1. Introduction
Glioblastoma multiforme (GBM) is certainly the most
frequent and malignant primary central nervous system (CNS) tumor
appearing in adults, as <20% of these survive ~1 year after
diagnosis (1).
The gold-standard management of GBM includes
post-operative radiotherapy (RT) with concurrent and adjuvant
temozolomide (TMZ) (2). However,
numerous elderly patients with glioblastoma are considered too
frail to tolerate the TMZ/RT combination (3). Furthermore, the absence of previous
tumor resection surgery leads to greater neurological instability
during treatment, indicating a clear need for alternative
methods.
Bevacizumab is a VEGF-targeting antibody and is
considered to be one of the most favorable candidate treatments to
improve the outcome of elderly patients with glioblastoma.
Nevertheless, in the first-line setting, only three trials favored
the advantage of bevacizumab in frail and elderly patients
(4-6).
The present meta-analysis study examined the
efficacy of the early administration of bevacizumab prior to
standard RT plus TMZ in managing patients with GBM and unfavorable
prognostic factors.
2. Sources and data extraction
Literature search strategy
The present study searched comparative articles
involving standard RT plus TMZ and RT/TMZ accompanied by
bevacizumab treatment in patients with GBM through electronic
databases, including the Cochrane Library, Medline (1983-2020.8;
https://www.cochranelibrary.com/),
PubMed (1983-2020.8; https://pubmed.ncbi.nlm.nih.gov/), and EMBASE
(1983-2020.8; https://www.elsevier.com/solutions/embase-biomedical-research)
Preferred reporting items for systematic reviews and meta-analyses
(PRISMA) were applied for establishing protocol and manuscript
design (7). The present study used
the keywords ‘radiotherapy,’ ‘chemotherapy,’ ‘temozolomide,’
‘bevacizumab,’ and ‘chemoradiotherapy’ in the MeSH list.
Inclusion and exclusion criteria
The literature was included in the present
meta-analysis if the article met the following criteria, as
determined by PICOS: i) Population: Limited to patients with GMB;
ii) Intervention: For GBM, the standard RT/TMZ and bevacizumab plus
standard RT/TMZ treatment were used. iii) Comparison: the outcomes
were compared. Table I contains
detailed data on these articles.
 | Table IDesign and baseline characteristics of
included trials. |
Table I
Design and baseline characteristics of
included trials.
| | Sample size | Mean age (years) | Number of males | OS | PFS | |
|---|
| Trial, year | BEV | CG/Control | BEV | CG/Control | BEV | CG/Control | BEV | CG/Control | BEV | CG/Control | (Refs.) |
|---|
| Chinot et al,
2014 | 458 | 921 | 56 | 57 | NR | NR | 17 | 17 | 11 | 6 | (4) |
| Gilbert et al,
2014 | 320 | 637 | NR | NR | NR | NR | 16 | 16 | 11 | 7 | (5) |
| Balana et al,
2016 | 44 | 87 | 62.9 | 62 | 31 | 25 | 11 | 8 | 5 | 2 | (9) |
| Wirsching et
al, 2018 | 50 | 75 | 70 | 70 | 32 | 16 | 12 | 12 | 12 | 12 | (10) |
Outcome measures: It involved one of the primary
outcomes, including progression-free survival (PFS) and overall
survival (OS). To avoid publication bias, the final aim was to
collect a homogenous pool of manuscripts, including articles that
compared only two modalities: standard RT/TMZ or bevacizumab plus
standard RT/TMZ.
Articles that were excluded from that article pool
were those that were editorials, reviews, case reports, articles
focusing on the pediatric population, unrelated outcomes,
co-morbidities, experimental techniques, or one of the two
treatment modalities and all those that demonstrated mixed or
unclear results, being separated between standard RT plus TMZ
(CG/Control group) or bevacizumab plus standard RT/TMZ (BEV group)
treatment (Fig. 1).
Data extraction and definition of
outcomes
In the present study, two of the reviewers (GF and
VEG) independently extracted data from the included articles,
following the guidelines of the epidemiology of meta-analysis. The
following essential information was captured: The main authors,
year of publication, total case number in the BEV and CG/Control
groups, study type and outcome indicator. The extracted data were
entered into a designed, standardized table according to the
Cochrane Handbook. When there was disagreement, another authority
author had the final say.
The primary outcomes involved in the present study
included PFS and OS. PFS was defined as the time from inclusion to
the first documented progression or mortality from any cause. OS
was defined as the time from inclusion to mortality from any cause.
The outcomes reported by the included articles were assessed at
least six months after the treatment (standard RT plus TMZ or
bevacizumab plus standard RT/TMZ). Additionally, to decrease the
risk of bias in poor articles, a quality assessment tool (the
Newcastle-Ottawa Scale) was used (Table II) (8).
 | Table IINewcastle-Ottawa Scale quality
assessment of the final article pool. |
Table II
Newcastle-Ottawa Scale quality
assessment of the final article pool.
| | Newcastle-Ottawa
Scale |
|---|
| Trial, year | Study design | Selection | Comparability | Exposure | Total scores | (Refs.) |
|---|
| Chinot et
al, 2014 | prosp | 3 | 3 | 3 | 9 | (4) |
| Gilbert et
al, 2014 | prosp | 3 | 3 | 3 | 6 | (5) |
| Balana et
al, 2016 | prosp | 3 | 3 | 3 | 9 | (9) |
| Wirsching et
al, 2018 | prosp | 3 | 2 | 2 | 7 | (10) |
Additionally, the patients were divided into two
groups: Those receiving therapy with bevacizumab plus standard
RT/TMZ (BEV group) and those receiving therapy with standard RT
plus TMZ (CG/Control group).
Statistical analysis
All analyses were carried out using STATA, version
16 (StataCorp LLC). Heterogeneity across trials was identified
using 12 statistics; considering 12 >50% as high heterogeneity,
a meta-analysis was conducted using a random-effect model according
to the Cochrane Handbook for Systematic Reviews of Interventions
(version 5.1.0; www.cochrane-handbook.org). Otherwise, the
fixed-effect model was performed. The continuous outcomes were
expressed as a weighted mean difference with 95% confidence
intervals (CIs). For discontinuous variables, odds ratios (OR) with
95% CIs were applied for the assessment. P<0.05 was considered
to indicate a statistically significant difference.
3. Data on the comparison of the outcome
after bevacizumab administration at the temozolamide or/plus
radiosurgery treatment in patients with glioblastoma
After the initial search, 55 articles were eligible
for further analysis. Applying all exclusion and inclusion
criteria, four articles were left in the final article pool
(Fig. 1) (4,5,9,10).
The total number of patients included in those four
articles was 2,592 (872 in the BEV group and 1,720 in the
CG/Control group). The detailed results of these articles are
presented in Table III.
 | Table IIIMeta-analysis results. |
Table III
Meta-analysis results.
| | Groups | Overall effect | Heterogeneity |
|---|
| Outcomes | Trial, n=4 | BEV | CG/Control | Effect
estimate | CI 95% | P-value | I2
(%) | P-value |
|---|
| OS | 4 | 56 | 53 | 0.67 | (0.28-1.07) | <0.05 | -270.32 | 0.85 |
| PFS | 4 | 39 | 27 | 0.92 | (0.41-1.43) | <0.05 | -31.49 | 0.52 |
OS. Information regarding the OS was
available in all articles (4,5,9,10).
There were 109 patients in the total group of patients (109/2,592):
56 in the BEV group and 53 in the CG/Control group. The pooled
results demonstrated a statistically significant difference between
the BEV and CG/Control groups [OR 0.67, CI 95% (0.28-1.07), and
P<0.05] with no heterogeneity (P=0.85 and I2=-270.32%
(Fig. 2A and B).
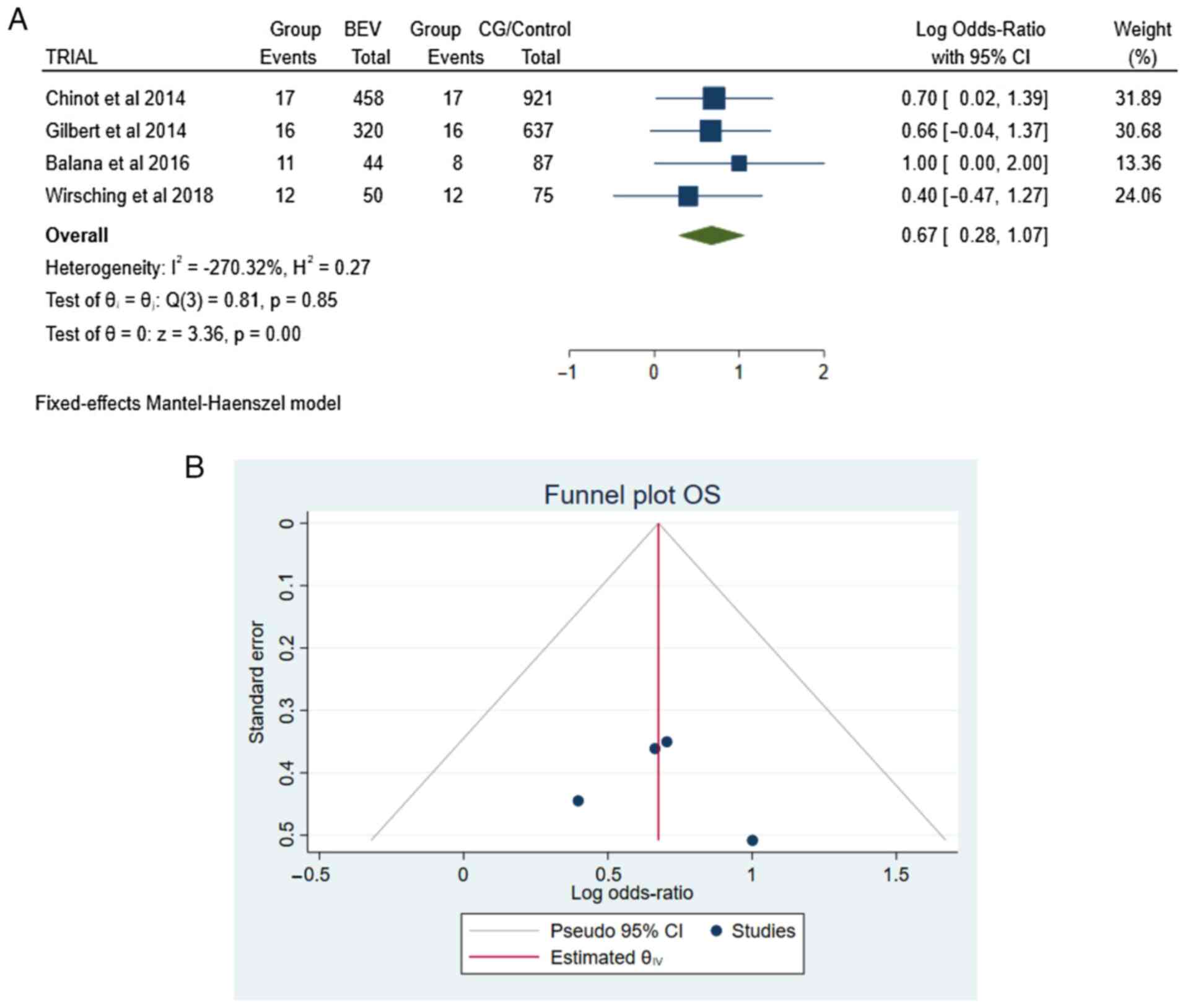 | Figure 2(A) Forest plot OS: Results
demonstrated a statistically significant difference between the BEV
and CG/Control groups [OR 0.67, CI 95% (0.28-1.07), and P<0.05.
(B) Funnel plot, testing the sensitivity with funnel plot for OS
there was no statistically significant superiority between groups,
with no heterogeneity (P=0.85 and I2=-270.32%. OS,
overall survival; RT, radiotherapy; TMZ, temozolomide; BEV,
bevacizumab plus standard RT/TMZ treatment; CG/Control, RT plus
TMZ; OR, Odds Ratio; I2 shows the percentage of total
variation across studies that is due to heterogeneity rather than
chance; CI, confidence interval. |
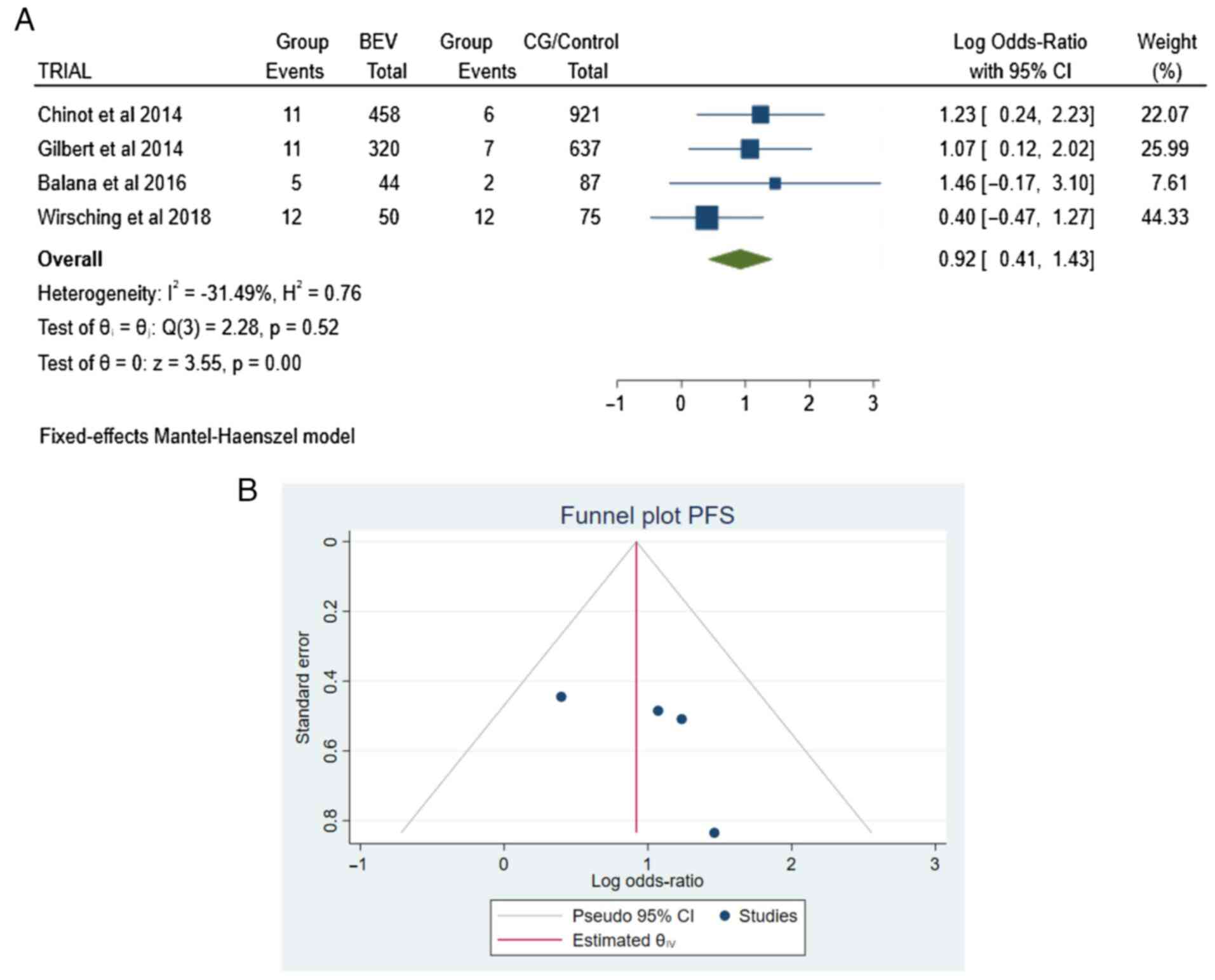
PFS. The four articles (4,5,9,10)
contained information about PFS. There were 66 patients in total
(66/2,592): 39 in the BEV group and 27 in the CG/Control group,
with no heterogeneity (P=0.52 and I2=-31.49%) (Fig. 3A and B).
4. Discussion
The present study suggested that bevacizumab
administration plus standard RT/TMZ (BEV group) treatment was
associated with increased survival of patients with GBM compared
with those treated with standard RT/TMZ (CG/Control group)
treatment alone. More precisely, OS and PFS were statistically
significant parameters in patients with GBM, showing the
superiority of bevacizumab administration over the standard RT/TMZ
treatment. The findings of the present meta-analysis study
suggested that this treatment may benefit the management of
GBM.
According to reports with bevacizumab management in
GBM patients, the benefit may be pronounced in elderly and poor
patients (11-13).
In addition, a predisposition to extended OS has been noted in
patients with lower Karnofsky performance scores and those who did
not obtain additional medication at the time of cancer development
(14). However, these explanations
lack statistical significance.
Additionally, according to patients' accounts,
quality of life was preserved under bevacizumab treatment for at
least up to tumor development and more patients received corticoids
with bevacizumab (10). On the
other hand, in some studies, there is an association between
bevacizumab treatment and worse cognitive functioning, encouraging
the assumption of presumed neurotoxicity (13,15,16).
However, other causes possibly affecting cognitive function in
individual patients are the instabilities in cognitive behavior at
baseline, the extended cure, and unknown cancer evolution (13,15,16).
Glioblastoma is commonly an unoperated tumor with
residual mass (17-19),
and those patients have an unfortunate outcome (2).
In the TEMAVIR trial (20) with unresected GBM patients and
bevacizumab as first-line treatment, although PFS was longer in the
TMZ plus BEV arm, the trial did not achieve its main endpoint of an
increase from 50-66% in 6-month PFS.
Intriguingly, the objective response was associated
with extended survival in all patients receiving bevacizumab,
suggesting that reducing quantifiable illness can allow patients to
attain longer OS (21). A
randomized study also detected an association between the objective
response and OS (22). Although
objective response has never been measured as a good substitute for
extended survival in GBM, there are increasing signs that it can
have an affirmative effect on PFS or OS (23).
Although the present study provided evidence of
benefit with bevacizumab in combination with RT/TMZ, the effect of
bevacizumab may have been narrowed to a pseudo response, as has
been observed with other antiangiogenics (24).
There are several limitations to the present study.
First, even though all of the eligible reports that were included
were prospective, some heterogeneity was found among included
trials in the study protocols, patient characteristics, definitions
of clinical endpoints Additionally, in order to eliminate the bias,
the article pool was very small.
5. Conclusion
In conclusion, the current study added to the
evidence that additional treatment with bevacizumab in combination
with temozolomide may be more effective in terms of response and
tumor reduction than standard RT/TMZ alone in patients with
glioblastoma, with no negative impact on survival.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Authors' contributions
GF and VEG conceived the current study. VEG, AAF,
KT, IT, DAS, GF and NT analyzed the data and wrote and prepared the
draft of the manuscript. VEG and GF provided critical revisions.
All authors contributed to manuscript revision and have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests. DAS is the Editor-in-Chief for the journal, but had no
personal involvement in the reviewing process, or any influence in
terms of adjudicating on the final decision, for this article.
References
|
1
|
Alexiou GA, Tsiouris S, Kyritsis AP,
Fotakopoulos G, Goussia A, Voulgaris S and Fotopoulos AD: The value
of 99mTc-tetrofosmin brain SPECT in predicting survival in patients
with glioblastoma multiforme. J Nucl Med. 51:1923–1926.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Herrlinger U, Scha¨fer N, Steinbach J,
Weyerbrock A, Hau P, Goldbrunner R, Leutgeb B, Urbach H, Stummer W
and Glas M: The randomized, multicenter Glarius trial investigating
bevacizumab/irinotecan vs standard temozolomide in newly diagnosed,
MGMT-non-methylated glioblastoma patients: Final survival results
and quality of life. Neuro Oncol. 16:ii23–ii24. 2014.
|
|
7
|
Foster RL: Reporting guidelines: CONSORT,
PRISMA, and SQUIRE. J Spec Pediatr Nurs. 17:1–2. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M, Tugwell P, Ga SW, Zello GA and Petersen JA: The
Newcastle-Ottawa scale (NOS) for assessing the quality of
nonrandomised studies in meta-analyses. 2014.
|
|
9
|
Balana C, De Las Penas R, Sepúlveda JM,
Gil-Gil MJ, Luque R, Gallego O, Carrato C, Sanz C, Reynes G,
Herrero A, et al: Bevacizumab and temozolomide versus temozolomide
alone as neoadjuvant treatment in unresected glioblastoma: The
GENOM 009 randomized phase II trial. J Neurooncol. 127:569–579.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wirsching HG, Tabatabai G, Roelcke U,
Hottinger AF, Jörger F, Schmid A, Plasswilm L, Schrimpf D, Mancao
C, Capper D, et al: Bevacizumab plus hypofractionated radiotherapy
versus radiotherapy alone in elderly patients with glioblastoma:
The randomized, open-label, phase II ARTE trial. Ann Oncol.
29:1423–1430. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nghiemphu PL, Liu W, Lee Y, Than T, Graham
C, Lai A, Green RM, Pope WB, Liau LM, Mischel PS, et al:
Bevacizumab and chemotherapy for recurrent glioblastoma: A
single-institution experience. Neurology. 72:1217–1222.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lai A, Tran A, Nghiemphu PL, Pope WB,
Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, et al:
Phase II study of bevacizumab plus temozolomide during and after
radiation therapy for patients with newly diagnosed glioblastoma
multiforme. J Clin Oncol. 29:142–148. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al:
Phase II trial of single-agent bevacizumab followed by bevacizumab
plus irinotecan at tumor progression in recurrent glioblastoma. J
Clin Oncol. 27:740–745. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Friedman HS, Prados MD, Wen PY, Mikkelsen
T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen
R, et al: Bevacizumab alone and in combination with irinotecan in
recurrent glioblastoma. J Clin Oncol. 27:4733–4740. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Buie LW and Valgus J: Bevacizumab: A
treatment option for recurrent glioblastoma multiforme. Ann
Pharmacother. 42:1486–1490. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bauchet L, Mathieu-Daudé H, Fabbro-Peray
P, Rigau V, Fabbro M, Chinot O, Pallusseau L, Carnin C, Lainé K,
Schlama A, et al: Oncological patterns of care and outcome for 952
patients with newly diagnosed glioblastoma in 2004. Neuro Oncol.
12:725–735. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chang SM, Parney IF, Huang W, Anderson FA
Jr, Asher AL, Bernstein M, Lillehei KO, Brem H, Berger MS and Laws
ER: Patterns of care for adults with newly diagnosed malignant
glioma. JAMA. 293:557–564. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Graus F, Bruna J, Pardo J, Escudero D,
Vilas D, Barceló I, Brell M, Pascual C, Crespo JA, Erro E, et al:
Patterns of care and outcome for patients with glioblastoma
diagnosed during 2008-2010 in Spain. Neuro Oncol. 15:797–805.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chauffert B, Feuvret L, Bonnetain F,
Taillandier L, Frappaz D, Taillia H, Schott R, Honnorat J, Fabbro
M, Tennevet I, et al: Randomized phase II trial of irinotecan and
bevacizumab as neo-adjuvant and adjuvant to temozolomide-based
chemoradiation compared with temozolomide-chemoradiation for
unresectable glioblastoma: Final results of the TEMAVIR study from
ANOCEF†. Ann Oncol. 25:1442–1447. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Taal W, Oosterkamp HM, Walenkamp AM,
Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D,
de Vos FY, et al: Single-agent bevacizumab or lomustine versus a
combination of bevacizumab plus lomustine in patients with
recurrent glioblastoma (BELOB trial): A randomised controlled phase
2 trial. Lancet Oncol. 15:943–953. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Prados M, Cloughesy T, Samant M, Fang L,
Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, et
al: Response as a predictor of survival in patients with recurrent
glioblastoma treated with bevacizumab. Neuro Oncol. 13:143–151.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pérez-Larraya J, Lahutte M, Petrirena G,
Reyes-Botero G, González-Aguilar A, Houillier C, Guillevin R,
Sanson M, Hoang-Xuan K and Delattre JY: Response assessment in
recurrent glioblastoma treated with irinotecan-bevacizumab:
Comparative analysis of the Macdonald, RECIST, RANO, and RECIST + F
criteria. Neuro Oncol. 14:667–673. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kazazi-Hyseni F, Beijnen JH and Schellens
JH: Bevacizumab. Oncologist. 15:819–825. 2010.PubMed/NCBI View Article : Google Scholar
|