Introduction
Superselective intra-arterial chemotherapy combined
with radiation therapy (SSIACRT) has recently received attention
for its favorable results in oral cancer (1-4).
The method can be performed either by catheterization in a
retrograde fashion via the superficial temporal artery (STA) and
occipital artery (OA) (3) or by
catheterization via the femoral artery (FA) using the Seldinger
technique (4). In the latter
method, inserting a catheter into multiple arteries is possible;
however, since the catheter passes through the common carotid
artery (CCA), catheter operation-related neurological complications
can occur, which has had a 3% incidence rate (5). The former method is associated with a
lower risk of cerebrovascular disease, but only one peripheral
feeding artery can be selected at a time. Most advanced head and
neck cancers, including oral cancers, receive blood flow and
nutrition from multiple arteries, aptly called tumor-feeding
arteries. Therefore, when the catheter needs to be inserted into
two routes, that is, the lingual and the facial arteries, the
catheter must be inserted into each of the STA and OA (3). In intra-arterial chemotherapy (IACT)
via the STA, vascular selectivity is a significant factor affecting
prognosis (1,2). Level II metastatic lymph nodes are
often fed from sternocleidomastoid branches from OA. Which would
otherwise be difficult to perform with conventional retrograde
intra-arterial chemoradiotherapy without changing catheters.
SSIACRT with the external carotid artery sheath (ECAS) can treat
cervical lymph node metastasis at level Ⅱ without changing
catheters. The ECAS system was first reported in 2017 for its
improvements to vascular selectivity (6). Here, we report two cases in which we
were able to control a primary tumor and metastatic lymph nodes in
the ⅡA and IIB region. We used ECAS to superselectively administer
anticancer agents via three or more routes including OA for oral
cancers with lymph node metastasis in the level ⅠB to ⅡB
regions.
Case report
Case 1
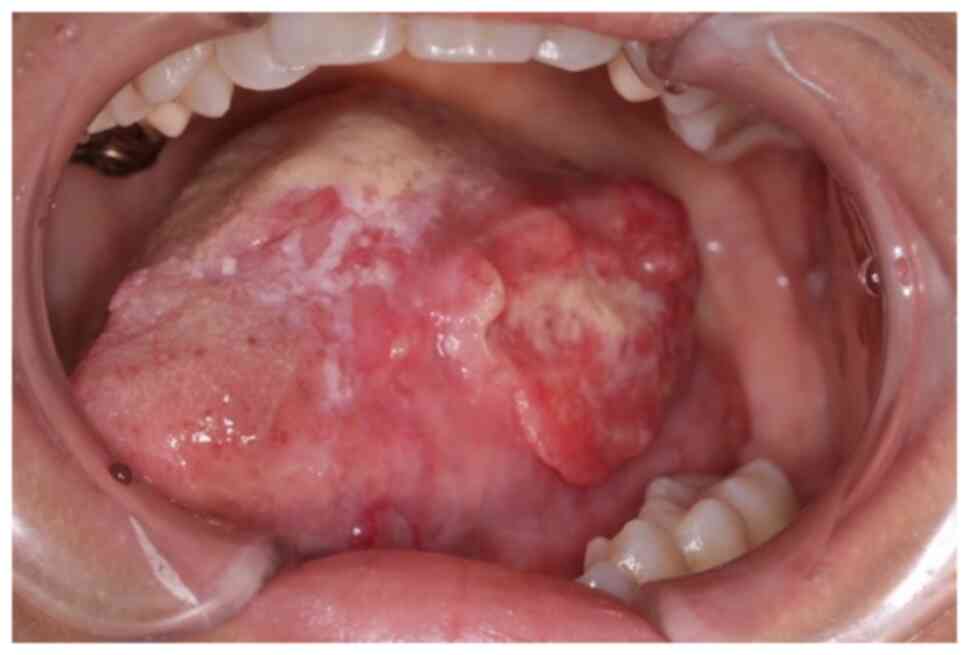
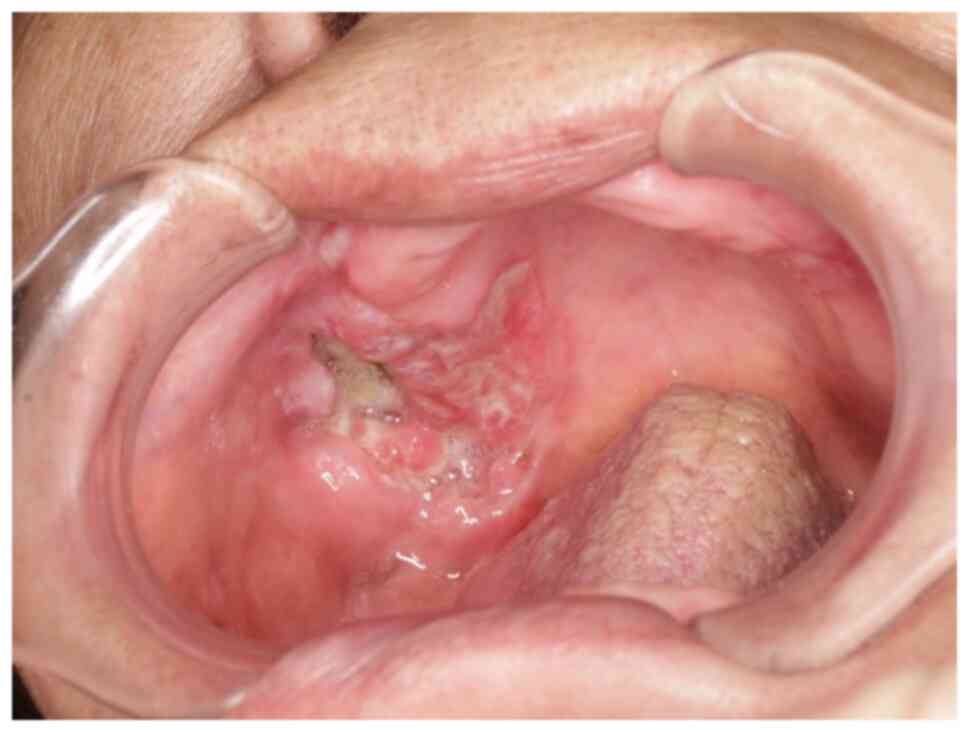
A 42-year-old female with increasing left tongue
pain was referred to our institution. On initial examination, the
patient had an ulcerative mass with induration at the left tongue
(Fig. 1), as well as a neck mass
on the left side. The tongue mass was diagnosed as a
well-differentiated squamous cell carcinoma (SCC) by biopsy.
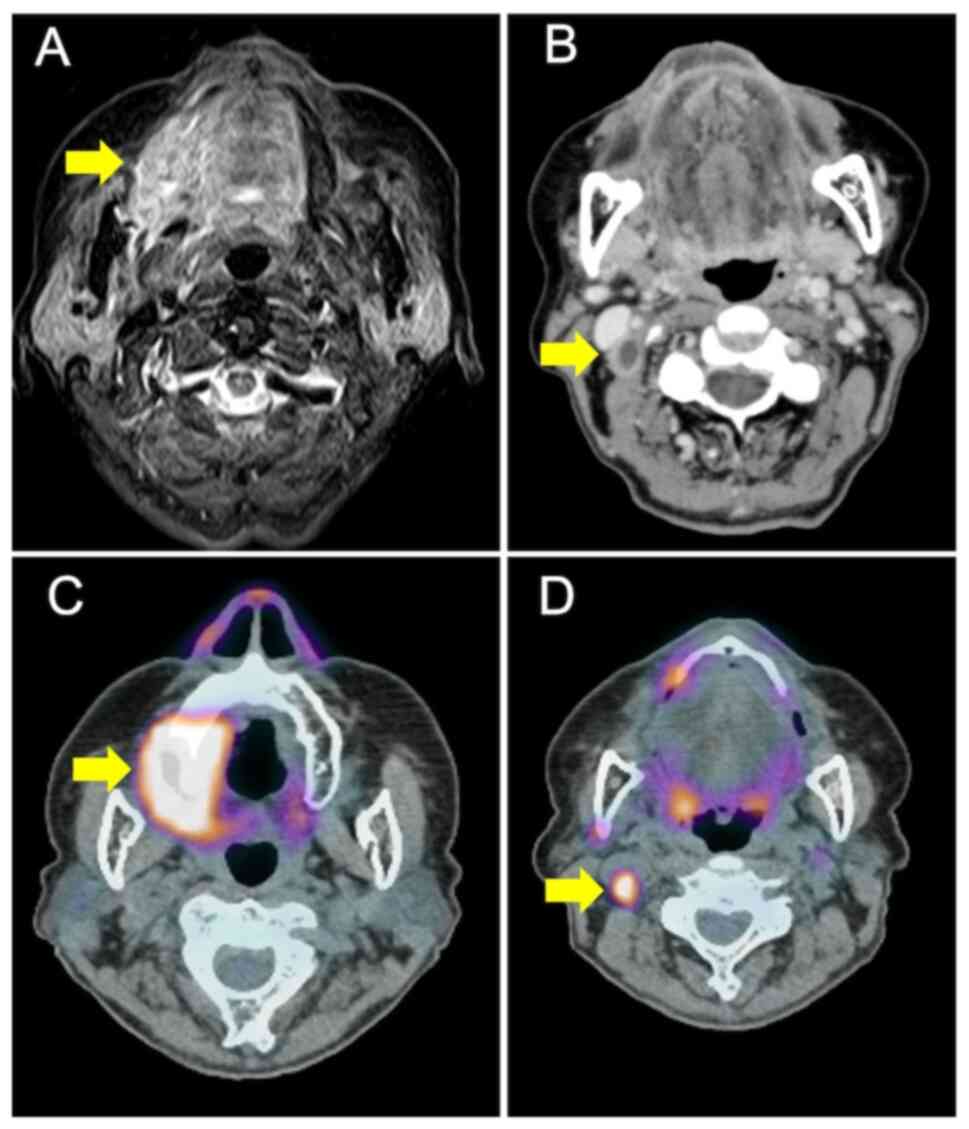
Magnetic resonance imaging (MRI) showed that the tumor extended to
near the center of the tongue (Fig.
2A). Contrast-enhanced computed tomography (E-CT) showed a
rim-enhanced mass at level II of the left cervical area, which was
located inside the sternocleidomastoid muscle (Fig. 2B). Subsequently,
18-fluorodeoxyglucose-positron emission tomography/computed
tomography (FDG-PET/CT) demonstrated high FDG uptake at the primary
tumor and cervical lymph nodes (maximum standardized uptake
values=15.5 and 7.4, respectively) (Fig. 2C). Results of the distant
metastasis workup was negative. Based on these findings, the
diagnosis was SCC of the left tongue (T3N2bM0: Stage IVA).
The patient refused treatment by surgical resection
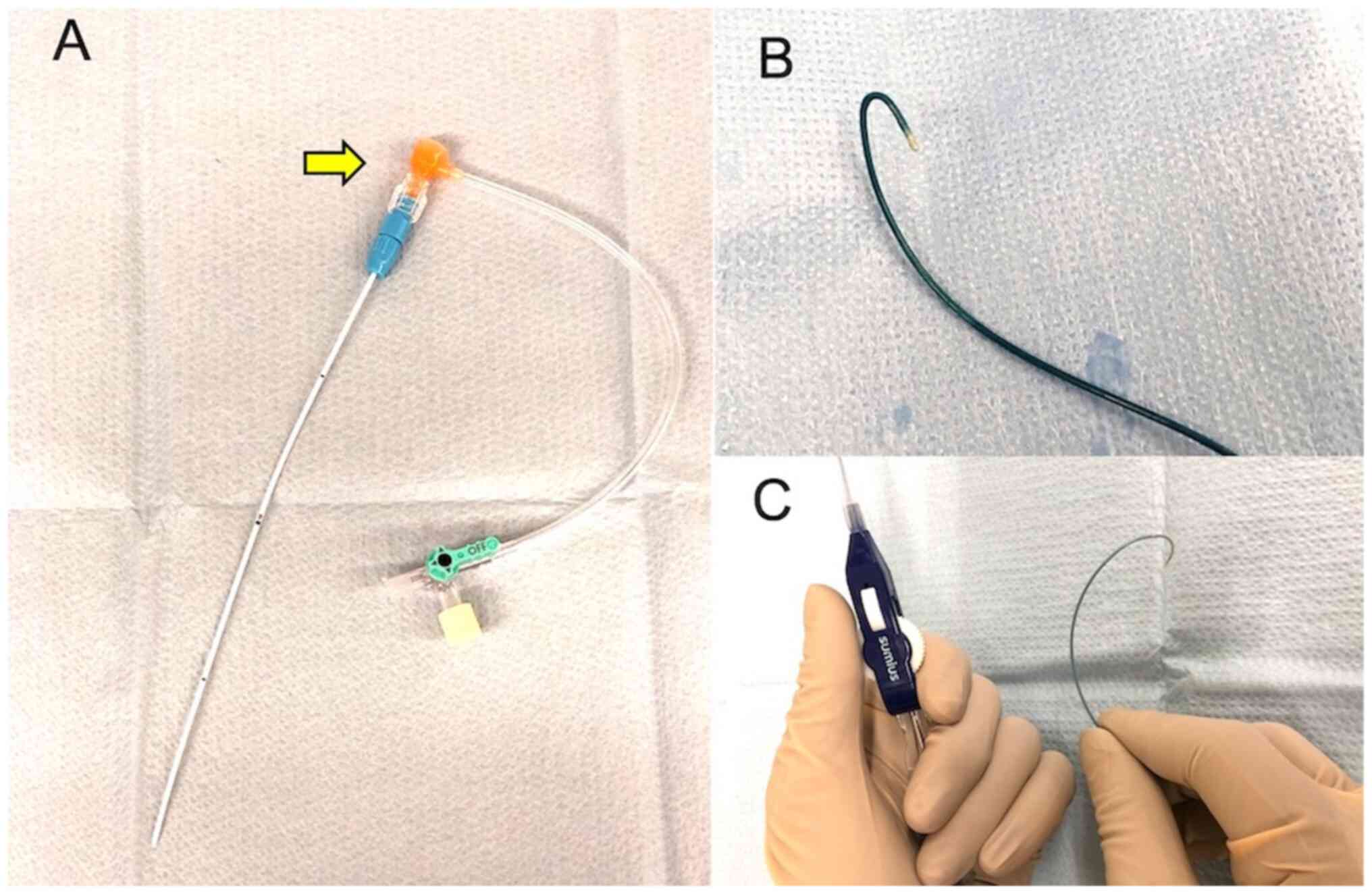
and instead underwent retrograde intra-arterial CRT using ECAS. The
ECAS (15 cm long and 5 Fr outer diameter; Toray Medical Co., Ltd.,
Tokyo, Japan) was made of polyurethane and surface-coated with
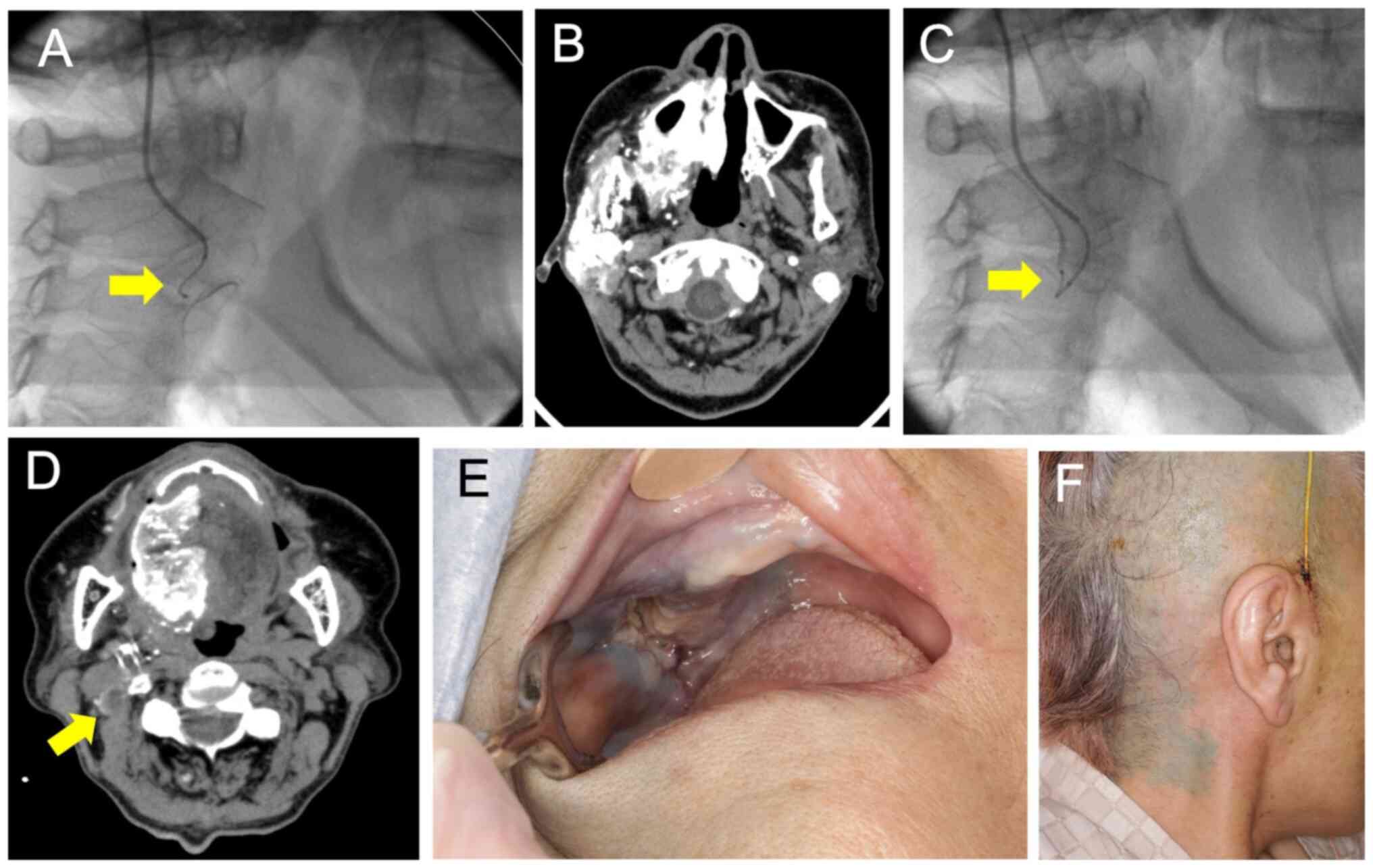
heparin resin to prevent thrombus formation A backflow valve can be
attached at its distal end (Fig.
3A), and a guidewire and microcatheter can be inserted through
the valve into the ECAS (7). The
ECAS was inserted in retrograde fashion through the STA, and its
tip was placed between the maxillary and facial arteries. The ECAS
remained indwelling during the entire 7-week course of IACT.
Heparin diluted in saline was continuously pumped into the ECAS to
prevent occlusion. Each weekly cycle of IACT was performed under
fluoroscopic guidance. First, a contrast medium was injected
through the ECAS, and a roadmap was created to identify the
position of the target arteries using digital subtraction
angiography (DSA). The feeding arteries of the primary tumor were
the left lingual artery (lt.LA) and the left facial artery (lt.FA).
The N2b (levels ⅠB and ⅡA areas) metastatic lymph nodes were
considered the vegetative arteries of the sternocleidomastoid
branch of the left facial artery and left OA (lt.OA). Hook-type
microcatheters (50 cm long and 2.3 Fr distal outer diameter; Toray
Medical Co., Ltd.) were employed to select the tumor-feeding
arteries (Fig. 3B). A guidewire
for the microcatheter (0.016 inch in diameter, Toray Medical Co.,
Ltd.) was inserted into the CCA through the ECAS under fluoroscopy
in a retrograde fashion. The microcatheter was then inserted along
the guidewire into the CCA, and the guidewire was removed. Although
not used in this case, if the external carotid artery is
significantly tortuous, it may be selected with a steerable
microcatheter (Merit SwiftNINJA®, Merit Medical System,
Inc.) with a remotely operated flexible tip. Unlike the hook-type
microcatheter, this catheter does not require a guidewire (Fig. 3C). The microcatheter was then
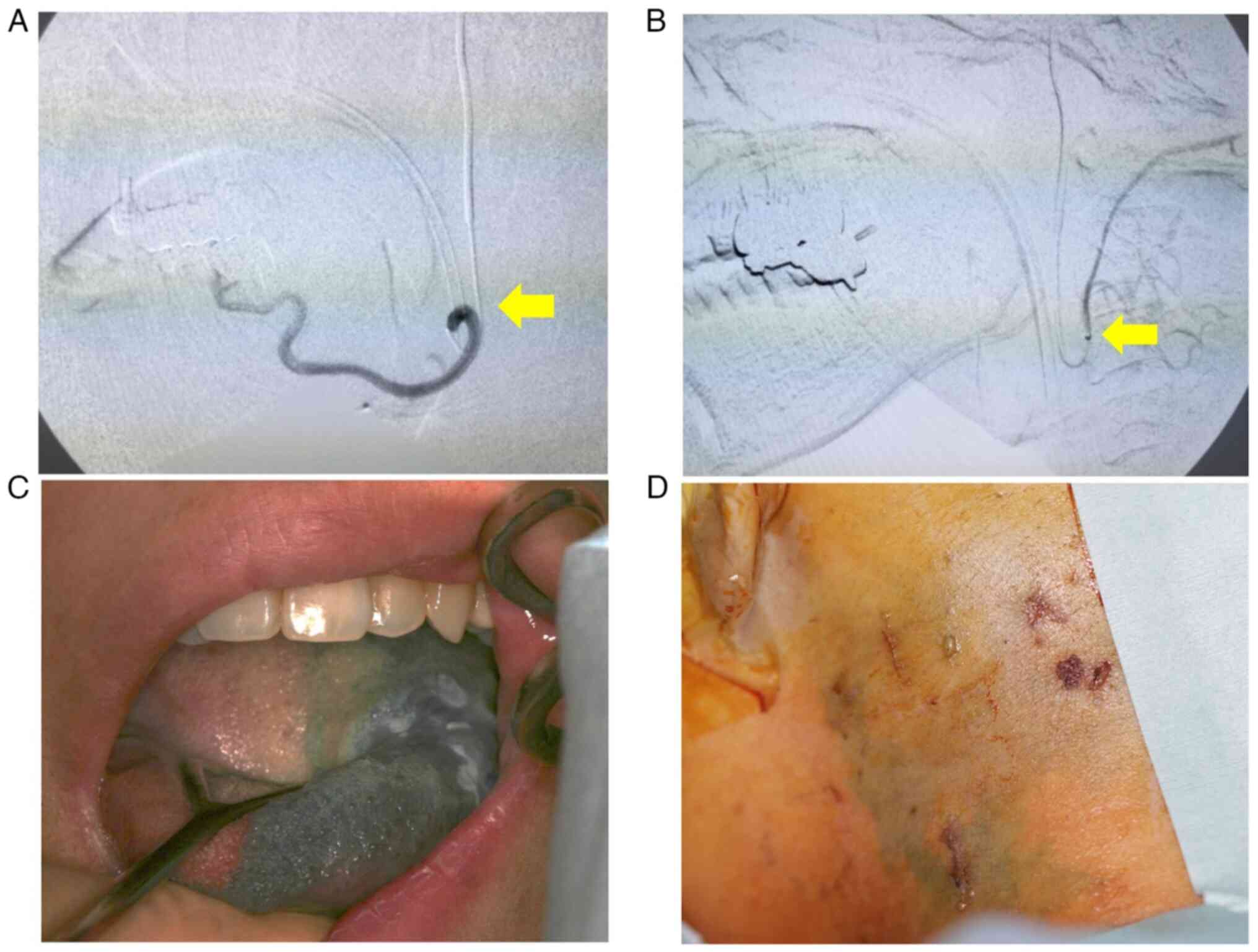
pulled back to select the target arteries using the roadmap
(Fig. 4A and B). Cisplatin was manually injected at a
total dose of 50 mg/m2 into each tumor-feeding artery at
5 ml/min once a week. IACT was performed once a week for a total of
seven cycles per week. Sodium thiosulfate (STS), a cisplatin
neutralizing agent, was intravenously administered at 0.4 g/mg
cisplatin over 8 h from 1 h prior to cisplatin administration.
Catheterization at the STA was performed using ECAS. A catheter was
inserted superselectively into the left OA via the STA. The
anticancer drug was administered through the OA at a total of 105
mg/body targeting the lymph node metastasis in the ⅡA to ⅡB
cervical region. The tongue lesions were treated with cisplatin at
235 mg/body from LA and 190 mg/body from FA to the oral floor and
tongue base. After catheterization, flow check DSA was performed to
ensure appropriate catheter placement. Indigotindisulfonate sodium
(indigocarmine) was used to dye the tongue and skin surface of the
level ⅡA to ⅡB cervical lymph nodes as confirmation of the
treatment (Fig. 4C and D). External irradiation was planned after
appropriate immobilization using a thermoplastic mask and
three-dimensional CT-based techniques. We performed irradiation on
the primary lesion and both sides of the neck. The total dose
delivered to the primary tumor and the metastatic cervical lymph
node sites was 68 Gy/34 fractions. Treatment proceeded as
indicated.
The acute adverse events (classified according to
the National Cancer Institute-Common Toxicity Criteria for Adverse
Events v. 4.0) observed within 1 month after treatment included
grade 3 oral mucositis and dermatitis, grade 2 neutropenia, and
grade 1 paronychia. No major complications such as cerebral
infarctions or other neurological complications were observed.
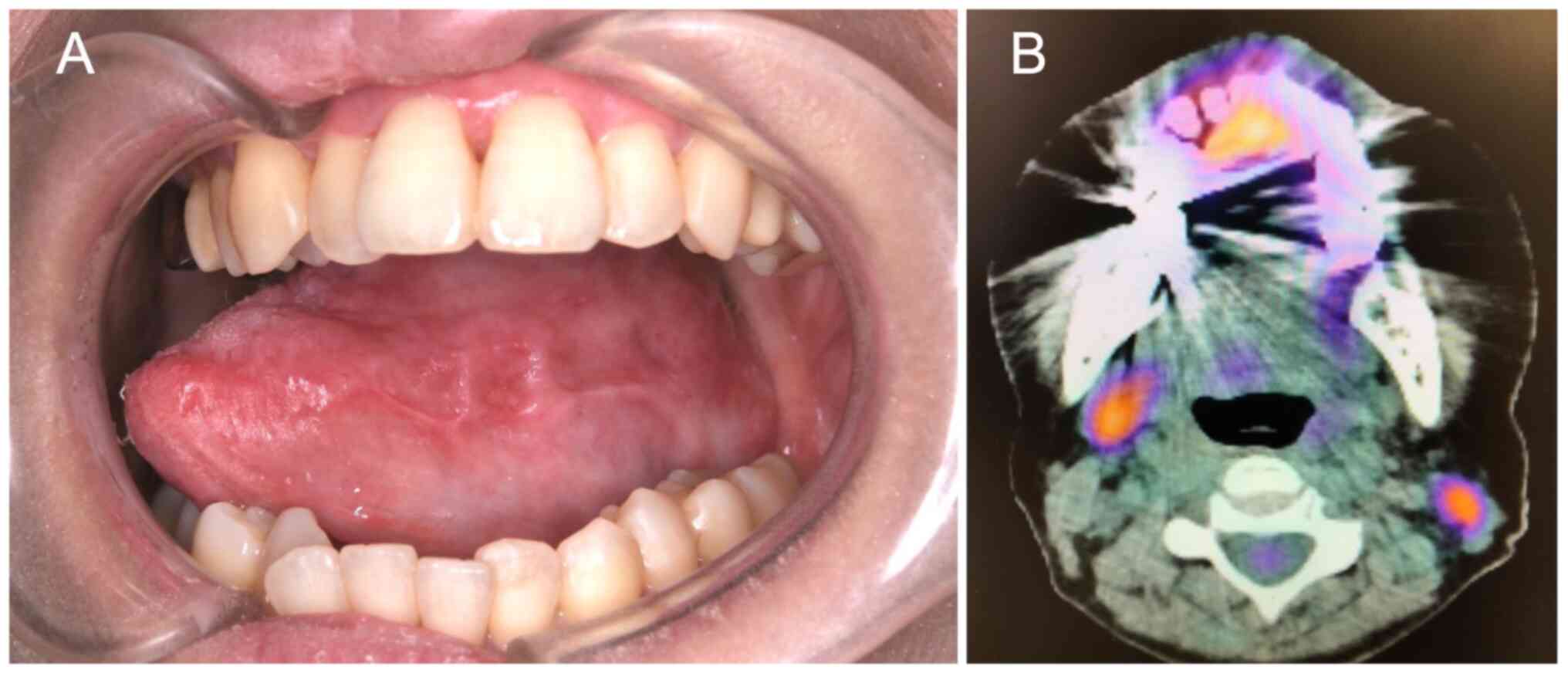
During the follow-up period, E-CT, FDG-PET/CT, and MRI of the
primary lesion were performed to evaluate the treatment outcomes.
E-CT and MRI showed indistinctive masses of the primary lesion and
nodal metastases. FDG-PET/CT showed a lack of FDG uptake in both
the primary tumor and cervical lymph nodes (Fig. 5A and B). Overall, the imaging findings indicate
that the primary lesion and cervical lymph nodes were effectively
controlled with the treatment. The patient has shown no evidence of
disease progression, both in the primary lesion and the cervical
lymph node area, or distant metastasis 5 years after the
treatment.
Case 2
A 76-year-old female with an enlarging right upper
gingival ulcer was referred to our institution. On initial
examination, the patient had an ulcerative mass with induration at
the right upper gingiva (Fig. 6),
as well as a neck mass on the right side. The gingival ulcer was
diagnosed as a well-differentiated SCC by biopsy. MRI showed that
the tumor extended to the right lateral pterygoid (Fig. 7A). E-CT showed a rim-enhanced mass
at levelⅡB of the right cervical area, which was located inside the
sternocleidomastoid muscle (Fig.
7B). FDG-PET/CT demonstrated high FDG uptake at the primary
tumor and cervical lymph nodes (Fig.
7C and D). Results of the
distant metastasis workup was negative. The diagnosis was SCC of
the right upper gingiva (T4aN2bM0: Stage IVA). The patient
underwent retrograde intra-arterial CRT using ECAS as in Case 1.
Since the external carotid artery was significantly tortuous, the
FA and OA were selected using a Merit SwiftNINJA®
steerable microcatheter (Merit Medical system, Inc.) with a
remote-controlled flexible tip in this case with reference to the
report by Nomura et al (7)
(Fig. 8A). Cisplatin at 50
mg/m2 was manually infused into each tumor-feeding
artery selected once per week in the operating room at a rate of 5
ml/min. SSIACRT was performed once a week for a total of seven
cycles per week. After the catheter was inserted into the feeding
vessel, flow check was performed by DSA and contrast-enhanced CT to
ensure proper catheter placement (Fig.
8A-D). Indigocarmine was used to confirm staining of the skin
surface of the maxillary gingival tumors and level IIb cervical
lymph nodes (Fig. 8E and F). We administered cisplatin at 280
mg/body through the MA and 120 mg/body through the FA to the
primary tumor. For the metastatic lymph nodes in the IIb region,
cisplatin was administered through the OA at 60 mg/body. External
irradiation was planned after appropriate immobilization using a
thermoplastic mask and three-dimensional CT-based techniques. The
primary lesion was irradiated with 60 Gy/30 times and the right
neck was irradiated with 40 Gy/20 times. The acute adverse events
observed in Case 2 within 1 month after treatment included grade 3
oral mucositis and dermatitis and grade 2 neutropenia. No major
complications were observed. During the follow-up period, e-CT,
MRI, and tissue biopsy of the primary lesion were performed to
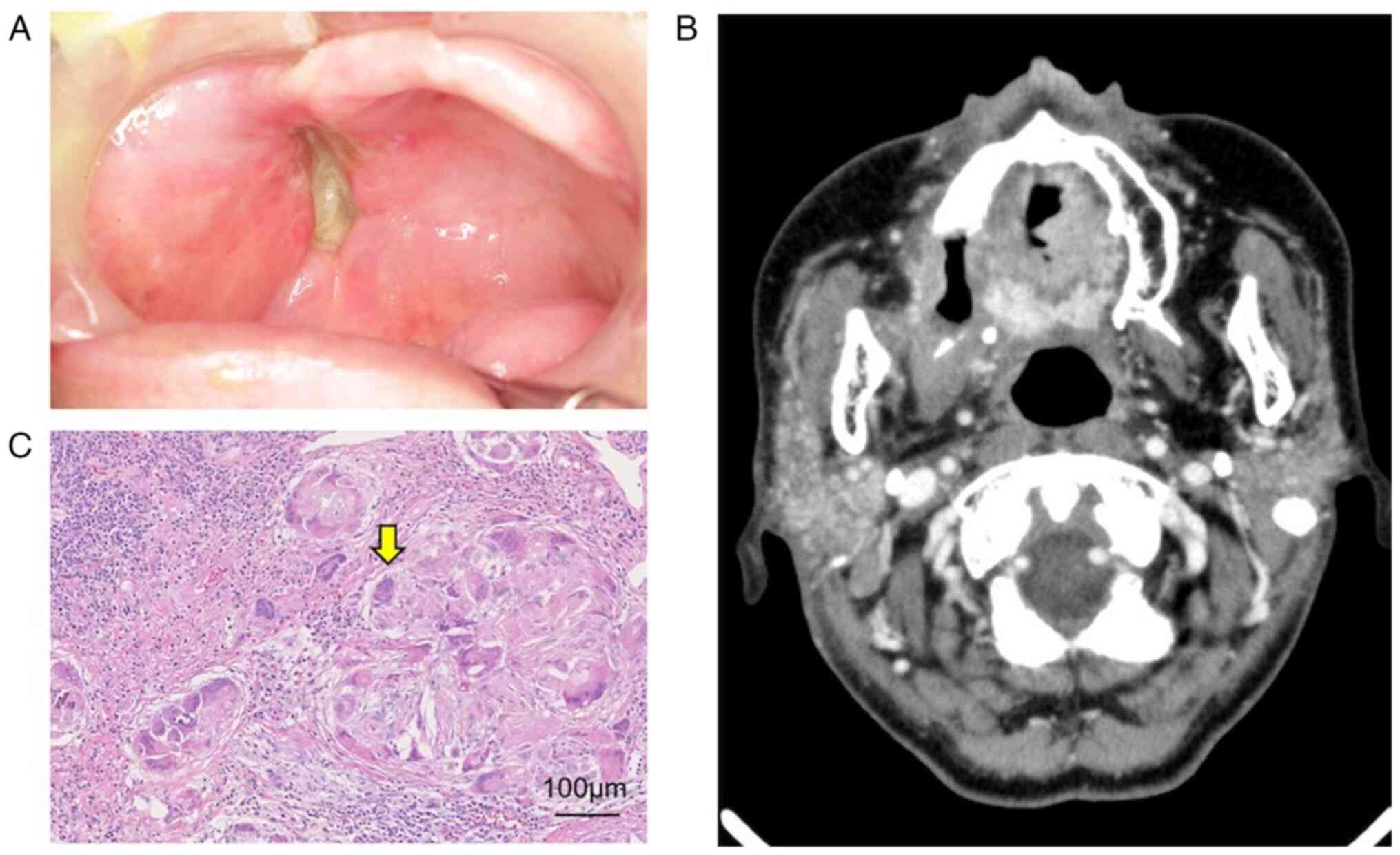
evaluate treatment outcomes. The maxillary gingival tumor showed no
viable tumor cells on biopsy, and e-CT showed no tumor recurrence
(Fig. 9A and B). The overall follow-up evaluation
indicates that the primary lesion and cervical lymph nodes were
effectively controlled with the treatment. Right side modified
radical neck dissection was performed after intra-arterial catheter
treatment. The histopathological findings of the dissected specimen
showed that the lymph nodes were grade Ⅲ (Fig. 9C).
Discussion
Oral cancer with cervical lymph node metastasis is
generally treated by surgery, however dysfunction due to surgical
resection is unavoidable. Our case report involved a patient who
refused surgical resection (Case 1) and another patient whose case
was considered difficult to operate. Good treatment results were
achieved in both cases by selecting multiple branches of the
external carotid artery from the STA on the same day and flushing
out the chemotherapeutic agent from the body. Treatment of the
include cervical lymph nodes using three routes simultaneously from
the STA without replacing the catheter has not been previously
reported. Given its potential efficacy, the technique should be
considered in future treatment strategies.
SSIACRT can be performed using the Seldinger method
(4,5) performed from the femoral artery or
the HFT method of placing the catheter retrogradely from the STA
(1,2,8). The
advantage of the HFT method is that it allows long-term catheter
placement without the intervention of a radiologist, and it can be
used concurrently radiation, so that a high antitumor effect can be
expected (1-3).
Mitsudo et al (9) reported
that the 3-year OS and locoregional control rates were 81.5% (Stage
III, 94.7%; Stage IV, 64.9%) and 80.3% (Stage III, 89.7%; Stage IV,
72.1%), respectively, for tongue cancer of 95 patients who
underwent daily retrograde IACRT with the two-channel method
combined with RT. Meanwhile, Takayama et al (10) reported that the 3-year OS, PFS, and
LC rates were 87.0, 74.1, and 86.6%, respectively, for tongue
cancer 33 patients who underwent weekly conventional retrograde
IACRT combined with proton beam therapy and systemic chemotherapy.
Another advantage is the very low intracerebral complication rates
due to thrombus because the catheter is not manipulated in the
common carotid artery. However, multiple feeding arteries
necessitate catheter replacement and complicates management
(3,11).
In arterial infusion chemotherapy for oral cancer,
vascular selectivity has been reported to influence prognosis
(1). Mitsudo et al
(3) performed SSIACRT from two
routes, STA and OA, and obtained a high therapeutic effect against
T3 and T4 oral cancers. However, in cervical lymph node metastasis,
the therapeutic effect varies with the metastatic level.
Furthermore, when a catheter is placed in the FA, flow is observed
in cervical I and IIa regions of the ipsilateral neck. In many
cases, an effect of Grade III or higher on the Ooboshi-Shimosato
classification was obtained. On the contrary, the therapeutic
effect is poor at levels IIb, III, and below where no flow is
observed. Furthermore, SSIACRT is ineffective in cervical lymph
node metastasis in areas IIb, III, and above (3). The reason for this is the difficulty
in selecting the feeding artery with a catheter. In our previous
report, blood flow in the level II region was dominated by the OA,
which makes conventional SSIACRT effective. However, during
treatment via OA and LA, administration of therapy to FA becomes
impossible (11). The ECAS system
we performed used a 5 Fr P-U catheter in the ECA between the STA
and FA or MA, and through a valve attached to its distal end, a
hook-type or steerable microcatheter can be inserted into ECAS.
Therefore, we could administer treatment via the STA to LA, FA, MA,
and OA on the same day, without needing to replace the catheter. In
the two cases treated in the present study, CDDP could be dispensed
to the sternocleidomastoid muscle branch of the OA, so that the Ⅱb
region could be treated. In our previous report, we described a
case in which N3 lymph node metastasis was drained to the OA and
cured, but in reality, several catheter replacements were
necessary, which was a rather complicated procedure (11). Compared with that case, using the
ECAS system facilitated the chemotherapeutic agent to the arteries
via three routes without changing the catheter. This method could
be introduced in hospitals where facilities and manpower are
insufficient. Furthermore, as mentioned above, the two types of
catheters can be used according to the degree of meandering of the
ECA (6,7,12). A
steerable microcatheter that can control the tip of the catheter
180 degrees at hand has been effective for blood vessels with a
meandering ECA that cannot be selected with a hook-type catheter
(7,13). In Case 1 of the present report, the
external carotid artery only meandered slightly, thus, the
hook-type microcatheter was used in the treatment. However, in Case
2, the meandering of the ECA was extensive, thus requiring the use
of a steerable microcatheter to facilitate artery selection.
Nomura et al (7) reported that blood vessel selectivity
was 88% for hook-type microcatheters and 94% for steerable
microcatheters. However, the steerable microcatheter is relatively
more expensive. The issue of cost should be addressed in the
future. In conclusion, SSIACT via the ECAS system effectively
treated oral cancer with cervical lymph node metastasis. Since a
steerable microcatheter or a hook-type microcatheter was used, LA,
FA, OA, and MA were selected from STA, avoiding catheter
replacement, which is necessary in conventional retrograde
arterial. It was thought that oral cancer with cervical lymph node
metastasis can be sufficiently treated by using the ECAS system
only with the approach from the superficial temporal artery.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KS, TK, TM, NF and AT made substantial contributions
to the conception and design, acquisition of data, and analysis and
interpretation of data. KS and TK confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patients provided written informed consent for
the publication of data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fuwa N, Kodaira T, Furutani K, Tachibana
H, Nakamura T, Nakahara R, Tomoda T, Inokuchi H and Daimon T:
Intra-arterial chemoradiotherapy for locally advanced oral cavity
cancer: Analysis of therapeutic results in134 cases. Br J Cancer.
98:1039–1045. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fuwa N, Kodaira T, Furutani K, Tachibana
H, Nakamura T, Nakahara R, Tomoda T, Inokuti H and Daimon T:
Arterial chemoradiotherapy for locally advanced tongue cancer:
Analysis of retrospective study of therapeutic results in 88
patients. Int J Radiat Oncol Biol Phys. 72:1090–1100.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mitsudo K, Koizumi T, Iida M, Iwai T,
Nakashima H, Oguri S, Kioi M, Hirota M, Koike I, Hata M and Tohnai
I: Retrograde superselective intra-arterial chemotherapy and daily
concurrent radiotherapy for stage III and IV oral cancer: Analysis
of therapeutic results in 112 cases. Radiother Oncol. 111:306–310.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Robbins KT, Storniolo AM, Kerber C,
Seagren S, Berson A and Howell SB: Rapid superselective high-dose
cisplatin infusion for advanced head and neck malignancies. Head
Neck. 14:364–371. 1992.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Robbins KT, Kumar P, Wong FS, Hartsell WF,
Flick P, Palmer R, Weir AB III, Neill HB, Murry T, Ferguson R, et
al: Targeted chemoradiation for advanced head and neck cancer:
Analysis of 213 patients. Head Neck. 22:687–693. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ii N, Fuwa N, Toyomasu Y, Takada A, Nomura
M, Kawamura T, Sakuma H and Nomoto Y: A novel external carotid
arterial sheath system for intra-arterial infusion chemotherapy of
head and neck cancer. Cardiovasc Intervent Radiol. 40:1099–1104.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nomura M, Fuwa N, Toyomasu Y, Takada A, Ii
N, Nomura J and Yamada H: A comparison of two types of
microcatheters used for a novel external carotid arterial sheath
system for intra-arterial chemotherapy of head and neck cancer. Jpn
J Radiol. 36:622–628. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tohnai I, Fuwa N, Hayashi Y, Kaneko R,
Tomaru Y, Hibino Y and Ueda M: New superselective intra-arterial
infusion via superficial temporal artery for cancer of the tongue
and tumour tissue platinum concentration after carboplatin (CBDCA)
infusion. Oral Oncol. 34:387–390. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mitsudo K, Hayashi Y, Minamiyama S, Ohashi
N, Iida M, Iwai T, Oguri S, Koizumi T, Kioi M, Hirota M, et al:
Chemoradiotherapy using retrograde superselective intra-arterial
infusion for tongue cancer: Analysis of therapeutic results in 118
cases. Oral Oncol. 79:71–77. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Takayama K, Nakamura T, Takada A, Makita
C, Suzuki M, Azami Y, Kato T, Hayashi Y, Ono T, Toyomasu Y, et al:
Treatment results of alternating chemoradiotherapy followed by
proton beam therapy boost combined with intra-arterial infusion
chemotherapy for stage III-IVB tongue cancer. J Cancer Res Clin
Oncol. 142:659–667. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sakuma K, Koizumi T, Mitsudo K, Ueda J,
Hayashi Y, Iwai T, Hirota M, Kioi M, Yoshii H, Kaizu H, et al:
Retrograde superselective intra-arterial chemoradiotherapy combined
with hyperthermia and cetuximab for carcinoma of the buccal mucosa
with N3 lymph node metastasis: A case report. Oral Radiol. 2:1–7.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Inaba Y, Arai Y, Sone M, Aramaki T, Osuga
K, Tanaka H and Kanemasa K: Experiments for the development of a
steerable microcatheter. Cardiovasc Intervent Radiol. 40:1921–1926.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Soyama T, Yoshida D, Sakuhara Y, Morita R,
Abo D and Kudo K: The steerable microcatheter: A new device for
selective catheterisation. Cardiovasc Intervent Radiol. 40:947–952.
2017.PubMed/NCBI View Article : Google Scholar
|