Introduction
Renal cell carcinoma (RCC) is a heterogeneous and
complex disease with numerous pathophysiological variants. Despite
the fact that it is the most common type of renal cancer, it
comprises only ~3% of malignant tumors in adults (1,2). RCC
develops from the renal ductal epithelium and is classified into
four major subtypes: i) clear cell RCC, ii) papillary RCC, iii)
chromophobe RCC and iv) collecting duct carcinoma (1,3).
This type of cancer can be associated with several localized and
systemic symptoms, including an abdominal mass, pain, hematuria,
anorexia, weight loss and several paraneoplastic syndromes, but the
symptoms may arise in the late stages of the disease. ~50 to 60% of
the cases are at risk of distant metastasis, with a quarter of them
being detected at presentation (4,5).
Despite the various treatment options, surgery remains the most
effective curative modality. Partial nephrectomy is the standard
option to manage T1a tumors and achieve cancer control while
preserving optimal renal function, whereas total nephrectomy is the
benchmark treatment for T1b-T4 tumors (6). Because ~40% of the cases succumb due
to cancer progression, RCC has become the most fatal of the common
urologic malignancies (7).
Prognostic factors are markers of disease progression, and the
precise determination of these factors is important for evaluating
and managing patients with RCC (8). Various prognostic factors for RCC
have been discussed in the literature, including clinical,
anatomical, and molecular parameters, but none have been
successfully validated so far (1,9).
Pathological stage, lymph node status and histological grade are
the prognostic factors that currently attract the attention of
scholars in this field (10). The
present study aimed to determine and find associations among the
histopathologic features of RCCs and their impact on survival and
metastasis.
Patients and methods
Study design
This is a cross-sectional study of patients with RCC
whose surgical specimens were evaluated at the pathology laboratory
of the Shorsh General Teaching Hospital in Sulaimani, Iraq, from
March 2008 to October 2021. Written informed consent was obtained
from the patients, and the study was approved (approval no.
130/2021) by the ethical committee of the University of
Sulaimani.
Inclusion criteria
The study included all the cases that met the
following criteria: Kidneys affected by any subtype of RCC
(including clear cell RCC, papillary RCC, chromophobe RCC and
collecting duct carcinoma); underwent partial or radical
nephrectomy; availability of nephrectomy specimens with detailed
clinical and histopathological reports.
Exclusion criteria
Cases that were excluded included those in which the
pathology report did not specify whether the specimen was a
nephrectomy or a core biopsy and those in which the excision was
for tumor recurrence if the primary excision report was already
included in the study sample.
Data collection
The data on the cases of RCC were collected from the
digital archive of the pathology laboratory of the Shorsh General
Teaching Hospital using a custom-built application that allowed
searching of the entire content of all the reports. The main
keyword for searching was ‘RCC,’ supplemented by searches for
specific tumor types (‘chromophobe’, ‘mucinous’, ‘tubular’ and
‘collecting duct’). A total of 228 eligible cases were identified,
which included both in-house and consult cases. The available data
from the studies on demographics and clinical and pathological
parameters were collected and tabulated into a spreadsheet.
Information regarding survival, recurrence, metastasis, and time
and presumed cause of death (for the cases that had passed away)
was obtained by contacting the patients via telephone and reviewing
their records at the Hiwa Cancer Hospital. The data were classified
into four major categories including demographic data, clinical and
gross parameters (procedure, laterality, site, focality, and size
of tumor), histopathological parameters (morphologic subtype,
grade, the presence of necrosis, sarcomatoid change, rhabdoid
change, and microvascular invasion, invasion of the renal capsule,
renal sinus, perinephric fat, Gerota's fascia, pelvicalyceal
system, renal vein, status of ureteric, vascular, parenchymal
margins and tumor stage) and follow-up information (survival,
recurrence, metastasis and the time and cause of death). The tumors
were graded according to the Fuhrman and International Society of
Urological Pathology (ISUP) grading systems and were staged
according to the American Joint Committee on Cancer (AJCC) and
Tumor, Node and Metastasis (TNM) staging systems (11-13).
Statistical analysis
The obtained data were entered into the Statistical
Package for the Social Sciences (v.25; IBM Corp.), and the
variables were optimized for analysis. Descriptive statistics were
generated, followed by correlation testing; the Chi-squared (Χ²)
and Fisher's exact tests were used to find correlations among the
various parameters. Kaplan-Meier analysis was used to identify the
relationship of the different parameters with survival and Cox
regression analysis was used to determine the relationship of the
parameters with the risk of metastasis as well as for multivariate
analysis. P≤0.05 was considered to indicate a statistically
significant difference.
Results
The age of the patients ranged from 4 to 90 years
old, with a mean and median of 51 years each. The majority of the
cases (43.9%) were between the ages of 45 and 64. The sex
distribution was skewed towards men (60.5%), with a men-to-women
ratio of 1.5:1. Almost 58% of the cases had a radical nephrectomy,
with the right-side tumors predominating (Table I). The tumors in most of the cases
(98.7%) were unifocal, with sizes ranging from 1.0 to 22.5 cm
(mean=6.4 cm). The major histologic type was clear cell RCC
(71.1%), followed by papillary RCC (13.6%) and chromophobe RCC
(11%) (Table I). More than half
(50.9%) of them were classified as grade II tumors. Necrosis, as
the most common aggressive factor, was found in 30.7% of the cases,
followed by renal capsule invasion (14.9%) and renal sinus invasion
(14.9%). Out of the partial nephrectomies, seven cases (8.1%) had
an involved parenchymal margin, and five cases (3.8%) of radical
nephrectomies had an involved renal vein margin (data not shown).
In 10 cases, the margin status was unclear because the procedure
type was not specified (Table I).
TNM stage was determined for all the tumors except three cases in
which the tumor size was missing from the reports and there was no
invasion of the renal sinus or other structures to upgrade them to
a T3 or T4 stage.
 | Table IBaseline characteristics. |
Table I
Baseline characteristics.
| Variables |
Percentages/Frequency |
|---|
| Age | |
|
≤18
years | 1.8% |
|
>18 and
≤44 years | 32% |
|
>44 and
≤64 years | 43.9% |
|
>64 and
≤84 years | 21.1% |
|
>84
years | 1.3% |
| Mean and median age
(min-max) | 51 (4-90
years) |
| Sex | |
|
Male | 138 (60.5%) |
|
Female | 90 (39.5%) |
| Clinical and gross
parameters | |
| Procedure | |
|
Partial
nephrectomy | 86 (37.7%) |
|
Radical
nephrectomy | 132 (57.9%) |
|
N/A | 10 (4.4%) |
| Laterality | |
|
Right
side | 115 (50.4%) |
|
Left
side | 99 (43.4%) |
| N/A | 14 (6.2%) |
| Site | |
|
Upper | 59 (25.9%) |
|
Lower | 69 (30.3%) |
|
Middle | 33 (14.5%) |
|
Pan (Entire
Kidney) | 7 (3.1%) |
|
N/A | 60 (26.2%) |
| Focality | |
|
Unifocal | 225 (98.7%) |
|
Multifocal | 2 (0.9%) |
|
N/A | 1 (0.4%) |
| Tumor size | |
|
≤4 cm | 83 (36.4%) |
|
>4 and ≤7
cm | 63 (27.6%) |
|
>7 and
≤10 cm | 46 (20.2%) |
|
>10
cm | 30 (13.2%) |
|
N/A | 6 (2.6%) |
| Histopathologic
parameters | |
| Tumor
morphotypes | |
|
Clear cell
RCC | 162 (71.1%) |
|
Papillary
RCC | 31 (13.6%) |
|
Papillary
RCC, type 1 | 11 (4.8%) |
|
Papillary
RCC, type 2 | 9 (3.9%) |
|
Papillary
RCC, not specified | 11 (4.8%) |
|
Chromophobe
RCC | 25 (11.0%) |
|
Translocation
RCC | 3 (1.3%) |
|
Clear cell
papillary RCC | 2 (0.9%) |
|
RCC,
unclassified | 2 (0.9%) |
|
Collecting
duct carcinoma | 1 (0.4%) |
|
Fumarate
hydratase-deficient RCC | 1 (0.4%) |
|
Mucinous,
tubular, and spindle cell RCC | 1 (0.4%) |
| Histopathologic
grade | |
|
1 | 50 (21.9%) |
|
2 | 116 (50.9%) |
|
3 | 23 (10.1%) |
|
4 | 17 (7.5%) |
|
N/A | 22 (9.6%) |
| Markers of
Aggressiveness and Invasiveness | |
|
Necrosis | 70 (30.7%) |
|
Sarcomatoid
change | 15 (6.6%) |
|
Rhabdoid
change | 10 (4.4%) |
|
Microvascular
invasion | 33 (14.5%) |
|
Renal sinus
invasion | 34 (14.9%) |
|
Renal
capsule invasion | 34 (14.9%) |
|
Perinephric
fat invasion | 27 (11.8%) |
|
Gerota's
fascia invasion | 9 (3.9%) |
|
Pelvicalyceal
invasion | 22 (9.6%) |
|
Renal vein
invasion | 20 (8.8%) |
| TNM staging | |
|
T1 | 121 (53.0%) |
|
T1a | 81 (35.5%) |
|
T1b | 40 (17.5%) |
|
T2 | 46 (20.2%) |
|
T2a | 29 (12.7%) |
|
T2b | 17 (7.5%) |
|
T3a | 48 (21.1%) |
|
T4 | 10 (4.4%) |
|
N/A | 3 (1.3%) |
| Follow-up
outcomes | |
| Survival
status | |
|
Deceased | 33 (14.5%) |
|
Cancer-related
complication | 23 (69.7%) |
|
Unrelated
cancer cause | 9 (27.3%) |
|
Unknown | 1 (3%) |
|
Alive | 154 (67.5%) |
|
N/A | 41 (18.0%) |
| Recurrence
status | |
|
Recurrence | 6 (2.6%) |
|
No
recurrence | 180 (78.9%) |
|
N/A | 42 (18.5%) |
| Metastasis
status | |
|
Metastasis | 36 (15.8%) |
|
No
metastasis | 150 (65.8%) |
|
N/A | 42 (18.4%) |
Follow-up data
A total of 187 cases (82%) could be reached when
contacted to obtain information about survival. Data about
recurrence, metastasis, and time and cause of death (if applicable)
were retrieved in 186 cases (81.6%). The follow-up data for the
remaining cases were missing as the patients were lost to follow-up
(Table I). The duration of
follow-up in patients with retrieved data ranged from 21 days to
13.1 years, with a mean of 3.9 years. Among the cases, 33 were
deceased; 23 cases (69.7%) succumbed to cancer-related
complications (metastasis), nine cases (27.3%) succumbed to
unrelated causes (other malignancies or chronic illnesses), and the
cause was unknown in one patient (3%).
Correlation of histologic
parameters
Out of all the gross and histological parameters
studied, sex showed a significant association with tumor necrosis
(P=0.002), with tumors in males revealing a higher association with
tumor necrosis. Tumors removed with a radical nephrectomy were more
significantly associated with all the markers of aggressiveness and
invasion (MAI) than those removed with a partial nephrectomy
(P<0.05). Out of all MAI, laterality was only significantly
correlated with renal sinus invasion (P=0.024), with the latter
being more common in right-sided tumors (data not shown). Tumor
size and tumor grade were both significantly correlated with each
other and with all MAI and tumor stage (Tables II, III). There was a significant
bidirectional association among numerous histological parameters,
including necrosis, sarcomatoid change, rhabdoid change,
microvascular invasion, renal sinus invasion, pelvicalyceal system
invasion, renal capsule invasion, and perinephric fat invasion
(P≤0.05). Most of these factors also had a significant association
with Gerota fascia invasion and renal vein invasion. Moreover, all
MAI, except rhabdoid change, were significantly associated with
increasing tumor stage (Table
IV).
 | Table IICorrelation of tumor size with
parameters of aggressiveness and invasiveness, tumor grade, and TNM
stage. |
Table II
Correlation of tumor size with
parameters of aggressiveness and invasiveness, tumor grade, and TNM
stage.
| | Tumor size | |
|---|
| Variables | Total | ≤4 cm | >4 and ≤7
cm | >7 and ≤10
cm | >10 cm | P-value |
|---|
| Necrosis | | | | | | <0.001 |
|
No | 154 | 73 | 48 | 25 | 8 | |
|
Yes | 68 | 10 | 15 | 21 | 22 | |
| Sarcomatoid
change | | | | | | <0.001 |
|
No | 207 | 83 | 60 | 41 | 23 | |
|
Yes | 15 | 0 | 3 | 5 | 7 | |
| Rhabdoid
change | | | | | | 0.012 |
|
No | 213 | 83 | 61 | 43 | 26 | |
|
Yes | 9 | 0 | 2 | 3 | 4 | |
| Microvascular
invasion | | | | | | <0.001 |
|
No | 192 | 82 | 54 | 36 | 20 | |
|
Yes | 30 | 1 | 9 | 10 | 10 | |
| Renal sinus
invasion | | | | | | <0.001 |
|
No | 189 | 82 | 50 | 34 | 23 | |
|
Yes | 33 | 1 | 13 | 12 | 7 | |
| Renal capsule
invasion | | | | | | <0.001 |
|
No | 192 | 79 | 57 | 37 | 19 | |
|
Yes | 30 | 4 | 6 | 9 | 11 | |
| Perinephric fat
invasion | | | | | | <0.001 |
|
No | 198 | 81 | 57 | 39 | 21 | |
|
Yes | 24 | 2 | 6 | 7 | 9 | |
| Gerota fascia
invasion | | | | | | 0.037 |
|
No | 213 | 82 | 61 | 44 | 26 | |
|
Yes | 9 | 1 | 2 | 2 | 4 | |
| Pelvicalyceal
invasion | | | | | | 0.017 |
|
No | 200 | 81 | 56 | 39 | 24 | |
|
Yes | 22 | 2 | 7 | 7 | 6 | |
| Renal vein
invasion | | | | | | 0.002 |
|
No | 203 | 81 | 58 | 36 | 28 | |
|
Yes | 19 | 2 | 5 | 10 | 2 | |
| Tumor Grade | | | | | | <0.001 |
|
Low-grade (1
and 2) | 164 | 79 | 42 | 27 | 16 | |
|
High-grade
(3 and 4) | 37 | 2 | 10 | 13 | 12 | |
| TNM stage | | | | | | <0.001 |
|
≤T2a | 150 | 78 | 45 | 27 | 0 | |
|
≥T2b | 72 | 5 | 18 | 19 | 30 | |
 | Table IIICorrelation of tumor grade with
parameters of aggressiveness and invasiveness, tumor size, and TNM
stage. |
Table III
Correlation of tumor grade with
parameters of aggressiveness and invasiveness, tumor size, and TNM
stage.
| | Tumor grade | |
|---|
| Parameters of
aggressiveness and invasiveness | Total | 1 | 2 | 3 | 4 | P-value |
|---|
| Necrosis | | | | | | <0.001 |
|
No | 140 | 43 | 84 | 10 | 3 | |
|
Yes | 66 | 7 | 32 | 13 | 14 | |
| Sarcomatoid
change | | | | | | <0.001 |
|
No | 192 | 50 | 115 | 22 | 5 | |
|
Yes | 14 | 0 | 1 | 1 | 12 | |
| Rhabdoid
change | | | | | | <0.001 |
|
No | 196 | 50 | 116 | 21 | 9 | |
|
Yes | 10 | 0 | 0 | 2 | 8 | |
| Microvascular
invasion | | | | | | <0.001 |
|
No | 176 | 50 | 106 | 16 | 4 | |
|
Yes | 30 | 0 | 10 | 7 | 13 | |
| Renal sinus
invasion | | | | | | <0.001 |
|
No | 176 | 49 | 103 | 14 | 10 | |
|
Yes | 30 | 1 | 13 | 9 | 7 | |
| Renal capsule
invasion | | | | | | <0.001 |
|
No | 176 | 48 | 108 | 15 | 5 | |
|
Yes | 30 | 2 | 8 | 8 | 12 | |
| Perinephric fat
invasion | | | | | | <0.001 |
|
No | 182 | 49 | 111 | 16 | 6 | |
|
Yes | 24 | 1 | 5 | 7 | 11 | |
| Gerota fascia
invasion | | | | | | 0.006 |
|
No | 198 | 50 | 113 | 21 | 14 | |
|
Yes | 8 | 0 | 3 | 2 | 3 | |
| Pelvicalyceal
invasion | | | | | | 0.047 |
|
No | 185 | 47 | 105 | 21 | 12 | |
|
Yes | 21 | 3 | 11 | 2 | 5 | |
| Renal vein
invasion | | | | | | <0.001 |
|
No | 188 | 50 | 108 | 16 | 14 | |
|
Yes | 18 | 0 | 8 | 7 | 3 | |
| TNM stage | | | | | | <0.001 |
|
T1a | 76 | 34 | 41 | 1 | 0 | |
|
T1b | 35 | 7 | 25 | 1 | 2 | |
|
T2a | 25 | 3 | 16 | 5 | 1 | |
|
T2b | 15 | 2 | 9 | 3 | 1 | |
|
T3a | 44 | 3 | 21 | 10 | 10 | |
|
T4 | 9 | 0 | 3 | 3 | 3 | |
 | Table IVCorrelation among the parameters of
aggressiveness and invasiveness and with TNM stage. |
Table IV
Correlation among the parameters of
aggressiveness and invasiveness and with TNM stage.
| Part A. |
|---|
| | Sarcomatoid
change | | Rhabdoid
change | | Microvascular
invasion | | Renal sinus
invasion | | Renal capsule
invasion |
|---|
| Variables | No | Yes | P-value | | No | Yes | P-value | | No | Yes | P-value | | No | Yes | P-value | | No | Yes | P-value |
|---|
| Necrosis | | | <0.001 | | | | <0.001 | | | | <0.001 | | | | 0.003 | | | | 0.009 |
|
No | 155 | 3 | | | 157 | 1 | | | 145 | 13 | | | 142 | 16 | | | 141 | 17 | |
|
Yes | 58 | 12 | | | 61 | 9 | | | 50 | 20 | | | 52 | 18 | | | 53 | 17 | |
| Sarcomatoid
change | | | | | | | <0.001 | | | | <0.001 | | | | 0.013 | | | | <0.001 |
|
No | | | | | 209 | 4 | | | 189 | 24 | | | 185 | 28 | | | 188 | 25 | |
|
Yes | | | | | 9 | 6 | | | 6 | 9 | | | 9 | 6 | | | 6 | 9 | |
| Rhabdoid
change | | | | | | | | | | | <0.001 | | | | 0.045 | | | | 0.008 |
|
No | | | | | | | | | 192 | 26 | | | 188 | 30 | | | 189 | 29 | |
|
Yes | | | | | | | | | 3 | 7 | | | 6 | 4 | | | 5 | 5 | |
| Microvascular
invasion | | | | | | | | | | | | | | | <0.001 | | | | <0.001 |
|
No | | | | | | | | | | | | | 182 | 13 | | | 179 | 16 | |
|
Yes | | | | | | | | | | | | | 12 | 21 | | | 15 | 18 | |
| Renal sinus
invasion | | | | | | | | | | | | | | | | | | | <0.001 |
|
No | | | | | | | | | | | | | | | | | 174 | 20 | |
|
Yes | | | | | | | | | | | | | | | | | 20 | 14 | |
| Part B. |
| | Perinephric fat
invasion | Gerota fascia
invasion | Pelvicalyceal
invasion | Renal vein
invasion | TNM stage |
| Variables | No | Yes | P-value | No | Yes | P-value | No | Yes | P-value | No | Yes | P-value | T1a | T1b | T2a | T2b | T3a | T4 | P-value |
| Necrosis | | | 0.004 | | | 0.284 | | | 0.004 | | | 0.048 | | | | | | | <0.001 |
|
No | 146 | 12 | | 153 | 5 | | 149 | 9 | | 148 | 10 | | 70 | 31 | 19 | 6 | 24 | 5 | |
|
Yes | 55 | 15 | | 66 | 4 | | 57 | 13 | | 60 | 10 | | 8 | 11 | 11 | 11 | 24 | 5 | |
| Sarcomatoid
change | | | <0.001 | | | 0.015 | | | 0.008 | | | 0.387 | | | | | | | 0.003 |
|
No | 194 | 19 | | 207 | 6 | | 196 | 17 | | 195 | 18 | | 78 | 40 | 29 | 15 | 40 | 8 | |
|
Yes | 7 | 8 | | 12 | 3 | | 10 | 5 | | 13 | 2 | | 0 | 2 | 1 | 2 | 8 | 2 | |
| Rhabdoid
change | | | 0.020 | | | 0.663 | | | 0.060 | | | 0.215 | | | | | | | 0.128 |
|
No | 195 | 23 | | 209 | 9 | | 199 | 19 | | 200 | 18 | | 78 | 40 | 29 | 16 | 43 | 9 | |
|
Yes | 6 | 4 | | 10 | 0 | | 7 | 3 | | 8 | 2 | | 0 | 2 | 1 | 1 | 5 | 1 | |
| Microvascular
invasion | | | <0.001 | | | 0.004 | | | 0.001 | | | 0.001 | | | | | | | <0.001 |
|
No | 184 | 11 | | 191 | 4 | | 182 | 13 | | 184 | 11 | | 78 | 40 | 29 | 15 | 24 | 6 | |
|
Yes | 17 | 16 | | 28 | 5 | | 24 | 9 | | 24 | 9 | | 0 | 2 | 1 | 2 | 24 | 4 | |
| Renal sinus
invasion | | | <0.001 | | | 0.030 | | | <0.001 | | | <0.001 | | | | | | | <0.001 |
|
No | 180 | 14 | | 189 | 5 | | 182 | 12 | | 186 | 8 | | 78 | 41 | 30 | 17 | 18 | 7 | |
|
Yes | 21 | 13 | | 30 | 4 | | 24 | 10 | | 22 | 12 | | 0 | 1 | 0 | 0 | 30 | 3 | |
| Renal capsule
invasion | | | <0.001 | | | <0.001 | | | 0.028 | | | 0.004 | | | | | | | <0.001 |
|
No | | | | 194 | 0 | | 179 | 15 | | 182 | 12 | | 76 | 42 | 28 | 17 | 28 | 1 | |
|
Yes | | | | 25 | 9 | | 27 | 7 | | 26 | 8 | | 2 | 0 | 2 | 0 | 20 | 9 | |
| Perinephric fat
invasion | | | | | | <0.001 | | | 0.007 | | | 0.001 | | | | | | | <0.001 |
|
No | | | | 201 | 0 | | 186 | 15 | | 189 | 12 | | 78 | 42 | 30 | 17 | 30 | 1 | |
|
Yes | | | | 18 | 9 | | 20 | 7 | | 19 | 8 | | 0 | 0 | 0 | 0 | 18 | 9 | |
| Gerota fascia
invasion | | | | | | | | | 0.606 | | | 0.181 | | | | | | | <0.001 |
|
No | | | | | | | 198 | 21 | | 201 | 18 | | 78 | 42 | 30 | 17 | 46 | 3 | |
|
Yes | | | | | | | 8 | 1 | | 7 | 2 | | 0 | 0 | 0 | 0 | 2 | 7 | |
| Pelvicalyceal
invasion | | | | | | | | | | | | 0.006 | | | | | | | <0.001 |
|
No | | | | | | | | | | 192 | 14 | | 78 | 42 | 28 | 17 | 29 | 9 | |
|
Yes | | | | | | | | | | 16 | 6 | | 0 | 0 | 2 | 0 | 19 | 1 | |
| Renal vein
invasion | | | | | | | | | | | | | | | | | | | <0.001 |
|
No | | | | | | | | | | | | | 78 | 41 | 30 | 16 | 33 | 7 | |
|
Yes | | | | | | | | | | | | | 0 | 1 | 0 | 1 | 15 | 3 | |
Survival, recurrence, and
metastasis
Survival analysis using a life table showed overall
survival rates at one, five, and ten-year intervals of 87, 79, and
55%, respectively. Sex did not influence survival using log-rank
analysis (P=0.726) (data not shown). Age had a significant impact
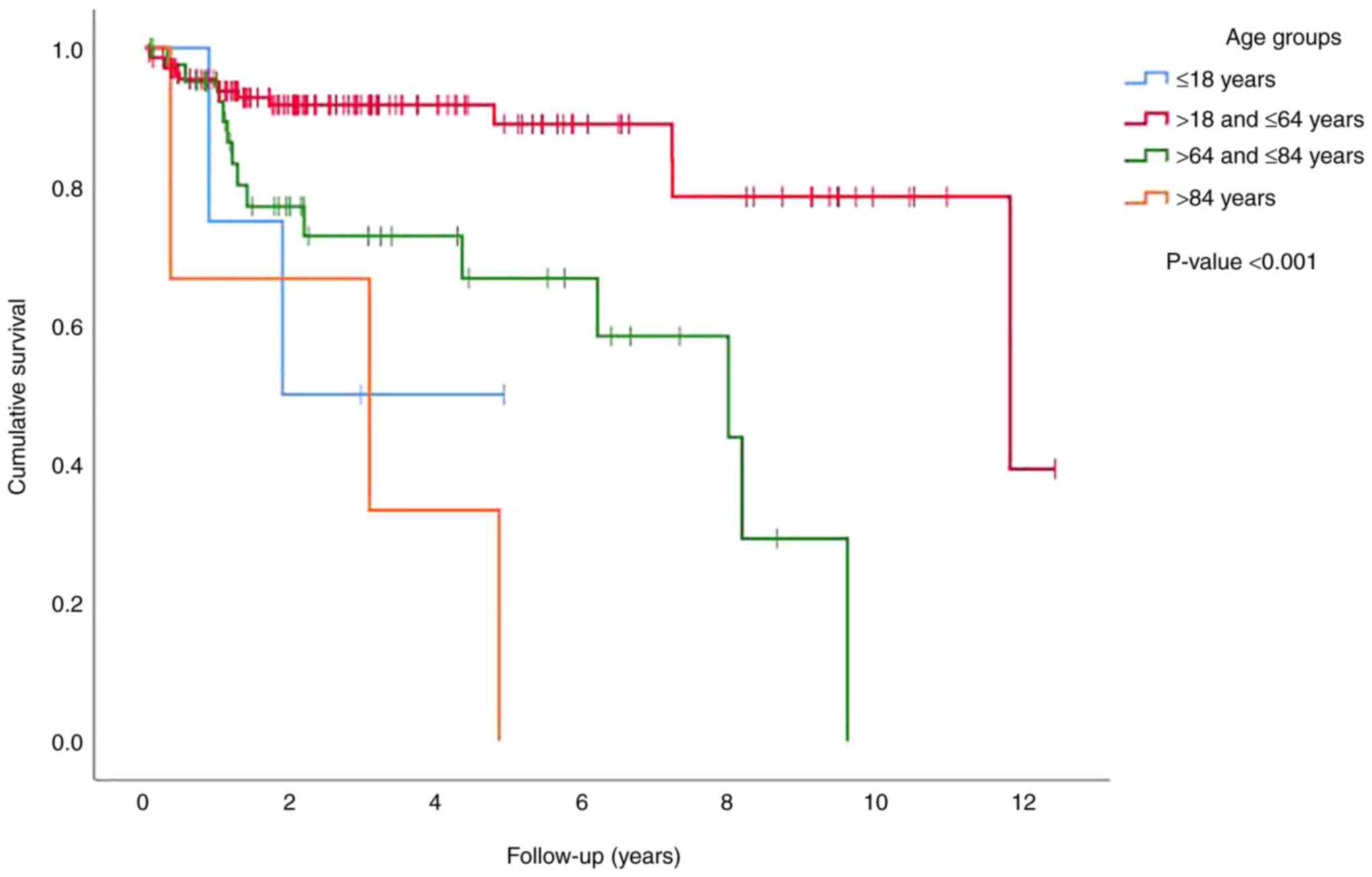
on survival (P<0.001) when divided into four age groups (≤18,
>18 and ≤64, >64 and ≤84, and >84 years), with patients in
the groups of ≤18 and >84 years having the worst survival rate,
followed by those in the >64 and ≤84 years group. The patients
in the group of >18 and ≤64 years had the best survival rate
(Fig. 1). Similar to its
association with MAI, radical nephrectomy had a negative impact on
survival (P=0.003) (data not shown).
Tumor laterality had no impact on survival (P=0.523)
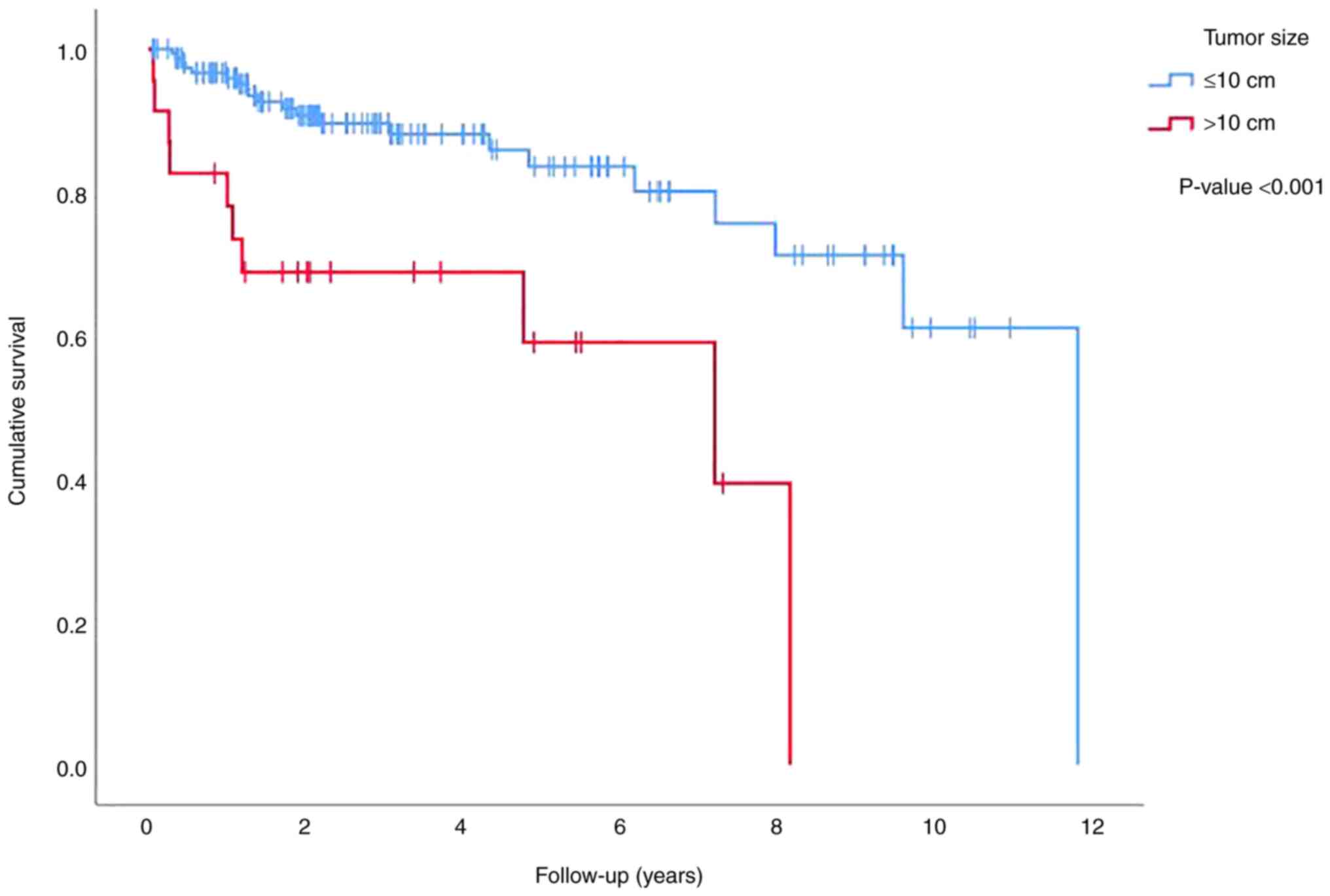
(data not shown). Tumor size had an impact on survival only when
divided into two groups of tumors with the sizes of ≤10 and >10
cm, in which patients with a tumor size of >10 cm had poorer
survival (P<0.001) (Fig. 2).
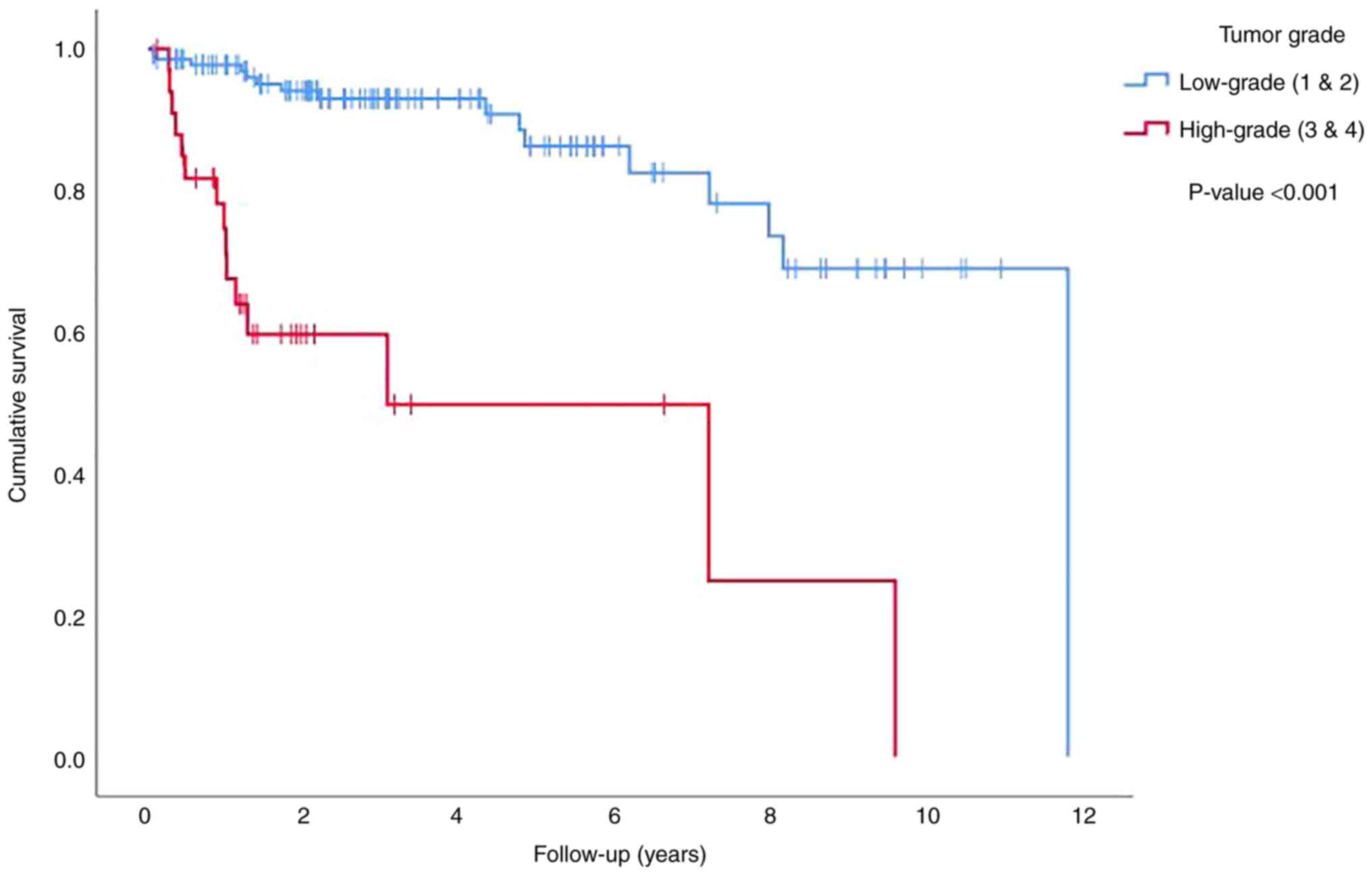
Tumor grade also affected survival when it was divided into
low-grade (grades 1 and 2 in the ISUP grading scheme) and
high-grade (grades 3 and 4 in the ISUP grading scheme) tumors, with
high-grade tumors having an adverse impact on survival (P<0.001)
(Fig. 3). Tumor size and tumor
grade had a significant impact on survival in univariate analysis
and retained their individual significance when combined in
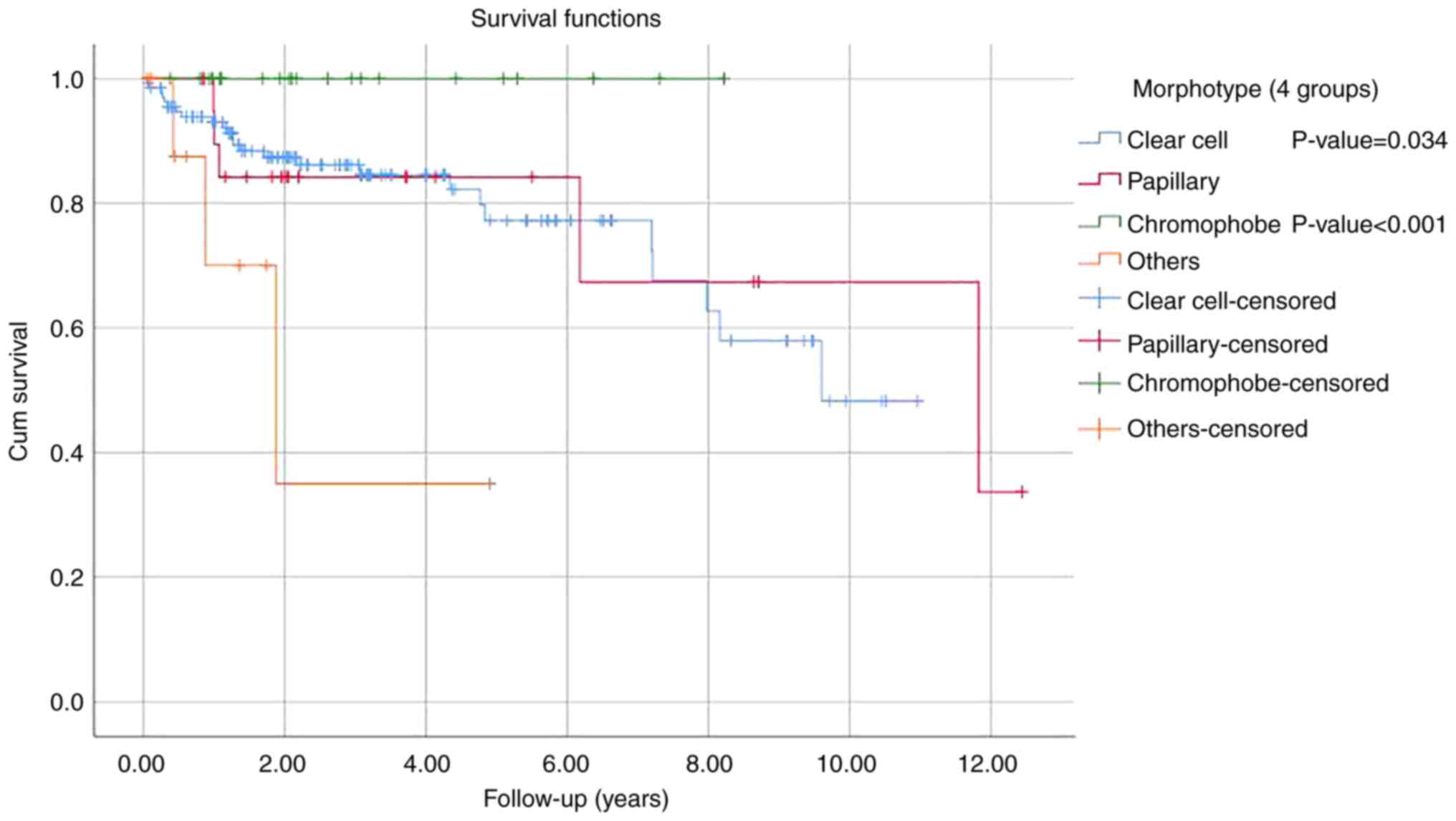
multivariate regression analysis (Tables V and VI). Analysis of survival by tumor type
revealed no significant difference among clear cell RCC, papillary
RCC and chromophobe RCC. The only significant difference pertained
to the worse outcome for the remaining histologic types grouped
together as compared with clear cell carcinoma (P=0.034) and
chromophobe carcinoma (P<0.001) (Fig. 4).
 | Table VUnivariate analysis of survival
correlation with tumor size, tumor grade, aggressiveness, and
invasiveness parameters. |
Table V
Univariate analysis of survival
correlation with tumor size, tumor grade, aggressiveness, and
invasiveness parameters.
| | Correlation with
survival |
|---|
| | | 95.0% CI for
Exp(B) |
|---|
| Variables in the
equation | B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper |
|---|
| Necrosis | 0.616 | 0.360 | 2.923 | 1 | 0.087 | 1.851 | 0.914 | 3.748 |
| Sarcomatoid
change | 2.146 | 0.427 | 25.231 | 1 | <0.001 | 8.552 | 3.702 | 19.759 |
| Rhabdoid
change | 1.297 | 0.622 | 4.345 | 1 | 0.037 | 3.658 | 1.081 | 12.380 |
| Microvascular
invasion | 2.163 | 0.393 | 30.213 | 1 | <0.001 | 8.694 | 4.021 | 18.797 |
| Renal sinus
invasion | 1.281 | 0.385 | 11.094 | 1 | 0.001 | 3.602 | 1.694 | 7.656 |
| Renal capsule
invasion | 1.190 | 0.368 | 10.479 | 1 | 0.001 | 3.288 | 1.599 | 6.761 |
| Perinephric fat
invasion | 1.953 | 0.387 | 25.469 | 1 | <0.001 | 7.047 | 3.301 | 15.044 |
| Gerota fascia
invasion | 1.226 | 0.541 | 5.131 | 1 | 0.024 | 3.409 | 1.180 | 9.849 |
| Pelvicalyceal
invasion | 1.021 | 0.501 | 4.152 | 1 | 0.042 | 2.775 | 1.040 | 7.406 |
| Renal vein
invasion | 0.991 | 0.497 | 3.986 | 1 | 0.046 | 2.695 | 1.018 | 7.132 |
| Tumor grade
[Low-grade (1 and 2) and High-grade (3 and 4)] | 1.962 | 0.376 | 27.267 | 1 | <0.001 | 7.117 | 3.407 | 14.865 |
| Tumor size (≤10 cm
and >10 cm) | 1.393 | 0.393 | 12.581 | 1 | <0.001 | 4.027 | 1.865 | 8.694 |
 | Table VIMultivariate analysis of survival
correlation with tumor size, tumor grade, aggressiveness and
invasiveness parameters. |
Table VI
Multivariate analysis of survival
correlation with tumor size, tumor grade, aggressiveness and
invasiveness parameters.
| | Correlation with
survival |
|---|
| | | 95.0% CI for Exp
(B) | |
|---|
| Variables | B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper | Covariate
means |
|---|
| Multivariate Cox
regression analysis for tumor size and grade (both two-tiered) as
covariates |
| Size (≤10 cm and
>10 cm) | 0.943 | 0.425 | 4.917 | 1 | 0.027 | 2.567 | 1.116 | 5.907 | 0.133 |
| Grade [Low-grade (1
and 2) and High-grade (3 and 4)] | 1.673 | 0.401 | 17.375 | 1 | <0.001 | 5.330 | 2.427 | 11.708 | 0.188 |
| Multivariate Cox
regression analysis stratified for tumor grade (two-tiered) |
| Necrosis | -0.031 | 0.465 | 0.004 | 1 | 0.948 | 0.970 | 0.390 | 2.413 | 0.320 |
| Sarcomatoid
change | 0.969 | 0.641 | 2.288 | 1 | 0.130 | 2.636 | 0.751 | 9.252 | 0.071 |
| Rhabdoid
change | -0.836 | 0.753 | 1.233 | 1 | 0.267 | 0.434 | 0.099 | 1.895 | 0.053 |
| Microvascular
invasion | 1.357 | 0.515 | 6.948 | 1 | 0.008 | 3.884 | 1.416 | 10.654 | 0.160 |
| Multivariate Cox
regression analysis stratified for tumor grade (two-tiered) |
| Renal sinus
invasion | 0.676 | 0.498 | 1.841 | 1 | 0.175 | 1.967 | 0.740 | 5.224 | 0.166 |
| Renal capsule
invasion | -9.035 | 110.183 | 0.007 | 1 | 0.935 | 0.000 | 0.000 | 7.321E+89 | 0.148 |
| Perinephric fat
invasion | 10.031 | 110.185 | 0.008 | 1 | 0.927 | 22,726.533 | 0.000 | 1.400E+98 | 0.124 |
| Gerota fascia
invasion | -0.583 | 0.766 | 0.579 | 1 | 0.447 | 0.558 | 0.125 | 2.504 | 0.041 |
| Pelvicalyceal
invasion | 0.165 | 0.568 | 0.085 | 1 | 0.771 | 1.180 | 0.388 | 3.591 | 0.107 |
| Renal vein
invasion | -0.498 | 0.639 | 0.607 | 1 | 0.436 | 0.608 | 0.174 | 2.127 | 0.089 |
| Multivariate Cox
regression analysis stratified for tumor size (two-tiered) |
| Renal sinus
invasion | 0.884 | 0.492 | 3.226 | 1 | 0.072 | 2.420 | 0.923 | 6.348 | 0.181 |
| Renal capsule
invasion | -9.096 | 105.708 | 0.007 | 1 | 0.931 | 0.000 | 0.000 | 1.068E+86 | 0.147 |
| Perinephric fat
invasion | 10.407 | 105.709 | 0.010 | 1 | 0.922 | 33,076.445 | 0.000 | 3.159E+94 | 0.124 |
| Gerota fascia
invasion | -0.564 | 0.694 | 0.662 | 1 | 0.416 | 0.569 | 0.146 | 2.215 | 0.045 |
| Pelvicalyceal
invasion | 0.214 | 0.576 | 0.138 | 1 | 0.710 | 1.238 | 0.400 | 3.831 | 0.107 |
| Renal vein
invasion | -0.108 | 0.643 | 0.028 | 1 | 0.866 | 0.897 | 0.254 | 3.164 | 0.090 |
Regarding the effects of MAI on survival, necrosis
had a significant impact only in patients with clear cell RCC
(P=0.026) (data not shown). In addition, univariate analysis
demonstrated a significant impact on survival in the presence of
sarcomatoid change (P<0.001), rhabdoid change (P=0.037), and
microvascular invasion (P<0.001) (Table V). However, on multivariate
analysis, when these three factors, along with tumor necrosis, were
stratified for tumor grade, only microvascular invasion retained a
significant impact (P=0.008) (Table
VI). On univariate analysis, there was, likewise, a significant
impact on survival in the presence of invasion of the renal sinus
(P=0.001), renal capsule (P=0.001), perinephric fat (P<0.001),
Gerota fascia (P=0.024), pelvicalyceal system (P=0.042), and renal
vein (P=0.046) (Table V). However,
multivariate analysis for all of these parameters, combined and
stratified against tumor grade and tumor size, revealed no
significant impact for any individual parameter (Table VI). Analysis of the effect of
tumor stage on survival only showed a significant difference
between tumors that were at stage T2a or lower and tumors that were
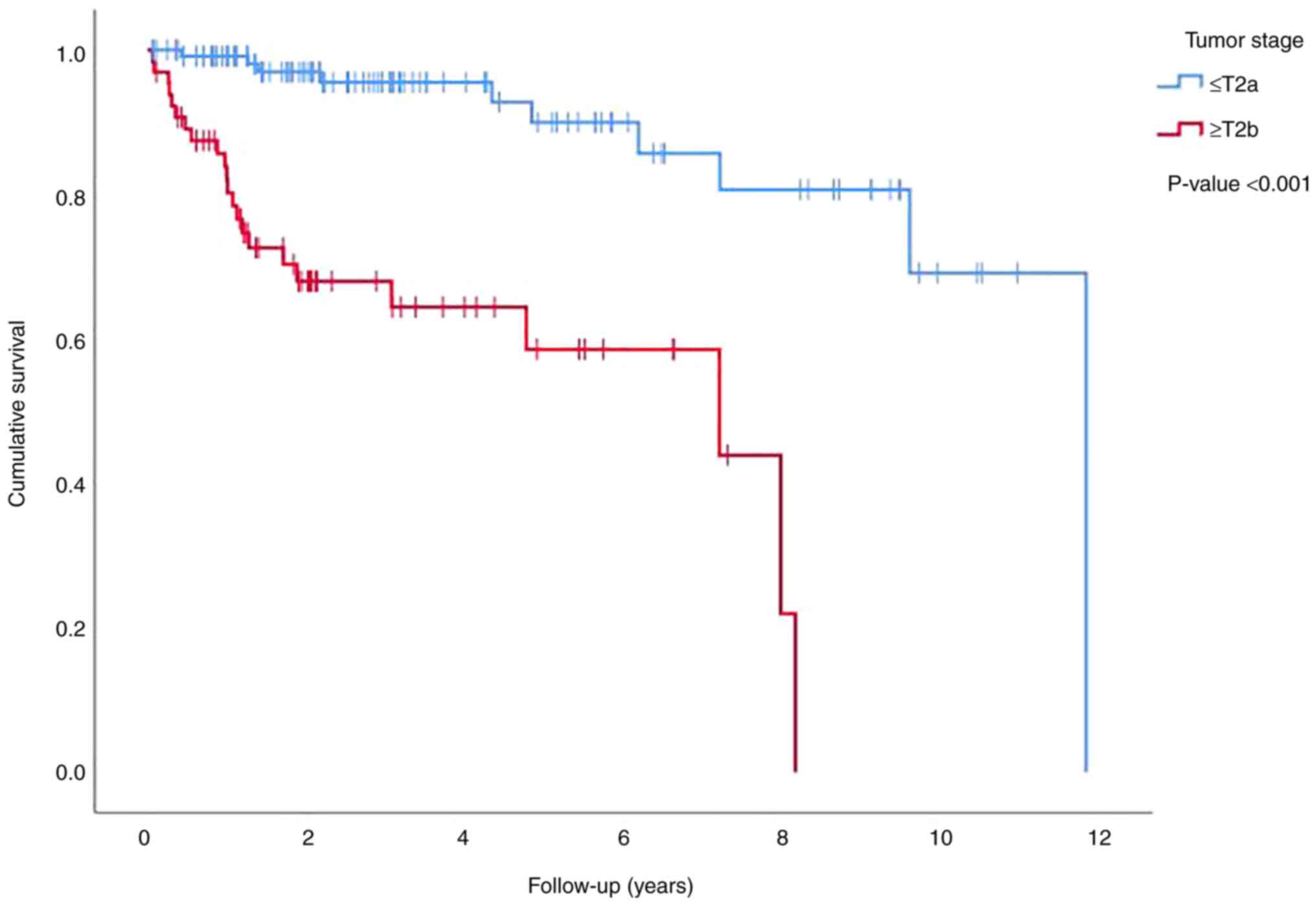
at stage T2b or higher (P<0.001) (Fig. 5).
Using Cox regression analysis, the rate of
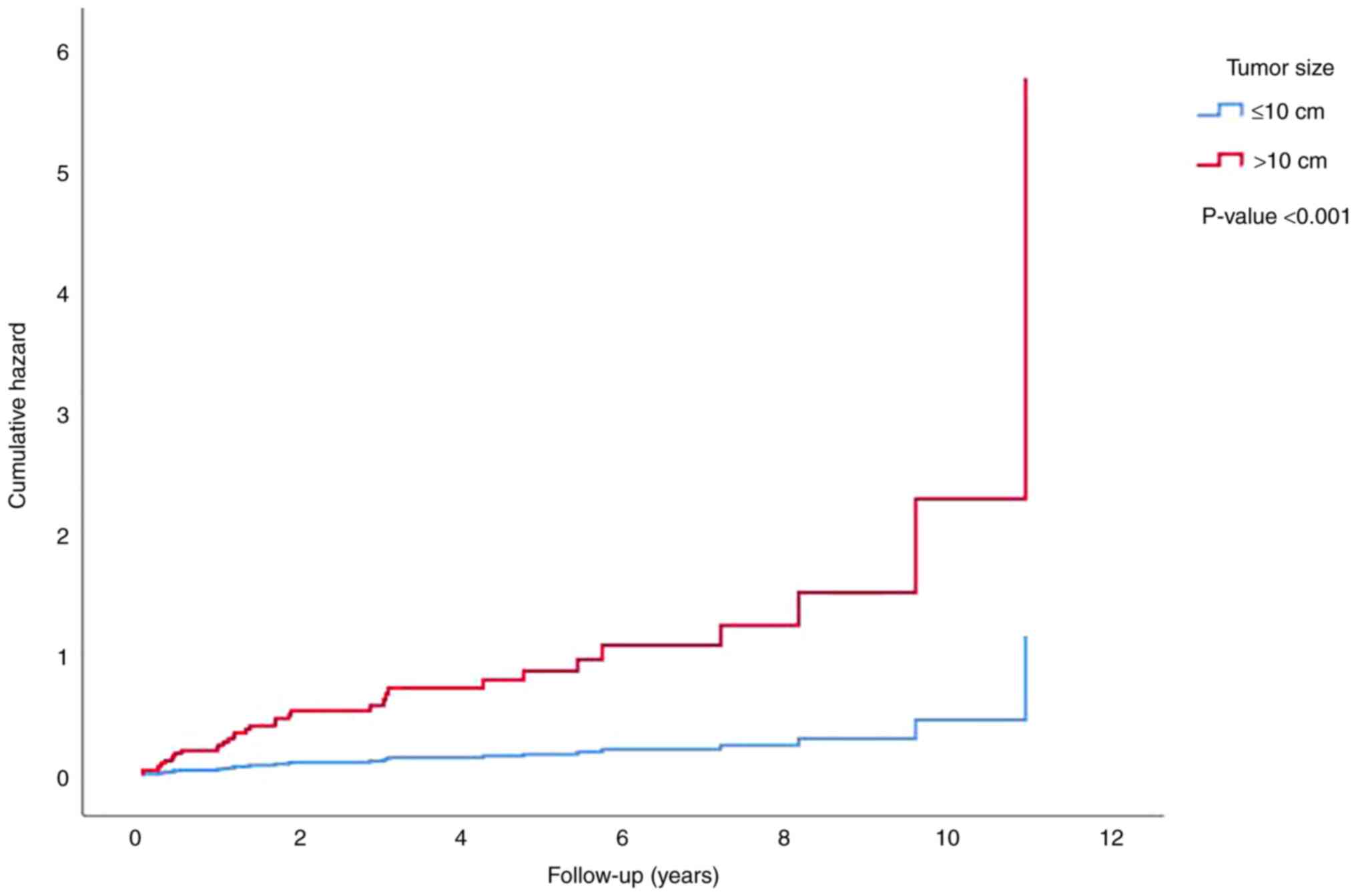
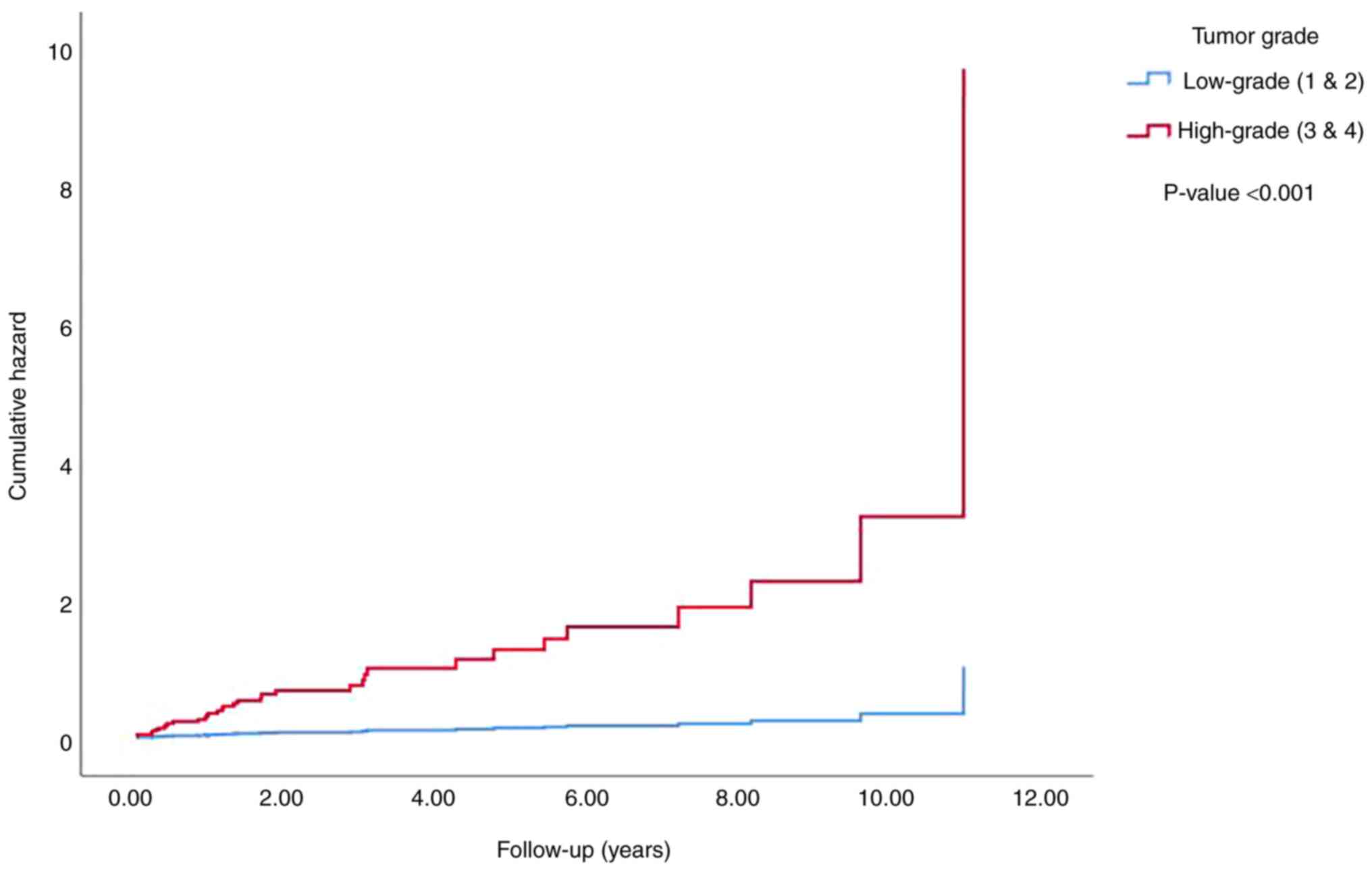
metastasis was demonstrated to be significantly increased
(P<0.001) with a higher tumor size (Fig. 6), grade (Fig. 7) and stage (data not shown) using
the two-tiered groupings mentioned previously for each parameter.
Furthermore, all MAI significantly increased the risk of metastasis
(Table VII).
 | Table VIIUnivariate analysis of metastasis
correlation with tumor stage and aggressiveness and invasiveness
parameters. |
Table VII
Univariate analysis of metastasis
correlation with tumor stage and aggressiveness and invasiveness
parameters.
| | Correlation with
metastasis |
|---|
| | | 95.0% CI for Exp
(B) |
|---|
| Variables in the
Equation | B | SE | Wald | df | Sig. | Exp (B) | Lower | Upper |
|---|
| Necrosis | 0.933 | 0.336 | 7.699 | 1 | 0.006 | 2.542 | 1.315 | 4.914 |
| Sarcomatoid
change | 2.301 | 0.389 | 35.057 | 1 | <0.001 | 9.982 | 4.661 | 21.379 |
| Rhabdoid
change | 1.967 | 0.465 | 17.869 | 1 | <0.001 | 7.151 | 2.872 | 17.802 |
| Microvascular
invasion | 2.304 | 0.363 | 40.232 | 1 | <0.001 | 10.010 | 4.912 | 20.397 |
| Renal sinus
invasion | 1.342 | 0.359 | 13.948 | 1 | <0.001 | 3.828 | 1.893 | 7.745 |
| Renal capsule
invasion | 1.390 | 0.344 | 16.330 | 1 | <0.001 | 4.013 | 2.045 | 7.874 |
| Perinephric fat
invasion | 1.944 | 0.356 | 29.733 | 1 | <0.001 | 6.984 | 3.473 | 14.045 |
| Gerota fascia
invasion | 1.272 | 0.487 | 6.825 | 1 | 0.009 | 3.567 | 1.374 | 9.261 |
| Pelvicalyceal
invasion | 1.062 | 0.457 | 5.400 | 1 | 0.020 | 2.892 | 1.181 | 7.083 |
| Renal vein
invasion | 1.368 | 0.410 | 11.164 | 1 | 0.001 | 3.929 | 1.761 | 8.766 |
| Tumor Stage | 2.298 | 0.432 | 28.250 | 1 | <0.001 | 9.952 | 4.265 | 23.222 |
Discussion
In the Surveillance, Epidemiology, and End Results
(SEER) data, the most frequent age group for all cases of kidney
and pelvis cancer was the 65-74 age group, with a median age of 64
years (14). In the present study,
the most frequent group was the 45-64 age group, with a mean age of
51 years, which is in line with a previous local study that
reported mean age of 54.3 in patients with metastatic RCC (15). This difference regarding the
aforementioned study and the present study compared with SEER data
may reflect the difference in life expectancy between the studied
populations as well as the differences in risk factor exposure.
The sex distribution in the incidence of RCC was
positively skewed towards males, with a male-to-female ratio of
1.8:1 in the SEER data (14),
although, a previous study revealed female preponderance in West
Africa (16). The findings of the
present study were concordant with the results of the SEER data
(14) and the aforementioned local
study (15), with a male-to-female
ratio of 1.5:1.
Aron et al (17) also reported significant sex
differences in other parameters, including larger tumor size,
higher grade, higher incidence of metastasis, and shorter overall
survival for males. However, in the data of the present study,
there was no significant sex difference in tumor size and grade, or
overall survival. More studies are required to determine whether
there are consistent differences between the sex in tumor
characteristics and whether there are genetic and biochemical bases
for these differences.
Performing radical or partial nephrectomies is based
on specific selection criteria for each procedure, and these can be
affected by the preference of the surgeon. Partial nephrectomy is
usually performed for smaller tumors without invasion of adjacent
structures, while radical nephrectomy is chosen for larger tumors,
tumors in the mid portion of the kidney, and tumors that have
invaded adjacent structures (6).
Kattan et al (18) excluded
the type of procedure from their postoperative nomogram for RCC due
to the lack of agreement on policy and fixed selection criteria for
nephron-sparing surgery among their surgeons. These criteria can
explain the reason that in the present study, the cases that
underwent radical nephrectomy were significantly more likely to
have markers of aggressiveness, whereas those for which partial
nephrectomy had been performed were less likely to have the
histologic parameters of aggressiveness. This simply reflects the
fact that the tumors that have a larger size or are determined by
imaging to be invading beyond the kidney or into the sinus or other
structures are more likely to be removed with a radical
nephrectomy. This also explains the worse survival rates observed
in patients for whom a radical nephrectomy had been performed, as
these tumors were larger and had a significantly higher likelihood
of invasion beyond the kidney or into the renal hilum.
A meta-analysis on the long-term outcome of partial
and radical nephrectomies for tumors 4-7 cm in size that included
sixteen studies with a total of 13,016 patients demonstrated a
higher rate of radical nephrectomies (88%) (19). They revealed that the 5- and
10-year cancer-specific survival was improved in the radical
nephrectomy group than in the partial nephrectomy group. The
present study did not show a significant difference in outcome
between radical and partial nephrectomy for any tumor size group in
particular. There was only an overall difference in the outcome,
with patients who underwent radical nephrectomy having worse
survival than those who underwent partial nephrectomy. This is
congruent with the fact that radical nephrectomies are performed
for higher-stage tumors, and these have a poorer prognosis due to
their other histologic parameters of aggressiveness, while the
better survival noted by the aforementioned study may be due to
performing partial nephrectomies for cases which should have been
treated by radical nephrectomy, leading to recurrence and
metastasis due to inadequate treatment.
In the present study, 50.4% of the tumors were
right-sided, and Guo et al (20) reported a similar rate of 50.6% from
their analysis of the SEER data, however, a local study reported a
higher percentage (64.8%) (15).
The present results showed a significantly higher rate of renal
sinus invasion in right-sided tumors, while Guo et al
(20) reported that right-sided
tumors were more likely to have favorable pathologic features,
including improved cancer-specific survival. Further studies are
required to identify consistent differences in tumor
characteristics and behavior with regard to sidedness and in
identifying an anatomical basis for them.
Different rates of tumor multifocality, including 5
to 25%, have been reported in the literature. Sargin et al
(21) reported a rate of 13.1% in
a total of 122 specimens (21).
They revealed a significant association of multifocality with tumor
stage and grade but not with tumor morphotype or other parameters.
In the present study, only 0.9% of the tumors were multifocal,
preventing firm conclusions from being drawn about their
significance. Variations in the rates of multifocality are partly
explained by differences in the meticulousness of grossing
technique, partly by differences in early detection of cancer due
to more frequent usage of abdominal imaging techniques, and partly
due to regional differences in the rates of papillary RCC and other
aggressive histological subtypes that may more frequently be
presented as multiple masses.
With regards to tumor size, Zhang et al
(22) reported frequencies of
tumor size according to the AJCC staging cut-offs of 49, 33.5,
12.9, and 4.6% for the group sizes of ≤4 cm, 4.1-7 cm, 7.1-10 cm,
and >10 cm, respectively (22).
This demonstrated a higher proportion of tumors in the ≤4 cm
category compared with the results of the present study, which
demonstrated 36.4, 27.6, 20.2 and 13.2% for the respective
categories, indicating a higher proportion of tumors >7 cm in
size in the current cases. They also revealed a significant
association between increasing tumor size and tumor grade, stage,
and invasiveness, similar to the findings of the present study.
Zhang et al (22) also
revealed in their study that the probability of clear cell
carcinoma increased as the tumor size increased, unfortunately, a
significant relationship between tumor size and morphotype could
not be demonstrated in the present study.
The three most common histologic types of RCC are
clear cell, papillary and chromophobe in decreasing order of
frequency, but the exact percentages vary among the different
studies (23,24). The findings of the present study
were concordant with the ordering of these three types, with
frequencies of 71.1, 13.6 and 11% respectively. Patard et al
(25) reported frequencies of
87.7, 9.7 and 2.5%, while Cheville et al (23) reported frequencies of 83.2, 11.3
and 4.3% for the three types (23), highlighting a lower rate of the
chromophobe subtype and a higher rate of the clear cell subtype
compared with the findings of the present study.
Patard et al (25) observed a significant survival
difference among the three histologic types only on univariate
analysis, with chromophobe carcinoma having a more favorable
outcome. Cheville et al (23) demonstrated a significant difference
in the outcome of the three types on both univariate and
multivariate analysis, with clear cell RCC having a worse outcome
than papillary RCC and chromophobe RCC. The results of the present
study revealed no significant survival difference between patients
with clear cell RCC and papillary RCC. The only significant
observation was that the remaining tumor types as a group had a
worse outcome than each of clear cell RCC and chromophobe RCC.
There was a trend toward better survival in type 1 papillary RCC
compared with type 2 papillary RCC, but this did not reach
statistical significance. It is difficult to draw conclusions from
this since a large proportion of the papillary tumors in the
present study were neither specified as type 1 nor type 2, and the
difficulties in making this assignment are also recognized in the
literature by the wide variation in the proportions of the two
tumor types (26). Any attempt to
compare the survival outcomes of the two types requires accurate
assignment to the appropriate category. This distinction also
affects the survival properties of papillary RCC, as some of the
tumors previously designated as type 2 papillary RCC are now
reclassified as other subtypes based on immunohistochemical
findings, and this partly explains the lack of a significant
difference in survival between papillary RCC and the other subtypes
in the data of the present study.
Grading of RCC was largely based on the Fuhrman
system until it was superseded by the ISUP grading scheme (12,13).
Considering the period in which the tumors in the present study
were reported, some have been graded using the Fuhrman system,
while the more recent tumors were graded using the ISUP system.
However, there is a close similarity between the two systems,
particularly in the first three grades, thus no attempt was made to
distinguish between them. The relative frequencies for the 4 grades
in the data of the present study were, in order, 21.9, 50.9, 10.1
and 7.5%. In a previous study by Dagher et al (24) regarding 374 tumors, the frequencies
of the 4 grades consecutively were 9.3, 50.3, 24.1 and 16.3%,
highlighting a lower rate of grade 1 tumors and higher rates of
grade 3 and 4 tumors compared with the findings of the present
study. This difference in the frequency of the nuclear grades can
partly be explained by interobserver variation in assigning a
nuclear grade and partly by selection bias (later detection of
renal cancer has been observed in the aforementioned studies due to
differences in risk factors and less liberal use of imaging
modalities for abdominal symptoms).
Delahunt et al (27), in a study of 121 cases, also
reported frequencies of 14, 74.3 and 11.6% for the first 3 grades
based on nucleolar prominence (27). In another study conducted on 125
patients with papillary RCC of both types, rates of 31, 36, 32, and
1% for the 4 grades in order were observed. These two studies show
the differences in the relative frequencies of the four grades when
tumors are selected by histological type (28).
The prognostic significance of these various grades
has been presented in several studies to various degrees (24,27,29).
Delahunt et al (27)
identified a statistically significant difference in survival
between grades 2 and 3 based on the worst nucleolar grade in both
univariate and multivariate analyses.
Khor et al demonstrated no significant
difference in outcome among grades 1, 2 and 3, but the difference
for grade 4 was significant (29).
Dagher et al (24) showed
significant differences in cancer-free survival among grades 2, 3,
and 4. In the results of the present study, the impact of tumor
grade on survival was only significant when the tumors were grouped
into a low-grade tier (ISUP grades 1 and 2) and a high-grade tier
(ISUP grades 3 and 4), negating the significance of any difference
between grades 1 and 2 on the one hand and grades 3 and 4 on the
other hand. These differences may pertain to the accuracy of
nuclear grading in these various studies and the lack of a
significant difference in behavior and outcome between grades 1 and
2 on the one hand and between grades 3 and 4 on the other hand.
Tumor necrosis was present in 30.7% of the cases of
the present study, and this is comparable to what others have
reported, including 30% by Katz et al (30), 30.4% by Sengupta et al
(31), 27% by Lee et al
(32) and 37.2% by Zhang et
al (22). Lee et al
(32) revealed that the presence
of tumor necrosis was more likely to be associated with increasing
tumor size, higher tumor stage, higher tumor grade, microvascular
invasion, and the presence of sarcomatoid change (32). The present study also demonstrated
a significant association between tumor necrosis and tumor size,
stage, grade and MAI.
With regards to its impact on survival, Lee et
al (32) showed that the
presence of necrosis had a significant impact on non-metastatic,
clear cell RCC that persisted even on multivariate analysis, while
the effect was lost for metastatic tumors as well as for non-clear
cell tumors (32). Katz et
al (30) revealed that the
presence of tumor necrosis associated with survival on a
univariate, but not a multivariate, analysis. Sengupta et al
(31) observed that necrosis
significantly affected survival for all three major tumor types in
both univariate and multivariate analyses. In the present study,
tumor necrosis did not significantly affect survival when analyzed
for all the tumor types combined, but it did significantly reduce
survival for clear cell RCC in particular when the analysis was
stratified for tumor morphotype.
Sarcomatoid change was present in 5% of the tumors
studied by Cheville et al (33). On both univariate and multivariate
analysis, the authors observed a significant decrease in survival
in the presence of sarcomatoid change, regardless of the type of
RCC or tumor grade. In their study of 101 RCCs with sarcomatoid
change, De Peralta-Venturina et al (34) identified worse survival on both
univariate and multivariate analysis. The findings of the present
study were mostly concordant with the aforementioned studies, with
6.6% of the tumors in the present study having sarcomatoid change,
and this revealed a significant impact on survival on univariate
analysis but not on multivariate analysis when compared with the
presence of necrosis, rhabdoid change and microvascular invasion.
Sarcomatoid change was also associated with a higher rate of
metastasis.
Rhabdoid change has been reported with a frequency
of 4.7% by Gökden et al (35) and 4.5% by Leroy et al
(36). They both identified a
significant impact of rhabdoid change on the rate of survival and
metastasis. In the present study, 4.4% of the tumors demonstrated
rhabdoid change, and this was associated with a significant
decrease in survival and an increase in the rate of metastasis only
in univariate analysis.
Microvascular invasion has been reported at a wide
range of frequencies, with Kroeger et al (37) reported it in 18% of their cases and
demonstrating a strong association with increasing tumor size,
tumor grade and tumor stage. In the present study, the incidence of
microvascular invasion was 14.5%, with a similarly strong
association with tumor size, tumor grade and tumor stage, as well
as with MAI. Kroeger et al (37) demonstrated a higher rate of
metastasis and lower survival in the presence of microvascular
invasion, but the effect on survival was lost on multivariate
analysis. The present study showed similar findings, but the
significance was retained even on multivariate analysis with
necrosis, sarcomatoid change, and rhabdoid change.
In the present study, tumor invasion into the renal
sinus, pelvicalyceal system, renal capsule, perinephric fat, Gerota
fascia and the renal vein was significantly associated with
decreased survival and an increased risk of metastasis, in
consistency with previous studies (38-40).
Shah et al (41), however,
showed no significant impact on survival for isolated perinephric
fat invasion, renal sinus invasion, or renal vein invasion, but
there was decreased overall survival for patients with multiple
patterns of extrarenal extension.
Most of these invasion patterns, except that of the
renal capsule, are included in the TNM staging system, and they
raise the tumor stage to T3a or T4 accordingly (11). Tsui et al (42) demonstrated that as the overall TNM
stage increased, cancer-specific survival decreased, although there
was no independent effect for the tumor stage. The findings of the
present study also showed no independent effect of the tumor stage
on overall survival beyond the effect of tumor size, as there was a
significant difference in the outcome between tumors of stage T2a
or lower (which are ≤10 cm in size) and tumors of stage T2b or
higher (which are >10 cm), but there was no significant
difference between T2b tumors and tumors that were T3a or higher.
These findings demonstrated that tumor size had an independent
impact on survival, while tumor stage and invasion parameters did
not.
The value of these prognostic indicators can be
further refined by performing additional, functional studies on
similar datasets. These can include immunohistochemical testing for
the expression level and localization of various proteins,
including tumor suppressors, cell cycle regulators, and angiogenic
factors, and the association of these with the histopathologic
parameters that have been outlined in this study as well as with
survival and risk of metastasis. Other study designs of interest
can include tissue microarray testing for protein expression
patterns and mutation analysis to identify differences in the
genetic makeup of these tumors and how they associate with
differences in aggressiveness, survival, and metastasis. The small
sample size and data from a single center were the limitations to
the present study.
In conclusion, accurate assessment of the gross and
histologic parameters of RCC is essential for tumor
prognostication, as numerous of these histological features
significantly impact the overall survival and the risk of
metastasis. The most important of these parameters are tumor size,
grade, and microvascular invasion.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RA and DM are pathologists who examined the
specimens and contributed majorly in the conception of the study,
data analysis, as well as for the literature search for related
studies. RA and DM confirm the authenticity of all the raw data.
RB, SF, BA and SM were involved in literature review, data
collection and organization. FK and HA contributed in the
literature review, acquisition and interpretation of data and the
writing of the manuscript. ST, RR, CO and HR were involved in the
literature review, the design of the study and the critical
revision of the manuscript. FA and MK contributed in the processing
of the figures and the critical revision of the manuscript. All the
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
130/2021) by the ethical committee of the University of Sulaimani
(Sulaymaniyah, Iraq). Written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Belldegrun AS: Renal cell carcinoma:
Prognostic factors and patient selection. Eur Urol Suppl.
6:477–483. 2007.
|
|
2
|
Bapir R, Hammood ZD, Omar SS, Salih AM,
Kakamad FH, Najar KA, et al: Synchronous invasive ductal breast
cancer with clear cell renal carcinoma: A rare case report with
review of literature. IJS Short Reports. 7(59)2022.
|
|
3
|
Fateh SM, Arkawazi LA, Tahir SH, Rashid
RJ, Rahman DH, Aghaways I, Kakamad FH, Salih AM, Bapir R,
Fakhralddin SS, et al: Renal cell carcinoma T staging: Diagnostic
accuracy of preoperative contrast-enhanced computed tomography. Mol
Clin Oncol. 18(11)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Furniss D, Harnden P, Ali N, Royston P,
Eisen T, Oliver RT and Hancock BW: National Cancer Research
Institute Renal Clinical Studies Group. Prognostic factors for
renal cell carcinoma. Cancer Treat Rev. 34:407–426. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yurut-Caloglu V, Caloglu M, Kaplan M,
Oz-Puyan F, Karagol H, Ibis K, Cosar-Alas R, Kocak Z and Inci O:
Prognostic factors for renal cell carcinoma: Trakya University
experience from Turkey. Eur J Cancer Care (Engl). 19:656–663.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Klatte T, Rossi SH and Stewart GD:
Prognostic factors and prognostic models for renal cell carcinoma:
A literature review. World J Urol. 36:1943–1952. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kontak JA and Campbell SC: Prognostic
factors in renal cell carcinoma. Urol Clin North Am. 30:467–480.
2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Méjean A, Oudard S and Thiounn N:
Prognostic factors of renal cell carcinoma. J Urol. 169:821–827.
2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suárez C, Campayo M, Bastús R, Castillo S,
Etxanitz O, Guix M, Sala N and Gallardo E: Prognostic and
predictive factors for renal cell carcinoma. Target Oncol.
13:309–331. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Volpe A and Patard JJ: Prognostic factors
in renal cell carcinoma. World J Urol. 28:319–327. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual (8th edition).
Springer International Publishing: American Joint Commission on
Cancer; 2017.
|
|
12
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Delahunt B, Cheville JC, Martignoni G,
Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch
H, Grignon DJ, et al: The international society of urological
pathology (ISUP) grading system for renal cell carcinoma and other
prognostic parameters. Am J Surg Pathol. 37:1490–1504.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
‘SEER Cancer Stat Facts: Kidney and Renal
Pelvis Cancer’. National Cancer Institute. Bethesda, MD, 2021.
https://seer.cancer.gov/statfacts/html/kidrp.html.
|
|
15
|
Hardi Tariq Hama, Ismaeel Hama Ameen
Akhaways and Lusan Abdulhameed Arkawazi: Incidence of Metastatic
Renal Cell Carcinoma in Sulaimaniyah Government, Iraq. J Zankoy
Sulaimani. 24:102–112. 2022.
|
|
16
|
Weikert S and Ljungberg B: Contemporary
epidemiology of renal cell carcinoma: Perspectives of primary
prevention. World J Urol. 28:247–252. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aron M, Nguyen MM, Stein RJ and Gill IS:
Impact of gender in renal cell carcinoma: An analysis of the SEER
database. Eur Urol. 54:133–140. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kattan M, Reuter V, Motzer R, Katz J and
Russo P: A postoperative prognostic nomogram for renal cell
carcinoma. J Urol. 166:63–67. 2001.PubMed/NCBI
|
|
19
|
Jiang YL, Peng CX, Wang HZ and Qian LJ:
Comparison of the long-term follow-up and perioperative outcomes of
partial nephrectomy and radical nephrectomy for 4 cm to 7 cm renal
cell carcinoma: A systematic review and meta-analysis. BMC Urol.
19(48)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Guo S, Yao K, He X, Wu S, Ye Y, Chen J and
Wu CL: Prognostic significance of laterality in renal cell
carcinoma: A population-based study from the surveillance,
epidemiology, and end results (SEER) database. Cancer Med.
8:5629–5637. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sargin S, Ekmekcioglu O, Arpali E, Altinel
M and Voyvoda B: Multifocality incidence and accompanying
clinicopathological factors in renal cell carcinoma. Urol Int.
82:324–329. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang C, Li X, Hao H, Yu W, He Z and Zhou
L: The correlation between size of renal cell carcinoma and its
histopathological characteristics: A single center study of 1867
renal cell carcinoma cases. BJU Int. 110:E481–E485. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cheville JC, Lohse CM, Zincke H, Weaver AL
and Blute ML: Comparisons of outcome and prognostic features among
histologic subtypes of renal cell carcinoma. Am J Surg Pathol.
27:612–624. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dagher J, Delahunt B, Rioux-Leclercq N,
Egevad L, Srigley JR, Coughlin G, Dunglinson N, Gianduzzo T, Kua B,
Malone G, et al: Clear cell renal cell carcinoma: Validation of
World Health Organization/International society of urological
pathology grading. Histopathology. 71:918–925. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Patard JJ, Leray E, Rioux-Leclercq N,
Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani
W, Abbou CC, et al: Prognostic value of histologic subtypes in
renal cell carcinoma: A multicenter experience. J Clin Oncol.
23:2763–2771. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Srigley JR, Delahunt B, Eble JN, Egevad L,
Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, et
al: The international society of urological pathology (ISUP)
vancouver classification of renal neoplasia. Am J Surg Pathol.
37:1469–1489. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Delahunt B, Sika-Paotonu D, Bethwaite PB,
William Jordan T, Magi-Galluzzi C, Zhou M, Samaratunga H and
Srigley JR: Grading of clear cell renal cell carcinoma should be
based on nucleolar prominence. Am J Surg Pathol. 35:1134–1139.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cornejo KM, Dong F, Zhou AG, Wu CL, Young
RH, Braaten K, Sadow PM, Nielsen GP and Oliva E: MPapillary renal
cell carcinoma: Correlation of tumor grade and histologic
characteristics with clinical outcome. Hum Pathol. 46:1411–1417.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khor LY, Dhakal HP, Jia X, Reynolds JP,
McKenney JK, Rini BI, Magi-Galluzzi C and Przybycin CG: Tumor
necrosis adds prognostically significant information to grade in
clear cell renal cell carcinoma: A study of 842 consecutive cases
from a single institution. Am J Surg Pathol. 40:1224–1231.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Katz MD, Serrano MF, Grubb RL, Skolarus
TA, Gao F, Humphrey PA and Kibel AS: Percent microscopic tumor
necrosis and survival after curative surgery for renal cell
carcinoma. J Urol. 183:909–914. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sengupta S, Lohse CM, Leibovich BC, Frank
I, Thompson RH, Webster WS, Zincke H, Blute ML, Cheville JC and
Kwon ED: Histologic coagulative tumor necrosis as a prognostic
indicator of renal cell carcinoma aggressiveness. Cancer.
104:511–520. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lee SE, Byun SS, Oh JK, Lee SC, Chang IH,
Choe G and Hong SK: Significance of macroscopic tumor necrosis as a
prognostic indicator for renal cell carcinoma. J Urol.
176:1332–1338. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cheville JC, Lohse CM, Zincke H, Weaver
AL, Leibovich BC, Frank I and Blute ML: Sarcomatoid renal cell
carcinoma: An examination of underlying histologic subtype and an
analysis of associations with patient outcome. Am J Surg Pathol.
28:435–441. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
de Peralta-Venturina M, Moch H, Amin M,
Tamboli P, Hailemariam S, Mihatsch M, Javidan J, Stricker H, Ro JY
and Amin MB: Sarcomatoid differentiation in renal cell carcinoma: A
study of 101 cases. Am J Surg Pathol. 25:275–284. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gökden N, Nappi O, Swanson PE, Pfeifer JD,
Vollmer RT, Wick MR and Humphrey PA: Renal cell carcinoma with
rhabdoid features. Am J Surg Pathol. 24:1329–1338. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Leroy X, Zini L, Buob D, Ballereau C,
Villers A and Aubert S: Renal cell carcinoma with rhabdoid
features: An aggressive neoplasm with overexpression of p53. Arch
Pathol Lab Med. 131:102–106. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kroeger N, Rampersaud EN, Patard JJ,
Klatte T, Birkhäuser FD, Shariat SF, Lang H, Rioux-Leclerq N, Remzi
M, Zomorodian N, et al: Prognostic value of microvascular invasion
in predicting the cancer specific survival and risk of metastatic
disease in renal cell carcinoma: a multicenter investigation. J
Urol. 187:418–423. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Thompson RH, Leibovich BC, Cheville JC,
Webster WS, Lohse CM, Kwon ED, Frank I, Zincke H and Blute ML: Is
renal sinus fat invasion the same as perinephric fat invasion for
pT3a renal cell carcinoma? J Urol. 174:1218–1221. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yoo C, Song C, Hong J, Kim C and Ahn H:
Prognostic significance of perinephric fat infiltration and tumor
size in renal cell carcinoma. J Urol. 180:486–491. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tongaonkar H, Dandekar N, Dalal A,
Kulkarni J and Kamat M: Renal cell carcinoma extending to the renal
vein and inferior vena cava: Results of surgical treatment and
prognostic factors. J Surg Oncol. 59:94–100. 1995.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shah PH, Lyon TD, Lohse CM, Cheville JC,
Leibovich BC, Boorjian SA and Thompson RH: Prognostic evaluation of
perinephric fat, renal sinus fat, and renal vein invasion for
patients with pathological stage T3a clear-cell renal cell
carcinoma. BJU Int. 123:270–276. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tsui KH, Shvarts O, Smith RB, Figlin RA,
deKernion JB and Belldegrun A: Prognostic indicators for renal cell
carcinoma: A multivariate analysis of 643 patients using the
revised 1997 TNM staging criteria. J Urol. 163:1090–1295.
2000.PubMed/NCBI View Article : Google Scholar
|