Introduction
Hepatocellular carcinoma (HCC) is frequently
observed in patients with cirrhosis caused by persistent hepatitis
B virus (HBV) and hepatitis C virus (HCV) infection, alcohol
consumption, obesity and diabetes mellitus (DM)-related metabolic
disorders (1). With notable
advancements being made in the treatment of HCV with direct-acting
antiviral therapy (2) and the
marked increase in the number of obese patients worldwide (3), the number of cases of HCV-related HCC
has decreased, while that of non-viral HCC cases has increased
(4,5). In fact, the population of non-viral
HCC for all HCC cases is reported to be 59% in the USA and 28.8% in
Japan (5,6). Non-alcoholic fatty liver disease
(NAFLD), hepatic manifestations of obesity and metabolic disorders,
and non-alcoholic steatohepatitis (NASH), a progressive liver
disease with inflammation and fibrosis that can lead to cirrhosis,
are of interest in relation to HCC. Patients with NASH are reported
to be 5.29 per 1,000 person-years, with risks increasing as liver
pathology, such as fibrosis, progresses (7).
Sufficient surveillance with regard to the risk
factors for HCC (e.g., etiology, severity of hepatic fibrosis, and
its history) is recommended so that it may be detected at an early
stage when curative treatment is feasible (8-10).
However, screening high-risk groups for HCC among patients with
NAFLD/NASH is extremely difficult as their-related HCC tends to
appear in non-cirrhotic livers. It has been reported that only 46%
of all NAFLD/NASH-related HCC cases were complicated by cirrhosis
(6). Furthermore, the numbers of
obese patients or patients with NAFLD/NASH are currently markedly
higher those of patients with viral hepatitis (11). As a result, a vast number of
patients with non-viral hepatitis could be subjected to HCC
surveillance, and there is an urgent need to develop a simple,
efficient and non-invasive HCC surveillance system for patients
with non-viral hepatitis.
The severity of hepatic fibrosis is one of the most
critical risk factors for HCC (8-10).
The Child-Pugh score (CPS) and platelet counts are established
indicators of the severity of cirrhosis, liver functional reserve
and hepatic fibrosis. Recently, the albumin-bilirubin (ALBI) score,
which can be easily calculated only from serum levels of albumin
and total bilirubin, has been reported to have an improved
capability compared with the CPS to assess hepatic function reserve
in patients with HCC (12). The
fibrosis 4 (FIB-4) index, which is based on age, aspartate
aminotransferase (AST) levels, alanine aminotransferase (ALT)
levels and the platelet count, is a non-invasive scoring system
used to evaluate hepatic fibrosis (13). The NAFLD fibrosis score (NFS),
which is calculated by adding the body mass index (BMI) and the
presence of DM to the four parameters used in the FIB-4 index, is
also a fibrosis scoring system exclusively for patients with NAFLD
(14). The American, European and
Japanese clinical practice guidelines for NAFLD/NASH recommend that
the FIB-4 index or NFS should be used as the first step in
evaluating hepatic fibrosis as these two indicators have a high
negative predictive value for ruling out advanced fibrosis
(15-18).
However, it remains unclear as to which cut-off values for each of
these indicators would be the most suitable for screening high-risk
groups of HCC among non-viral hepatitis in a clinical setting.
In the present study, patients with non-viral HCC
treated in Gifu University Hospital (Gifu, Japan) were divided
according to the representative cut-off values of each indicator
described above. The present study aimed to identify the most
suitable indicator for screening high-risk groups of HCC by
comparing the possibility that HCC was overlooked in patients
outside the cut-off values.
Materials and methods
Enrolled patients
Between May, 2006 and December, 2021, 536 patients
with HCC were treated in Gifu University hospital; of these, 346
were found to have virus-related HCC (74 HBV-related, 269
HCV-related and 3 HBV/HCV overlaps). A total of 190 patients
(35.4%) with neither a hepatitis B surface antigen nor HCV antibody
diagnosed with HCC were enrolled in the present study. The mean age
of the enrolled patients was 72.9 years (range, 40-90 years). In
this cohort, 105, 100 and 36 patients had diabetes, hypertension
and hyperlipidemia, respectively. In total, 160 patients visited
the hospitals regularly to undergo treatment for the aforementioned
metabolic syndromes or other diseases prior to the diagnosis of
HCC. Moreover, 57, 44, 74, 8 and 7 patients underwent liver
resection, radiofrequency ablation, transcatheter arterial
chemoembolization, radiotherapy and systemic chemotherapy,
respectively. These initial treatments for HCC were determined
according to the applicable Japanese guidelines (8). All patients were administered
standard clinical treatment. Of the 190 patients, 126 patients (53
pathologically and 73 clinically) were diagnosed with NAFLD/NASH,
provided that they had metabolic syndromes, such as obesity or DM;
patients with other types of non-viral hepatitis, such as alcoholic
hepatitis, autoimmune hepatitis, or primary cholangitis were
excluded from the study.
HCC was diagnosed based on typical hypervascular
tumor staining on angiography and typical dynamic computed
tomography or magnetic resonance imaging findings of enhanced
staining in the early phase and attenuation in the delayed phase
(8). The authors were unable to
obtain written, informed consent in advance due to the
retrospective design of the study. Instead, by disclosing the
details of the study, the study participants were provided with an
opportunity to opt-out. The study design was reviewed and approved
by the Ethics Committee of the Gifu University School of Medicine
on September 5, 2022 (ethical protocol code: 2022-0193).
Study design and scoring
The ALBI score, FIB-4 index and NFS were calculated
using the following formulas, as previously described (12-14):
i) ALBI score={log10 [17.1 x total bilirubin (mg/dl)] x0.66} + [10x
albumin (g/dl) x -0.085]; ii) FIB-4 index=age (years) x AST
(U/l)/{platelet count (104/µl) x [√ALT (U/l)]}; iii)
NFS=-1.675 + 0.037 x age (years) + 0.094 x BMI (kg/m2) +
1.13x DM (yes=1, no=0) + 0.99 x AST (U/l)/ALT (U/;) -0.013x
platelet counts (104/µl) -0.66x albumin (g/dl).
Two cut-off values for low and high levels of
fibrosis, for CPS, platelet count, ALBI score, FIB-4 index and NFS
were set based on data at the time of the diagnosis of HCC. The
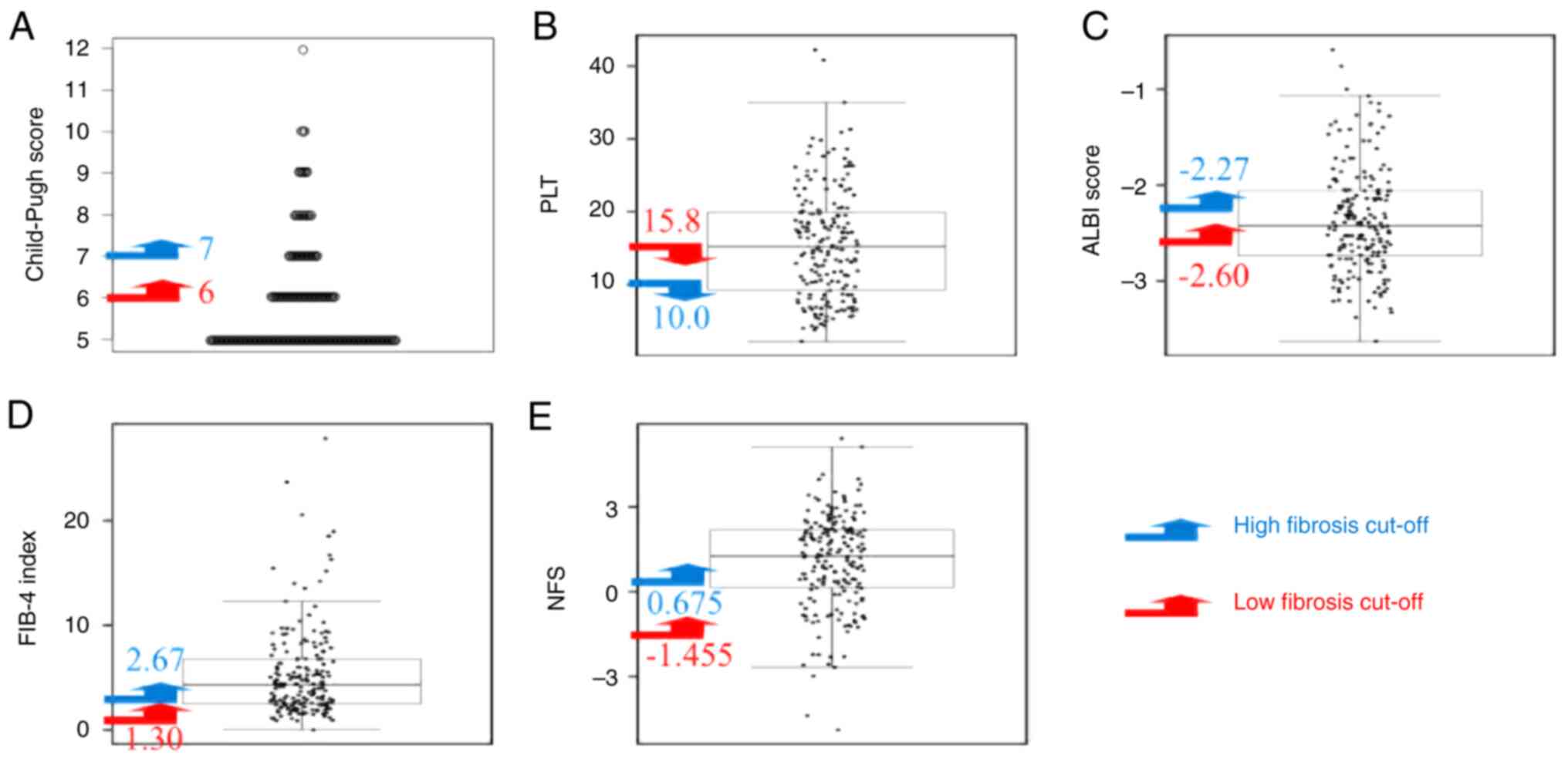
cut-off values for CPS were 6 (grade A) for low levels of fibrosis
and 7 (grade B) for levels of high fibrosis. The cut-off values for
platelet counts were 15.8x104/µl for low levels of
fibrosis and 10.0x104/µl for high levels of fibrosis.
These values were determined as a platelet count of
<10.0x104/µl is pathologically associated with stage
4 hepatic fibrosis in the case of hepatitis C (19,20)
and that of 15.8x104/µl is the lower limit of normal in
Gifu University Hospital. The ALBI score was set to low fibrosis at
-2.60 and the cut-off value between ALBI grades I and IIa. The
score for high fibrosis was set at -2.27 and the cut-off value for
ALBI grades IIa and IIb, respectively (12). The cut-off values of low fibrosis
and high fibrosis in the FIB-4 index (1.31 and 2.67) and those in
the NFS (-1.455 and 0.675) were determined, respectively, according
to the NAFLD/NASH guidelines (15).
Analysis of the cut-off values
The ratio of the number of patients who fell outside
the above cut-off values to the total number of patients was
defined as the overlooking rate. The most appropriate indicator for
HCC surveillance was examined in patients with non-viral hepatitis
by comparing the overlooking rates of low and high fibrosis cut-off
values for each indicator. These comparisons were performed without
the use of any formal statistical analyses. Instead, cut-off values
were considered preferable when they corresponded to lower
overlooking rates.
Results
Baseline characteristics of the
patients
The baseline characteristics of all the enrolled
patients (n=190) and patients with NAFLD/NASH (n=126) are presented
in Table I. Of all the patients,
112, 39, 18, 11, 6, 3 and 1 patients had a CPS of 5, 6, 7, 8, 9, 10
and 12, respectively. The mean platelet count, ALBI score, FIB-4
index and NFS were 15.6±7.5 (range, 9.3-42.2)x104/µl,
-2.37±0.55 (range, -3.63 to -0.59), 5.33±4.27 (range, 0.50-27.9)
and 1.063±1.668 (range, -4.897-5.429), respectively. Among the 126
patients with NAFLD/NASH, 76, 26, 12, 6, 5 and 1 patients had a CPS
of 5, 6, 7, 8, 9 and 10, respectively. The mean platelet count,
ALBI score, FIB-4 index and NFS in these patients were 16.7±7.4
(range, 3.8-42.2) x104/µl, -2.38±0.52 (range, -3.38 to
-0.760), 4.74±3.39 (range, 0.50-16.8) and 0.944±.656 (range,
-4.897-4.146), respectively.
 | Table IBaseline demographic and clinical
characteristics of the enrolled patients. |
Table I
Baseline demographic and clinical
characteristics of the enrolled patients.
| Variable | All patients
(n=190) | Patients with
NAFLD/NASH (n=126) |
|---|
| Sex
(male/female) | 135/55 | 82/44 |
| Age (years) | 72.9±9.1 | 74.3±9.0 |
| Etiology
(NAFLD/alcohol/others) | 126/50/14 | - |
| BMI
(kg/m2) | 24.7±3.7 | 24.8±3.4 |
| AST (U/l) | 53.3±45.7 | 52.1±38.3 |
| ALT (U/l) | 38.0±40.1 | 40.7±47.3 |
| ALB (g/dl) | 3.7±0.6 | 3.7±0.5 |
| T-Bil (mg/dl) | 1.2±1.2 | 1.2±1.3 |
| PLT
(x104/µl) | 15.6±7.5 | 16.7±7.4 |
| PT (%) | 87.7±19.9 | 88.8±20.7 |
| Child-Pugh score
(5/6/7/8/9/10/11/12) |
112/39/18/11/6/3/0/1 |
76/26/12/6/5/1/0/0 |
| ALBI score | -2.37±0.55 | -2.38±0.52 |
| FIB-4 index | 5.33±4.27 | 4.74±3.39 |
| NAFLD fibrosis
score | 1.063±1.668 | 0.944±1.656 |
| Diabetes mellitus
(yes/no) | 105/85 | 75/51 |
| AFP (ng/dl) | 10790±68264 | 6202±22585 |
| PIVKA-II
(mAU/ml) | 27207±196026 | 56733±298328 |
| Stage
(I/II/III/IV) | 24/66/66/34 | 12/45/43/26 |
The dot charts with low and high-fibrosis cut-off
values for CPS, platelet counts, ALBI score, FIB-4 index and NFS in
all the enrolled patients are presented in Fig. 1. The overlooking rates for CPS,
platelet counts, ALBI score, FIB-4 index and NFS on the low
fibrosis cut-off value were 41.0, 48.9, 35.8, 4.2 and 5.8%,
respectively, while those on the high fibrosis cut-off value were
79.5, 73.2, 62.6, 30.0 and 37.4%, respectively. Even when the
analysis was limited to the 126 cases of NAFLD/NASH, those for the
FIB-4 index and NFS on the low fibrosis cut-off value were 4.8 and
6.3%, which were markedly lower than those corresponding to the
other cut-off values (31.7 and 81.0%) (Table II). These findings suggest that
the overlooking rates of the FIB-4 index (4.2% for all patients and
4.8% for patients with NAFLD/NASH) and NFS (5.8% for all patients
and 6.3% for patients with NAFLD/NASH) at the low fibrosis cut-off
value were lower than those of CPS, platelet counts and ALBI
score.
 | Table IIOverlooking rates of the indicators
that represent liver functional reserve and fibrosis among all
patients and those with NAFLD. |
Table II
Overlooking rates of the indicators
that represent liver functional reserve and fibrosis among all
patients and those with NAFLD.
| | All patients
(n=190) | Patients with
NAFLD/NASH (n=26) |
|---|
| Variable | Overlooking rate (low
fibrosis) (%) | Overlooking rate
(high fibrosis) (%) | Overlooking rate (low
fibrosis) (%) | Overlooking rate
(high fibrosis) (%) |
|---|
| Child-Pugh score | 41.0 | 79.5 | 60.3 | 81.0 |
| PLT | 48.9 | 73.2 | 56.3 | 80.2 |
| ALBI score | 35.8 | 62.6 | 34.9 | 70.6 |
| FIB-4 index | 4.2 | 30.0 | 4.8 | 31.7 |
| NAFLD fibrosis
score | 5.8 | 37.4 | 6.3 | 38.9 |
Discussion
The results of the present study demonstrated that
the overlooking rates of the FIB-4 index and NFS using the low
fibrosis cut-off values set here were low enough to screen patients
with non-viral HCC, including NAFLD/NASH-related HCC. These cut-off
values (≥1.30 for the FIB-4 index or ≥-1.455 for NFS) may be used
to screen high-risk groups for HCC in patients with non-viral
hepatitis, including NAFLD/NASH. These findings reinforce the
findings of previous studies that have demonstrated that both the
FIB-4 index and NFS are promising non-invasive scoring systems for
assessing the risk of liver fibrosis and ultimately
NASH/NAFLD-related HCC (13-15).
It should also be noted that these cut-off values of the FIB-4
index and NFS are recommended for selecting cases of advanced
fibrosis in the Japanese NAFLD/NASH guidelines (15). A previous meta-analysis revealed
that the FIB-4 index and NFS were diagnostically sensitive to
advanced fibrosis in patients with NAFLD (21). The negative predictive value for a
diagnosis of advanced fibrosis with an FIB-4 index ≥1.30 is 99%
(13). These reports indicate
again that these systems may be useful in identifying groups at a
high risk of developing HCC in non-fibrotic, advanced cases,
without cirrhosis. Surveillance for HCC is difficult for patients
with non-viral hepatitis as they are rarely followed-up by
gastroenterologists, although are often followed-up by general
physicians or annual physical examinations. The FIB-4 index and NFS
can be easily calculated from AST, ALT, albumin, platelet count,
BMI, age and the presence of DM, all of which can be evaluated in
general clinical practice. Therefore, general physicians should
evaluate the risk of hepatic fibrosis and hepatocarcinogenesis in
non-viral hepatitis patients using the FIB-4 index or NFS and
consult a gastroenterologist if they find patients with FIB-4 index
≥1.30 or NFS ≥-1.455.
By contrast, in the present study, platelet counts,
CPS and ALBI scores were not appropriate indicators for screening
high-risk groups of HCC in patients with non-viral hepatitis. For
example, 48.9% of the patients with non-viral hepatitis and 56.3%
of those with NAFLD/NASH develop HCC before the platelet counts
decreases to <10.0x104/µl (Table II), which is a marker of
progression to cirrhosis and the appropriate cut-off value to
screen high-risk groups of HCC in HCV-positive patients (19,20).
These findings are consistent with those of a previous study which
demonstrated that approximately half of patients with non-viral
hepatitis had initial HCC emerging in non-cirrhotic livers
(6). On the other hand, patients
with an FIB-4 index <1.30 or NFS <-1.455 accounted for
approximately only 5% of all the patients HCC in the present study.
These results, therefore, suggest that high-risk groups could be
screened for non-viral HCC by not using platelet counts, CPS, or
ALBI, but the FIB-4 index or NFS. These results may help to improve
the management of non-viral hepatitis, as platelet counts or CPS,
the established risk factors for HCV-related HCC (19,20),
are still utilized by most general physicians. Furthermore, as
described above in the Materials and methods section, patients with
non-viral hepatitis are frequently followed-up by general
physicians for presence of metabolic syndrome or other diseases.
Among the 190 patients enrolled in the present study, 160 were
followed-up in hospitals other than that of the authors.
Obesity-related factors, such as adipose tissue
remodeling and pro-inflammatory adipokine secretion, have been
reported to function synergistically with liver fibrosis and
contribute to liver carcinogenesis (11). In fact, obesity-related disorders,
including insulin resistance, excessive accumulation of leptin and
increased levels of oxidative stress and visceral adipose tissue,
can promote the recurrence of HCC following curative treatment
(22-26).
Obesity is a global epidemic, including in Japan, and the number of
obese individuals is increasing as rapidly as the number of
patients with NAFLD/NASH (5).
Obese patients with metabolic diseases, such as DM and NAFLD/NASH,
tend to be followed-up by general physicians, but non-viral HCC
often develops in the non-cirrhotic liver of these patients.
General physicians can easily calculate the FIB-4 index and NFS.
Therefore, in patients with non-viral hepatitis, particularly those
with obesity or NAFLD/NASH, an appropriate HCC surveillance
strategy would be for the general physician to first evaluate
hepatic fibrosis using the FIB-4 index and NFS, and in the second
step for the gastroenterologist to screen for HCC with a detailed
examination using abdominal ultrasound and tumor markers.
The present study has some limitations. First, this
was a retrospective study that only included patients with HCC.
Furthermore, the FIB-4 index and the NFS were considered on data
based only at the time of the HCC diagnosis in the present study.
Sensitivity and the overlooking rate (false-negative rate) were
demonstrated, but specificity and a false-positive rate could not
be determined, particularly since the present study did not include
non-HCC patients with non-viral hepatitis. The receiver operating
characteristic curve and Youden index are generally used to
determine an optimal cut-off value (27,28).
However, these analyses could not be performed in the present study
as the specificity was not determined. To validate the efficacy of
the cut-off values obtained herein, a time-to-event plot and
analysis including non-viral hepatitis patients without HCC needs
to be performed. Second, the overlooking rate was simply determined
and may not have been sufficient to ensure a high diagnostic
accuracy. However, as a false-negative rate is more important than
a false-positive rate in disease screening, the low overlooking
rate obtained by setting a cutoff value of FIB-4 index ≥1.30 or NFS
≥1.455 was considered to be useful in screening patients at a high
risk of developing non-viral HCC, particularly when performed by
non-gastroenterologists. Third, it was necessary to verify whether
each cut-off value set at this time was appropriate. An FIB-4 of
≥1.30 and NFS of ≥1.455 were considered suitable cut-off values as
their corresponding overlooking rates were lower (~5%) than those
corresponding to the other cutoff values (from 30.0 to 79.5%).
Statistically significant differences were not evaluated in the
present. To determine the appropriate cut-off values, it is
necessary to consider not only the rate of missed cases, but also
the adequacy of the specificity and the economic burden of
healthcare.
In conclusion, in the present study, the cut-off
values of the FIB-4 index ≥1.30 or NFS ≥1.455 were associated with
a low false-negative rate (overlooking rate) of ~5% and may thus be
used to screen high-risk groups for HCC in patients with non-viral
hepatitis including NAFLD/NASH. A simple setting of cut-off values
may be particularly useful in clinical settings which often involve
non-gastroenterologists. However, the present study did not include
patients with non-viral hepatitis without HCC; thus, a long-term
prospective study including a sufficient cohort of such patients is
required to examine the usefulness of this cut-off value in the
real world.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Japan Agency for
Medical Research and Development (grant no. JP22fk0210113) and MHLW
Policy Research for Hepatitis Measures Program (grant no.
JPMH22HC1001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (KI, KT, SU, TM, TH, AS and MS) designed
the study. KI analyzed the data and drafted the manuscript. KT
supervised the treatment of the participants. KT, SU, TM, TH and AS
contributed to the selection of the participants and collected the
data. KT, SU, TM, TH and AS revised the manuscript, and MS mainly
reviewed and amended the manuscript. KT and MS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study design was reviewed and approved
by the Ethics Committee of the Gifu University School of Medicine
on September 5, 2022 (ethical protocol code: 2022-0193). The
authors were unable to obtain written informed consent in advance
due to the retrospective design of our study. Instead, by
disclosing the details of the study, the study participants were
provided with an opportunity to opt-out
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Serag HB: Hepatocellular carcinoma: An
epidemiologic view. J Clin Gastroenterol. 35:S72–S78.
2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Falade-Nwulia O, Suarez-Cuervo C, Nelson
DR, Fried MW, Segal JB and Sulkowski MS: Oral direct-acting agent
therapy for hepatitis c virus infection: A systematic review. Ann
Intern Med. 166:637–648. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Blüher M: Obesity: Global epidemiology and
pathogenesis. Nat Rev Endocrinol. 15:288–298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mittal S and El-Serag HB: Epidemiology of
hepatocellular carcinoma: Consider the population. J Clin
Gastroenterol. 47 (Suppl):S2–S6. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tateishi R, Uchino K, Fujiwara N, Takehara
T, Okanoue T, Seike M, Yoshiji H, Yatsuhashi H, Shimizu M, Torimura
T, et al: A nationwide survey on non-B, non-C hepatocellular
carcinoma in Japan: 2011–2015 update. J Gastroenterol. 54:367–376.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sanyal A, Poklepovic A, Moyneur E and
Barghout V: Population-based risk factors and resource utilization
for HCC: US perspective. Curr Med Res Opin. 26:2183–2191.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of nonalcoholic fatty
liver disease-Meta-analytic assessment of prevalence, incidence,
and outcomes. Hepatology. 64:73–84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kokudo N, Takemura N, Hasegawa K, Takayama
T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, et al:
Clinical practice guidelines for hepatocellular carcinoma: The
Japan society of hepatology 2017 (4th JSH-HCC guidelines) 2019
update. Hepatol Res. 49:1109–1113. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL clinical
practice guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Heimbach JK, Kulik LM, Finn RS, Sirlin CB,
Abecassis MM, Roberts LR, Zhu AX, Murad MH and Marrero JA: AASLD
guidelines for the treatment of hepatocellular carcinoma.
Hepatology. 67:358–380. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Karagozian R, Derdák Z and Baffy G:
Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism.
63:607–617. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hiraoka A, Kumada T, Michitaka K and Kudo
M: Newly proposed ALBI grade and ALBI-T score as tools for
assessment of hepatic function and prognosis in hepatocellular
carcinoma patients. Liver Cancer. 8:312–325. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono
M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, et al:
Validation of the FIB4 index in a Japanese nonalcoholic fatty liver
disease population. BMC Gastroenterol. 12(2)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Angulo P, Hui JM, Marchesini G, Bugianesi
E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, et
al: The NAFLD fibrosis score: A noninvasive system that identifies
liver fibrosis in patients with NAFLD. Hepatology. 45:846–854.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tokushige K, Ikejima K, Ono M, Eguchi Y,
Kamada Y, Itoh Y, Akuta N, Yoneda M, Iwasa M, Yoneda M, et al:
Evidence-based clinical practice guidelines for nonalcoholic fatty
liver disease/nonalcoholic steatohepatitis 2020. Hepatol Res.
51:1013–1025. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ: The diagnosis and
management of non-alcoholic fatty liver disease: Practice guideline
by the American association for the study of liver diseases,
American college of gastroenterology, and the American
gastroenterological association. Hepatology. 55:2005–2023.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Francque SM, Marchesini G, Kautz A,
Walmsley M, Dorner R, Lazarus JV, Zelber-Sagi S, Hallsworth K,
Busetto L, Frühbeck G, et al: Non-alcoholic fatty liver disease: A
patient guideline. JHEP Rep. 3(100322)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tokushige K, Ikejima K, Ono M, Eguchi Y,
Kamada Y, Itoh Y, Akuta N, Yoneda M, Iwasa M, Yoneda M, et al:
Evidence-based clinical practice guidelines for nonalcoholic fatty
liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol.
56:951–963. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yoshida H, Shiratori Y, Moriyama M,
Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, et
al: Interferon therapy reduces the risk for hepatocellular
carcinoma: National surveillance program of cirrhotic and
noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study
group. inhibition of hepatocarcinogenesis by interferon therapy.
Ann Intern Med. 131:174–181. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shiratori Y, Imazeki F, Moriyama M, Yano
M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G,
et al: Histologic improvement of fibrosis in patients with
hepatitis C who have sustained response to interferon therapy. Ann
Intern Med. 132:517–524. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiao G, Zhu S, Xiao X, Yan L, Yang J and
Wu G: Comparison of laboratory tests, ultrasound, or magnetic
resonance elastography to detect fibrosis in patients with
nonalcoholic fatty liver disease: A meta-analysis. Hepatology.
66:1486–1501. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Imai K, Takai K, Miwa T, Maeda T, Hanai T,
Shiraki M, Suetsugu A and Shimizu M: Increased visceral adipose
tissue and hyperinsulinemia raise the risk for recurrence of Non-B
Non-C hepatocellular carcinoma after curative treatment. Cancers
(Basel). 13(1542)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Imai K, Takai K, Maeda T, Watanabe S,
Hanai T, Suetsugu A, Shiraki M and Shimizu M: Increased visceral
fat volume raises the risk for recurrence of hepatocellular
carcinoma after curative treatment. Oncotarget. 9:14058–14067.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Imai K, Takai K, Hanai T, Suetsugu A,
Shiraki M and Shimizu M: Homeostatic model assessment of insulin
resistance for predicting the recurrence of hepatocellular
carcinoma after curative treatment. Int J Mol Sci.
20(605)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Suzuki Y, Imai K, Takai K, Hanai T,
Hayashi H, Naiki T, Nishigaki Y, Tomita E, Shimizu M and Moriwaki
H: Hepatocellular carcinoma patients with increased oxidative
stress levels are prone to recurrence after curative treatment: A
prospective case series study using the d-ROM test. J Cancer Res
Clin Oncol. 139:845–852. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Watanabe N, Takai K, Imai K, Shimizu M,
Naiki T, Nagaki M and Moriwaki H: Increased levels of serum leptin
are a risk factor for the recurrence of stage I/II hepatocellular
carcinoma after curative treatment. J Clin Biochem Nutr.
49:153–158. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fluss R, Faraggi D and Reiser B:
Estimation of the Youden Index and its associated cutoff point.
Biom J. 47:458–472. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cao L, Cheng H, Jiang Q, Li H and Wu Z:
APEX1 is a novel diagnostic and prognostic biomarker for
hepatocellular carcinoma. Aging (Albany NY). 12:4573–4591.
2020.PubMed/NCBI View Article : Google Scholar
|