Introduction
Prostate cancer (PCa) is the most common type of
cancer in men and external beam radiation therapy (EBRT) is one of
the useful treatment modalities for PCa (1,2). In
the last two decades, radiation technologies such as
intensity-modulated radiation therapy (IMRT) and image-guided
radiation therapy (IGRT) have advanced in the field of EBRT. They
have achieved dose escalation to target volumes and dose reduction
in normal organs. Furthermore, precise radiation technologies, such
as ultra-hypofractionated IMRT, are emerging (3,4).
Therefore, a subtle attentiveness is required.
In previous studies, a hydrogel spacer (HS; SpaceOAR
System, Augmenix, Inc., Waltham, MA, USA) was inserted between the
prostate and rectum to reduce rectal toxicity (5,6).
Though the safety and efficacy of HS in EBRT for PCa have been
reported in several studies (6,7), the
effectiveness presented in these studies reduced rectal toxicity.
Therefore, HS assumes importance as an IMRT tool for PCa,
especially in ultra-hypofractionated IMRT.
Additionally, the seminal vesicle (SV), one of the
targeted structures in EBRT for PCa, is an organ whose anatomical
position fluctuates with HS insertion. Nevertheless, to best our
knowledge, no studies have examined SV displacement in relation to
HS insertion. Therefore, in our present study, we aimed to examine
the SV displacement associated with HS insertion.
Materials and methods
Study population
Between March 2019 and March 2022, 95 patients were
treated with definitive IMRT for PCa at our institution. Of these,
patients with the following characteristics were excluded from the
study: i) No use of HS (n=56); ii) absence of computed tomography
(CT) and magnetic resonance (MR) imaging data before HS insertion
(n=6); and iii) large changes in bladder volume (>50
cm3) and rectal volume (>15 cm3) between
simulation CT images for planning after HS insertion and CT images
before HS insertion (n=10). Finally, twenty consecutive PCa
patients [median age (range): 71 years (61-84 years), median
pretreatment body mass index (range): 23.7 (18.1-30.3)] from the
twenty-three remaining patients were included in the analysis. When
SV invasion and tumor infiltration around SV, HS insertion is
prohibited because of the possibility of scattering the tumor
cells. All patients had bladder volumes of >50 cm3 on
simulation CT. None of the patients had received hormone therapy
because EBRT doses for intermediate-risk PCa in our institution are
sufficiently dose-escalated (8).
The baseline characteristics of the patients are shown in Table I. The Ethics Committee of the
National Hospital Organization Shikoku Cancer Center (Matsuyama,
Japan) approved the study protocol (approval no. Rin 202105). The
need for informed consent was waived due to the study's
retrospective nature.
 | Table IBaseline characteristics. |
Table I
Baseline characteristics.
| Characteristic | No. of patients |
|---|
| Prostate size,
cm3 | |
|
<38 | 10 |
|
≥38 | 10 |
| Rectum size,
cm3 | |
|
Before HS
insertion | |
|
<45 | 9 |
|
≥45 | 11 |
|
After HS
insertion | |
|
<45 | 10 |
|
≥45 | 10 |
| Bladder size,
cm3 | |
|
Before HS
insertion | |
|
<120 | 12 |
|
≥120 | 8 |
|
After HS
insertion | |
|
<120 | 13 |
|
≥120 | 7 |
| BMI | |
|
<23 | 7 |
|
≥23 | 13 |
| PSA at diagnosis | |
|
<8 | 10 |
|
≥8 | 10 |
| Biopsy Gleason
score | |
|
3 + 3 | 2 |
|
3 + 4 | 10 |
|
4 + 3 | 8 |
| Clinical T-stage | |
|
1 | 11 |
|
2 | 9 |
| Radiation dose | |
|
74-78
Gy/37-39 fractions | 11 |
|
70 Gy/28
fractions | 4 |
|
60 Gy/20
fractions | 5 |
HS insertion
Under general anesthesia and transrectal
ultrasound guidance, all patients underwent transperineal
insertion of two intra-prostatic gold seed markers (9) and HS at our institution.
Approximately 8-10 ml of HS was inserted into the anterior
perirectal space between the Denonvilliers' fascia and the anterior
rectal wall. A urologist performed the insertion of the gold seed
markers and HS.
Evaluation
CT images of the region, including the prostate and
SV, were collected at 2.5 mm between two slices (thickness of 2.5
mm). The images were loaded onto our Eclipse 3D treatment planning
system (TPS; Varian Medical Systems, Palo Alto, CA, USA), and a
radiation oncologist created each SV delineation. To obtain SV
delineation from the CT images, the radiation oncologist adhered to
the European Society for Therapeutic Radiology and Oncology (ESTRO)
Advisory Committee for Radiation Oncology Practice (ACROP)
consensus guideline and actual anatomy (10). Registration of the CT images before
and after HS insertion depended on the base of the prostate. The
amount of SV displacement owing to HS insertion was measured.
Displacement in the cranial, lateral, and anterior translation
directions was given positive values. In contrast, displacement was
given as negative values in the caudal, medial, and posterior
directions.
Statistical analysis
Statistical analyses were performed using the JMP
software (JMP version 14.3.0; SAS Institute, Cary, NC, USA). An
unpaired Student's t-test and Fisher's exact test were used to
assess the significance of group differences in the variables.
Results
Amount of SV displacement
The maximum cranial displacements mean was 0.16 cm
(range, -0.25-1.00 cm), the maximum anterior displacements mean it
was 0.00 cm (range, -0.45-1.14 cm), and the maximum lateral
displacements mean was 0.00 cm (range, -0.24-0.58 cm). Rectal wall
infiltration (RWI) score (10) was
1 (range, 0-3). Large displacements (≥0.5 cm) were observed in six
patients (cranial, 4; anterior, 1; cranial + anterior + lateral,
1).
SV cranial displacement
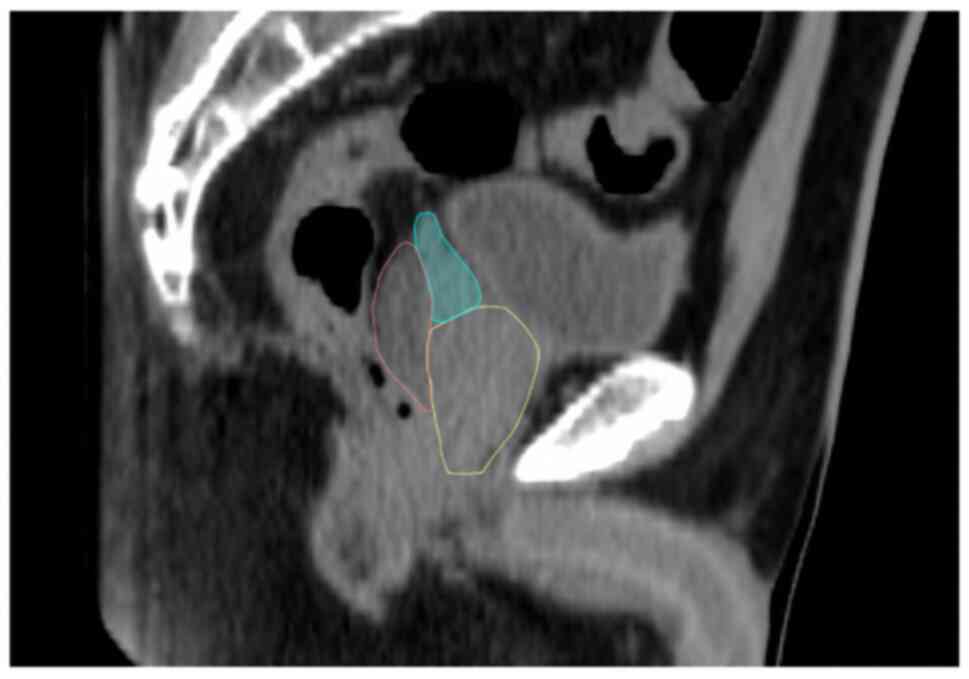
SV cranial displacements of 1.00 cm were observed in
5% (1/20) and 0.50-1.00 cm in 20% (4/20) of all patients. An
example of HS insertion is shown in Fig. 1.
An HS lateral distribution of ≥1.00 cm in the upper
two slices (midgland + superior) influenced the SV cranial
displacements (P<0.01) and influenced the large (≥0.5 cm)
SV cranial displacements (P<0.01, Table II). The HS cranial distribution,
where the evaluation point was the middle of the HS in the upper
slices, did not influence the SV cranial displacements (P=0.16). In
addition, HS thickness, as an indicator of anterior distribution,
did not influence SV cranial displacements (P=0.51).
 | Table IISeminal vesicle displacements
according to hydrogel spacer insertion. |
Table II
Seminal vesicle displacements
according to hydrogel spacer insertion.
| A, SV cranial
displacement |
|---|
| Factors | Mean displacement
(SE) | P-value | >0.5 cm
displacement (%) | P-value |
|---|
| HS lateral
distribution | | <0.01 | | <0.01 |
|
Upper 2
slices | 0.41 (0.10) | | 5/8 (62.5) | |
|
Lower 1
slice | 0 (0.08) | | 0/12 (0) | |
| HS cranio-caudal
distribution | | 0.16 | | 0.13 |
|
Midgland-surperior | 0.23 (0.09) | | 5/14 (35.7) | |
|
Inferior | 0 (0.13) | | 0/6 (0) | |
| HS thickness | | 0.51 | | 0.52 |
|
≥1.5 cm | 0.19 (0.09) | | 4/14 (28.6) | |
|
<1.5
cm | 0.08 (0.14) | | 1/6 (16.7) | |
| B, SV lateral
displacement |
| Factors | Mean displacement
(SE) | P-value | >0.5 cm
displacement (%) | P-value |
| HS lateral
distribution | | 0.50 | | 0.40 |
|
Upper 2
slices | 0.13 (0.06) | | 1/8 (12.5) | |
|
Lower 1
slice | 0.07 (0.05) | | 0/0 (0) | |
| HS cranio-caudal
distribution | | 0.95 | | 0.70 |
|
Midgland-surperior | 0.09 (0.05) | | 1/14 (7.1) | |
|
Inferior | 0.09 (0.07) | | 0/0 (0) | |
| HS thickness | | >0.99 | | 0.70 |
|
≥1.5 cm | 0.09 (0.07) | | 1/14 (7.1) | |
|
<1.5
cm | 0.09 (0.05) | | 0/0 (0) | |
| C, SV anterior
displacement |
| Factors | Mean displacement
(SE) | P-value | >0.5 cm
displacement (%) | P-value |
| HS lateral
distribution | | 0.70 | | 0.66 |
|
Upper 2
slices | 0.19 (0.12) | | 2/12 (16.7) | |
|
Lower 1
slice | 0.25 (0.10) | | 1/8 (12.5) | |
| HS cranio-caudal
distribution | | 0.36 | | 0.80 |
|
Midgland-surperior | 0.28 (0.09) | | 2/14 (14.3) | |
|
Inferior | 0.12 (0.14) | | 1/6 (16.7) | |
| HS thickness | | 0.75 | | 0.80 |
|
≥1.5 cm | 0.25 (0.09) | | 2/14 (14.3) | |
|
<1.5
cm | 0.19 (0.14) | | 1/6 (16.7) | |
SV lateral displacement
SV lateral displacements of 0.50-1.00 cm were
observed in only 5% (1/20) of the patients.
An HS lateral distribution of ≥1.00 cm in the upper
two slices (midgland + superior) did not influence the SV lateral
displacements (P=0.50, Table II).
The HS cranial distribution in the upper slices did not induce SV
lateral displacements (P=0.95). In addition, the HS thickness did
not affect the SV lateral displacements (P=0.99).
SV anterior displacement
SV anterior displacements of 1.00 cm were observed
in 5% (1/20) and 0.50-1.00 cm in 10% (2/20) of all patients.
An HS lateral distribution of ≥1.00 cm in the upper
two slices (midgland + superior) did not influence the SV lateral
displacements (P=0.70, Table II).
The cranial distribution of HS in the upper slices did not induce
SV lateral displacements (P=0.36). In addition, the HS thickness
did not affect the SV lateral displacements (P=0.75).
Discussion
This study investigated the influence of HS
distribution on SV position for patients with PCa treated with
IMRT. Our results indicated that HS distribution caused rare
clinically significant changes in SV position. We observed large SV
cranial displacements according to asymmetrical HS insertion [HS
lateral distribution of ≥1.00 cm in the upper two slices (midgland
+ superior)].
In radiotherapy for PCa, the rectum and bladder are
considered important organs at risk. Therefore, the use of HS in
IMRT planning for PCa significantly reduces the rectal dose,
toxicity, and quality of life (6).
However, in radiotherapy for PCa, the sigmoid colon and small bowel
often limit the dose distribution of the planning target volume
(11). This is attributed to these
organs receiving higher doses, which is also related to intestinal
toxicity (12). Although
Fischer-Valuck et al (13)
suggested that asymmetric HS insertion also leads to an adequate
reduction of rectal dose as symmetric HS insertion, they did not
evaluate the association between the sigmoid colon and the small
bowel. In our study, the incidence of SV cranial displacement was
associated with long-distance asymmetric HS insertion from the
superior to the midgland space. Symmetric HS insertion assumed
importance regarding a few SV cranial displacements.
Furthermore, in our study, SV cranial displacement
was associated with two factors (HS insertion in the upper two
slices + HS insertion in a lateral distribution of ≥1.00 cm). HS
insertion in the inferior perirectal space did not lead to SV
cranial displacement. Pinkawa et al (14) demonstrated that there was a
learning curve for symmetrical HS insertion (i.e., modifying the HS
lateral displacement), improved treatment planning, and less
treatment-related acute toxicity. Fukumitsu et al (15) proposed a new technique to enhance
HS craniocaudal displacement. Though this novel technique requires
further proficiency, it may prove useful in reducing the risk of SV
cranial displacement. Although multiple studies have investigated
the usefulness of hypofractionated radiation therapy for PCa
(16,17), a recent meta-analysis showed that
hypofractionated radiation therapy induced a significant risk of
acute gastrointestinal toxicity (18). Hence, appropriate HS insertion is
extremely important when a patient with PCa is treated with
hypofractionated radiation therapy. The sigmoid colon or small
bowel is depressed in rectovesical excavation; even so, the SV
needs to be included in the target volume. An expert physician with
proficiency in HS insertion is thus required for HS insertion.
This study had limitations associated with a small
sample size. We selected only twenty cases with acceptable
differences in bladder and rectum volume variations before and
after HS insertion. This is because many clinical cases did not
have an equivalent volume of bladder and rectum before and after HS
insertion. In our study, SV displacement was unaffected by volume
variation of the rectum or bladder (data not shown). In addition,
the inter- and intra-fractional motion of the SV was approximately
8 mm (19). Most of the results
were within the range of intra- and inter-fraction motion of the
SV; however, only SV cranial displacement correlated with the
position of HS insertion. Therefore, HS insertion has an impact on
the cranial displacement of the SV. Although our present study was
inadequate in concluding the influence of HS insertion and further
studies are needed, we suggest that HS insertion in the upper two
slices and the lateral distribution of ≥1.00 cm had increased the
dose constraint of the target volume when the sigmoid colon or
small bowel is depressed in the rectovesical excavation. SV needs
to be included in the target volume.
In conclusion, SV displacements were influenced by
the position of the inserted HS. HS insertion in the upper two
slices and lateral distribution of ≥1.00 cm had impacted the SV
cranial displacement. HS insertion must be carefully performed when
the sigmoid colon or small bowel is depressed during rectovesical
excavation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM, YH, HK and KN were involved in the conception
and design of the study. KM, YH, HK, KN and KH collected patient
data and drafted the manuscript. KM, YH, HK, KN and KH interpreted
the data. KM and YH prepared the manuscript, and HK, KN and KH
edited the manuscript. All authors confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were conducted according to the ethical standards of
the institutional research committee and The 1964 Declaration of
Helsinki and its later amendments or comparable ethical standards.
This retrospective study was approved by the institutional review
board of National Hospital Organization Shikoku Cancer Center
(Matsuyama, Japan; approval no. Rin 202105). The need for informed
consent was waived due to the study's retrospective nature.
Patient consent for publication
The need for informed consent was waived due to the
study's retrospective nature.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mottet N, Bellmunt J, Bolla M, Briers E,
Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau
S, et al: EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1:
Screening, diagnosis, and local treatment with curative intent. Eur
Urol. 71:618–629. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koontz BF, Bossi A, Cozzarini C, Wiegel T
and D'Amico A: A systematic review of hypofractionation for primary
management of prostate cancer. Eur Urol. 68:683–691.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jackson WC, Silva J, Hartman HE, Dess RT,
Kishan AU, Beeler WH, Gharzai LA, Jaworski EM, Mehra R, Hearn JWD,
et al: Stereotactic body radiation therapy for localized prostate
cancer: A systematic review and meta-analysis of over 6,000
patients treated on prospective studies. Int J Radiat Oncol Biol
Phys. 104:778–789. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Susil RC, McNutt TR, DeWeese TL and Song
D: Effects of prostate-rectum separation on rectal dose from
external beam radiotherapy. Int J Radiat Oncol Biol Phys.
76:1251–1258. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hamstra DA, Mariados N, Sylvester J, Shah
D, Karsh L, Hudes R, Beyer D, Kurtzman S, Bogart J, Hsi RA, et al:
Continued benefit to rectal separation for prostate radiation
therapy: Final results of a phase III trial. Int J Radiat Oncol
Biol Phys. 97:976–985. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chao M, Ho H, Chan Y, Tan A, Pham T,
Bolton D, Troy A, Temelcos C, Sengupta S, McMillan K, et al:
Prospective analysis of hydrogel spacer for patients with prostate
cancer undergoing radiotherapy. BJU Int. 122:427–433.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bolla M, Maingon P, Carrie C, Villa S,
Kitsios P, Poortmans PM, Sundar S, van der Steen-Banasik EM,
Armstrong J, Bosset JF, et al: Short androgen suppression and
radiation dose escalation for intermediate- and high-risk localized
prostate cancer: Results of EORTC trial 22991. J Clin Oncol.
34:1748–1756. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ng M, Brown E, Williams A, Chao M,
Lawrentschuk N and Chee R: Fiducial markers and spacers in prostate
radiotherapy: Current applications. BJU Int. 113 (Suppl 2):S13–S20.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Salembier C, Villeirs G, De Bari B, Hoskin
P, Pieters BR, Van Vulpen M, Khoo V, Henry A, Bossi A, De Meerleer
G and Fonteyne V: ESTRO ACROP consensus guideline on CT- and
MRI-based target volume delineation for primary radiation therapy
of localized prostate cancer. Radiother Oncol. 127:49–61.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
De Meerleer GO, Villeirs GM, Vakaet L,
Delrue LJ and De Neve WJ: The incidence of inclusion of the sigmoid
colon and small bowel in the planning target volume in radiotherapy
for prostate cancer. Strahlenther Onkol. 180:573–581.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fonteyne V, De Neve W, Villeirs G, De
Wagter C and De Meerleer G: Late radiotherapy-induced lower
intestinal toxicity (RILIT) of intensity-modulated radiotherapy for
prostate cancer: The need for adapting toxicity scales and the
appearance of the sigmoid colon as co-responsible organ for lower
intestinal toxicity. Radiother Oncol. 84:156–163. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fischer-Valuck BW, Chundury A, Gay H,
Bosch W and Michalski J: Hydrogel spacer distribution within the
perirectal space in patients undergoing radiotherapy for prostate
cancer: Impact of spacer symmetry on rectal dose reduction and the
clinical consequences of hydrogel infiltration into the rectal
wall. Pract Radiat Oncol. 7:195–202. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pinkawa M, Klotz J, Djukic V, Schubert C,
Escobar-Corral N, Caffaro M, Piroth MD, Holy R and Eble MJ:
Learning curve in the application of a hydrogel spacer to protect
the rectal wall during radiotherapy of localized prostate cancer.
Urology. 82:963–968. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fukumitsu N, Mima M, Demizu Y, Suzuki T,
Ishida T, Matsushita K, Yamaguchi R, Fujisawa M and Soejima T:
Separation effect and development of implantation technique of
hydrogel spacer for prostate cancers. Pract Radiat Oncol.
12:226–235. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Catton CN, Lukka H, Gu CS, Martin JM,
Supiot S, Chung PWM, Bauman GS, Bahary JP, Ahmed S, Cheung P, et
al: Randomized trial of a hypofractionated radiation regimen for
the treatment of localized prostate cancer. J Clin Oncol.
35:1884–1890. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Arcangeli G, Saracino B, Arcangeli S,
Gomellini S, Petrongari MG, Sanguineti G and Strigari L: Moderate
hypofractionation in high-risk, organ-confined prostate cancer:
Final results of a phase III randomized trial. J Clin Oncol.
35:1891–1897. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Datta NR, Stutz E, Rogers S and Bodis S:
Conventional versus hypofractionated radiation therapy for
localized or locally advanced prostate cancer: A systematic review
and meta-analysis and therapeutic implications. Int J Radiat Oncol
Biol Phys. 99:573–589. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brand VJ, Milder MTW, Christianen MEMC,
Hoogeman MS and Incrocci L: Seminal vesicle inter- and
intra-fraction motion during radiotherapy for prostate cancer: A
review. Radiother Oncol. 169:15–24. 2022.PubMed/NCBI View Article : Google Scholar
|