Introduction
Hepatocellular carcinoma (HCC) is one of the most
lethal malignancies; it has the sixth highest incidence rate and is
the third leading cause of cancer-associated death in the world in
2021(1). Despite the development
of treatment strategies for HCC, the overall survival rate is low
due to recurrence, aggressive growth, metastasis and
chemoresistance (2-4).
Accumulating evidence has shown that
epithelial-mesenchymal transition (EMT) serves an important role in
HCC invasion and metastasis and EMT-related markers are associated
with HCC metastasis (5). Among
them, the Wnt/β-catenin signaling pathway is highly conserved in
evolution and serves a vital role in tumor growth and metastasis
(6,7). In brief, when Wnt ligands combine
with Frizzled receptors, Dishevelled (DVL) is phosphorylated and
recruits Axis inhibition protein 1 and glycogen Synthase Kinase 3β
(GSK3β), thus inhibiting the formation of the degradation complex.
Finally, the activated β-catenin complex is transported to the
nucleus and promotes transcription of genes regulating cell
proliferation and metastasis (8).
Since the activation of the Wnt/β-catenin pathway induces HCC
proliferation, migration and invasion, therapeutic strategies
targeting this signaling cascade hold promise in HCC treatment.
Frankincense and myrrh (FM) are traditional Chinese
herbal medicines. Frankincense is resin exudated from the bark of
Boswellia of the Burseraceae family, while myrrh is dried
resin of species of the Commiphora family (9). Both compounds have been used as
anti-inflammatory and anti-cancer drugs. Extensive pharmacological
research has investigated the mechanisms underlying the antitumor
function of FM (10,11). β-elemene, one of the active
components of FM, has been reported to treat colon cancer by
inducing ferroptosis and inhibiting EMT (12). Gugulipid, a primary extract of the
Commiphora mukul tree, also induces apoptosis by targeting
the β-catenin signaling pathway (13).
In the present study, a water decoction extract of
FM was obtained. Considering the key role of Wnt/β-catenin
signaling in tumor progression and the potent antitumor ability of
FM (14), the mechanisms
underlying liver cancer progression and EMT were explored by
targeting the key EMT axis Wnt/β-catenin.
Materials and methods
Cell culture
Human HCC cell line HCC-LM3 and mouse HCC cell line
Hepa1-6 were purchased from the Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
All cells were cultured in complete Dulbecco's Modified Eagle
Medium (DMEM) containing 10% fetal bovine serum, 100 U/ml
penicillin, and 100 µg/ml streptomycin (all Thermo Fisher
Scientific, Inc.) and were maintained at 37˚C in humidified
air containing 5% CO2.
Reagents
FM was purchased from Jiangsu Province Hospital of
Traditional Chinese Medicine (Nanjing, China). Anti-N-cadherin
(cat. no. #13116), E-cadherin (cat. no. #14472), Vimentin (cat. no.
#46173), snail (cat. no. #3879), slug (#9585), twist1 (#90445),
Zinc Finger E-Box Binding Homeobox 1 (ZEB1) (#83243), Dishevelled
Segment Polarity Protein 2 (DVL-2) (cat. no. #3442), and β-catenin
(#8480) antibodies were purchased from Cell Signaling Technology,
Inc. Anti-GAPDH (MB001) was purchased from Bioworld Technology,
Inc. All antibodies were diluted at 1:1,000 in diluent (cat. no.
P0023A; Beyotime Institute of Biotechnology). The Wnt/β-catenin
agonists, SKL2001 (Cat.No.681667, Sigma, USA).
Water decoction extract of FM
The extract of FM was prepared as previously
described (15). Briefly, the dry
herb of frankincense (500 g) and myrrh (500 g) were extracted in a
1:1 ratio, boiled with 5 l water twice and filtered through gauze.
The extracted solution was evaporated for about 2 h in a rotary
evaporator under vacuum at 55˚C to obtain the frankincense
or myrrh powder.
UPLC-Q TOF/MS
The chromatogram column was Waters ACQUITY UPLC X
Bridge® BEH C18 Column (2.1x50 mm, 2.5 µm); Mobile
phase: A was 0.1% formic acid-water, B was B Nitrile, Volume flow:
0.3 ml/min; The column temperature is 35˚C; Gradient elution
conditions: 0~1 min: 5% B, 1~3 min: 5~50% B, 3~13 min: 50~85% B,
13~14 min: 85~95% B.
Mass spectrometry conditions: The scan range was
50-1500 m/z, the scan time was 0.2 sec, the collision voltage was
35 V, and the capillary voltage was 4500 V (negative ion) and 5500
V (positive ion). The ionophore source temperatures were 400˚C
(negative ions) and 550˚C (positive ions), and the de-cluster
voltage was 60 V. The curtain gas flow rate was 25 l/min and the
gas was nitrogen.
MTT assay
A total of ~5,000 HCC-LM3 or Hepa1-6 were cultured
in 96-well plates and treated in 37˚C for 12 or 24 h with FM
(0, 0.5, 1.0, 5.0, 20.0, 40.0 mg/ml). Following 12 or 24 h
incubation at 37˚C, 20 µl MTT reagent was added to each well
and cells were incubated at 37˚C for 3 h. Subsequently, the
supernatant was removed and 100 µl isopropanol was added to each
well, followed by shaking at room temperature for 10 min. The
absorbance at 570 nm was measured using a spectrophotometer.
Western blotting
Cells of HCC-LM3 or Hepa1-6 were lysed on ice with
RIPA peptide lysis buffer (Beyotime Institute of Biotechnology).
The concentration of each protein was detected by BCA Protein
Concentration Detection kit). 20 ug of protein were loaded and
subjected to 10% SDS-PGAE. Following electrophoresis, proteins were
transferred to PVDF membranes (Roche Diagnostics). The membrane was
incubated with specific antibodies (E-cadherin, N-cadherin,
Vimentin, Snail, Slug, Twist1, ZEB1, DVL-2, β-catenin or GAPDH) at
4˚C for 12 h following blocking with 5% non-fat milk at room
temperature for 1 h. After incubating with the appropriate
HRP-conjugated secondary antibodies at room temperature for 2 h,
the signal was determined using chemiluminescent detection
substrate (cat. no. WBKLS0050, MilliporeSigma) and visualized by a
Tanon 5200 imaging system (Tanon Science and Technology Co., Ltd.).
Protein concentrations relative to GAPDH were calculated by Image J
software (Version 1.8.0.112, National Institutes of Health).
Wound healing assay
HCC cells were seeded in 6-well plates. When the
cells reached 90-100% confluence in culture plate wells, 200-µl
tips were used to create wounds in a single confluent cell layer.
Then, cells were washed three times with PBS to remove crossed
cells and serum-free medium was added. After 12 or 24 or 48 h, the
width of the wound was photographed using a phase contrast
microscope (magnification, x4). Data were quantified by Image J
software (Version 1.8.0.112, National Institutes of Health).
Transwell invasion assay
Transwell chambers (pore size, 8 µm; Corning, Inc.)
were used to evaluate cell invasion. Matrigel was precoated in the
upper chamber at 37˚C for 3 h. Then, about 10,000 cells were
seeded in the diluted Matrigel-coated (BD Biosciences) upper
chamber with 200 µl serum-free DMEM, while the lower chamber
contained 10% DMEM with 10% fetal calf serum as the
chemoattractant. Following incubation at 37˚C for 24 h,
cells in the upper chamber were removed and migrated cells in the
lower chamber were fixed with 4% paraformaldehyde for 20-30 min at
room temperature, followed by staining with 0.1% crystal violet for
5-10 min at room temperature. Cells were photographed using a phase
contrast microscope with 10x ordinary light
Immunofluorescence
100,000 cHCC-LM3 or Hepa1-6 were seeded on
coverslips and fixed with 4% paraformaldehyde at room temperature
for 15 min. Following washing with PBS, cells were incubated with
1% BSA and 22.52 mg/ml glycine in PBST (PBS + 0.1% Tween 20) for 30
min at room temperature to block nonspecific binding of antibodies.
Then, cells were incubated with DVL-2 (ab228804) or β-catenin
(ab32572) at 4˚C overnight. After that, the cells were
incubated with Alexa Fluor-conjugated secondary antibodies
(ab150113), and the nuclei were counterstained with DAPI
(Sigma-Aldrich; Merck KGaA) for 5 min at room temperature and
images were visualized (magnification, x200) using a confocal
microscope (FV10i; Olympus Corporation).
Statistical analysis
All experiments are repeated three times and all
data are presented as the mean ± SD. Differences between groups
were estimated using paired Student's t test or one-way ANOVA
followed by Tukey's post hoc test. Graphs were generated with
GraphPad software (Version 5.0; Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
Cytotoxic effect of FM on HCC
cells
It was found by UPLC that the active components of
FM were boswellic acid and terpene. The non-toxic concentration of
FM on HCC cells was determined. The inhibitory effects of FM
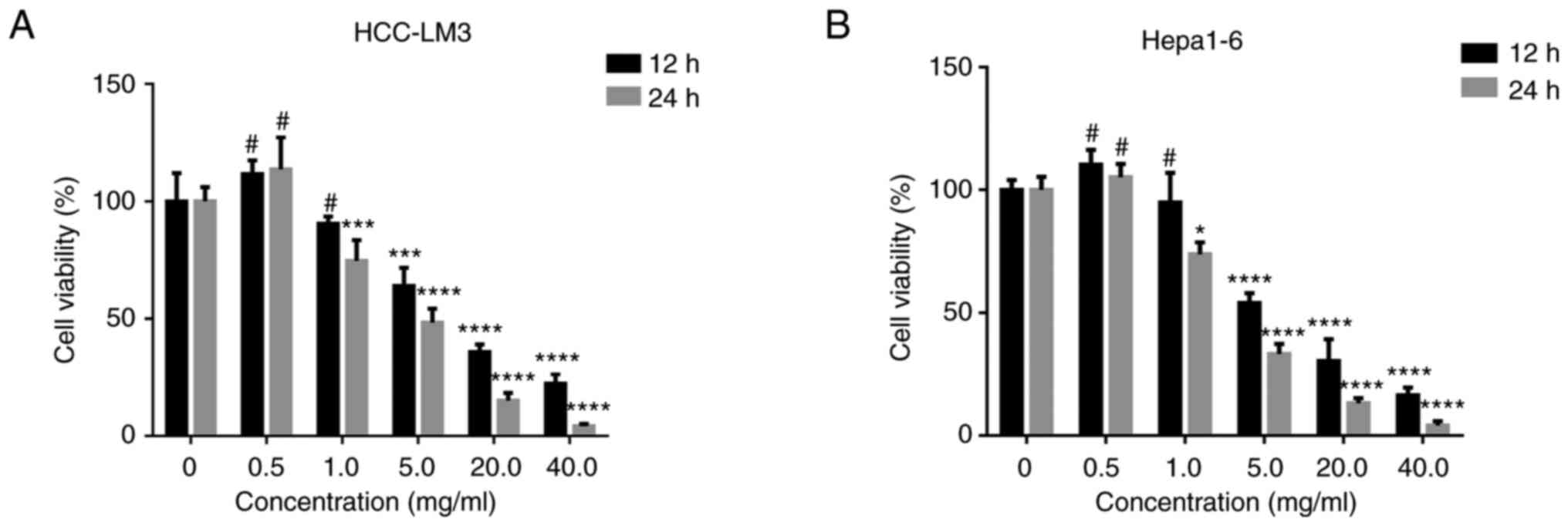
against HCC-LM3 or Hepa1-6 cells were evaluated. FM inhibited the
proliferation of human and murine cancer cells in a dose- and
time-dependent manner (Fig. 1A and
B). Moreover, FM showed
significant cytotoxicity on tumor cells at a concentration <5
mg/ml, while no inhibition was observed at FM concentrations <1
mg/ml. Therefore, 0.5 mg/ml FM was used in subsequent
experiments.
FM suppresses migration and invasion
of tumor cells
The human HCC-LM3 cell line and murine Hepa1-6 cells
were treated with 0.5 mg/ml FM to investigate the effect of FM on
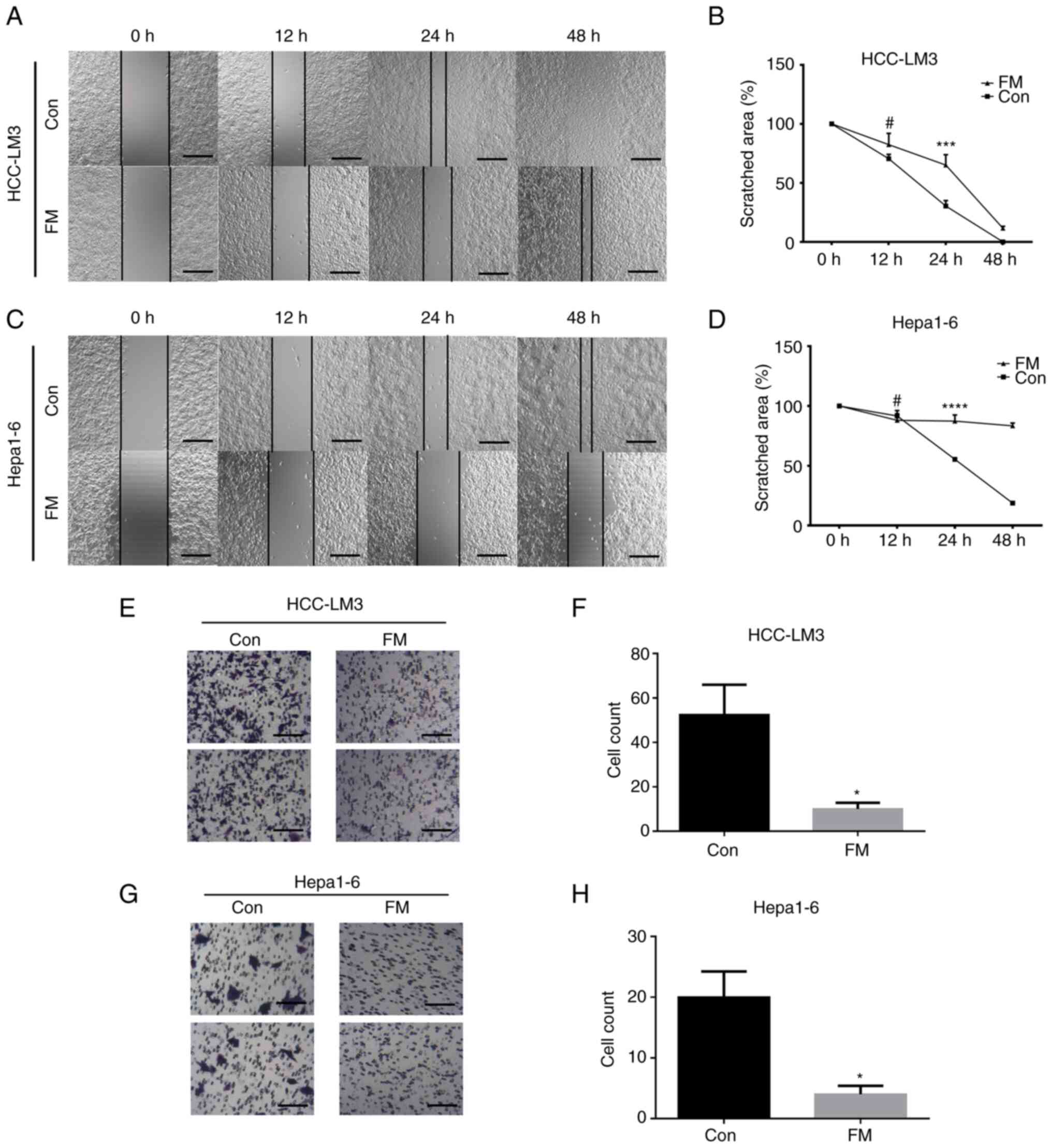
the migration and invasion of cancer cells. As shown in the wound
healing assay, cell migration was significantly inhibited in the
FM-treated group compared with the control group (Fig. 2A-D). Matrigel-coated Transwell
assay was performed to detect the inhibitory effect of FM on the
invasion of tumor cells. Cells were pretreated with 0.5 mg/ml FM
and the assay was performed for 24 h. The results showed a
significant decrease in invasion ability with FM treatment in both
human and murine cancer cell lines (Fig. 2E-H). These results indicated that
FM inhibited both the migration and invasion of cancer cells.
FM inhibits EMT in liver cancer
cells
The present study also investigated the mechanism
underlying FM suppression of liver cancer migration and invasion.
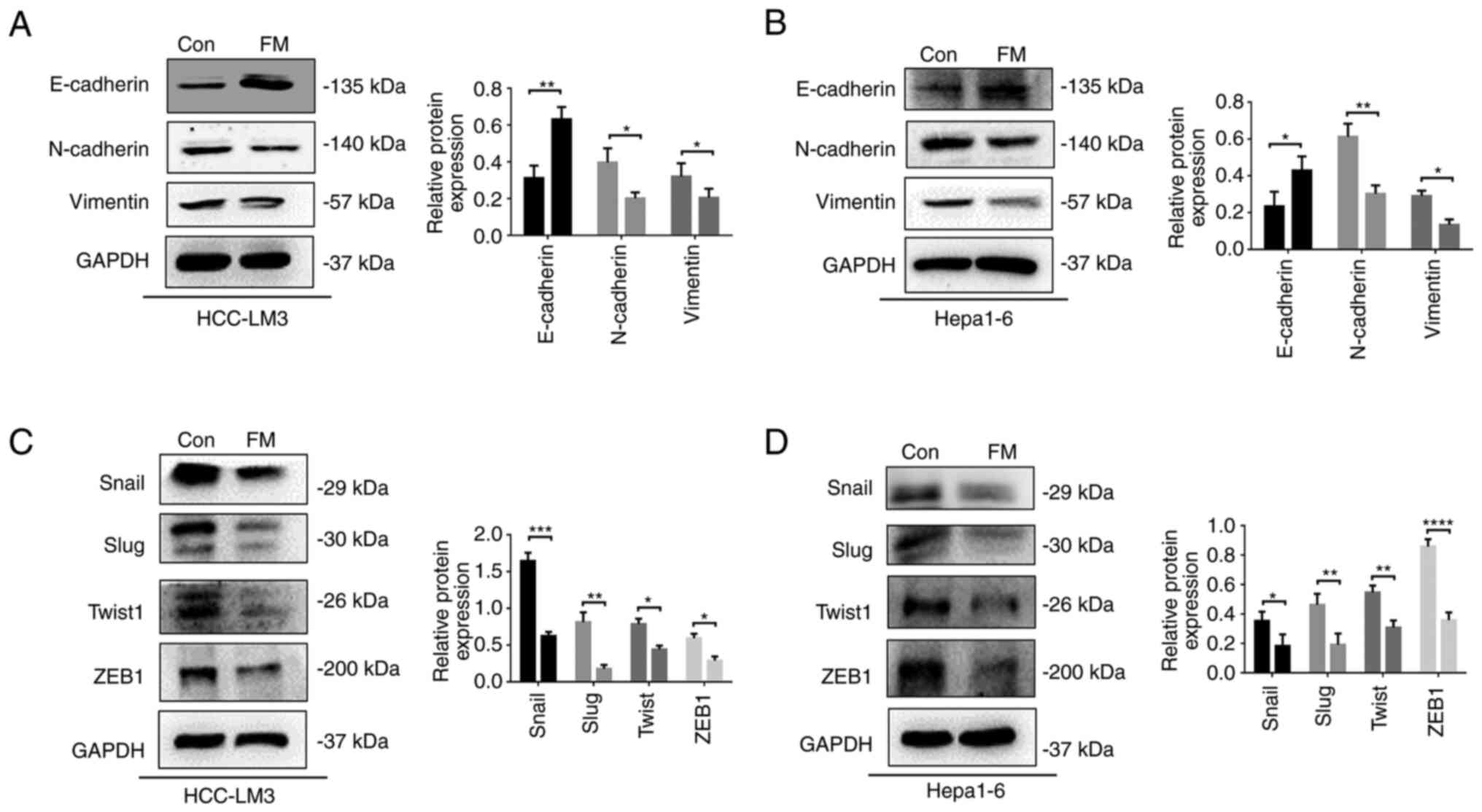
EMT markers of tumor cells were detected using western blot assay.
High levels of the epithelial marker E-cadherin and low expression
of the mesenchymal marker vimentin were observed in the FM-treated
group compared with the control (Fig.
3A and B). Moreover, EMT could
be regulated by several transcription factors (TFs), such as Snail,
Slug, Twist, and ZEB1(16). The
expression of Snail, Slug, Twist, and ZEB1 significantly decreased
in the FM-treated group compared with the control (Fig. 3C and D). These results indicated that FM
suppressed EMT by downregulating TFs such as Snail, Slug, Twist and
ZEB1.
FM inhibits EMT by disrupting
Wnt/β-catenin signaling in HCC cells
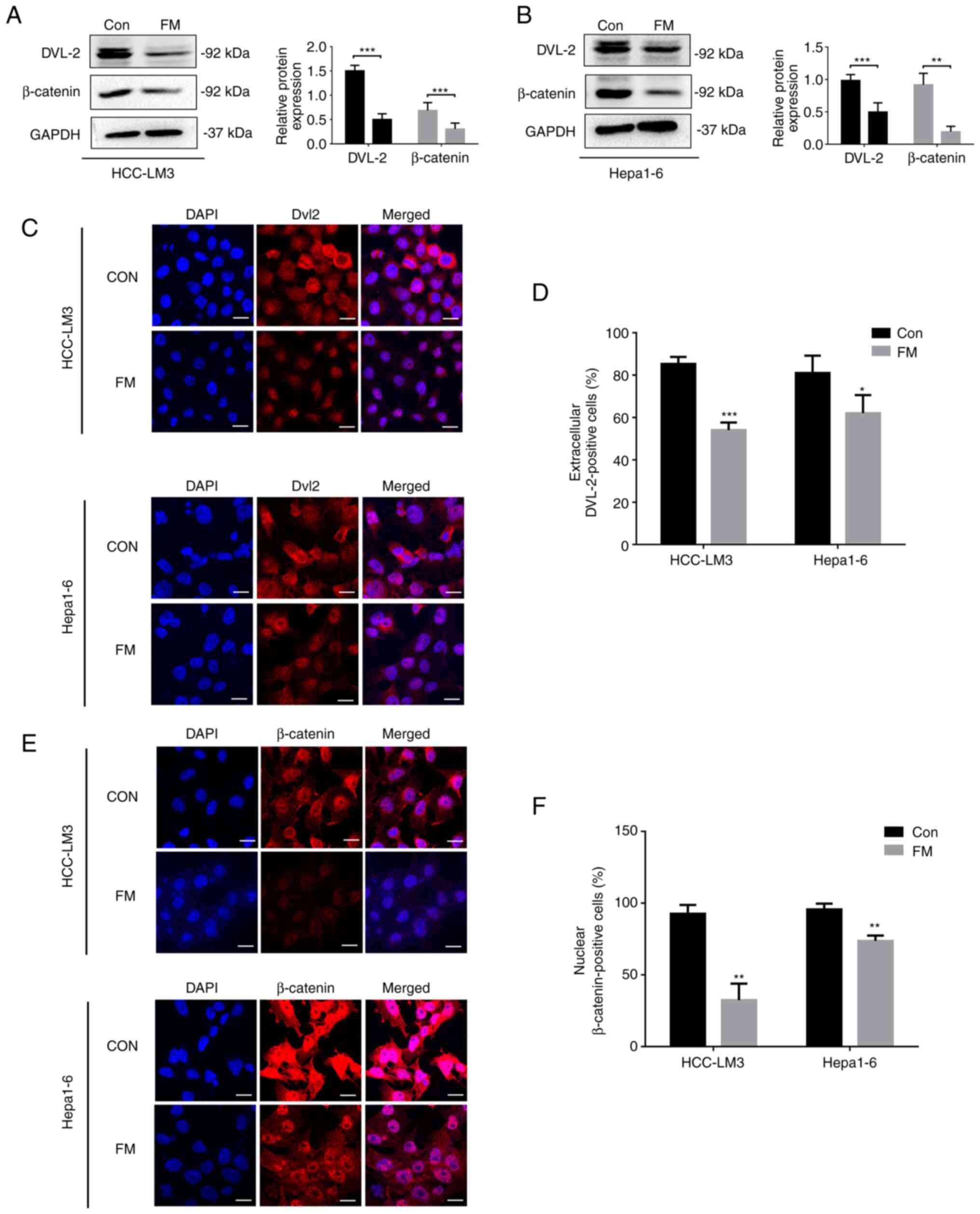
To determine the effect of FM on Wnt/β-catenin
signaling, human or murine HCC cells were treated with. FM
treatment reduced the protein levels of DVL-2 and β-catenin
(Fig. 4A and B). In addition, immunofluorescence assay
was performed to analyze the nuclear translocation of DVL-2 and
β-catenin. In the control group, DVL-2 protein was found in the
nuclei of nearly 80% of human and murine HCC cells. However, only
50-60% of FM-treated cells showed extracellular localization with
DVL-2 (Fig. 4C and D). Cells treated with FM revealed a
significant decrease in the nuclear staining of β-catenin (Fig. 4E and F). These results suggested FM inhibited
Wnt/β-catenin signaling in the HCC tumor cell lines.
FM regulates EMT in liver cancer cell
lines through Wnt signaling
In order to demonstrate that FM regulated EMT in
liver cancer cell lines through Wnt signaling, the Wnt/beta-catenin
agonists (SKL2001) in combination with FM were used in hepa1-6
cells. The results showed that FM could significantly inhibit
activation of β-catenin induced by SKL2001 (Fig. S1).
Discussion
FM is widely used in cancer treatment (17,18).
Here, FM inhibited invasion and migration in HCC cells. Moreover,
FM-suppressed cancer cell invasion and migration ability was
mediated by EMT. FM was shown to inhibit the Wnt/β-catenin
signaling pathway of EMT in HCC. The present findings show a novel
antitumor mechanism of this herbal extract, suggesting FM as a
potential strategy for clinical treatment of HCC.
The EMT pathway includes numerous signaling
pathways, such as TGF-β, Notch and Wnt (19). Among them, the Wnt signaling
pathway not only plays a role in embryonic development, but is
associated with occurrence and development of many human tumors,
such as HCC (6).
Acetyl-11-keto-β-boswellic acid (an active component of FM) can
effectively inhibit the Wnt/β-catenin signaling pathway in mouse
intestinal tumors, thereby inhibiting the proliferation and
metastasis of mouse intestinal cancer cells (20). To the best of our knowledge,
however, in HCC, there are few reports about FM and Wnt signaling
(13,21).
Preliminary in vitro and in vivo
studies have revealed that the water extract of FM inhibits cancer
progression: Ren et al (22) reported that frankincense suppresses
tumor progression by regulating the AMPK/mTOR pathway. Sun et
al (23) found that myrrh
inhibits the proliferation and migration of cancer cells by
regulating cyclooxygenase-2 expression. Moreover, the Xihuang pill,
primarily composed of FM, has been applied in cancer therapy for
>300 years (18).
FM at 0.5 mg/ml did not significantly inhibit
proliferation; therefore, this non-toxic concentration was used to
investigate whether FM inhibited the occurrence of EMT in liver
cancer by inhibiting Wnt/β-catenin signaling. In the MTT
experiment, at 0.5 mg/ml FM, the cell proliferation was >100%
and morphology was similar to the control group. It was
hypothesized that at low concentrations, FM promoted cell
viability, which is worth further study.
Furthermore, wound healing and Transwell assay
demonstrated that FM significantly decreased the migration and
invasion abilities of HCC cells. However, the mechanism underlying
FM suppression of the invasion and migration of HCC via
Wnt/β-catenin signaling remains unclear.
EMT is primarily mediated by core EMT TFs, including
Snail, ZEB and Twist family (24).
EMT is activated in tumor cells. EMT TFs and downstream regulated
genes influence multiple stages of cancer progression, including
cancer development and metastasis (25). As a member of the Snail family,
Snail1 overexpression is usually negatively associated with
E-cadherin expression and positively associated with tumor cell
migration, invasion and metastasis, which also predicts a poor
prognosis (26).
Similar to Snail1, Slug is also a major inducer of
EMT and an important mediator of Twist-induced EMT and tumor
metastasis (27). As a member of
the Twist family, Twist1 is a key factor inducing vimentin
expression, which is also associated with poor prognosis and high
metastasis rate (28). The Zeb
family consists of Zeb1 and Zeb2, which have similar functions and
increase proliferation and malignancy by inhibiting E-cadherin
(29). The present study
demonstrated that FM decreased expression of mesenchymal markers
(N-cadherin and Vimentin) and EMT-activating TFs (Snail, Slug,
Twist1 and ZEB1) while increasing the expression of the epithelial
marker E-cadherin.
Furthermore, the Wnt/β-catenin signaling is reported
to promote migration and invasion of cancer cells by mediating EMT
(30). When Wnt is activated, it
binds to Frizzled (FZD) receptor, resulting in the phosphorylation
of lipoprotein receptor-related protein 5/6 (LRP5/6) to form the
Wnt-FZD-LRP5/6 complex, which activates downstream DVL (31). The activated DVL, especially DVL-2,
protects against degradation of β-catenin in Wnt signaling and the
interaction of DVL and β-catenin promotes transcription of proteins
associated with Wnt signaling (32,33).
The present results showed that FM could suppress DVL-2 nuclear
translocation, resulting in lower protein levels and suggesting
that DVL-2 instability serves an essential role in regulating the
Wnt/β-catenin signaling pathway. Therefore, FM may inhibit
activation of DVL-2 by inhibiting binding of Wnt to the membrane
surface receptor protein FZD. This hypothesis deserves further
investigation.
The present study confirmed that FM can block EMT by
inhibiting the Wnt/β-catenin signaling pathway, thereby suppressing
the occurrence and development of HCC. These findings support use
of FM in the treatment of malignant tumors and may provide a novel
and practical strategy for clinical HCC therapy.
Supplementary Material
FM regulated EMT in liver cancer cell
line through Wnt signaling. Briefly, Hepa1-6 cells were treated
with 20 μm SKL2001 and/or 0.5 mg/ml FM for 24 h. Then, cells were
lysed for western blot to detect the specific protein. Relative
protein concentrations against GAPDH were calculated by Image J.
The data are shown as the means ± SD of three independent
experiments. **P<0.01 and ****P<0.0001
vs. SKL2001 group or control group.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 82200576), Dual Initiative
Plan of Jiangsu Province, China [grant no. 2019(30393)], Science
and Education Project of Suzhou (grant no. KJXW2019066), Natural
Science Foundation of Nanjing University of Traditional Chinese
Medicine (grant no. XZR2021075) and Suzhou Medical Health Science
and Technology Innovation (grant no. SKYD2022053).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL conceived the study, wrote and reviewed the
manuscript and designed the methodology. JM and YW designed the
methodology and reviewed the manuscript. YH visualized data and
performed the experiments. MG analyzed and interpreted data. XL and
JM confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Akateh C, Black SM, Conteh L, Miller ED,
Noonan A, Elliott E, Pawlik TM, Tsung A and Cloyd JM: Neoadjuvant
and adjuvant treatment strategies for hepatocellular carcinoma.
World J Gastroenterol. 25:3704–3721. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nakagawa S, Wei L, Song WM, Higashi T,
Ghoshal S, Kim RS, Bian CB, Yamada S, Sun X, Venkatesh A, et al:
Molecular liver cancer prevention in cirrhosis by organ
transcriptome analysis and lysophosphatidic acid pathway
inhibition. Cancer Cell. 30:879–890. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Han TS, Hur K, Cho HS and Ban HS:
Epigenetic associations between lncRNA/circRNA and miRNA in
hepatocellular carcinoma. Cancers (Basel). 12(2622)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Giannelli G, Koudelkova P, Dituri F and
Mikulits W: Role of epithelial to mesenchymal transition in
hepatocellular carcinoma. J Hepatol. 65:798–808. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
He S and Tang S: WNT/β-catenin signaling
in the development of liver cancers. Biomed Pharmacother.
132(110851)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Perugorria MJ, Olaizola P, Labiano I,
Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L and Banales JM:
Wnt-β-catenin signalling in liver development, health and disease.
Nat Rev Gastroenterol Hepatol. 16:121–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu C, Xu Z, Zhang Y, Evert M, Calvisi DF
and Chen X: β-Catenin signaling in hepatocellular carcinoma. J Clin
Invest. 132(e154515)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
El Ashry ESH, Rashed N, Salama OM and
Saleh A: Components, therapeutic value and uses of myrrh.
Pharmazie. 58:163–168. 2003.PubMed/NCBI
|
|
10
|
Efferth T and Oesch F: Anti-inflammatory
and anti-cancer activities of frankincense: Targets, treatments and
toxicities. Semin Cancer Biol. 80:39–57. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Suliman RS, Alghamdi SS, Ali R, Aljatli D,
Aljammaz NA, Huwaizi S, Suliman R, Kahtani KM, Albadrani GM,
Barhoumi T, et al: The role of myrrh metabolites in cancer,
inflammation, and wound healing: Prospects for a multi-targeted
drug therapy. Pharmaceuticals (Basel). 15(944)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen P, Li X, Zhang R, Liu S, Xiang Y,
Zhang M, Chen X, Pan T, Yan L, Feng J, et al: Combinative treatment
of β-elemene and cetuximab is sensitive to KRAS mutant colorectal
cancer cells by inducing ferroptosis and inhibiting
epithelial-mesenchymal transformation. Theranostics. 10:5107–5119.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang G, Xiao X, Zeng Y, Nagabhushanam K,
Majeed M and Xiao D: Targeting beta-Catenin signaling to induce
apoptosis in human breast cancer cells by z-Guggulsterone and
Gugulipid extract of Ayurvedic medicine plant Commiphora
mukul. BMC Complement Altern Med. 13(203)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu
C, Wang C and Ye L: Wnt/β-catenin signaling in cancers and targeted
therapies. Signal Transduct Target Ther. 6(307)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu D, Wang C, Li F, Su S, Yang N, Yang Y,
Zhu C, Shi H, Yu L, Geng X, et al: A combined water extract of
frankincense and myrrh alleviates neuropathic pain in mice via
modulation of TRPV1. Neural Plast. 2017(3710821)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brabletz S, Schuhwerk H, Brabletz T and
Stemmler MP: Dynamic EMT: A multi-tool for tumor progression. EMBO
J. 40(e108647)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cao B, Wei XC, Xu XR, Zhang HZ, Luo CH,
Feng B, Xu RC, Zhao SY, Du XJ, Han L and Zhang DK: Seeing the
unseen of the combination of two natural resins, frankincense and
myrrh: Changes in chemical constituents and pharmacological
activities. Molecules. 24(3076)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo Q, Lin J, Liu R, Gao Y, He S, Xu X,
Hua B, Li C, Hou W, Zheng H and Bao Y: Review on the applications
and molecular mechanisms of Xihuang pill in tumor treatment. Evid
Based Complement Alternat Med. 2015(854307)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7(re8)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu HP, Gao ZH, Cui SX, Wang Y, Li BY, Lou
HX and Qu XJ: Chemoprevention of intestinal adenomatous polyposis
by acetyl-11-keto-beta-boswellic acid in APC(Min/+) mice. Int J
Cancer. 132:2667–2681. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mohamed EA, Ahmed HI, Zaky HS and Badr AM:
Boswellic acids ameliorate neurodegeneration induced by AlCl3: The
implication of Wnt/β-catenin pathway. Environ Sci Pollut Res Int.
29:76135–76143. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ren P, Ren X, Cheng L and Xu L:
Frankincense, pine needle and geranium essential oils suppress
tumor progression through the regulation of the AMPK/mTOR pathway
in breast cancer. Oncol Rep. 39:129–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun M, Hua J, Liu G, Huang P, Liu N and He
X: Myrrh induces the apoptosis and inhibits the proliferation and
migration of gastric cancer cells through down-regulating
cyclooxygenase-2 expression. Biosci Rep.
40(BSR20192372)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Tang X, Sui X, Weng L and Liu Y: SNAIL1:
Linking tumor metastasis to immune evasion. Front Immunol.
12(724200)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu QQ, Ma C, Wang Q, Song Y and Lv T: The
role of TWIST1 in epithelial-mesenchymal transition and cancers.
Tumour Biol. 37:185–197. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Peiffer DS, Wyatt D, Zlobin A, Piracha A,
Ng J, Dingwall AK, Albain KS and Osipo C: DAXX suppresses
tumor-initiating cells in estrogen receptor-positive breast cancer
following endocrine therapy. Cancer Res. 79:4965–4977.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Z, Li Z, Wu Q, Li C, Li J, Zhang Y,
Wang C and Sun S and Sun S: DNER promotes epithelial-mesenchymal
transition and prevents chemosensitivity through the Wnt/β-catenin
pathway in breast cancer. Cell Death Dis. 11(642)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y and Wang X: Targeting the
Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol.
13(165)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gan X, Wang J, Xi Y, Wu Z, Li Y and Li L:
Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to
stabilization of beta-catenin-TCF interaction. J Cell Biol.
180:1087–1100. 2008.PubMed/NCBI View Article : Google Scholar
|