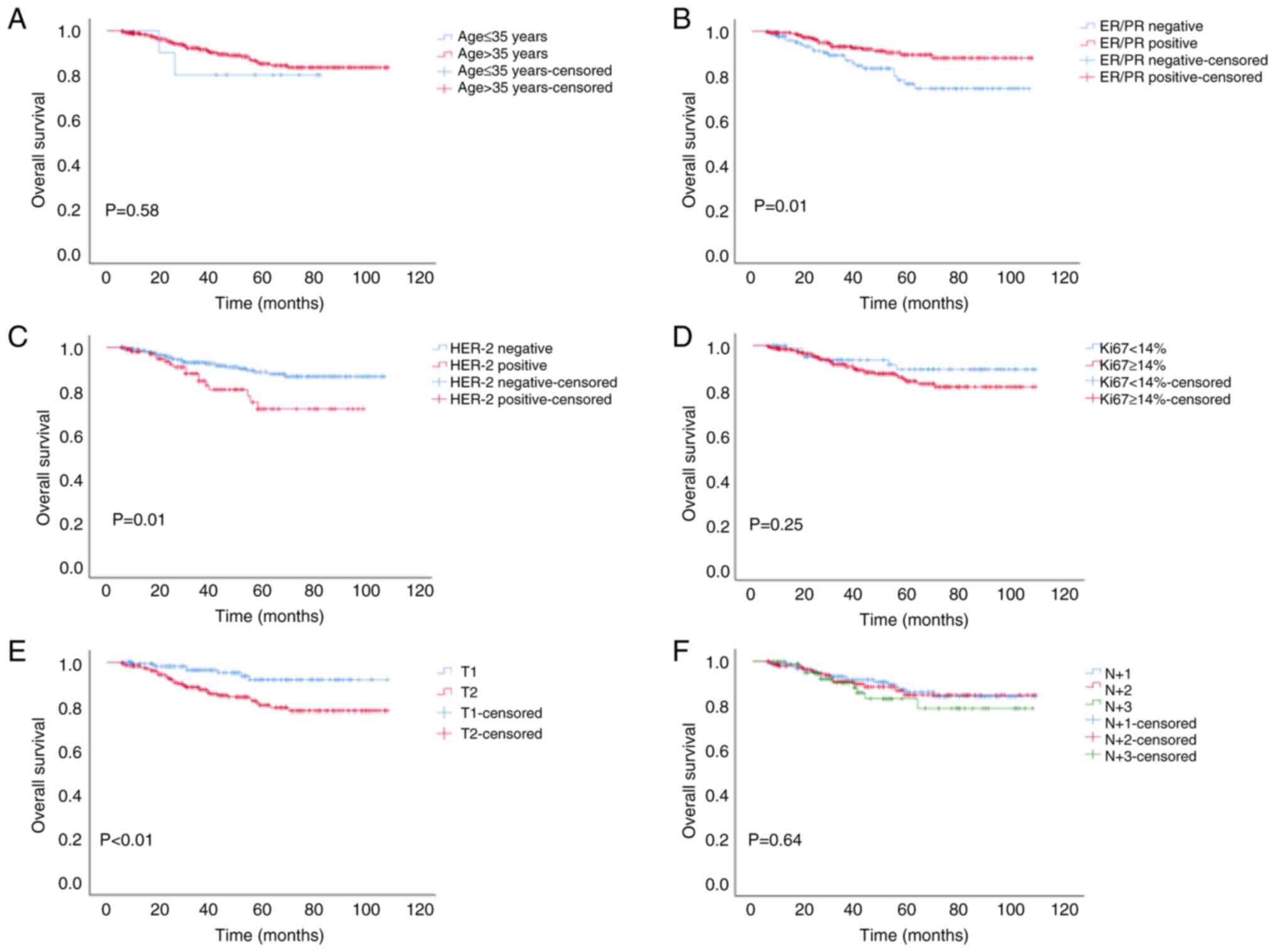
(Fig. 1). Further stratified
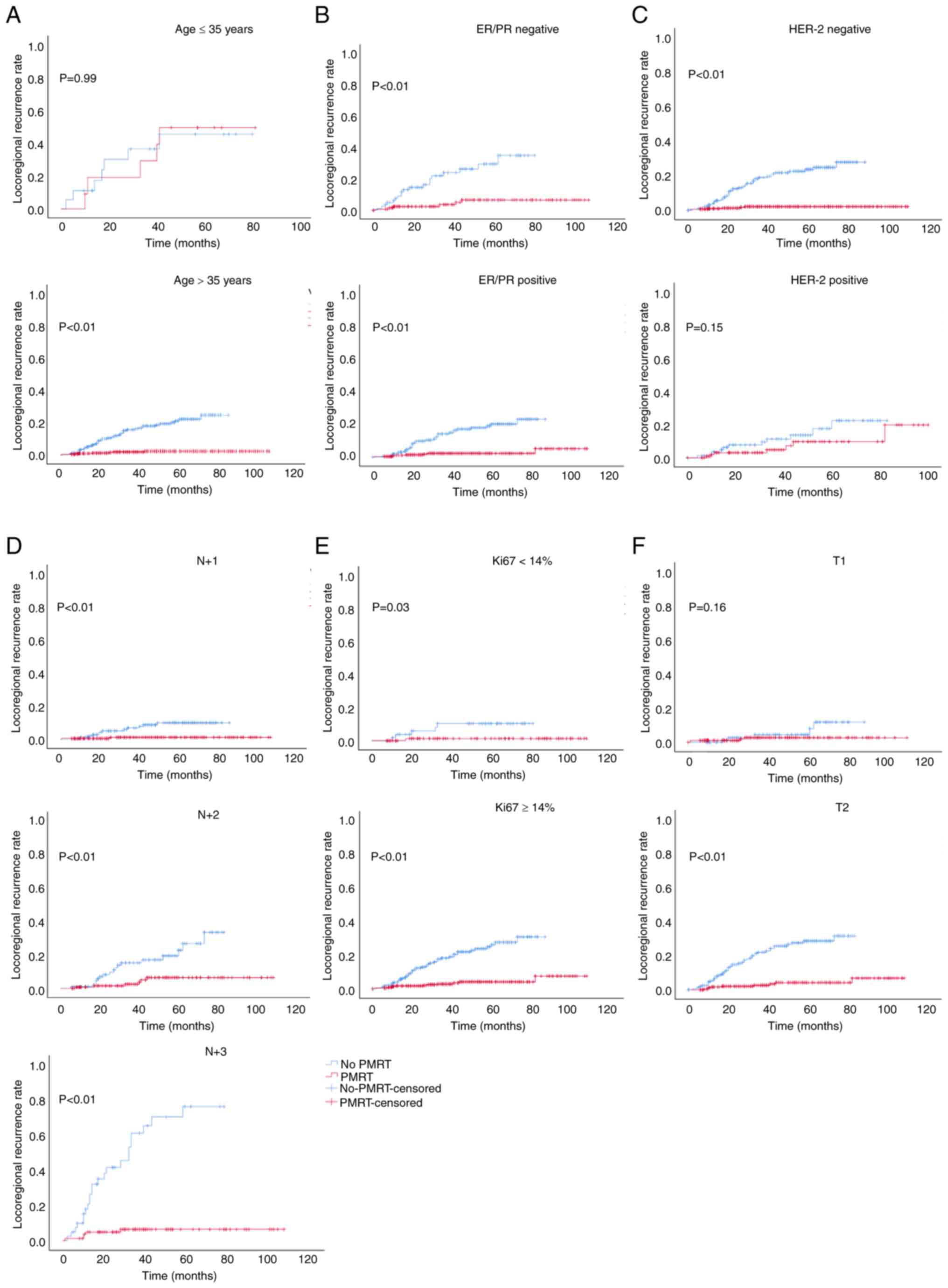
analysis revealed that PMRT did not reduce the LRR of patients aged
≤35 years, or in those with a positive HER-2 status or T1 stage
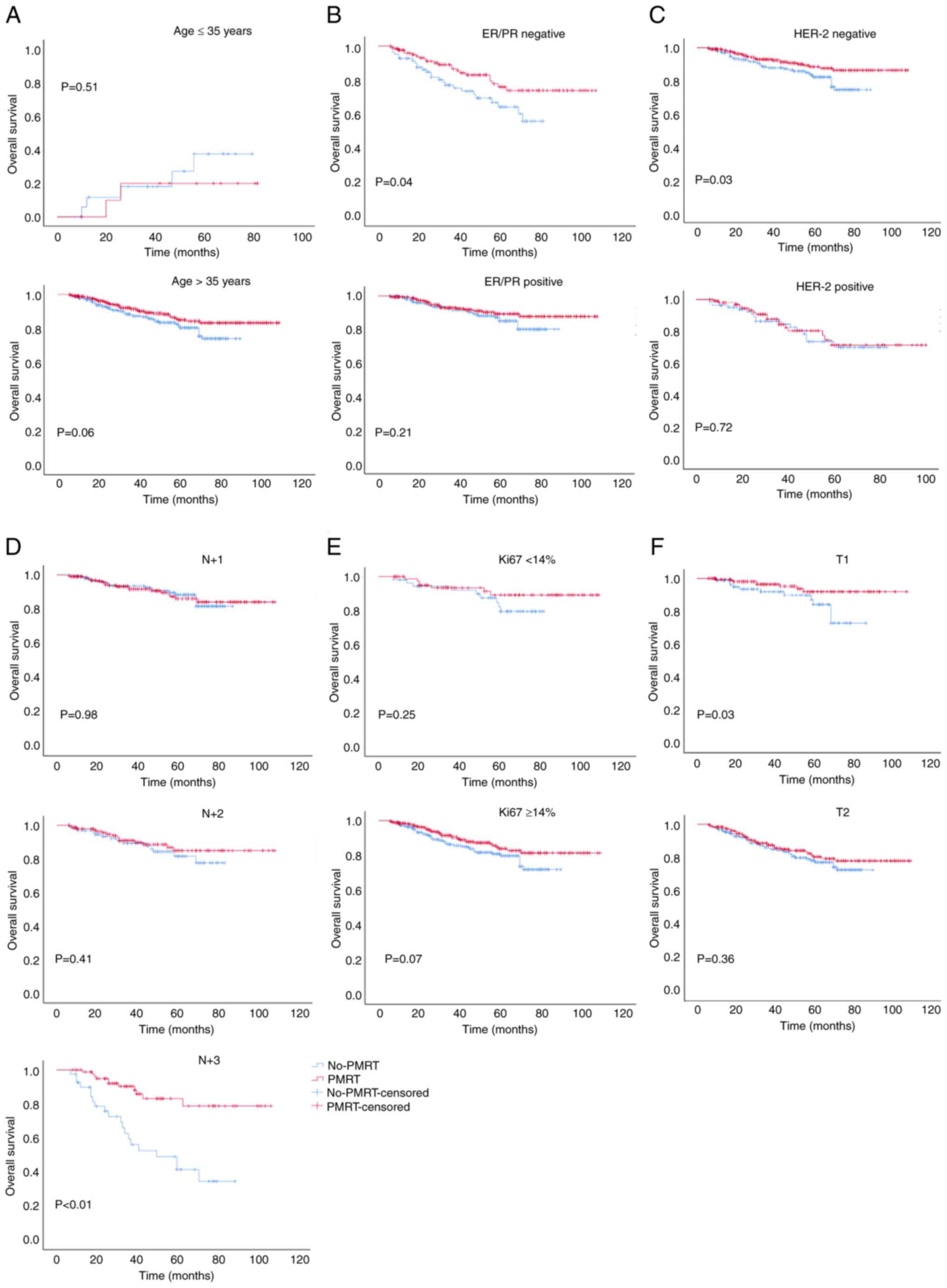
(Fig. 2); it also did not improve
the OS of patients aged ≤35 years, or in those who were had a
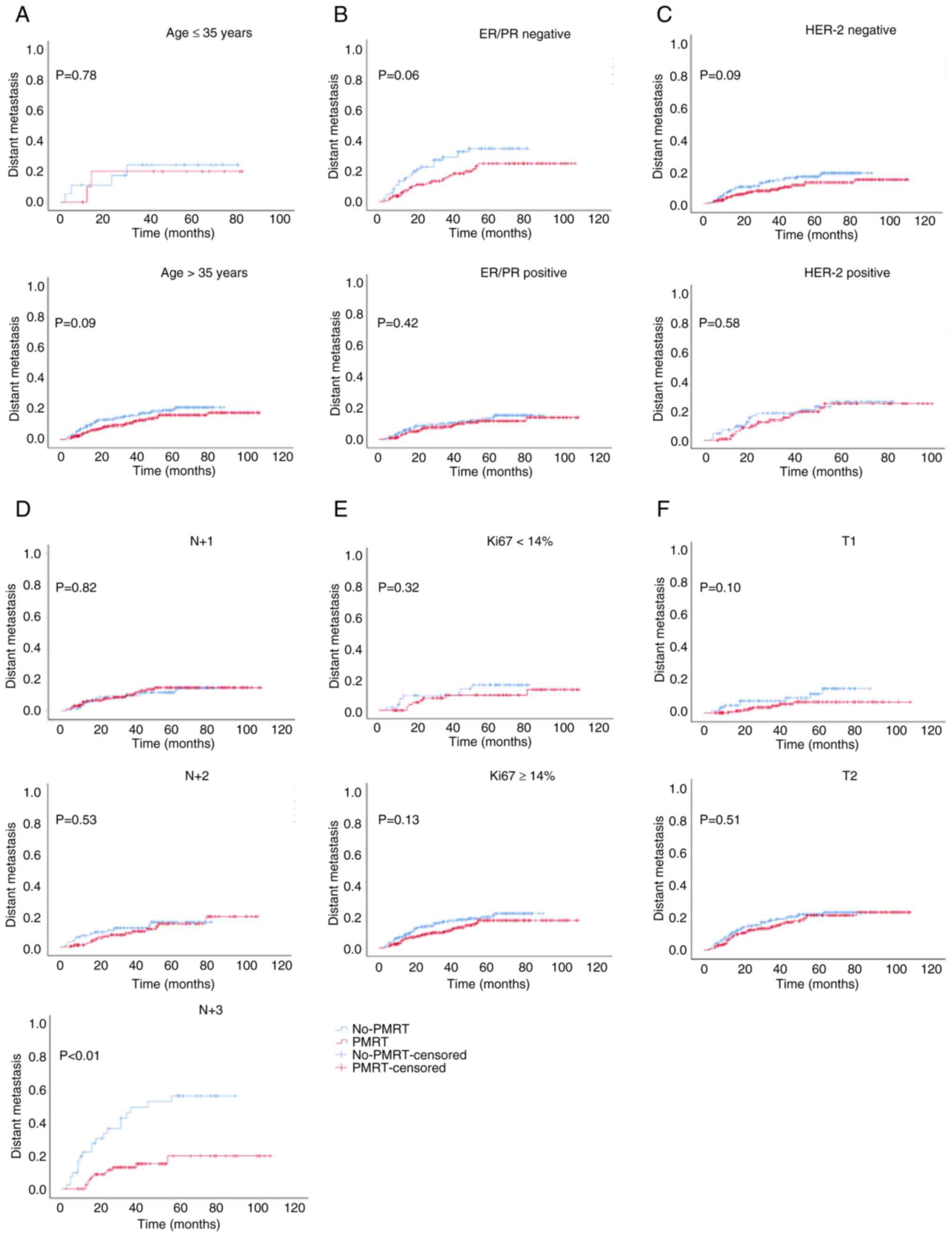
positive ER/PR or HER-2 status, or T2, N+1 or N+2 stage (Fig. 3). Moreover, PMRT did not reduce the
DM of patients, apart from those who had N+3 stage cancer (Fig. 4). These results suggested that
there was no marked difference in the LRR, DM and OS of patients
aged ≤35 years or in those with a positive HER-2 status between the
PMRT and non-PMRT groups.
Patients aged ≤35 years or with a
positive HER-2 status are more likely to experience local
recurrence even following PMRT
Following the analysis of 438 patients with PMRT, it
was found that patients aged ≤35 years were more likely to
experience local recurrence compared with patients aged >35
years (P<0.01). Moreover, similar results were obtained for
patients with a positive HER-2 status (P=0.03; Table II). Even following PMRT, the
prognoses of patients with ER/PR- (vs. ER/PR+, HR=0.483,
95% CI=0.278-0.839, P=0.01), HER-2+ (vs.
HER-2-, HR=1.804, 95% CI=1.006-3.232, P=0.01) and T2
(vs. T1, HR=3.828, 95% CI=1.799-8.144, P<0.01) were poor
(Fig. 5). These results indicated
that patients aged ≤35 years or those with a positive HER-2 status
are more likely to experience local recurrence even following
PMRT.
 | Table IIFactors affecting the LRR of patients
following post-mastectomy radiotherapy (n=438). |
Table II
Factors affecting the LRR of patients
following post-mastectomy radiotherapy (n=438).
| | LRR | |
|---|
| Characteristic | n (%) | + (n=13) | - (n=425) | χ2 | P-value |
|---|
| Age, years | | | | | <0.01 |
|
≤35 | 11 (2.51) | 5 | 6 | 56.40 | |
|
>35 | 427 (97.49) | 8 | 419 | | |
| ER/PR | | | | | 0.33 |
|
Negative | 132 (30.14) | 6 | 126 | 0.94 | |
|
Positive | 306 (69.86) | 7 | 299 | | |
| HER-2 | | | | | 0.03 |
|
Negative | 330 (75.34) | 6 | 324 | 4.63 | |
|
Positive | 108 (24.66) | 7 | 101 | | |
| Ki67 | | | | | 0.45 |
|
≤14 | 87 (19.86) | 1 | 86 | 0.58 | |
|
>14 | 351 (80.14) | 12 | 339 | | |
| T stage | | | | | 0.40 |
|
T1 | 185 (42.24) | 4 | 181 | 0.72 | |
|
T2 | 253 (57.76) | 9 | 244 | | |
| Positive lymph
nodes, n | | | | | 0.04 |
|
1 | 204 (46.57) | 2 | 202 | 6.25 | |
|
2 | 149 (34.02) | 6 | 143 | | |
|
3 | 85 (19.41) | 5 | 80 | | |
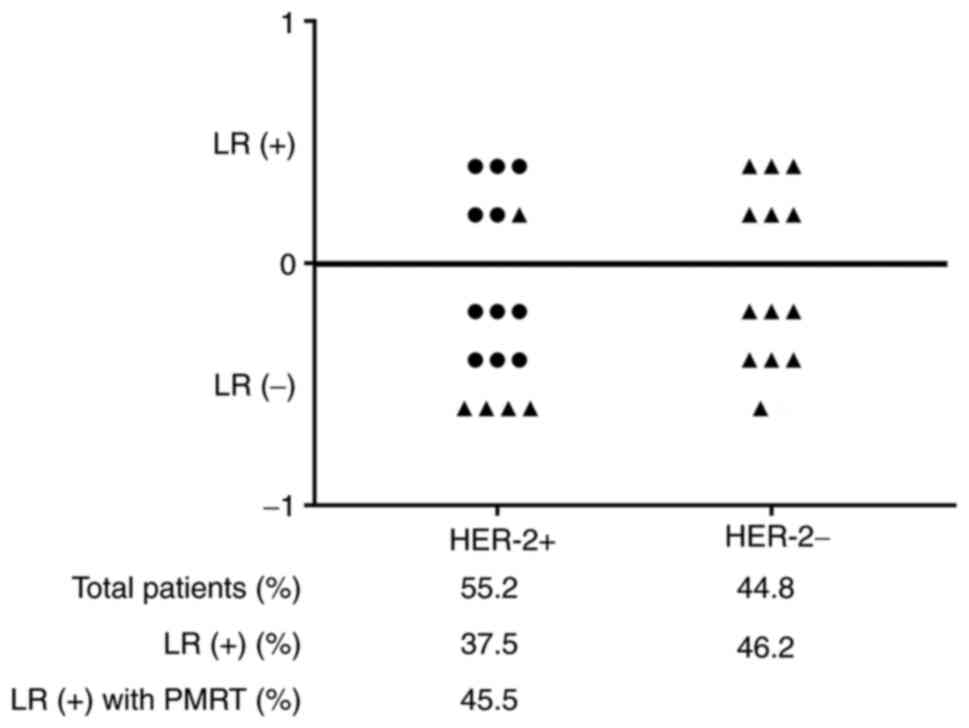
A total of 29 patients aged ≤35 years were included
in the present study, and 41.4% (12/29) of the patients experienced
local recurrence (Fig. 6). Of
these, patients with a positive HER-2 status accounted for 55.2%
(16/29), and all 11 patients who received PMRT had a positive HER-2
status. However, 45.5% of the patients with a positive HER-2 status
who received PMRT experienced local recurrence. These results
suggested that patients aged ≤35 years and those with a positive
HER-2 status have a higher rate of local recurrence.
Discussion
Breast cancer is ranked second among the most common
causes of cancer-related mortality in women worldwide (16). According to the results of the
EBCTCG (7), PMRT is highly
recommended for patients with one to three positive nodes. The aim
of the present study was to determine whether the use of PMRT may
be omitted in patients with one to three positive lymph nodes. The
present study included 728 post-operative patients with T1-2N1
breast cancer at the Second Affiliated Hospital, Medical School of
Xi'an Jiaotong University. All factors were equally distributed in
the PMRT and non-PMRT groups, apart from age and T stage.
The EBCTCG previously updated its PMRT meta-analysis
and provided evidence recommending the use of PMRT for decreasing
the 5-year LRR (PMRT, 2.8%; non-PMRT, 16.5%) among breast cancer
patients with one to three involved nodes (7). The present study suggested that
patients with T1-2N1 breast cancer benefited from PMRT, with a
reduced 3-year LRR (PMRT, 4.0%; non-PMRT, 17%; P<0.01) and an
improved 3-year OS (PMRT, 91%; non-PMRT, 87%; P=0.03); however,
PMRT had no significant effect on DM. The LRR in the PMRT group in
the present study was similar to that of a previous study (7), whereas it was higher in the non-PMRT
group. This may be related to the fact that only 290 patients were
included in the non-PMRT group. The data presented herein also
suggested that patients with T1-2N1 breast cancer may benefit from
PMRT.
The results of the present study demonstrated that
PMRT did not reduce the LRR and DM, or improve OS in patients aged
≤35 years or in those with a positive HER-2 status. Thus, it is
still necessary to explore and consider whether PMRT can be
omitted, and whether systemic treatment methods may be used, for
this group of patients. It was further revealed that patients aged
≤35 years or with a positive HER-2 status were more prone to local
recurrence than patients aged >35 years or in those with a
negative HER-2 status, even following PMRT. These results further
suggested that PMRT had no significant effect on local control in
this group of patients. Further studies are required to reduce the
LRR in patients aged ≤35 years or in those with a positive HER-2
status by changing the scope of surgery, altering the conventional
radiotherapy modality, scope or dosing.
Approximately one in 40 women diagnosed with
early-stage breast cancer are very young (<35 years) and this
age group has a worse prognosis (17,18);
these patients deserve special attention as there are differences
in the prognosis, histopathology, systemic and loco-regional
treatment options, and outcomes in this specific age group. In a
retrospective Danish cohort study, Kroman et al (19) concluded that women <35 years of
age diagnosed with breast cancer should be regarded as high-risk
according to age alone. In a large Korean study, the 5-year OS rate
of women diagnosed at <35 years of age was 81.5% compared with
89.4% for women aged 35-50 years (P<0.0001) (20). Furthermore, breast cancer in very
young women more frequently exhibits HER-2 upregulation compared
with tumors in older women (18).
The upregulation of HER-2 in breast cancer has been shown to be
associated with a more aggressive tumor subtype, a poorer prognosis
and a shorter OS rate (21). The
very young patients (<35 years) with a positive HER-2 status in
the present study accounted for 55.2% of the study population, and
exhibited a high rate of local recurrence even following PMRT.
However, the number of patients included in the present study was
small and further studies are thus required to confirm the
findings.
Hagio et al (22) recruited 13 women aged <35 years
at diagnosis with early-stage breast cancer, and performed genomic
DNA testing. This previous study detected germline gene alterations
in all patients, with the exception of one (22). This finding suggests the need for
genetic testing in younger patients with breast cancer in order to
develop more personalized treatments. In addition, the fear of
cancer recurrence is more intense in younger women and they may
require targeted mental health intervention (23). Attention to appropriate
psychosocial support is critical due to the potential for distress
and reduced compliance with therapy in very young patients
diagnosed with early-stage breast cancer (24). A more comprehensive evaluation and
a more individualized treatment plan is required for young patients
with breast cancer.
In conclusion, the findings of the present
retrospective study suggested that further studies are required to
confirm the need for the stratification of patients with T1-2N1
breast cancer in order to determine whether they should undergo
PMRT. It is hoped that further studies will be conducted to perform
more in-depth analyses and allow patients to avoid non-essential
treatments, and thus reduce the side effects of treatments and
improve the quality of life of patients.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
Natural Science Foundation of Shaanxi Province (Youth Project)
(grant no. 2021JQ-423) and was also supported by the foundation of
the Second Affiliated Hospital of Xi'an Jiaotong University [grant
no. C(XM)201706].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW and ZW confirm the authenticity of all the raw
data, made substantial contributions to conception and design, and
wrote the main manuscript text. YW was responsible for the
statistical analysis. FX was responsible for table and figure
generation, and analyzed data. HR and JC were responsible for
collecting patient information, conducting follow-up visits and
acquisition of data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study followed The Declaration of
Helsinki and was approved by the Second Affiliated Hospital of
Xi'an Jiaotong University Medical Ethics Committee (approval no.
2022258). All subjects included in this study orally agreed to
participate. All methods were carried out in accordance with
relevant guidelines and regulations.
Patient consent for publication
The publication of the research received oral
consent from all subjects. All forms of personally identifiable
data, including biomedical, clinical and biometric data, are not
included in the manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Borm KJ, Oechsner M, Combs SE and Duma MN:
Deep-Inspiration breath-hold radiation therapy in breast cancer: A
word of caution on the dose to the axillary lymph node levels. Int
J Radiat Oncol Biol Phys. 100:263–269. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241.
2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Truong PT, Olivotto IA, Whelan TJ and
Levine M: Steering Committee on Clinical Practice Guidelines for
the Care and Treatment of Breast Cancer. Clinical practice
guidelines for the care and treatment of breast cancer: 16.
Locoregional post-mastectomy radiotherapy. CMAJ. 170:1263–1273.
2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Overgaard M, Hansen PS, Overgaard J, Rose
C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen
MB and Zedeler K: Postoperative radiotherapy in high-risk
premenopausal women with breast cancer who receive adjuvant
chemotherapy Danish breast cancer cooperative Group 82b trial. N
Engl J Med. 337:949–955. 1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Atkins CD: Postoperative radiotherapy in
high-risk postmenopausal breast cancer. Lancet. 354(865): author
reply 866. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ragaz J, Olivotto IA, Spinelli JJ,
Phillips N, Jackson SM, Wilson KS, Knowling MA, Coppin CM, Weir L,
Gelmon K, et al: Locoregional radiation therapy in patients with
high-risk breast cancer receiving adjuvant chemotherapy: 20-year
results of the British Columbia randomized trial. J Natl Cancer
Inst. 97:116–126. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
EBCTCG (Early Breast Cancer Trialists'
Collaborative Group). McGale P, Taylor C, Correa C, Cutter D, Duane
F, Ewertz M, Gray R, Mannu G, Peto R, et al: Effect of radiotherapy
after mastectomy and axillary surgery on 10-year recurrence and
20-year breast cancer mortality: Meta-analysis of individual
patient data for 8135 women in 22 randomised trials. Lancet.
383:2127–2135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Recht A, Comen EA, Fine RE, Fleming GF,
Hardenbergh PH, Ho AY, Hudis CA, Hwang ES, Kirshner JJ, Morrow M,
et al: Postmastectomy radiotherapy: An American society of clinical
oncology, American society for radiation oncology, and society of
surgical oncology focused guideline update. Pract Radiat Oncol.
6:e219–e234. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McBride A, Allen P, Woodward W, Kim M,
Kuerer HM, Drinka EK, Sahin A, Strom EA, Buzdar A, Valero V, et al:
Locoregional recurrence risk for patients with T1,2 breast cancer
with 1-3 positive lymph nodes treated with mastectomy and systemic
treatment. Int J Radiat Oncol Biol Phys. 89:392–398.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang N, Zhang J, Zhang H, Liu Y, Zhao W,
Wang L, Chen B, Moran MS, Haffty BG and Yang Q: Individualized
prediction of survival benefit from postmastectomy radiotherapy for
patients with breast cancer with one to three positive axillary
lymph nodes. Oncologist. 24:e1286–e1293. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gradishar WJ, Anderson BO, Abraham J, Aft
R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD,
et al: Breast cancer, version 3.2020, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 18:452–478.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members. Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St. Gallen International expert consensus on the primary
therapy of early breast cancer. Ann Oncol. 22:1736–1747.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stuart-Harris R, Caldas C, Pinder SE and
Pharoah P: Proliferation markers and survival in early breast
cancer: A systematic review and meta-analysis of 85 studies in
32,825 patients. Breast. 17:323–334. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Inwald EC, Klinkhammer-Schalke M,
Hofstädter F, Zeman F, Koller M, Gerstenhauer M and Ortmann O:
Ki-67 is a prognostic parameter in breast cancer patients: Results
of a large population-based cohort of a cancer registry. Breast
Cancer Res Treat. 139:539–552. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lin L, Hu P, Shi J, Appleton CM, Maslov K,
Li L, Zhang R and Wang LV: Single-breath-hold photoacoustic
computed tomography of the breast. Nat Commun.
9(2352)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen LJ and Chang YJ and Chang YJ:
Treatment and long-term outcome of breast cancer in very young
women: Nationwide population-based study. BJS Open.
5(zrab087)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Francis PA: Optimal adjuvant therapy for
very young breast cancer patients. Breast. 20:297–302.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kroman N, Jensen MB, Wohlfahrt J,
Mouridsen HT, Andersen PK and Melbye M: Factors influencing the
effect of age on prognosis in breast cancer: Population based
study. BMJ. 320:474–478. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ahn SH, Son BH, Kim SW, Kim SI, Jeong J,
Ko SS and Han W: Korean Breast Cancer Society. Poor outcome of
hormone receptor-positive breast cancer at very young age is due to
tamoxifen resistance: Nationwide survival data in Korea-a report
from the Korean Breast Cancer Society. J Clin Oncol. 25:2360–2368.
2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hrynchak I, Santos L, Falcão A, Gomes CM
and Abrunhosa AJ: Nanobody-Based theranostic agents for
HER2-Positive breast cancer: Radiolabeling strategies. Int J Mol
Sci. 22(10745)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hagio K, Hatanaka KC, Amano T, Matsuno Y,
Hatanaka Y and Yamashita H: Genetic heterogeneity during breast
cancer progression in young patients. Breast. 60:206–213.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schapira L, Zheng Y, Gelber SI, Poorvu P,
Ruddy KJ, Tamimi RM, Peppercorn J, Come SE, Borges VF, Partridge AH
and Rosenberg SM: Trajectories of fear of cancer recurrence in
young breast cancer survivors. Cancer. 128:335–343. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bajpai J, Ventrapati P, Joshi S,
Wadasadawala T, Rath S, Pathak R, Nandhana R, Mohanty S, Chougle Q,
Engineer M, et al: Unique challenges and outcomes of young women
with breast cancers from a tertiary care cancer centre in India.
Breast. 60:177–184. 2021.PubMed/NCBI View Article : Google Scholar
|