Introduction
Nasopharyngeal carcinoma (NPC) is a type of cancer
that arises from the nasopharyngeal epithelium. In 2020, ~133,354
new cases of NPC were diagnosed in 185 countries worldwide
accounting for 0.7% of all cancer cases (1). The incidence of NPC has an
age-standardized rate of 3.0/100,000 individuals in China and
0.4/100,000 individuals in predominantly Caucasian populations.
Notably, its incidence has gradually but progressively declined,
and NPC-associated mortality has been substantially reduced
(2). However, some individuals,
including the Bidayuh ethnic group in Borneo, the Naga ethnic group
in northern India and the Inuit ethnic group in the Arctic regions
have higher incidences of NPC, with an age-standardized incidence
of >16/100 000 person-years in men (3). The hypothesis that NPC may originate
from the Baiyue ethnic groups may account for the high incidence of
NPC in a number of diverse populations worldwide, whose ancestry
can be traced back to this population (4). However, how these genetic factors,
which are probably carried by Baiyue women, actually contributes to
the carcinogenesis of NPC remains unknown (4). Keratinizing squamous,
non-keratinizing and basaloid squamous NPC are the subtypes of this
type of cancer, according to the World Health Organization
(2). The improved survival of
patients with NPC has been suggested to be due to the widespread
application of intensity modulated radiotherapy and optimization of
chemotherapy strategies; however, further research should focus on
biomarkers related to the diagnosis and prognostic risk of NPC
(2).
Aldo-keto reductase family 1 member B10 (AKR1B10)
was identified and characterized in 1998. Two previous studies
independently identified a nicotinamide adenine dinucleotide
phosphate (NADP+)-dependent gene whose sequence
exhibited high homology with the corresponding region of human
aldose reductase (5,6). The AKR superfamily reduces
NADP+-dependent oxidoreductases that function in
elimination reactions by modifying carbonyl groups on aldehyde or
ketones to form primary or secondary alcohols, which are then
conjugated with sulfates or glucuronide for excretion (7). AKR1B10 is a key member of the AKR
superfamily and an essential enzyme in the metabolism of carbonyls,
retinal and farnesal/geranylgeraniol for detoxifying active
carbonyls, maintaining cellular homeostasis of retinal-retinoid
acid, and recycling farnesal/geranylgeraniol, the key intermediate
products of cholesterol synthesis (8). AKR1B10 is primarily expressed in the
human colon, small intestine and adrenal gland, with a low level
also present in the liver (9).
AKR1B10 is a protein enzyme originally identified in
human hepatocellular carcinoma (HCC) (5,6). A
large-scale multicenter study validated that AKR1B10 could be a
novel prevalent serum marker for HCC (10). AKR1B10 has also been reported to be
associated with other tumors. AKR1B10 has been shown to be highly
expressed, and to exert oncogenic or metastasis-promoting roles in
the carcinogenesis in of a number of tumors, such as breast cancer
(11-13),
gastric cancer (14), lung cancer
(15-17),
pancreatic carcinoma (8,18), oral squamous cell carcinoma (OSCC)
(19-21)
and laryngeal squamous cell carcinoma (22). However, AKR1B10 exerts opposing
roles in colorectal cancer (23,24)
and endometrial cancer (25,26).
Notably, different results have been reported in NPC. Guo et
al (27) suggested that low
expression of AKR1B10 may be an independent prognostic indicator in
NPC, and that AKR1B10 could be involved in regulating the
proliferation and migration of NPC cells. By contrast, another
study revealed that AKR1B10 is overexpressed in nasopharyngeal
hyperplasia, benign tumors and NPC (28).
To explore the role and diagnostic value of AKR1B10
in NPC, bioinformatics analysis was performed and clinical serum
samples were analyzed in the present study.
Materials and methods
Ethics approval
The present study protocol was approved by the
Ethics Committee of Zhuhai People's Hospital (Zhuhai, China). The
present study followed the ethical standards on human
experimentation of the institution. Written informed consent was
obtained from the patients.
Mining analysis using The Cancer
Genome Atlas (TCGA) database
AKR1B10 Mrna expression data from patients with head
and neck squamous cell carcinoma (HNSCC) were downloaded from
TCGA-HNSC dataset (http://gdc.cancer.gov). AKR1B10 RNA-seq data were
obtained from 504 HNSCC tissues and 44 adjacent normal tissues from
the patients with HNSCC; 43 pairs of tumor tissues and their
corresponding normal tissues were selected according to TCGA
labels. The extracted data were normalized by log2 transformation
using R software version 4.2.1 (R Core Team). Clinical information
from 502 HNSCC patients, including sex, age at initial pathological
diagnosis, smoking history, alcohol history, pathological stage,
TNM stage (2), overall survival
(OS) status and OS times were also download from TCGA-HNSC
dataset.
Mining analysis using the Gene
Expression Omnibus (GEO) database
The GSE53819(29)
dataset consisting of data from 18 NPC primary tumors and 18
non-cancerous nasopharyngeal tissues; the GSE61218(30) dataset consisting of data from 10
NPC and six normal healthy nasopharyngeal tissue specimens; and the
GSE103611(31) dataset consisting
of data from 24 locoregionally advanced NPC (LA-NPC) tumor tissues
with distant metastasis after radical treatment and 24 LA-NPC tumor
tissues without distant metastasis after radical treatment were all
downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). The extracted data
were normalized by log2 transformation using R software version
4.2.1.
Clinical serum samples
A total of 71 patients pathologically diagnosed with
NPC and 30 healthy individuals without known diseases were selected
from Sun Yat-sen University Cancer Center (Guangzhou, China)
between December 2019 and May 2020 Serum samples were obtained by
venipuncture from subjects after an overnight fast between 09:00
and 10:00 a.m. under standard conditions. Samples were clotted at
4-8˚C, and then centrifuged at 1,006 x g for 10 min at room
temperature. The collected serum was distributed in 500-µl aliquots
and stored at -80˚C until needed.
Measurement of AKR1B10
AKR1B10 ELISA kits (cat. no. E-EL-H5453) were
purchased from Elabscience Biotechnology, Inc. and AKR1B10 was
detected according to the manufacturer's instructions. All samples
were analyzed simultaneously. Serum concentrations of AKR1B10 were
determined using standard curves and expressed as unit per liter
(ng/l). The linear ranges for AKR1B10 were 0-5,000 ng/l.
Statistical analysis
All statistical analyses were performed using SPSS
software 18.0 (IBM Corp.). Comparisons between groups regarding
AKR1B10 levels and clinical features were performed using unpaired
or paired Student's t-tests. OS was analyzed using Kaplan-Meier
analysis with log-rank test. Univariate and multivariate Cox
proportional hazards models were used to evaluate the relative risk
factors associated with OS, and hazard ratio with 95% confidence
interval was obtained for each variable. Receiver operating
characteristic (ROC) curves were generated and area under the curve
(AUC) was calculated to determine the diagnostic power of AKR1B10
in NPC. The cut-off point was defined as the maximum Youden index.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of AKR1B10 in patients with
HNSCC
HNSCC includes cancer of the oral cavity, tongue,
hypopharynx, nasopharynx, larynx and thyroid; therefore, the
present study first analyzed the Mrna expression levels of AKR1B10
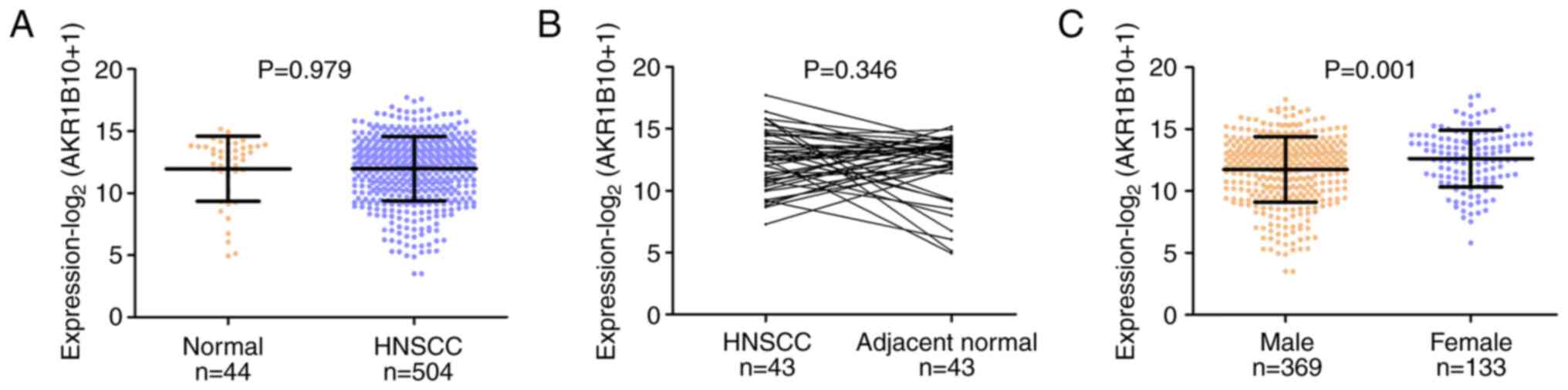
in a HNSCC cohort obtained from TCGA database. The results showed
that there was no significant difference in the expression of
AKR1B10 between HNSCC tissues (n=504) and normal epithelial tissues
(n=44) (P=0.979; Fig. 1A). To
further investigate the expression of AKR1B10, 43 pairs of HNSCC
and adjacent normal tissues were analyzed. Similarly, there was no
significant difference in the expression levels of AKR1B10 in HNSCC
tissues compared with those in the adjacent normal tissues
(P=0.346; Fig. 1B).
Relationship between AKR1B10
expression levels and clinicopathological factors in HNSCC
The present study next examined the association
between AKR1B10 expression and the clinicopathological features of
patients with HNSCC. In TCGA database, AKR1B10 expression was not
associated with age, smoking history, alcohol history, pathological
stage, tumor stage or nodal stage (Table I). However, AKR1B10 expression was
significantly higher in female patients with HNSCC than that in
male patients (P=0.001; Fig.
1C).
 | Table IAssociation between AKR1B10 expression
and clinicopathological features of patients with head and neck
squamous cell carcinoma in The Cancer Genome Atlas database. |
Table I
Association between AKR1B10 expression
and clinicopathological features of patients with head and neck
squamous cell carcinoma in The Cancer Genome Atlas database.
| Characteristics | n | AKR1B10 expression,
mean ± SD | P-value |
|---|
| Sex | | | |
|
Male | 369 | 11.743±2.643 | 0.001a |
|
Female | 133 | 12.620±2.293 | |
| Age | | | |
|
<60
years | 222 | 11.731±2.538 | 0.060 |
|
≥60
years | 279 | 12.169±2.610 | |
|
Not
available | 1 | | |
| Smoking
history | | | |
|
Yes | 287 | 11.984±2.629 | 0.931 |
|
No | 215 | 11.964±2.525 | |
| Alcohol
history | | | |
|
Yes | 333 | 11.827±2.639 | 0.063 |
|
No | 158 | 12.290±2.415 | |
|
Not
available | 11 | | |
| Pathological
stage | | | |
|
I + II | 95 | 12.378±2.543 | 0.200 |
|
III +
IV | 339 | 11.997±2.564 | |
|
Not
available | 68 | | |
| Tumor stage | | | |
|
T0 + T1 +
T2 | 180 | 11.863±2.664 | 0.195 |
|
T3 + T4 | 267 | 12.186±2.514 | |
|
Not
available | 55 | | |
| Nodal stage | | | |
|
N0 | 171 | 12.328±2.527 | 0.073 |
|
N1 + N2 +
N3 | 238 | 11.873±2.522 | |
|
Not
available | 93 | | |
High AKR1B10 expression is not
associated with poor OS in patients with HNSCC
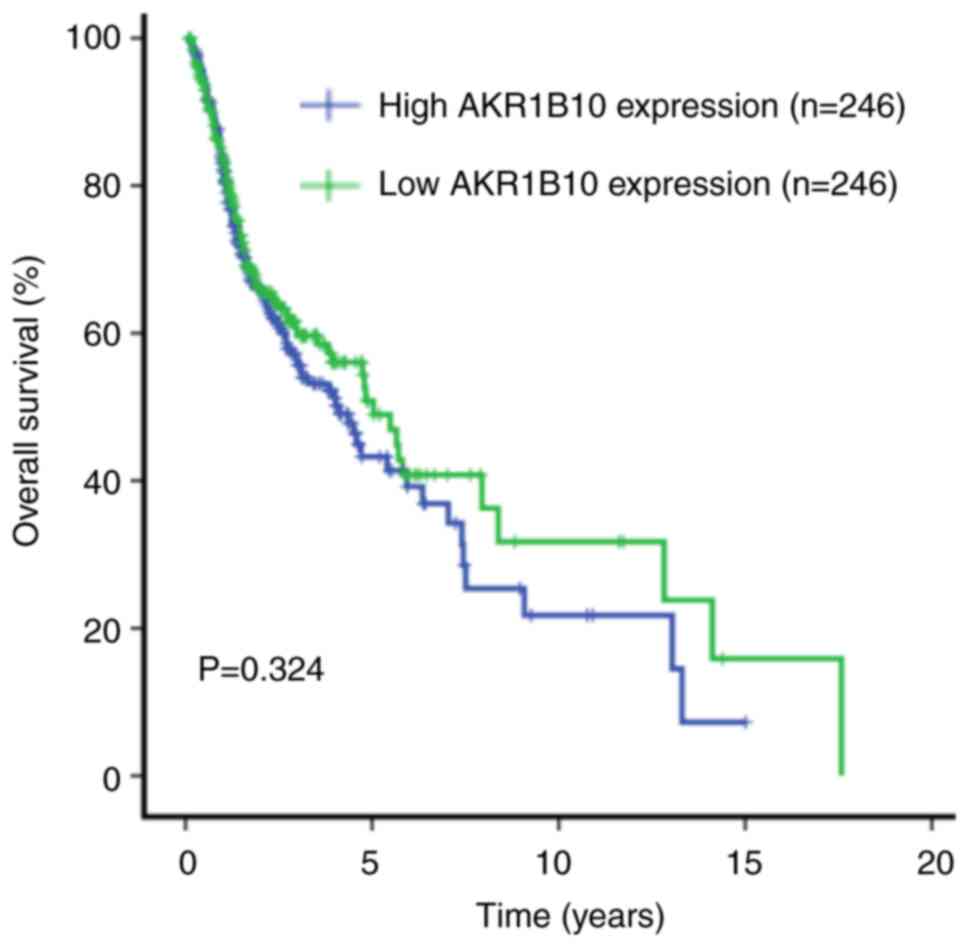
To investigate the prognostic value of AKR1B10 in
HNSCC, Kaplan-Meier analysis was performed. Patients were divided
into high expression and low expression groups according to the
median mRNA expression level. The Kaplan-Meier curve showed that
the mRNA expression levels of AKR1B10 were not associated with the
OS rate (P=0.324; Fig. 2). This
finding was further confirmed by univariate and multivariate Cox
proportional hazard regression analyses of clinicopathological
features and AKR1B10 expression for OS (Table II). Univariate analysis showed
that sex (P=0.047), pathological stage (P=0.005), tumor stage
(P<0.001) and nodal stage (P<0.001) were associated with OS
in patients with HNSCC. Subsequently, multivariate analysis showed
that tumor stage (P=0.004) and nodal stage (P=0.007) were
independent risk factor for OS in patients with HNSCC. However,
AKR1B10 expression, similar to other clinical features (age,
smoking history and alcohol history), was not associated with OS in
patients with HNSCC.
 | Table IIUnivariate and multivariate Cox
proportional hazard regression analyses of clinicopathological
features and AKR1B10 expression for overall survival. |
Table II
Univariate and multivariate Cox
proportional hazard regression analyses of clinicopathological
features and AKR1B10 expression for overall survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<60 vs. ≥60
years) | 0.800 | 0.610-1.062 | 0.120 | 0.826 | 0.587-1.163 | 0.272 |
| Sex (male vs.
female) | 0.740 | 0.560-0.996 | 0.047a | 0.689 | 0.472-1.004 | 0.052 |
| Smoking history
(Yes vs. No) | 1.100 | 0.840-1.546 | 0.300 | 0.410 | 0.613-1.319 | 0.587 |
| Alcohol history
(Yes vs. No) | 1.000 | 0.770-1.389 | 0.810 | 1.202 | 0.832-1.738 | 0.328 |
| Pathological stage
(I + II vs. III + IV) | 0.560 | 0.380-0.838 | 0.005a | 0.958 | 0.418-2.194 | 0.920 |
| Tumor stage (T0 +
T1 + T2 vs. T3 + T4) | 0.510 | 0.370-0.709 |
<0.001a | 0.467 | 0.280-0.799 | 0.004a |
| Nodal stage
(Negative vs. Positive) | 0.520 | 0.370-0.730 |
<0.001a | 0.567 | 0.376-0.856 | 0.007a |
| AKR1B10 (High vs.
Low) | 1.149 | 0.873-1.515 | 0.320 | 1.130 | 0.816-1.566 | 0.462 |
Expression of AKR1B10 in patients with
NPC
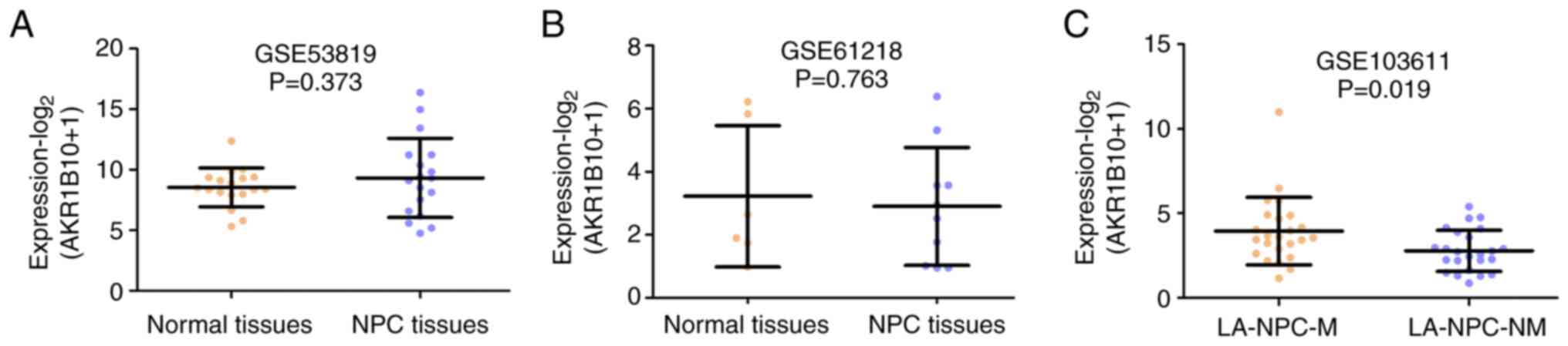
Since differences exist between NPC and other
epithelial tumors in the head and neck region, the present study
subsequently assessed the expression of AKR1B10 in NPC (3). The GEO database was used to analyze
AKR1B10 expression in NPC. Two gene expression profile datasets
(GSE53819 and GSE61218) demonstrated that the expression levels of
AKR1B10 were not significantly different in NPC tissues compared
with those in normal tissues (P=0.373; Fig. 3A; P=0.763; Fig. 3B). Notably, analysis of the
GSE103611 dataset showed that the tumors from patients with LA-NPC
and distant metastasis exhibited higher AKR1B10 expression than
those without distant metastasis after radical treatment (P=0.019;
Fig. 3C).
Diagnostic value of serum AKR1B10 in
NPC
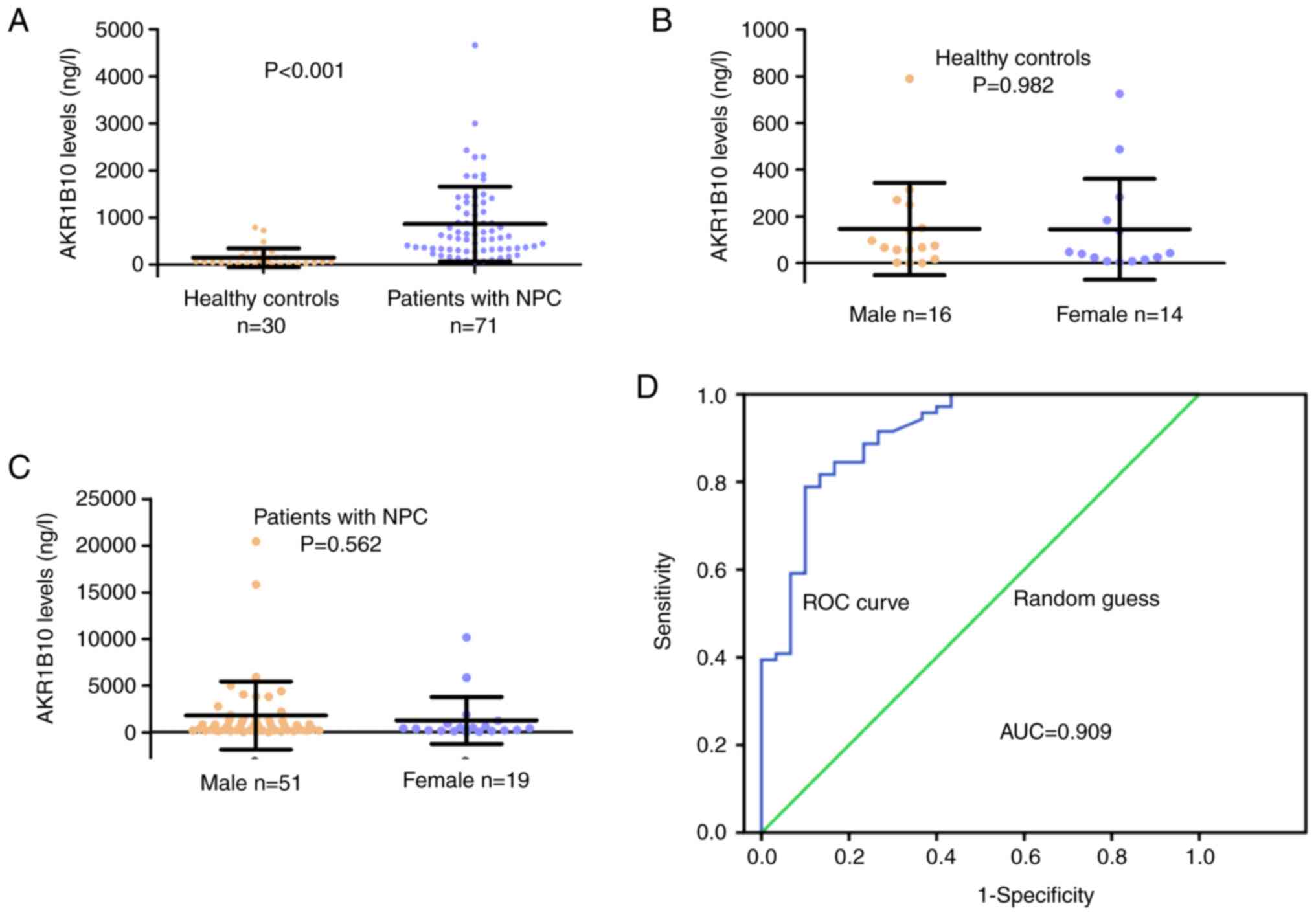
Serum samples were collected from healthy controls
(n=30) and patients with NPC (n=71). The levels of AKR1B10 in the
serum were measured using ELISA kits. The results revealed that
AKR1B10 levels were significantly increased in the serum samples
from patients with NPC compared with those from health controls
(P<0.001; Fig. 4A). Since
AKR1B10 expression was higher in HNSCC tissues from female patients
compared with those from male patients, the present study analyzed
the serum levels of AKR1B10; the results revealed that sex did not
affect AKR1B10 levels in healthy controls (P=0.982 Fig. 4B) or in patients with NPC (P=0.562;
Fig. 4C). A ROC curve was
generated and the AUC was calculated to evaluate the diagnostic
value of AKR1B10. The AUC was 0.909 (Fig. 4D). When the cutoff value (318.01
ng/l) was used, the sensitivity and specificity were 0.789 and
0.900. These results indicated the potential of serum AKR1B10 in
NPC diagnosis.
Discussion
Recently, the role of AKR1B10 has been described in
several types of cancer, including breast cancer, gastric cancer,
lung cancer, pancreatic carcinoma, oral squamous cell carcinoma and
laryngeal squamous cell carcinoma (8,11-21,32).
HNSCC comprises a heterogeneous group of tumors that arise from the
mucosal epithelium in the oral cavity, pharynx and larynx (33). The roles of AKR1B10 in HNSCC have
been assessed in previous years. It has been reported that high
AKR1B10 expression is associated with reduced survival in patients
with OSCC (19,21). Notably, salivary AKR1B10 levels may
be a promising biomarker for screening high-risk patients with OSCC
and monitoring the progression of OSCC (20). Similarly, AKR1B10 may be related to
the development of laryngeal carcinoma and could be used as a
prognostic indicator for laryngeal carcinoma (22). However, using TCGA database, the
present study revealed that there were no significant differences
in the expression of AKR1B10 between HNSCC tissues and adjacent
normal tissues, and high expression of AKR1B10 was not associated
with poor OS in HNSCC. These conflicting results may be due to the
different samples assessed. Numerous agents and factors, such as
ethoxyquin, MG-132, doxorubicin and smoking history, have
previously been reported to induce upregulation of AKR1B10
(7,32); however, the present study did not
verify the regulation of AKR1B10 by smoking history. Notably,
although most of the clinicopathological features of patients with
HNSCC were not associated with the AKR1B10 expression, the levels
of AKR1B10 were higher in tissues, but not serum samples, from
female patients with HNSCC patients compared with in male patients.
To the best of our knowledge, no previous studies have assessed the
sex differences of AKR1B10 expression and the mechanism is unclear;
therefore, our future research will focus on this.
NPC is a type of HNSCC, which is distinctly
different from other types of HNSCC. Epstein-Barr virus infection
is perhaps the most studied etiological factor for NPC (3). Lifestyle and environmental changes,
enhanced understanding of the pathogenesis and risk factors of NPC,
population screening, advancements in imaging techniques, and
individualized comprehensive chemoradiotherapy strategies may be
the reason for the declined incidence and mortality of NPC
(2,3). Identifying biomarkers related to the
diagnosis and prognostic risk of NPC should be the focus in the
next few decades. Notably, contrasting roles of AKR1B10 in NPC have
been reported in previous studies: a previous study suggested that
low expression of AKR1B10 may be an independent prognostic
indicator in NPC (27), whereas
another study revealed that AKR1B10 is overexpressed in
nasopharyngeal hyperplasia, benign tumors and NPC (28). Another study reported that AKR1B10
could induce radiotherapy resistance and promote cell survival in
NPC (34). In the present study,
analysis of GEO datasets suggested that the expression levels of
AKR1B10 were not significantly different in NPC tissues compared
with those in normal tissues. However, the serum levels of AKR1B10
were revealed to be higher in patients with NPC than in healthy
controls and the ROC curve analysis indicated that AKR1B10 may be a
potential marker for NPC diagnosis.
Besides the diagnostic and prognostic value of
AKR1B10, other roles of AKR1B10 have been reported. In gastric
cancer, AKR1B10 has been shown to participate in the chemotherapy
resistance of gastric cancer (14,35,36)
and human lung cancer (37).
Similarly, AKR1B10 confers resistance to radiotherapy in NPC
(34). Using the GSE103611
dataset, the present study indicated that AKR1B10 may be associated
with distant metastasis after radical treatment of NPC. The
mechanism underlying the effects of AKR1B10 on NPC require further
investigation. A number of pathways are involved in AKR1B10
regulation in other types of cancer; notably, AKR1B10 can active
the diacylglycerol second messenger (13) and PI3K/AKT/NF-Κb pathway (12) in breast cancer; and can regulate
the fibroblast growth factor 1-dependent pathway in colorectal
cancer (38). Furthermore, AKR1B10
can mediate liver cancer cell proliferation through
sphingosine-1-phosphate (39).
The present study has some limitations. First,
tissues were not collected from patients with NPC and healthy
controls. Second, a larger cohort should be used to assess the
value of serum AKR1B10. Finally, the mechanism underlying the
effects of AKR1B10 on NPC require further investigation. Taken
together, the present study revealed that serum levels of AKR1B10
in patients with NPC were relatively higher than those in healthy
controls; therefore, AKR1B10 may be a potential marker for NPC
diagnosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Guangzhou
Science and Technology Plan Project (grant no. 202201020039), the
Zhuhai Science and Technology Plan Project (grant no.
ZH2202200030HJL) and the Zhuhai People's Hospital Cultivation Plan
Project (grant no. 2019PY-29).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL analyzed the public data and wrote the
manuscript. TK collected the serum used in the experiments. ZZ
designed the project and performed the ELISA experiments. JL and ZZ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhuhai People's Hospital (approval no. ZY2019-26).
Patients and healthy controls provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wee JT, Ha TC, Loong SL and Qian CN: Is
nasopharyngeal cancer really a ‘Cantonese cancer’? Chin J Cancer.
29:517–526. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cao D, Fan ST and Chung SS: Identification
and characterization of a novel human aldose reductase-like gene. J
Biol Chem. 273:11429–11435. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Scuric Z, Stain SC, Anderson WF and Hwang
JJ: New member of aldose reductase family proteins overexpressed in
human hepatocellular carcinoma. Hepatology. 27:943–950.
1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang R, Wang G, Ricard MJ, Ferris B,
Strulovici-Barel Y, Salit J, Hackett NR, Gudas LJ and Crystal RG:
Smoking-Induced Upregulation of AKR1B10 expression in the airway
epithelium of healthy individuals. Chest. 138:1402–1410.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang W, Li H, Yang Y, Liao J and Yang GY:
Knockdown or inhibition of aldo-keto reductase 1B10 inhibits
pancreatic carcinoma growth via modulating Kras-E-cadherin pathway.
Cancer Lett. 355:273–280. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang C, Yan R, Luo D, Watabe K, Liao DF
and Cao D: Aldo-keto reductase family 1 member B10 promotes cell
survival by regulating lipid synthesis and eliminating carbonyls. J
Biol Chem. 284:26742–26748. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ye X, Li C, Zu X, Lin M, Liu Q, Liu J, Xu
G, Chen Z, Xu Y, Liu L, et al: A large-scale multicenter study
validates aldo-keto reductase family 1 member B10 as a prevalent
serum marker for detection of hepatocellular carcinoma. Hepatology.
69:2489–2501. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Weverwijk A, Koundouros N, Iravani M,
Ashenden M, Gao Q, Poulogiannis G, Jungwirth U and Isacke CM:
Metabolic adaptability in metastatic breast cancer by
AKR1B10-dependent balancing of glycolysis and fatty acid oxidation.
Nat Commun. 10(2698)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qu J, Li J, Zhang Y, He R, Liu X, Gong K,
Duan L, Luo W, Hu Z, Wang G, et al: AKR1B10 promotes breast cancer
cell proliferation and migration via the PI3K/AKT/NF-kappaB
signaling pathway. Cell Biosci. 11(163)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang C, Cao Z, Ma J, Shen Y, Bu Y,
Khoshaba R, Shi G, Huang D, Liao DF, Ji H, et al: AKR1B10 activates
diacylglycerol (DAG) second messenger in breast cancer cells. Mol
Carcinog. 57:1300–1310. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Ahmed SMU, Jiang ZN, Zheng ZH, Li Y, Wang
XJ and Tang X: AKR1B10 expression predicts response of gastric
cancer to neoadjuvant chemotherapy. Oncol Lett. 17:773–780.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hung JJ, Yeh YC and Hsu WH: Prognostic
significance of AKR1B10 in patients with resected lung
adenocarcinoma. Thorac Cancer. 9:1492–1499. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu W, Song J, Du X, Zhou Y, Li Y, Li R,
Lyu L, He Y, Hao J, Ben J, et al: AKR1B10 (Aldo-keto reductase
family 1 B10) promotes brain metastasis of lung cancer cells in a
multi-organ microfluidic chip model. Acta Biomater. 91:195–208.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fukumoto S, Yamauchi N, Moriguchi H, Hippo
Y, Watanabe A, Shibahara J, Taniguchi H, Ishikawa S, Ito H,
Yamamoto S, et al: Overexpression of the aldo-keto reductase family
protein AKR1B10 is highly correlated with smokers' non-small cell
lung carcinomas. Clin Cancer Res. 11:1776–1785. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chung YT, Matkowskyj KA, Li H, Bai H,
Zhang W, Tsao MS, Liao J and Yang GY: Overexpression and oncogenic
function of aldo-keto reductase family 1B10 (AKR1B10) in pancreatic
carcinoma. Mod Pathol. 25:758–766. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ko HH, Cheng SL, Lee JJ, Chen HM, Kuo MY
and Cheng SJ: Expression of AKR1B10 as an independent marker for
poor prognosis in human oral squamous cell carcinoma. Head Neck.
39:1327–1332. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ko HH, Peng HH, Cheng SJ and Kuo MY:
Increased salivary AKR1B10 level: Association with progression and
poor prognosis of oral squamous cell carcinoma. Head Neck.
40:2642–2647. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fang CY, Lin YH and Chen CL:
Overexpression of AKR1B10 predicts tumor recurrence and short
survival in oral squamous cell carcinoma patients. J Oral Pathol
Med. 48:712–719. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu J, Ban H, Liu Y and Ni J: The
expression and significance of AKR1B10 in laryngeal squamous cell
carcinoma. Sci Rep. 11(18228)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Taskoparan B, Seza EG, Demirkol S, Tuncer
S, Stefek M, Gure AO and Banerjee S: Opposing roles of the
aldo-keto reductases AKR1B1 and AKR1B10 in colorectal cancer. Cell
Oncol (Dordr). 40:563–578. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zu X, Yan R, Pan J, Zhong L, Cao Y, Ma J,
Cai C, Huang D, Liu J, Chung FL, et al: Aldo-keto reductase 1B10
protects human colon cells from DNA damage induced by electrophilic
carbonyl compounds. Mol Carcinog. 56:118–129. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Sinreih M, Stupar S, Cemazar L, Verdenik
I, Frkovic Grazio S, Smrkolj S and Rizner TL: STAR and AKR1B10 are
down-regulated in high-grade endometrial cancer. J Steroid Biochem
Mol Biol. 171:43–53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hojnik M, Frkovic Grazio S, Verdenik I and
Rizner TL: AKR1B1 and AKR1B10 as prognostic biomarkers of
endometrioid endometrial carcinomas. Cancers (Basel).
13(3398)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guo Y, Luo W, Hu Z, Li J, Li X, Cao H, Li
J, Wen B, Zhang J, Cheng H, et al: Low expression of Aldo-keto
reductase 1B10 is a novel independent prognostic indicator for
nasopharyngeal carcinoma. Cell Biosci. 6(18)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
He YC, Shen Y, Cao Y, Tang FQ, Tian DF,
Huang CF, Tao H, Zhou FL, Zhang B, Song L, et al: Overexpression of
AKR1B10 in nasopharyngeal carcinoma as a potential biomarker.
Cancer Biomark. 16:127–135. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bao YN, Cao X, Luo DH, Sun R, Peng LX,
Wang L, Yan YP, Zheng LS, Xie P, Cao Y, et al: Urokinase-type
plasminogen activator receptor signaling is critical in
nasopharyngeal carcinoma cell growth and metastasis. Cell Cycle.
13:1958–1969. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Fan C, Wang J, Tang Y, Zhang S, Xiong F,
Guo C, Zhou Y, Li Z, Li X, Li Y, et al: Upregulation of long
non-coding RNA LOC284454 may serve as a new serum diagnostic
biomarker for head and neck cancers. BMC Cancer.
20(917)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tang XR, Li YQ, Liang SB, Jiang W, Liu F,
Ge WX, Tang LL, Mao YP, He QM, Yang XJ, et al: Development and
validation of a gene expression-based signature to predict distant
metastasis in locoregionally advanced nasopharyngeal carcinoma: A
retrospective, multicentre, cohort study. Lancet Oncol. 19:382–393.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Endo S, Matsunaga T and Nishinaka T: The
Role of AKR1B10 in physiology and pathophysiology. Metabolites.
11(332)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6(92)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu X, Hu Z, Qu J, Li J, Gong K, Wang L,
Jiang J, Li X, He R, Duan L, et al: AKR1B10 confers resistance to
radiotherapy via FFA/TLR4/NF-κB axis in nasopharyngeal carcinoma.
Int J Biol Sci. 17:756–767. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Matsunaga T, Suzuki A, Kezuka C, Okumura
N, Iguchi K, Inoue I, Soda M, Endo S, El-Kabbani O, Hara A and
Ikari A: Aldo-keto reductase 1B10 promotes development of cisplatin
resistance in gastrointestinal cancer cells through down-regulating
peroxisome proliferator-activated receptor-ү-dependent mechanism.
Chem Biol Interact. 256:142–153. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Morikawa Y, Kezuka C, Endo S, Ikari A,
Soda M, Yamamura K, Toyooka N, El-Kabbani O, Hara A and Matsunaga
T: Acquisition of doxorubicin resistance facilitates migrating and
invasive potentials of gastric cancer MKN45 cells through
up-regulating aldo-keto reductase 1B10. Chem Biol Interact.
230:30–39. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Matsunaga T, Yamaji Y, Tomokuni T, Morita
H, Morikawa Y, Suzuki A, Yonezawa A, Endo S, Ikari A, Iguchi K, et
al: Nitric oxide confers cisplatin resistance in human lung cancer
cells through upregulation of aldo-keto reductase 1B10 and
proteasome. Free Radic Res. 48:1371–1385. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yao Y, Wang X, Zhou D, Li H, Qian H, Zhang
J, Jiang L, Wang B, Lin Q and Zhu X: Loss of AKR1B10 promotes
colorectal cancer cells proliferation and migration via regulating
FGF1-dependent pathway. Aging (Albany NY). 12:13059–13075.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jin J, Liao W, Yao W, Zhu R, Li Y and He
S: Aldo-keto reductase family 1 member B 10 mediates liver cancer
cell proliferation through sphingosine-1-phosphate. Sci Rep.
6(22746)2016.PubMed/NCBI View Article : Google Scholar
|