1. Introduction
Thyroid cancer (TC) accounts for >90% of all
endocrine cancer types and is therefore the most frequently
occurring endocrine-related malignancy (1). Most thyroid tumors are pathologically
differentiated cancers with a good prognosis and a 5-year survival
rate of >98% (2). Papillary TC
(PTC) accounts for ~80% of all TC cases and is the most frequently
occurring differentiated TC (DTC). The other types of DTC are
follicular TC (FTC) and medullary TCs (MTC), and these cancer types
come from cells in the follicle and the area around the follicle
(3). There is also a small subset
of TC, also known as anaplastic TC (ATC). ATC is a rare and
aggressive tumor arising from the follicular cells of the thyroid
gland, similar to well-differentiated TC (WDTC). However, ATC cells
lack any of the biological characteristics of the original
follicular cells, such as iodine uptake and thyroglobulin synthesis
(1).
The 2022 World Health Organization classification of
Endocrine Tumors subdivides thyroid neoplasms into specific
categories based on their cell of origin (4). Malignant follicular cell-derived
thyroid tumors include PTC, FTC, high-grade thyroid carcinomas
(poorly differentiated and differentiated subtypes) and ATC
(undifferentiated). On the other hand, MTC falls in the C
cell-derived thyroid carcinoma category. Giant-cell, spindle-cell
and squamoid-cell tumors are among the histological patterns of
ATC. ATC may form spontaneously, although it is more likely to
emerge from a pre-existing DTC, particularly the follicular variant
(5,6).
ATC incidence rates have been reported as unchanged
over the last 30 years, and the disease occurs most frequently in
older people but has been described in all age groups (1). Due to the unresectability of most ATC
cases, the diagnosis is typically made by fine-needle aspiration
(FNA). High-grade features such as pleomorphism, necrosis, mitotic
figures and spindle cells are characteristic cytological findings.
Immunohistochemical findings include positivity for keratin in most
cases, but no or focal immunoreactivity for thyroid-specific
markers such as thyroid-transcription factor-1 and thyroglobulin
(usually no expression) (4,5).
The majority of patients with ATC have local
symptoms such as dysphagia, dysphonia, stridor and dyspnea, as well
as neck discomfort and soreness. The tumor infiltrates adjacent
tissues such as fat, muscle, tracheal, esophageal and laryngeal
tissues in >70% of patients (7). The clinical history of a rapidly
expanding tumor that is solid and attached to surrounding tissues
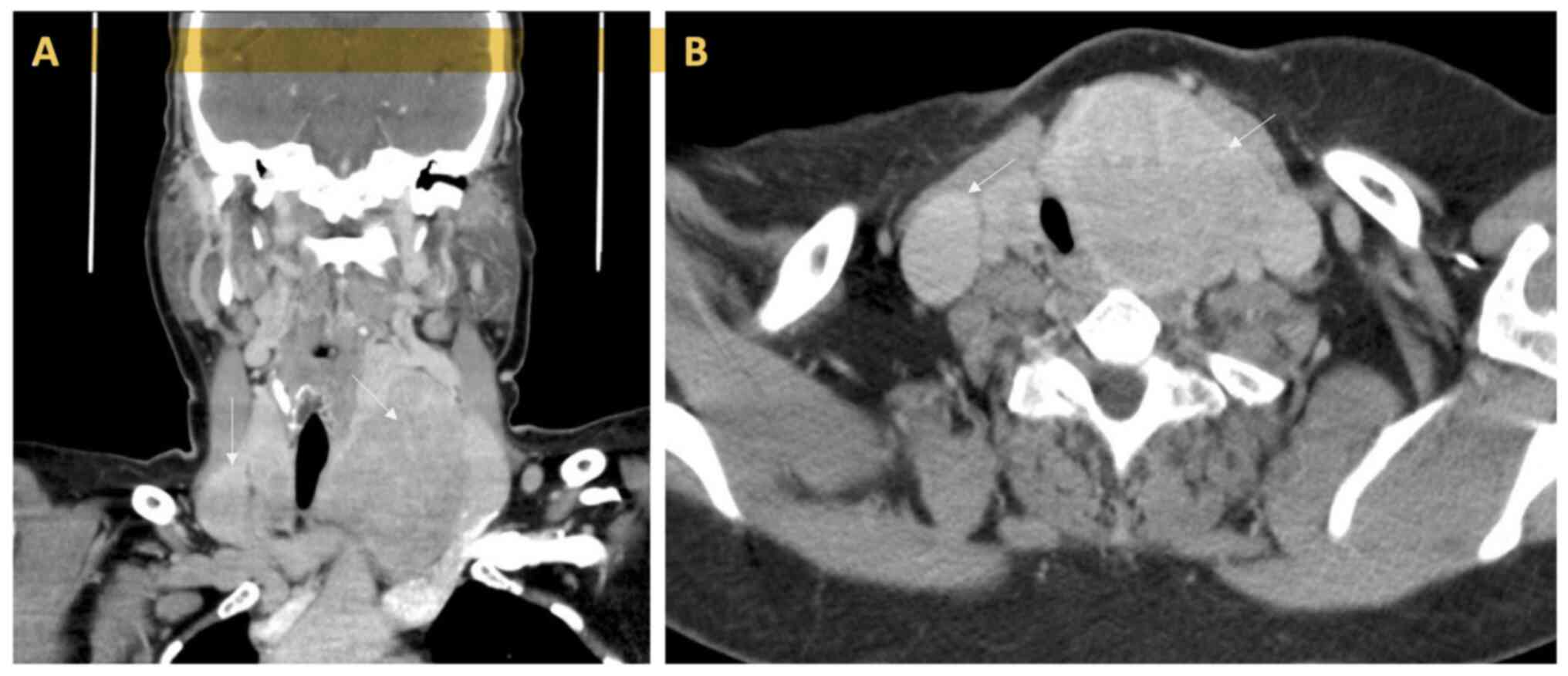
is particularly indicative of ATC (Fig. 1). Approximately 70% of ATCs spread
to nearby tissues, such as the trachea, the esophagus and the
larynx. The lungs, bones and brain are also common locations to
which ATC metastasizes. FNA cytology in uncertain situations, and
histology on core biopsy, may confirm the diagnosis (7). In addition, positron emission
tomography, computed tomography scans and magnetic resonance
imaging are effective for defining the local area and finding
distant metastases (7).
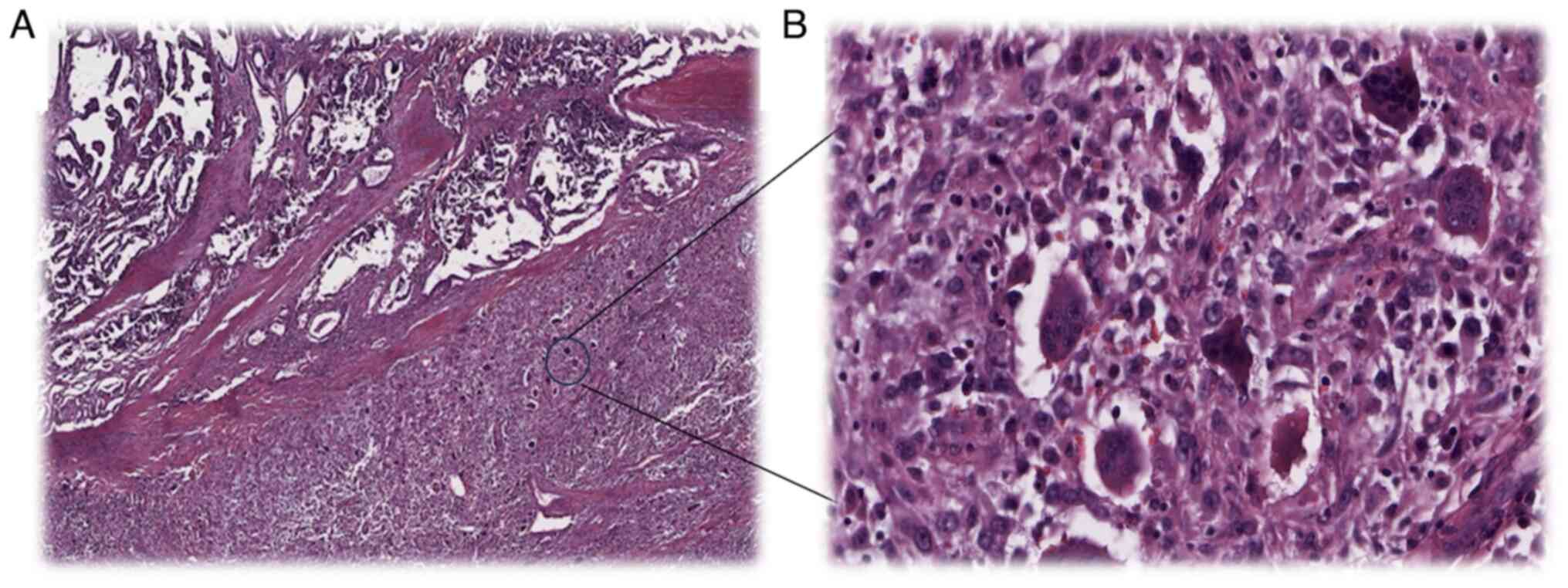
At the microscopic level, ATC appears to be made up
of anaplastic cells that have obvious cytological atypia and a high
level of mitotic activity. Necrosis of the tumor and vascular
invasion are prevalent. The presence of coexisting areas of WDTC in
approximately one-third of instances of ATC is one piece of
evidence that lends support to the concept that ATC develops from
WDTC. Spindle cells, gigantic cells and squamoid cells are all
examples of different histological patterns. Other patterns, such
as angiomatoid, carcinosarcoma, lymphoepithelioma-like and
adenosquamous patterns, have been described. Undifferentiated
carcinoma of the thyroid, also known as ATC, needs to be
distinguished from other high-grade tumors that originate from
nearby structures in the neck and have a microscopic appearance
that is comparable (Fig. 2). On
occasion, this distinction can only be made on the basis of
clinical or anatomical evidence (8).
The newest cancer staging manual (the 8th edition of
the American Joint Committee on Cancer classification) defines all
ATCs as stage IV tumors due to their aggressive nature at
diagnosis, regardless of their total tumor burden. Most patients
die within 6 months of diagnosis, largely due to local tumor
invasion (9). Treatment for ATC
has not been standardized, since it is unclear if medication is
helpful in extending survival time. Surgery, radiation and
chemotherapy are seldom enough to achieve overall disease control
when used alone, but a combination of these therapies may enhance
local control (10). If the tumor
is intra-thyroidal, surgical therapy for the local illness may
provide a chance to prolong survival time (10). The surgical approach to ATC, when
the tumor is extra-thyroidal, is controversial. Although ATC forms
only 2% of all TC cases, it accounts for >50% of all
TC-associated deaths (1). ATC
continues to rank as one of the deadliest diseases worldwide and
carries a very poor prognosis. The mortality rate is close to 100%,
with most patients already having metastatic disease by the time of
diagnosis (9).
Recent research using high-throughput sequencing has
shed light on the genetic signature of ATC. A high mutational load
as a result of the accumulation of various somatic mutations is an
essential trait. Besides BRAF and TP53 mutations,
which are considered as the genetic characteristics of ATC, various
additional genetic abnormalities, including RAS, PTEN and
RET mutations, which also occur in DTC, have been found in
ATC. As a result, it is conceivable for DTC to have given rise to a
portion of ATC, when histology shows that DTC is present in 30-50%
of ATC cases (11).
No standardized therapy for ATC has yet been shown
to improve overall survival (OS). However, several guidelines, such
as those from the American Thyroid Association and the British
Thyroid Association, discuss the various treatment options, which
include surgery, radiotherapy or chemotherapy (12). The effectiveness of these
therapeutic techniques has been published in the form of case
studies. In addition, there is the possibility that new therapeutic
approaches, such as the use of checkpoint inhibitors, may open new
therapeutic avenues (13). The
present review will summarize our current knowledge on
pathogenesis, prognostic factors and the genetic landscape of ATC.
The development of therapies targeting pathways currently being
tested in clinical studies will also be discussed.
2. Prognostic factors and treatment
approaches for ATC
ATC typically presents in patients as an invasive
and rapidly growng neck mass, with involvement of the regional
cervical lymph nodes. Distant metastatic disease will also be found
in ~50% of patients at presentation (14). This is in contrast to
differentiated TC, which has a favorable prognosis and is managed
primarily through surgery. Consequently, individuals with ATC are
often incurable when they are first diagnosed, and they have
typically been treated palliatively or sent to intensive care. The
OS times reported in several retrospective studies that analyzed
the course of the illness and aimed to discover prognostic
indicators for ATC varied highly, with times ranging from a few
weeks to a few years (7). This
suggests that risk stratification might be beneficial, despite the
fact that ATC results in death in the majority of cases. This
would, however, make it possible to distinguish between patients
who should get possible supportive care and those who could be
candidates for more aggressive therapy.
Due to the low occurrence of ATC, few institutions
over the world have case series with more than 100 individuals.
Analysis of prognostic markers is only feasible in larger series,
as most of the patients pass away within the first few weeks or
months following diagnosis, creating a false impression that
patients with ATC have no meaningful chance of survival.
Significant survival has been seen in broader series, allowing
investigation of prognostic markers linked with prolonged life in
patients with ATC (6). In 2013, a
study by Haymart et al (15) analyzed 699 patients with ATC who
were diagnosed between 2003 and 2008, and found that patients with
stage IVA illness had a 9-month survival rate, while those with
stage IVB disease had a 4.8-month survival rate and those with
stage IVC disease had a 3-month survival rate on average (15).
There have been various retrospective studies
describing negative prognostic factors such as older age, early
distant metastatic disease, a large primary tumor diameter (>5
cm) and an increased white blood count (15,16).
In a retrospective German multicenter study of 100 ATC patients
pretreatment between 2000 and 2015, it was found that the
prognostic factors for survival were an age >70 years and
presence of distant metastases, which were all independent risk
factors of an unfavorable outcome. By applying multivariate
analysis on the cohort, OS rates similar to earlier reported data
were found. By contrast, patients <70 years old, with slowly
growing tumors limited to the thyroid and without distant
metastases, had the best prognosis (7).
Unexpectedly, one previous study reported that a
full thyroidectomy had no association with an extended OS time
(17). However, the importance of
a total thyroidectomy is still debatable due to the high percentage
of unresectable tumors at first diagnosis and the significant
morbidity associated with intensive local resection (17). Surgical intervention is typically
not recommended for individuals with ATC owing to substantial
metastases, but full surgical resection is recommended for
localized disease when achievable, with little morbidity (14). When used alone to treat ATC,
surgery, radiotherapy or chemotherapy rarely have an impact on OS
rates. Accordingly, multimodal therapy is increasingly being
applied as the treatment of choice for ATC, as the disease is
commonly advanced at the time of diagnosis and usually cannot be
resected completely. Further postoperative radiotherapy is also
sought-after (14). Improved OS
times were associated with the use of definitive or adjuvant
radiation therapy, a younger patient age and a lack of distant
metastases, according to the findings of a retrospective case
series study that was conducted on a large institutional cohort of
97 patients who were treated for ATC over the course of 21 years
(17).
The majority of patients with ATC require
chemotherapy due to the existence of advanced regional disease or
distant metastasis at the time of presentation. Doxorubicin is a
frequently used regimen, whether combined with cisplatin or used
alone, but the response rate is typically low (18). Second-line treatments are targeted
inhibitors that act on a specific target molecule, thus preventing
the growth and progression of cancer. Such inhibitors typically
target overactive or mutant molecules active in certain signaling
pathways of cancer cells. Examples of these inhibitors are
incorporating targeted therapies, such as tyrosine kinase
inhibitors, anti-angiogenic drugs, and agonists, and multi-kinase
inhibitors (MKIs) targeting hyperactive BRAF gene, mTOR,
and/or BCR-ABL. For BRAFV600E mutation-positive
tumors, vemurafenib, as well as the combined use of dabrafenib and
trametinib have shown promising responses in treating ATC (19). In a phase 2 multicenter
investigation of BRAF-mutated tumors, including 7 patients
with BRAFV600E-mutated ATC, the BRAF inhibitor
vemurafenib, which had been previously licensed to treat
BRAF-mutated melanoma, was assessed. There was one complete
response (CR) and one partial response (PR) among the participants
in this cohort, which resulted in an overall response rate of 69%.
More importantly, a single patient experienced a sustained response
that lasted >1 year (20). This
and other anecdotal evidence led to the investigation of the
BRAF inhibitor dabrafenib and the MEK inhibitor trametinib
in a phase 2 multicenter trial for
BRAFV600E-mutated ATC (19). Dabrafenib and trametinib were
administered daily to patients with ATC and
BRAFV600E mutations. Among the 16 participants,
the vast majority had already undergone some form of surgical
procedure and/or external beam radiation. Overall, 1 patient (6%)
achieved a CR and 10 patients (63%) achieved a PR at a median
follow-up time of 47 weeks (range, 4-120 weeks), yielding an
overall response rate (ORR) of 69%. Furthermore, 19% of patients
experienced stable disease.
In 2018, dabrafenib/trametinib for
BRAFV600E-mutated ATC was authorized by the Food
and Drug Administration (FDA) as a result of the aforementioned
findings (19). However,
generally, single-targeted inhibitors have not exhibited
substantial therapeutic effects in patients with ATC, prompting the
adoption of MKIs. MKIs have the ability to act on two or more
targets at the same time. MKIs such as sorafenib, axitinib,
pazopanib and sunitinib have been studied in preclinical models, as
well as a clinical trial, with promising results (21). The use of sorafenib also resulted
in a progression-free survival time of ~5 months in a phase III
clinical trial, with patients experiencing manageable toxicities in
comparison with the placebo group (22). Additionally, patients with
unresectable or metastatic BRAFV600E-positive ATC
have a treatment option, including the BRAF inhibitor dabrafenib
and the MEK inhibitor trametinib (23).
Neo-adjuvant therapy refers to the administration of
systemic treatment before surgery with the goal of downsizing the
tumor, improving surgical outcomes and potentially increasing
survival rates. Implementation of neo-adjuvant BRAF-directed
therapy before surgery may result in improved OS times for patients
with BRAFV600E-mutated ATC (24). BRAF-directed therapy is highly
effective, and subsequent to using these drugs, surgically
unresectable tumors may become resectable, although drug resistance
may also develop. The traditional trimodal therapy of surgery,
adjuvant chemotehrapy and adjuvant radiotherapy could in future be
substituted with up-front BRAF inhibitors, subsequent surgery and
possible adjuvant chemoradiation in patients with stage IVB and IVC
BRAFV600E-mutated ATC (24). Additionally, neo-adjuvant therapy
could serve as a ‘test-of-time’ for individual tumor response to
targeted therapy, allowing for treatment tailoring and
identification of patients who may benefit the most from surgical
intervention (24).
While specific data on the impact of neo-adjuvant
BRAF-directed therapy followed by surgery in ATC is limited,
emerging evidence from case reports and small studies suggests
encouraging outcomes. One study reported significant tumor
shrinkage, improved resectability and even a complete pathological
response following neo-adjuvant targeted therapy (25). These findings indicate the
potential for improved OS times and disease control in patients
with ATC. Recently, molecularly targeted treatments for ATC have
been created. Lenvatinib successfully increased progression-free
survival time in patients with both DTC and ATC. In 2020 and 2023,
Niu and Xing conducted comprehensive literature searches for
ATC-targeted drug studies and provided a summary of their efficacy
and adverse effects. It was stated that in comparison to other
targeted medications, the pairing of dabrafenib and trametinib
stood out as the most effective treatment discovered so far
(26,27). A study by Ferrari et al
(28) found that despite the
recognition of doxorubicin, docetaxel/paclitaxel and cisplatin by
American Thyroid Association guidelines for the treatment of ATC,
there has been no improvement in the survival rate of patients with
advanced ATC. Additionally, the use of combination therapy,
particularly involving TKIs, is considered a highly significant
area for future research. Moreover, it is essential to acknowledge
that drug resistance poses a substantial challenge for researchers
and clinicians. However, studies have indicated that combining
anticancer-targeted drugs with other specific medications could
potentially mitigate drug resistance. All in all, targeted agents
show great promise as an approach for treating ATC (26,29).
3. Genetic landscape and molecular
pathogenesis of ATC
Tumor development is associated with the
accumulation of mutations in the genome. It has been hypothesized
that ATC progresses from DTC through genetic alterations causing
anaplastic transformation (8).
When differentiated and anaplastic components of TC are
co-localized inside the same thyroid gland, both tumors usually
have the same oncogenic mutations in BRAFV600E or
RAS (28). This further
supports the hypothesis that DTC cells undergo anaplastic
transition. Nonetheless, most thyroid tumors with BRAF or
RAS mutations are at low risk, demonstrating that these
basic oncogenic mutations are insufficient to trigger ATC (11). Therefore, multiple genetic hits are
believed to be necessary for the development of ATC; a mutation in
one of the key oncogenic driver genes responsible for
differentiated thyroid carcinoma and additional deleterious genetic
alterations will lead to ATC transformation.
The Cancer Genome Atlas has made it clear that
molecular profiles such as those of RAS and
BRAFV600E are linked to follicular growth, and
also to papillary growth (30).
For example, RAS gene mutations are strongly associated with
follicular architecture, regardless of whether the cancer cells
have invaded. The number of follicular adenomas, follicular
carcinomas and PTCs that have RAS mutations is the same. The
same is true for other molecular changes that happen less often,
such as BRAF mutations that are not V600E (like
BRAFK601E) or PTEN mutations, which are
all found in tumors with follicular architecture at approximately
the same rate in each histological type (31,32).
On the other hand, ‘conventional’ PTC is marked by the presence of
neoplastic papillae and the molecular change
BRAFV600E, and similar changes such as RET
and NTRK rearrangements (32). In poorly differentiated TC and ATC,
RAS-like and BRAFV600E-like signatures are partly
kept, as mutations of the BRAF and RAS genes are
found in a subset of cases at prevalence rates that are not very
different from those of WDTC and FTC (30,31).
TERT promoter mutations, on the other hand, are associated
with invasive growth in PTC and FTC, and their rate of occurrence
goes up in PDTC and ATC (33).
Consistent with the multiple hit hypothesis, the ATC mutation
burden is highest among TC subtypes. In a study on a mouse model
with thyrocyte-specific expression of BRAFV600E,
the need for several genetic mutations for anaplastic
transformation was validated. In the absence of any further genetic
alterations, the BRAFV600E-induced PTC in mice
exhibited an indolent nature and did not commonly lead to the
development of a progressively fatal condition. Likewise, the
absence of TP53 alone is inadequate to produce ATC. However,
ATC is almost fatal when a BRAFV600E transgene is
produced in combination with a TP53 deletion, and/or with
inactivation of both PTEN and TP53 or mutationally
activated PIK3CA (32,34).
The TP53 mutation is more common in ATC than in all other
advanced/aggressive TC types, including both PDTC and high-grade
PTC (35).
Compared with advanced DTC, ATC had a significantly
higher prevalence of genetic changes in cell cycle genes (13 and
29%, respectively), such as inactivating mutations in the cell
cycle regulators CDKN2A and CDKN2B and copy number
gains of CCNE1 (35). These
changes are necessary for the cell cycle G1/S
transition. The most important copy number loss location in ATC is
the deletion of the 9p21.3 locus, which contains the CDKN2A
and CDKN2B genes, and is frequently observed in TC cell
lines (35). Gene expression
variations are correlated with changes in CDKN2A,
CDKN2B and CCNE1 copy number (34,35).
Cell cycle gene mutations are compatible with ATC progression. Loss
of PTEN expression is also observed in a fraction of TCs,
and promoter methylation of PTEN is prevalent in FTC and ATC
(34). Mutations of different
RAS isoforms, PIK3CA mutations and amplifications,
and PTEN mutations have all been linked to methylation of
the PTEN gene in thyroid tumors, including ATC.
TERT promoter mutations cause telomerase to
reactivate and have been linked to advanced DTC. A study by
Pozdeyev et al (35) in
2018 found that TERT promoter mutations were quite common in
their large-scale investigation of advanced DTC genetics. The study
showed that TERT promoter mutations were more frequent in
ATC than in DTC. Six investigations, totaling 252 cases with ATC,
searched for TERT promoter mutations. Among these tumors,
100 (or 39.7%) had TERT promote (36,37).
However, TERT promoter mutations alone do not cause
anaplastic transformation, but they do contribute to the aggressive
phenotype, which is susceptible to conversion to ATC if one of the
ATC-related second genetic alteration events occurs (38,39).
The PDTC phenotype is susceptible to transformation to ATC when one
of the ATC-related ‘second hit’ genetic events, such as TERT
mutation, occurs. The prevalence of TERT mutation is
significantly higher in high-grade PTC and PDTC, approaches >70%
in ATC (11-13),
and is associated with a poor prognosis. TERT mutations
correlate with a higher frequency of metastasis, prolonged disease
and a lower survival rate in patients with PTC, particularly when
they co-occur with oncogenic mutations such as
BRAFV600E or RAS (14-17).
Given that anaplastic transition requires many
genetic changes, most ATC cases are expected to undergo the
differentiated phase, whether or not DTC is detected through
histological investigation. However, the lack of typical oncogenic
mutations in these tumors and the rapid accumulation of numerous
mutations in tumors with DNA repair deficiencies may encourage the
de novo generation of ATC from healthy follicular cells
(8,9).
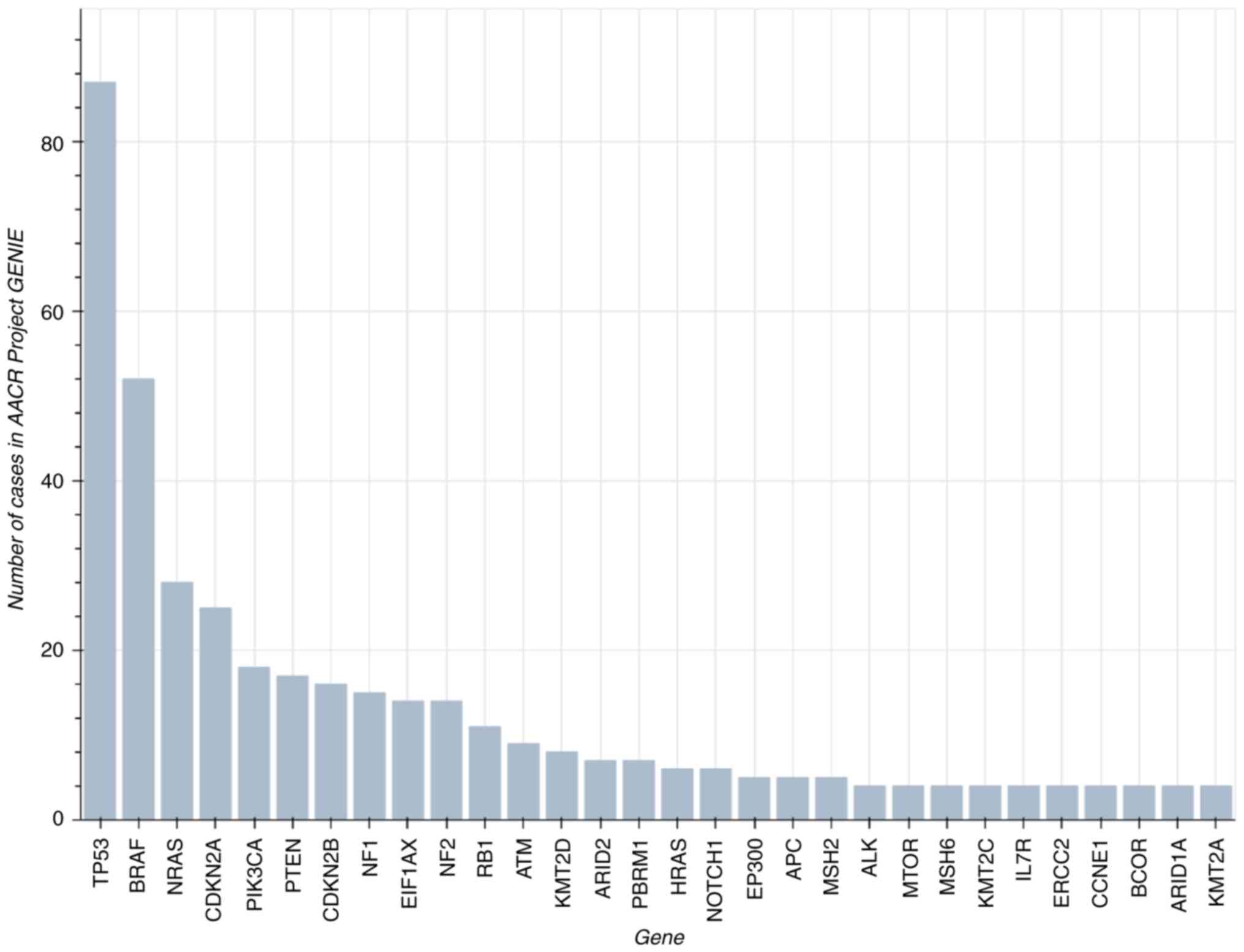
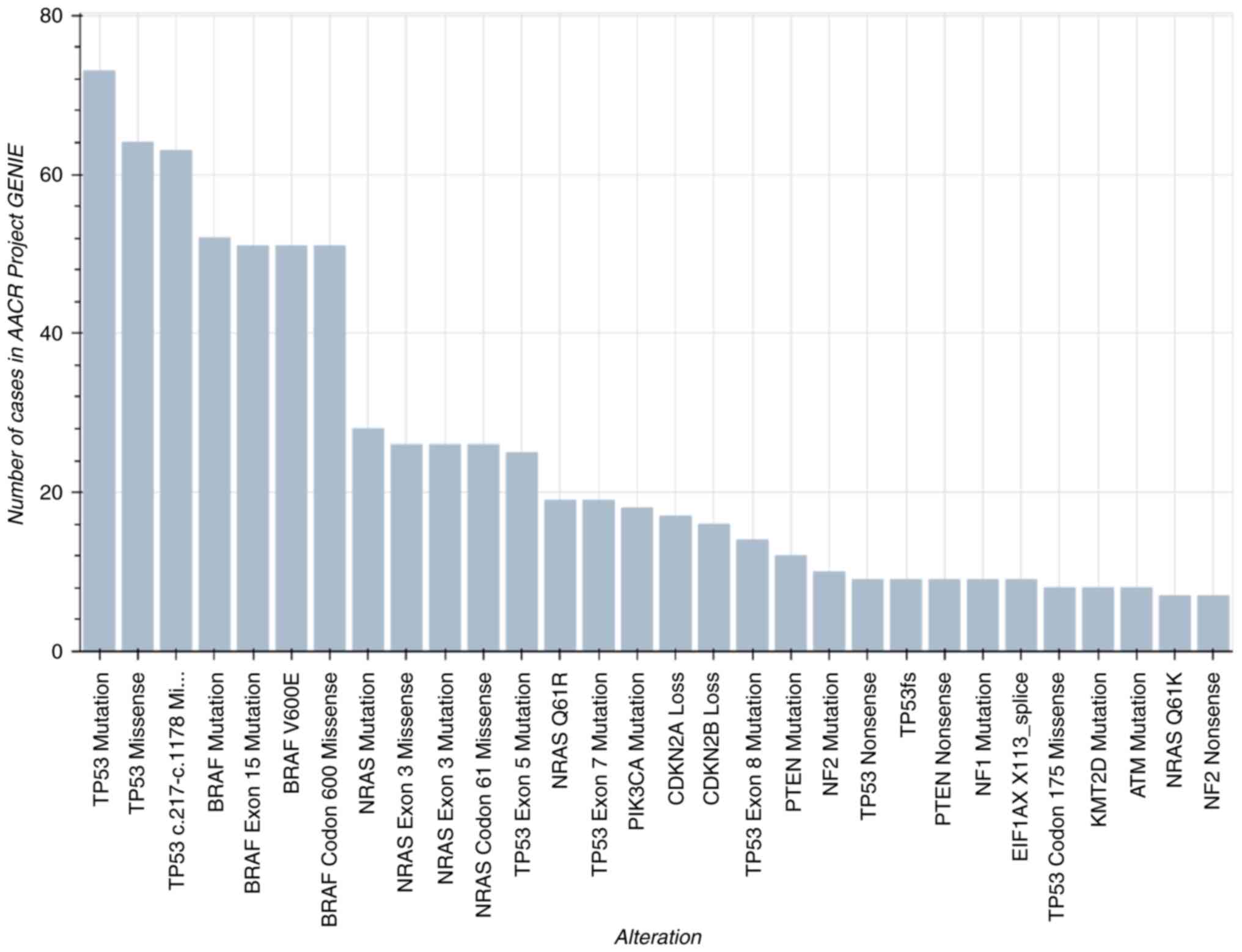
According to the AACR Project GENIE Consortium
dataset version 8 (https://www.aacr.org/professionals/research/aacr-project-genie/aacr-project-genie-data/),
ATC most frequently harbors alterations in TP53,
BRAF, NRAS, CDKN2A and PIK3CA (Figs. 3 and 4) (40).
In summary, ATC is a genetically complicated
malignancy that is derived from numerous unique DTC subtypes. This
complexity is reflected in its etiology. Our knowledge of the
genetic processes that can lead to anaplastic transformation has
substantially expanded as a result of the development of more
sophisticated tools for genotyping and the growing application of
genetic information in clinical care. However, the mechanisms and
risk factors that lead to a subset of TCs transforming into the
most aggressive form of human cancer remain largely unknown.
Additionally, a true tailored therapy is now more feasible due to
the recent development of innovative and affordable diagnostic
tools, such as individual mutational testing and in vitro
analysis of patient ATC cells. This is completed in an effort to
improve therapeutic success and prevent the use of harmful and
ineffective treatments.
4. Conclusion
In conclusion, ATC has a complex genetic landscape
and molecular pathogenesis that includes a variety of genetic
modifications, epigenetic changes and signaling pathway
activations. For the purpose of creating novel and efficient ATC
treatments, it is essential to understand these underlying
mechanisms, and continued research in this field is required to
enhance the prognosis for those who have this aggressive type of
TC. Due to the advancement of genotyping methods and the increased
utilization of genetic data, better knowledge now exists with
regard to the genetic mechanisms that lead to the anaplastic
transformation of ATC. Frequent and quick molecular testing is
performed to look for actionable oncogenic mutations. ATC is
characterized by genomic instability, which leads to mutations in
the RET, BRAF, RAS, PTEN, PIK3CA
and TP53 genes. Given the complexity of the genetic
alterations linked to ATC, a tailored treatment plan that strictly
limits critical pathways may enhance therapeutic results. In the
future, a multidisciplinary approach to these treatments might be
successful. Additionally, for patients with the
BRAFV600E mutation, the usage of neo-adjuvant
BRAF-directed therapy, followed by surgery, may result in
significant improvements to OS. However, despite these important
developments in the pathophysiology of ATC and its potential
translational uses, little is known about the mechanisms through
which these mutations contribute to the carcinogenesis of ATC. In
addition, the advancement of care for patients diagnosed with ATC
will largely depend on how seriously enrollment in clinical trials
is taken into consideration.
Acknowledgements
No applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AGA draft the manuscript. OA reviewed the manuscript
and provide Fig. 1. YA reviewed
the manuscript and provided Fig.
2. All authors have read and approved the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent was obtained from the patients for
the use of the images in Figs. 1
and 2.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rao SN and Smallridge RC: Anaplastic
thyroid cancer: An update. Best Pract Res Clin Endocrinol Metab.
37(101678)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fagin JA and Wells SA Jr: Biologic and
clinical perspectives on thyroid cancer. N Engl J Med.
375(2307)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fahiminiya S, de Kock L and Foulkes WD:
Biologic and clinical perspectives on thyroid cancer. N Engl J Med.
375:2306–2307. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jung CK, Bychkov A and Kakudo K: Update
from the 2022 World Health Organization Classification of Thyroid
Tumors: A standardized diagnostic approach. Endocrinol Metab
(Seoul). 37:703–718. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Molinaro E, Romei C, Biagini A, Sabini E,
Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini
A, Torregrossa L, et al: Anaplastic thyroid carcinoma: From
clinicopathology to genetics and advanced therapies. Nat Rev
Endocrinol. 13:644–660. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American Thyroid Association guidelines for management
of patients with anaplastic thyroid cancer. Thyroid. 22:1104–1139.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wendler J, Kroiss M, Gast K, Kreissl MC,
Allelein S, Lichtenauer U, Blaser R, Spitzweg C, Fassnacht M,
Schott M, et al: Clinical presentation, treatment and outcome of
anaplastic thyroid carcinoma: Results of a multicenter study in
Germany. Eur J Endocrinol. 175:521–529. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ragazzi M, Ciarrocchi A, Sancisi V,
Gandolfi G, Bisagni A and Piana S: Update on anaplastic thyroid
carcinoma: Morphological, molecular, and genetic features of the
most aggressive thyroid cancer. Int J Endocrinol.
2014(790834)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chiacchio S, Lorenzoni A, Boni G, Rubello
D, Elisei R and Mariani G: Anaplastic thyroid cancer: Prevalence,
diagnosis and treatment. Minerva Endocrinol. 33:341–357.
2008.PubMed/NCBI
|
|
10
|
Corrigan KL, Williamson H, Elliott Range
D, Niedzwiecki D, Brizel DM and Mowery YM: Treatment Outcomes in
Anaplastic Thyroid Cancer. J Thyroid Res.
2019(8218949)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guerra A, Di Crescenzo V, Garzi A, Cinelli
M, Carlomagno C, Tonacchera M, Zeppa P and Vitale M: Genetic
mutations in the treatment of anaplastic thyroid cancer: A
systematic review. BMC Surg. 13 (Suppl 2)(S44)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Coca-Pelaz A, Rodrigo JP, Lopez F, Shah
JP, Silver CE, Al Ghuzlan A, Menke-van der Houven van Oordt CW,
Smallridge RC, Shaha AR, Angelos P, et al: Evaluating new
treatments for anaplastic thyroid cancer. Expert Rev Anticancer
Ther. 22:1239–1247. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brauner E, Gunda V, Vanden Borre P,
Zurakowski D, Kim YS, Dennett KV, Amin S, Freeman GJ and Parangi S:
Combining BRAF inhibitor and anti PD-L1 antibody dramatically
improves tumor regression and anti tumor immunity in an
immunocompetent murine model of anaplastic thyroid cancer.
Oncotarget. 7:17194–17211. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bible KC, Kebebew E, Brierley J, Brito JP,
Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T,
Kasperbauer J, et al: 2021 American Thyroid Association guidelines
for management of patients with anaplastic thyroid cancer. Thyroid.
31:337–386. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Haymart MR, Banerjee M, Yin H, Worden F
and Griggs JJ: Marginal treatment benefit in anaplastic thyroid
cancer. Cancer. 119:3133–3139. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Orita Y, Sugitani I, Amemiya T and
Fujimoto Y: Prospective application of our novel prognostic index
in the treatment of anaplastic thyroid carcinoma. Surgery.
150:1212–1219. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chintakuntlawar AV, Foote RL, Kasperbauer
JL and Bible KC: Diagnosis and Management of anaplastic thyroid
cancer. Endocrinol Metab Clin North Am. 48:269–284. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Koda K, Katoh M and Yasuhara K: Management
of anaplastic thyroid cancer and proposed treatment guidelines-A
5-year case series study. Cancer Rep (Hoboken).
5(e1727)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-Mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hyman DM, Puzanov I, Subbiah V, Faris JE,
Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, et al:
Vemurafenib in multiple nonmelanoma cancers with BRAF V600
Mutations. N Engl J Med. 373:726–736. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Brose MS, Smit JWA, Lin CC, Tori M, Bowles
DW, Worden F, Shen DH, Huang SM, Tsai HJ, Alevizaki M, et al:
Multikinase inhibitors for the treatment of asymptomatic
radioactive iodine-refractory differentiated thyroid cancer: Global
noninterventional study (RIFTOS MKI). Thyroid. 32:1059–1068.
2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saini S, Tulla K, Maker AV, Burman KD and
Prabhakar BS: Therapeutic advances in anaplastic thyroid cancer: A
current perspective. Mol Cancer. 17(154)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Crispo F, Notarangelo T, Pietrafesa M,
Lettini G, Storto G, Sgambato A, Maddalena F and Landriscina M:
BRAF inhibitors in thyroid cancer: Clinical impact, mechanisms of
resistance and future perspectives. Cancers (Basel).
11(1388)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cabanillas ME, Ferrarotto R, Garden AS,
Ahmed S, Busaidy NL, Dadu R, Williams MD, Skinner H, Gunn GB, Grosu
H, et al: Neoadjuvant BRAF- and immune-directed therapy for
anaplastic thyroid carcinoma. Thyroid. 28:945–951. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao X, Wang JR, Dadu R, Busaidy NL, Xu L,
Learned KO, Chasen NN, Vu T, Maniakas A, Eguia AA, et al: Surgery
After BRAF-Directed Therapy is associated with improved survival in
BRAFV600E mutant anaplastic thyroid cancer: A single-center
retrospective cohort study. Thyroid. 33:484–491. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Niu Y, Ding Z, Deng X, Guo B, Kang J, Wu B
and Fan Y: A novel multimodal therapy for anaplastic thyroid
carcinoma: 125I seed implantation plus apatinib after
surgery. Front Endocrinol (Lausanne). 11(207)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xing Y, Wang Y and Wu X: Radiotherapy
combined with immunotherapy successfully treated one case of
anaplastic thyroid cancer: A case report. Front Oncol.
13(1125226)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ferrari SM, Elia G, Ragusa F, Ruffilli I,
La Motta C, Paparo SR, Patrizio A, Vita R, Benvenga S, Materazzi G,
et al: Novel treatments for anaplastic thyroid carcinoma. Gland
Surg. 9 (Suppl 1):S28–S42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oishi N, Kondo T, Ebina A, Sato Y, Akaishi
J, Hino R, Yamamoto N, Mochizuki K, Nakazawa T, Yokomichi H, et al:
Molecular alterations of coexisting thyroid papillary carcinoma and
anaplastic carcinoma: Identification of TERT mutation as an
independent risk factor for transformation. Mod Pathol.
30:1527–1537. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Khatami F and Tavangar SM: A review of
driver genetic alterations in thyroid cancers. Iran J Pathol.
13:125–135. 2018.PubMed/NCBI
|
|
31
|
Song YS and Park YJ: Genomic
characterization of differentiated thyroid carcinoma. Endocrinol
Metab (Seoul). 34:1–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kasaian K, Wiseman SM, Walker BA, Schein
JE, Zhao Y, Hirst M, Moore RA, Mungall AJ, Marra MA and Jones SJ:
The genomic and transcriptomic landscape of anaplastic thyroid
cancer: Implications for therapy. BMC Cancer.
15(984)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Man J, Nicolson N, Gibson C and Carling T:
TERT promoter mutations in thyroid cancer: Growing evidence for a
predictor of poor outcome. Gland Surg. 8:301–303. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Landa I and Knauf JA: Mouse models as a
tool for understanding progression in Braf V600E-Driven
thyroid cancers. Endocrinol Metab (Seoul). 34:11–22.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pozdeyev N, Gay LM, Sokol ES, Hartmaier R,
Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, et
al: Genetic analysis of 779 advanced differentiated and anaplastic
thyroid cancers. Clin Cancer Res. 24:3059–3068. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Landa I, Pozdeyev N, Korch C, Marlow LA,
Smallridge RC, Copland JA, Henderson YC, Lai SY, Clayman GL, Onoda
N, et al: Comprehensive genetic characterization of human thyroid
cancer cell lines: A validated panel for preclinical studies. Clin
Cancer Res. 25:3141–3151. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu R and Xing M: TERT promoter mutations
in thyroid cancer. Endocr Relat Cancer. 23:R143–R155.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ravi N, Yang M, Gretarsson S, Jansson C,
Mylona N, Sydow SR, Woodward EL, Ekblad L, Wennerberg J and
Paulsson K: Identification of targetable lesions in anaplastic
thyroid cancer by genome profiling. Cancers (Basel).
11(402)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hou P, Ji M and Xing M: Association of
PTEN gene methylation with genetic alterations in the
phosphatidylinositol 3-kinase/AKT signaling pathway in thyroid
tumors. Cancer. 113:2440–2447. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Alzahrani AS, Alsaadi R, Murugan AK and
Sadiq BB: TERT promoter mutations in thyroid cancer. Horm Cancer.
7:165–177. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
The AACR Project GENIE Consortium. AACR
Project GENIE: Powering precision medicine through an International
Consortium. Cancer Discov. 7:818–831. 2017.PubMed/NCBI View Article : Google Scholar
|