Introduction
Lung squamous cell carcinoma (LSCC) is a subtype of
non-small cell lung carcinoma (NSCLC) and accounts for 20-30% of
all lung cancers (1). LSCC is
known to be associated with a poor prognosis; the 5-year overall
survival rate of LSCC patients with clinical stages I and II is
approximately 40%, while that of patients with clinical stages III
and IV is less than 5% (2). Recent
developments in genetic and molecular techniques has allowed the
identification of driver mutations in epidermal growth factor
receptors (EGFR), anaplastic lymphoma kinase (ALK), and c-ROS
oncogene 1 (ROS1) with regards to NSCLC (3-6).
Subsequent analyses revealed that these mutations were frequently
observed in adenocarcinoma, another subtype of NSCLC, but were
rarely detected in LSCC. For example, activating mutations in the
EGFR gene were detected in 30% of adenocarcinomas, while less than
3 % of SCC patients were identified as having these mutations
(7). Therefore, based on these
findings, molecular-targeted drugs have been proven to be effective
for lung adenocarcinoma. However, advances in the treatment of LSCC
are lacking as compared to those of lung adenocarcinoma, and there
is currently no definite clinical implication for LSCC (8). Furthermore, few regimens, including
the VEGF-A-targeting monoclonal antibody bevacizumab, cannot be
selected for LSCC. Based on the responses to these treatments and
the results of clinical trials for immune checkpoint inhibitors,
NSCLC is currently divided into SCC and non-SCC (9-11).
Therefore, identification of LSCC-specific clinicopathological
features is necessary to improve the cure and survival rates of
LSCC patients.
Human tissue kallikreins (KLKs) is a group of 15
members of the serine-protease family. KLKs are present in a
variety of healthy human tissues, including airway tissues, and
play crucial roles in the pathophysiology of chronic, infectious,
and tumor lung diseases (12). One
of the 15 kallikrein subfamily members, KLK5, has been shown to
contribute to the remodeling of the airway epithelium in patients
with chronic obstructive pulmonary disease (COPD) (13), while KLK13 has been known to
enhance the malignancy of lung adenocarcinoma (14). In contrast, a recent study showed
that the downregulation of KLK13 is correlated with a poor
prognosis in several carcinomas, such as bladder cancer (15), oral squamous cell carcinoma
(16), breast cancer (17), and colorectal cancer (18). KLK13 has diverse physiological
functions, including many cancer-related processes, and whether
KLK13 expression promotes or suppresses tumor progression may
depend on the context (19).
However, the expression and clinicopathological features of KLK13
in LSCC remain unknown.
In this study, we immunohistochemically examined
LSCC specimens for KLK13 expression, and found that the KLK13
expression was limited to keratinizing cells in LSCC. We further
assessed 94 cases with detailed clinical information and
retrospectively analyzed the relationship between KLK13 expression
and the clinicopathological parameters of LSCC. Our results
demonstrated that the KLK13 expression in LSCC correlated with the
absence of lymphatic vessel invasion.
Materials and methods
Patients
We examined 94 patients diagnosed with LSCC who
underwent lobectomy, segmentectomy, or wedge resection between
January 2011 and September 2018 at the National Center for Global
Health and Medicine (NCGM). This study was approved by the NCGM
Research Ethics Committee (2417), and the requirement for consent
was waived and a poster was displayed before the start of the
study. Formalin-fixed paraffin-embedded sections of surgical
specimens were used for immunohistochemical analyses.
Immunohistochemical analysis
Formalin-fixed paraffin-embedded sections of
surgically resected LSCC specimens were deparaffinized and
rehydrated. Target Retrieval Solution (Dako, Glostrup, Denmark) was
used to retrieve the antigens. Sections were stained with
anti-KLK13 antibody (Sigma-Aldrich, Inc., St. Louis, MO, USA) and
an ImmPACTTM DAB peroxidase Substrate Kit (Vector Laboratories,
Burlingame, CA, USA), and counterstaining was performed using
hematoxylin. Anti-human papillomavirus (HPV) type 16 L1 antibody
(GeneTex Inc. CA, USA) was used to examine the HPV status of LSCC
patients. For double staining with KLK13, the MACH 2 Double Stain 1
Kit (Biocare Medical, Concord, CA) and Warp Red Chromogen Kit
(Biocare Medical) were used to detect the anti-p40 (Biocare
Medical) or anti-E-cadherin antibody (Santa Cruz Biotechnology,
Inc., Dallas, TX) signals. Regarding the expression of E-cadherin,
we classified the LSCC cases into E-cadherin-positive and -negative
groups based on the localization of E-cadherin at the membranes of
tumor cells in dominant area. Two observers reviewed all slides,
without any access to clinical or pathological data, exhibiting
high inter-observer reliability with a clear difference between
KLK13-positive/negative samples.
Statistical analysis
Each tumor was classified based on its location,
size, pathology, condition of the lymph nodes, and degree of
metastasis (pTNM, 8th edition, 2017) (20). The KLK13 staining results were
compared using Fisher’s exact tests for sex, pT status, pN status,
pM status, cancer stage, vessel invasion, lymphatic vessel
invasion, pathological differentiation, E-cadherin expression, and
HPV expression. In using the Fisher’s exact tests, we re-classified
datasets of pT, pN, and stage into 2 groups. Unpaired t-test was
used for comparison of age between the groups. Statistical analyses
were performed using Prism 8 (GraphPad Software Inc., La Jolla, CA,
USA). All tests were two-tailed, and P values <0.05 were
considered statistically significant.
Results
KLK13 was limited in the keratinized
tumor cells of LSCC
Although prior studies have previously reported the
mRNA expression of KLK13 in normal lung tissues (21), its protein expression has never
been reported before. Therefore, we first performed
immunohistochemical analyses to investigate the protein expression
and localization of KLK13 in normal lung tissue and LSCC tissue.
The demographic features of the 94 patients are summarized in
Table I. This study included 79
male and 15 female patients, all of whom had a smoking history. In
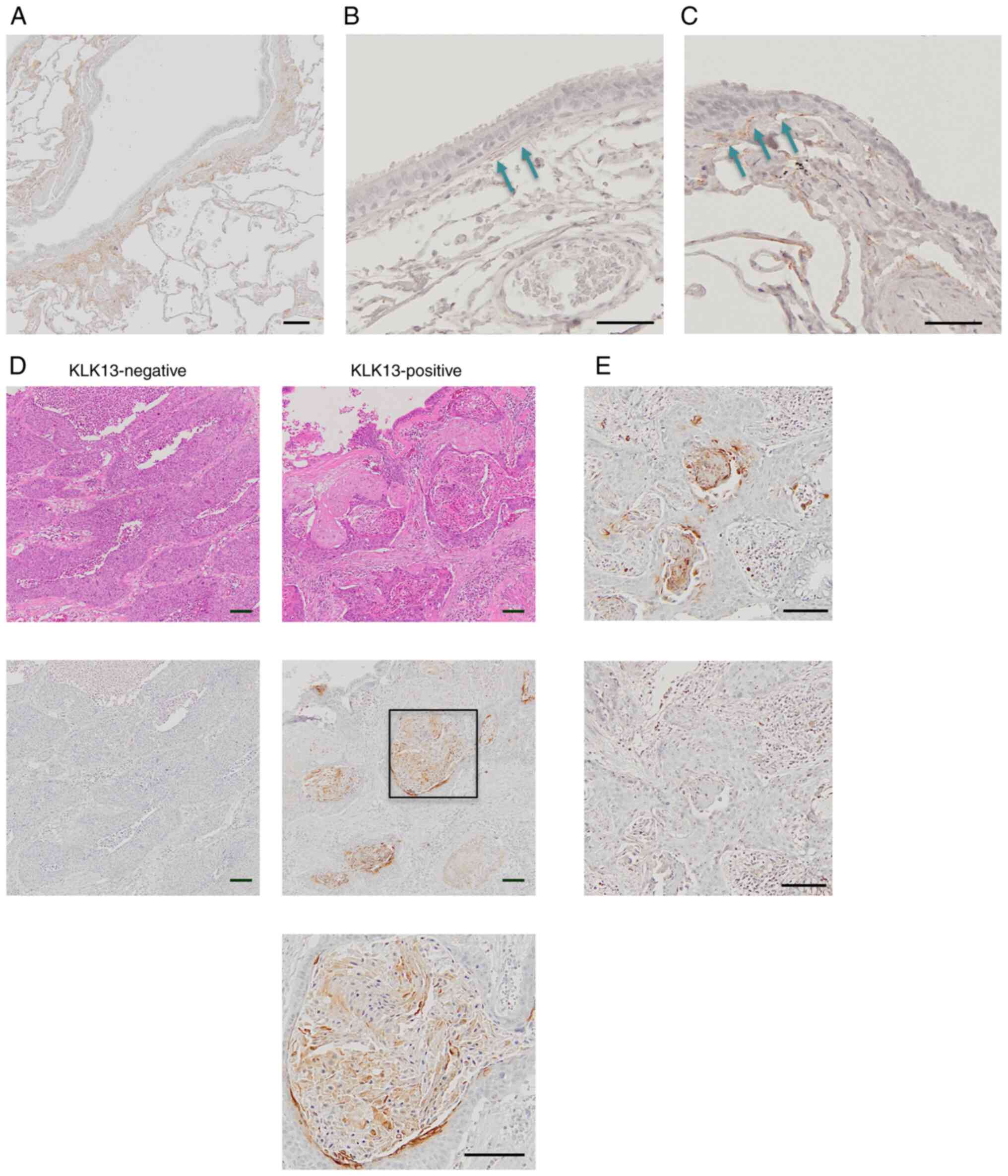
normal lungs, the protein expression of KLK13 was detected in the
connecting tissue between epithelium and lamina propria in the
proximal airway (Fig. 1A and
B), as well as in the bronchiole
(Fig. 1C). In LSCC, 70 of the 94
samples did not express KLK13 proteins, whereas the remaining 24
tumor samples showed an obvious KLK13 expression. In KLK13-positive
LSCC, the protein expression of KLK13 was focal and restricted to
the cytoplasm and cellular membrane of keratinized cells, but was
not detected in the nuclei of keratinized cells or fibrous and
stromal cells in LSCC (Fig. 1D).
The specificity of the staining signals observed in tumors was
confirmed by their disappearance in the presence of blocking
peptides (Fig. 1E).
 | Table IPatient characteristics (n=94). |
Table I
Patient characteristics (n=94).
| Characteristics | Value |
|---|
| Mean age ± SD,
years | 71.64±7.43 |
| Sex, n (%) | |
|
Male | 79(84) |
|
Female | 15(16) |
| Smoking history, n
(%) | |
|
Positivea | 94(100) |
|
Negative | 0 (0) |
| pT classification, n
(%) | |
|
T1 | 43(46) |
|
T2 | 30(32) |
|
T3 | 14(15) |
|
T4 | 7(7) |
| pN classification, n
(%) | |
|
N0 | 80(85) |
|
N1 | 8(9) |
|
N2 | 6(6) |
| pM classification, n
(%) | |
|
M0 | 91(97) |
|
M1 | 3(3) |
| Cancer
stageb, n (%) | |
|
I | 55(58) |
|
II | 23(25) |
|
III | 13(14) |
|
IV | 3(3) |
| Differentiation, n
(%) | |
|
Keratinizing | 72(77) |
|
Non-keratinizing | 22(23) |
| Lymphatic vessel
invasion, n (%) (n=93) | |
|
Positive | 11(12) |
|
Negative | 82(88) |
| Vessel invasion, n
(%) (n=93) | |
|
Positive | 47(51) |
|
Negative | 46(49) |
Associations between KLK13
immunoreactivity and LSCC clinicopathological features
To analyze the relationship between KLK13 expression
and the clinicopathological features of LSCC tumors, we classified
LSCC cases into the KLK13-negative and KLK13-positive groups
(Table II). There were no
significant differences between the groups with respect to sex, pT,
pN, pM, cancer stage, or vessel invasion. In contrast, KLK13
expression in tumors trended to associate with keratinization
(P=0.052) and no lymphatic vessel invasion (P=0.0603). Because
KLK13 inhibits the cell invasion and migration due to the
upregulation of E-cadherin in oral squamous cell carcinoma
(16), we examined the relation
between the expression of KLK13 and of E-cadherin.
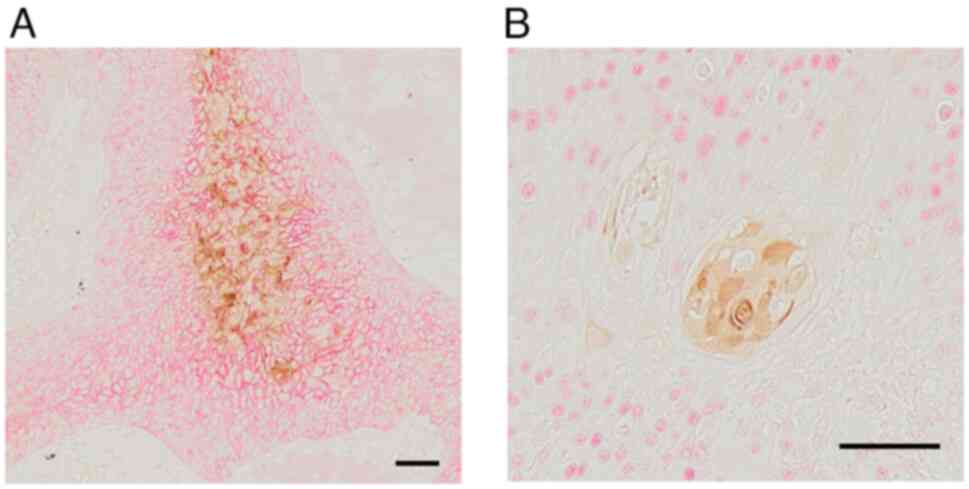
Immunohistochemically, the membrane expression of E-cadherin was
observed in KLK13-positive LSCC, whereas its expression levels was
decreased in tumor cells without KLK13 expression (Fig. 2A). KLK13 expression was
significantly associated with E-cadherin expression (P=0.0072,
Table II). Furthermore, we
examined the relationship between KLK13 expression and HPV status
because well differentiated-LSCC had high prevalence of HPV
(22). Although there was no
significant difference between the KLK13-positive groups and
KLK13-negative groups (34.21% vs. 19.64%, P=0.1488, Table II), cases of KLK13-positive tumors
tended to have an HPV-positive status. We further conducted double
staining for KLK13 with p40 (ΔNp63), which is widely used as an
LSCC diagnostic marker and is seen in cells with stemness (23). Tumor cells expressing KLK13 were
negative for p40 nuclear staining, but were surrounded by
p40-expressing cells (Fig.
2B).
 | Table IIClinicopathological features of
patients with KLK13-positive and KLK13-negative lung squamous cell
carcinoma. |
Table II
Clinicopathological features of
patients with KLK13-positive and KLK13-negative lung squamous cell
carcinoma.
|
Characteristics | Total (n=94) | KLK13-negative
(n=70) | KLK13-positive
(n=24) | P-value |
|---|
| Mean age ± SD,
years | 72.00±7.40 | 71.64±7.43 | 70.29±7.05 | 0.3054 |
| Sex, n (%) | | | | 0.3395 |
|
Male | 79 (84.0) | 57 (81.4) | 22 (91.7) | |
|
Female | 15 (16.0) | 13 (18.6) | 2 (8.3) | |
| pT classification,
n (%) | | | | 0.4393 |
|
T1-2 | 73 (77.7) | 53 (75.7) | 20 (83.3) | |
|
T3-4 | 21 (22.3) | 17 (24.3) | 4 (16.7) | |
| pN classification,
n (%) | | | | 0.7072 |
|
N0 | 80 (85.1) | 59 (84.3) | 21 (87.5) | |
|
N1-2 | 14 (14.9) | 11 (15.7) | 3 (12.5) | |
| pM classification,
n (%) | | | | 0.1592 |
|
M0 | 91 (96.8) | 69 (98.6) | 22 (91.7) | |
|
M1 | 3 (3.2) | 1 (1.4) | 2 (8.3) | |
| Cancer
stagea, n (%) | | | | 0.9573 |
|
I-II | 78 (83.0) | 58 (82.9) | 20 (83.3) | |
|
III-IV | 16 (17.0) | 12 (17.1) | 4 (16.7) | |
| Differentiation, n
(%) | | | | 0.0521 |
|
Keratinizing | 72 (76.6) | 50 (71.4) | 22 (91.7) | |
|
Non-keratinizing | 22 (23.4) | 20 (28.6) | 2 (8.3) | |
| Lymphatic vessel
invasion, n (%) (n=93) | | | | 0.0603 |
|
Positive | 11 (11.8) | 11 (15.9) | 0 (0.0) | |
|
Negative | 82 (87.2) | 58 (84.1) | 24(100) | |
| Vessel invasion, n
(%) (n=93) | | | | >0.9999 |
|
Positive | 47 (50.5) | 35 (50.7) | 12 (50.0) | |
|
Negative | 46 (49.5) | 34 (49.3) | 12 (50.0) | |
| E-cadherin
expression, n (%) | | | | 0.0143b |
|
Positive | 37 (39.4) | 22 (31.4) | 15 (62.5) | |
|
Negative | 57 (60.6) | 48 (68.6) | 9 (37.5) | |
| HPV, n (%) | | | | 0.1488 |
|
Positive | 38 (40.4) | 25 (35.7) | 13 (54.2) | |
|
Negative | 56 (59.6) | 45 (64.3) | 11 (45.8) | |
Discussion
In the present study, we observed an ectopic
expression of KLK13 in keratinized LSCC cells. Although a previous
study reported that KLK13 was associated with cell invasion and
migration in lung adenocarcinoma (14), KLK13 expression in LSCC was
associated with negative lymphatic vessel invasion. Invasion of
cancer cells into the surrounding tissue and infiltration into the
lymphatic and blood vessels are key features of cancer progression
and metastasis. Lymphatic vessel invasion has been reported to be
associated with tumor recurrence and prognosis in NSCLC (24); few reports have argued that NSCLC
patients with lymphatic vessel invasion would require more
aggressive treatment after surgery (25). In the present study, KLK13
expression was negatively correlated with lymphatic vessel
invasion. Furthermore, cases of KLK13-positive tumors tended to
have a better postoperative prognosis than those with a negative
expression of KLK13, although this difference is not statistically
significant (P=0.18) because of the specific feature of the cases
examined in this retrospective study. All tumors were in the early
stages, and the patients had absolute indications for surgical
intervention of the (stage I + II) LSCC. Additional studies should
include a higher number of cases and clarify the biological roles
of KLK13 in established cancer cells to validate KLK13 as a
clinical prognostic marker in LSCC. Planque et al (26) reported that NSCLC patients with
high KLK13 expression at the mRNA trended to have lower overall
survival, although the difference were marginally significant.
Since their results were contrary to our present finding that cases
of KLK13 immunoreactivity-positive tumors tended to have a better
postoperative prognosis than those with a negative expression of
KLK13 protein, we suspected that protein levels of KLK13 is
post-transcriptionally regulated. Therefore, further study to
clarify the molecular mechanisms regulating the protein levels of
KLK13 may be required to explain this discrepancy. In 2015, the
World Health Organization classified the subtyping of LSCC into
keratinizing, non-keratinizing, and basaloid subtypes (27). Recent studies have revealed that
there is no significant difference in the survival indications
between keratinizing SCC and non-keratinizing SCC (28). Furthermore, Chen et al
(29) reported that lymphatic
vessel invasion was not affected by the keratinizing status in
LSCC. We also analyzed the relationship between keratinization and
lymphatic vessel invasion in the 94 LSCC cases in this study and
found no significant association between them (P=0.29, data not
shown). Therefore, we suspect that the expression of KLK13 may
influence the lymphatic vessel invasion or the prognosis of LSCC
not via the induction of keratinization, although KLK13 was
expressed in a part of keratinized cells (Table II).
The results of the present study implied that KLK13
may exert a protective role in lymphatic vessel invasion and
eventually cancer progression in LSCC. Although these results
depict a diametrically opposed role of KLK13 than is observed for
adenocarcinoma, prior research has reported that KLK13 has diverse
physiological functions in carcinogenesis and cancer progression.
Indeed, in the oral and the esophageal squamous cell lines, the
overexpression of KLK13 inhibits the cell invasion and migration
(16,30), possibly due to the upregulation of
adhesion molecules such as E-cadherin, α-catenin, β-catenin,
desmoglein3, and desmoplakin. In the present study, we found the
significant association between the expression of KLK13 and of
E-cadherin (Fig. 2A and Table II). Generally, E-cadherin is known
as a marker of differentiated epithelial cells and is involved in
cell–to–cell adhesion. The disruption of the E-cadherin-mediated
cell–cell adhesion is often observed in malignant cancer
progression such as tumor invasion and metastasis. Therefore,
E-cadherin expression may explain the findings of our study, where
KLK13 expression inhibits lymphatic vessel invasion in LSCC. Hural
et al (31) reported that
KLK4 has the potential to be useful as a vaccine for prostate
cancer. Additionally, several kallikrein proteins, such as KLK3,
KLK5, KLK6, KLK10, and KLK14, have been proposed as serum markers
for diagnosis and prognosis of prostate and breast carcinomas
(32); however, we did not assess
the KLK13 levels using serum samples in the present study. Although
additional studies to clarify the biological roles of KLK13 in
established cancer cells as well as in sera will refine the value
of KLK13 expression as a molecular target in LSCC, the previous
study findings, as well as ours, encourage us to further
investigate the therapeutic application of KLK13 as a
molecular-target drug or peptide vaccine against LSCC. Because
KLK13 has diverse functions, including the promotion of tumor cell
motility via the induction of N-cadherin and vimentin in lung
adenocarcinoma (14), it may be
necessary to assess side effects of molecular targeted drugs
modulating the expression of KLK13 for clinical applications in
LSCC.
Acknowledgements
The authors would like to thank Dr Miwa
Tamura-Nakano and Dr Chinatsu Oyama at the National Center for
Global Health and Medicine Electronic Microscope Support Unit
(Tokyo, Japan) for their technical support with histological
analysis. The authors would also like to thank Ms. Yasuko Nozaki
(Communal Laboratory, Research Institute, National Center for
Global Health and Medicine, Tokyo, Japan) for technical
assistance.
Funding
Funding: This work was supported by JSPS KAKENHI (grant no.
JP19K08457) and by grants from the National Center for Global
Health and Medicine (grant nos. 29-1019 and 19A1021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YIK conceived and designed the study. RS, TH and YIK
acquired data. RS, TH, KY and YIK analyzed and interpreted the
data. RS, SN, HM, TI and KY provided clinical material support and
analyzed clinicopathological data. RS and YIK drafted the
manuscript. RS and YIK confirm the authenticity of all the raw
data. RS, SN, KY and YIK obtained funding. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved (2417) by the Research
Ethics Committee of National Center for Global Health and Medicine
(Tokyo, Japan). The requirement for written informed consent was
waived by the Institutional Review Board of National Center for
Global Health and Medicine (Tokyo, Japan) because the present study
was retrospective and non-interventional in nature. Therefore, the
poster was displayed before this study had started in accordance
with our hospital’s ethics. In addition, it has been described in
the poster that all the patients can refuse to participate in this
study at any time.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Senoo S, Ninomiya K, Hotta K and Kiura K:
Recent treatment strategy for advanced squamous cell carcinoma of
the lung in Japan. Int J Clin Oncol. 24:461–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu D, Huo C, Jiang S, Huang Y, Fang X, Liu
J, Yang M, Ren J, Xu B and Liu Y: Exostosin1 as a novel prognostic
and predictive biomarker for squamous cell lung carcinoma: A study
based on bioinformatics analysis. Cancer Med. 10:2787–2801.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rafei H, El-Bahesh E, Finianos A,
Nassereddine S and Tabbara I: Immune-based therapies for non-small
cell lung cancer. Anticancer Res. 37:377–387. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Goldstraw P, Ball D, Jett JR, Chevalier
TL, Lim E and Nicholson AG: Non-small-cell lung cancer. Lancet.
378:1727–1740. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sholl LM: Biomarkers in lung
adenocarcinoma: A decade of progress. Arch Pathol Lab Med.
139:469–480. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and radical disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun Y, Yin X, Wen MM, Zhang J, Wang XJ,
Xia JH, Zhang YN, Zhang ZP and Li XF: EGFR mutations subset in
Chinese lung squamous cell carcinoma patients. Mol Med Rep.
17:7575–7584. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cancer Genome Atlas Research Network.
Comprehensive genomic characterization of squamous cell lung
carcinoma. Nature. 489:519–525. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
West H, McCleod M, Hussein M, Morabito A,
Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et
al: Atezolizumab in combination with carboplatin plus
nab-paclitaxel chemotherapy compared with chemotherapy alone as
first-line treatment for metastatic non-squamous non-small-cell
lung cancer (Impower130): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:924–937. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: Carboplatin and pemetrexed with
or without pembrolizumab for advanced, non-squamous non-small-cell
lung cancer: A randomised, phase 2 cohort of the open-label
KEYNOTE-021 study. Lancet Oncol. 17:1497–1508. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reck M, Shankar G, Lee A, Coleman S,
McCleland M, Papadimitrakopoulou VA, Socinski MA and Sandler A:
Atezolizumab in combination with bevacizumab, paclitaxel and
carboplatin for the first-line treatment of patients with
metastatic non-squamous non-small cell lung cancer, including
patients with EGFR mutations. Expert Rev Respir Med. 14:125–136.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bonda WLM, Iochmann S, Magnen M, Courty Y
and Reverdiau P: Kallikrein-related peptidases in lung diseases.
Biol Chem. 399:959–971. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lenga Ma Bonda W, Lavergne M, Vasseur V,
Brisson L, Roger S, Legras A, Guillon A, Guyétant S, Hiemstra PS,
et al: Kallikrein-related peptidase 5 contributes to the remodeling
and repair of bronchial epithelium. FASEB J.
35(e21838)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chou RH, Lin SC, Wen HC, Wu CW and Chang
WS: Epigenetic activation of human kallikrein 13 enhances
malignancy of lung adenocarcinoma by promoting N-cadherin
expression and laminin degradation. Biochem Biophys Res Commun.
409:442–447. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tokas T, Avgeris M, Alamanis C, Scorilas
A, Stravodimos KG and Constantinides CA: Downregulated KLK13
expression in bladder cancer highlights tumor aggressiveness and
unfavorable patients’ prognosis. J Cancer Res Clin Oncol.
143:521–532. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ishige S, Kasamatsu A, Ogoshi K, Saito Y,
Usukura K, Yokoe H, Kouzu Y, Koike H, Sakamoto Y, Ogawara K, et al:
Decreased expression of kallikrein-related peptidase 13: Possible
contribution to metastasis of human oral cancer. Mol Carcinog.
53:557–565. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Chang A, Yousef GM, Scorilas A, Grass L,
Sismondi P, Ponzone R and Diamandis EP: Human kallikrein gene 13
(KLK13) expression by quantitative RT-PCR: An independent indicator
of favourable prognosis in breast cancer. Br J Cancer.
86:1457–1464. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Björkman K, Mustonen H, Kaprio T, Haglund
C and Böckelman C: Mucin 16 and Kallikrein 13 as potential
prognostic factors in colon cancer: Results of an oncological
92-multiplex immunoassay. Tumour Biol.
41(1010428319860728)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nohara K, Yamada K, Yamada L, Hagiwara T,
Igari T, Yokoi C, Soma D, Yamashita S, Dohi T and Kawamura YI:
Expression of kallikrein-related peptidase 13 is associated with
poor prognosis in esophageal squamous cell carcinoma. Gen Thorac
Cardiovasc Surg. 66:351–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brierley JD, Gospodarowicz MK and
Wittekind C: UICC International Union Against Cancer (eds). TNM
classification of malignant tumours. 8th edition. WileyBlackwell,
NY, 2017.
|
|
21
|
Gueugnon F, Barascu A, Mavridis K,
Petit-Courty A, Marchand-Adam S, Gissot V, Scorilas A, Guyetant S
and Courty Y: Kallikrein-related peptidase 13: An independent
indicator of favorable prognosis for patients with nonsmall cell
lung cancer. Tumour Biol. 36:4979–4986. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Miyagi J, Tsuhako K, Kinjo T, Iwamasa T
and Hirayasu T: Recent striking in histological differentiation and
rate of human papillomavirus infection in squamous cell carcinoma
of the lung in Okinawa, a subtropical island in southern Japan. J
Clin Pathol. 53:676–684. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Affandi KA, Tizen NMS, Mustangin M and Zin
RRMRM: P40 immunohistochemistry is an excellent marker in primary
lung squamous cell carcinoma. J Pathol Transl Med. 52:283–289.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Araki K, Adachi Y, Metsugi H and Tokushima
T: Prognostic implication of lymphatic vessel invasion in stage IB
(pT2aN0M0) non-small cell lung cancer. Gen Thorac Cardiovasc Surg.
59:605–608. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang J, Wang B, Zhao W, Guo Y, Chen H, Chu
H, Liang X and Bi J: Clinical significance and role of lymphatic
vessel invasion as a major prognostic implication in non-small cell
lung cancer: A meta-analysis. PLoS One. 7(e52704)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Planque C, Bléchet C, Ayadi-Kaddour A,
Heuzé-Vourc’h N, Dumont P, Guyétant S, Diamandis EP, El Mezni F and
Courty Y: Quantitative RT-PCR analysis and immunohistochemical
localization of the kallikrein-related peptidases 13 and 14 in
lung. Biol Chem. 389:781–786. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 world health organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
An N, Leng X, Wang X, Sun Y and Chen Z:
Survival comparison of three histological subtypes of lung squamous
cell carcinoma: A population-based propensity score matching
analysis. Lung Cancer. 142:13–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen R, Ding Z, Zhu L, Lu S and Yu Y:
Correlation of clinicopathologic features and lung squamous cell
carcinoma subtypes according to the 2015 WHO. classification.
43:2308–2314. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin Q, Mao W, Wu Q, He X, Li S, Fan Y,
Chen J, Feng T and Cao X: Downregulation of KLK13 promotes the
invasiveness and metastasis of oesophageal squamous cell carcinoma.
Biomed Pharmacother. 96:1008–1015. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hural JA, Friedman RS, McNabb A, Steen SS,
Henderson RA and Kalos M: Identification of naturally processed CD4
T cell epitopes from the prostate-specific antigen kallikrein 4
using peptide-based in vitro stimulation. J Immunol. 169:557–565.
2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Borgoño CA and Diamandis EP: The emerging
roles of human tissue kallikreins in cancer. Nat Rev Cancer.
4:876–890. 2004.PubMed/NCBI View
Article : Google Scholar
|