Introduction
Cancer of unknown primary (CUP), also known as
occult cancer, accounts for approximately 3-5% of all cancers and
is the fourth most common cause of mortality due to cancer in the
US (1,2). CUP is defined as a case of metastatic
cancer where the origination site cannot be determined (1). Median survival after CUP diagnosis is
approximately 3-4 months, with less than 25% of patients alive
after one year (1,2). Patients with CUP are categorized into
two prognostic subgroups according to their clinicopathologic
characteristics. The majority of patients with CUP (80-85%) belong
to unfavorable subsets (3). The
favorable risk cancer subgroup (15-20%) includes patients with
neuroendocrine CUP, peritoneal adenocarcinomatosis of a serous
papillary subtype, isolated axillary nodal metastases in females,
squamous cell carcinoma involving non-supraclavicular cervical
lymph nodes, single metastatic deposit from unknown primary and men
with blastic bone metastases and PSA expression (3). Very recently, new favorable subsets
of CUP seem to emerge including colorectal, lung and renal CUP
which underlies specific treatments (3).
Patients with CUP receive significantly less
treatment yet use more health services when compared to patients
with metastatic cancer of a known site (4). In the era of targeted therapies,
accurate histopathological and molecular classification of tumors
is essential to administer the best tailored therapeutic strategy
(5). Classifications based on
epigenetic alterations have served this purpose. Indeed, cancer
cells are characterized by a massive overall loss of DNA
methylation (20-60% overall decrease in 5-methylcytosine), and by
the simultaneous acquisition of specific patterns of
hypermethylation at CpG islands of certain promoters, which can
alter gene function, thereby contributing to cancer progression
(5).
Relatedly, pancreatic cancer accounts for
approximately 3% of all cancers and is the third most common cause
of mortality due to cancer in the U.S. (6). The most critical prognostic factor
for pancreatic cancer is stage at diagnosis (7). Median survival time after stage 1 and
stage 2 pancreatic cancer diagnosis is 4 months, with stage 3 and
stage 4 pancreatic cancer decreasing survival time to 2-3 months
(8). Approximately 53% of
pancreatic cancer patients are diagnosed after metastasis has
occurred (9). Since CUP and
metastatic pancreatic cancer are comparable in incidence and
survival outcome, there may be similarities in patient
characteristics of those initially misdiagnosed with CUP who
ultimately receive a diagnosis of pancreatic cancer. Definitive
diagnosis is crucial to the prognosis of patients diagnosed with
CUP, but studies examining characteristics of this outcome are
limited (10,11). Therefore, we sought to build upon
our previous research (12) to
examine patient characteristics associated with definitive
diagnosis of metastatic pancreatic cancer in older patients who
initially present with CUP.
Materials and methods
Study population
This retrospective cohort study uses 2010-2015
Surveillance, Epidemiology, and End Results (SEER)-Medicare data, a
national population-based cancer registry linked to Medicare
claims. The cohort consisted of patients identified in the SEER
dataset diagnosed with CUP, International Classification of
Diseases for Oncology, Third Edition (ICD-O-3) codes C80.9 and
those diagnosed with stage 3 and stage 4 pancreatic cancer (ICD-O-3
codes C250-C259), between January 1, 2010 and December 31, 2015.
Initial CUP diagnosis was defined by the date of the first biopsy
or date of ICD-O-3 diagnosis, whichever came first. Patients had to
be continuously enrolled in Medicare fee-for-service (both Part A
and B) beginning 1 year prior to diagnosis through the observation
period. Only the first reported primary cancer for each patient was
included, that is, this was the first time the patients had been
diagnosed with any type of cancer. Exclusion criteria were used to
maximize patients whose claims data were complete: patients were
excluded if enrolled in Medicare due to chronic disability, as well
as those diagnosed only on a death certificate, at autopsy, or in a
nursing home as their care was likely dissimilar to other patients.
Only claims paid by Medicare were included so as to avoid erroneous
billing codes. The final cohort consisted of 68,146 patients, of
which 17,565 were initially diagnosed with CUP prior to a
pancreatic cancer diagnosis.
Patient characteristics
Patient characteristics included sex, age in four
groups (65, 66-74, 75-84, 85 and older), race [White, Black, and
Other (which included Asian, American Indian/Alaska Native, Native
Hawaiian/Other Pacific Islander, and Multi-racial; per the race
variable definition in the SEER data dictionary)], ethnicity
(Latino or Non-Latino; per the ethnicity variable definition in the
SEER data dictionary), area of residence (rural or urban), and
histology of the primary tumor (adenocarcinoma, squamous cell
carcinoma, epithelial/unspecified, and neuroendocrine). Comorbidity
was assessed utilizing the Klabunde adaptation of the Charlson
comorbidity score (13).
Definitive diagnosis
Odd ratios (OR) and 95% confidence intervals (CI)
were calculated using two binary logistic regression models to
analyze the patient characteristics of who received definitive
diagnosis between the CUP-Pancreas group (those with an initial
diagnosis of CUP who eventually received a stage 3 or 4 pancreatic
cancer diagnosis) and the Pancreas group (those diagnosed with
stage 3 or 4 pancreatic cancer only). All analyses were conducted
using SAS, version 9.4 (Cary, NC).
Results
Patient characteristics
There were 68,146 patients who received a definitive
diagnosis of stage 3/4 pancreatic cancer between 2010-2015.
Approximately 74% were diagnosed with pancreatic cancer only
(n=50,581) and 26% started with an initial diagnosis of CUP
(n=17,565). The CUP-Pancreas group were 53.4% female, 37.3% were
between the ages of 75-84, 81.6% were White, 92.3% were non-Latino,
59.1% lived in an urban area, 42.6% had a Charlson comorbidity
score of 2 or higher, and 60.7% were histologically confirmed as
adenocarcinoma (Table I). Of the
cases diagnosed with pancreatic cancer only, characteristics were
generally similar to those initially diagnosed with CUP, however,
29.9% were between the ages of 75-84 and 31.9% had a Charlson
comorbidity score of 2 or higher.
 | Table IDescriptive characteristics of
definitive pancreatic cancer diagnosis in those initially diagnosed
with CUP compared to those diagnosed with pancreatic cancer only,
SEER-Medicare, 2010-2015. |
Table I
Descriptive characteristics of
definitive pancreatic cancer diagnosis in those initially diagnosed
with CUP compared to those diagnosed with pancreatic cancer only,
SEER-Medicare, 2010-2015.
| Characteristic | CUP-pancreas
(n=17,565) (%) | Pancreas (n=50,581)
(%) |
|---|
| Sex | | |
|
Female | 9,387 (53.4) | 25,897 (51.2) |
|
Male | 8,178 (46.6) | 24,684 (48.8) |
| Age at diagnosis,
years | | |
|
65 | 2,025 (11.5) | 12,038 (23.8) |
|
66-74 | 6,031 (34.3) | 16,237 (32.1) |
|
75-84 | 6,553 (37.3) | 15,123 (29.9) |
|
85+ | 2,956 (16.9) | 7,183 (14.2) |
| Race | | |
|
White | 14,329 (81.6) | 41,122 (81.3) |
|
Black | 2,123 (12.1) | 5,564 (11.0) |
|
Other | 1,113 (6.3) | 3,895 (7.7) |
| Ethnicity | | |
|
Non-Latino | 16,212 (92.3) | 46,180 (91.3) |
|
Latino | 1,353 (7.7) | 4,401 (8.7) |
| Urban | | |
|
Yes | 10,379 (59.1) | 30,602 (60.5) |
|
No | 7,186 (40.9) | 19,979 (39.5) |
| Charlson comorbidity
score | | |
|
0 | 5,337 (30.4) | 21,649 (42.8) |
|
1 | 4,748 (27.0) | 12,797 (25.3) |
|
2+ | 7,480 (42.6) | 16,135 (31.9) |
| Histology | | |
|
Adenocarcinoma | 10,661 (60.7) | 29,843 (59.0) |
|
Epithelial
unspecified | 3,627 (20.6) | 11,077 (21.9) |
|
Neuroendocrine | 3,070 (17.5) | 9,105 (18.0) |
|
Squamous
cell carcinoma | 207 (1.2) | 556 (1.1) |
Definitive diagnosis of CUP-Pancreas
group
CUP patients between the ages of 75-84 had lower
odds of definitive pancreatic cancer diagnosis [OR, 0.90 (0.83,
0.97)] compared to patients 85 years or older (Fig. 1). CUP patients had lower odds of
definitive pancreatic cancer diagnosis for those with a Charlson
comorbidity score of 0 [OR, 0.85 (0.79, 0.91)] or 1 [OR, 0.80
(0.74, 0.85)] compared to a score of 2 or higher; and lower odds of
definitive pancreatic cancer diagnosis for epithelial/unspecified
histology compared to all other histology types [OR, 0.76 (0.71,
0.82)]. CUP patients who identified as a race other than Black or
White had higher odds of definitive pancreatic cancer diagnosis
[OR, 1.27 (1.13, 1.43)] compared to White patients.
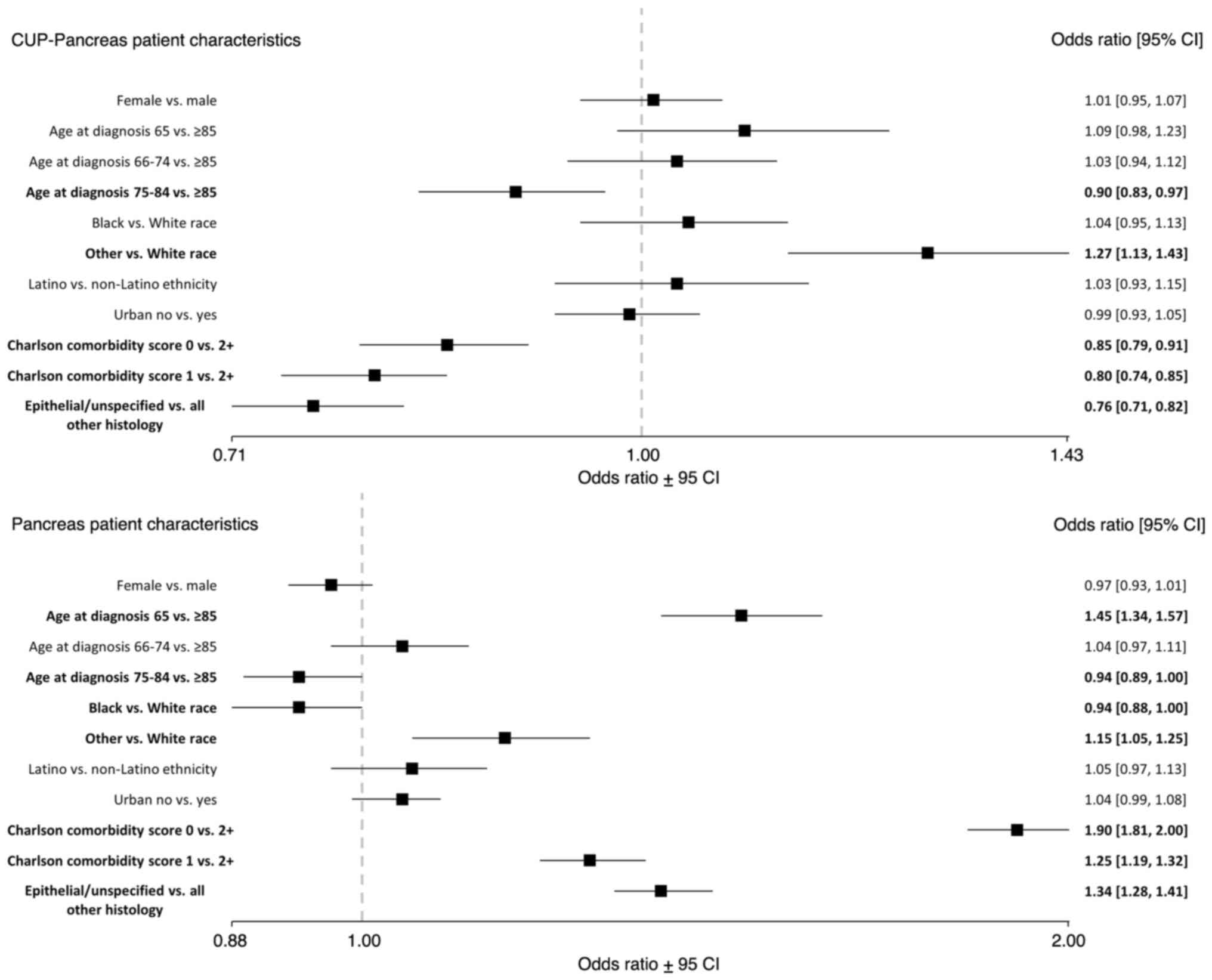 | Figure 1ORs of definitive pancreatic cancer
diagnosis in those initially diagnosed with CUP (n=17,565) compared
to those diagnosed with pancreatic cancer only (n=50,581) by
patient characteristics, SEER-Medicare, 2010-2015. Bold indicates
statistical significance (P<0.05). CUP, cancer of unknown
primary; CI, confidence interval; OR, odds ratio; SEER,
Surveillance, Epidemiology, and End Results. |
Definitive diagnosis of pancreas only
group
Pancreatic only patients between the ages of 75-84
had lower odds of definitive diagnosis [OR, 0.94 (0.89, 1.00)]
compared to patients 85 years or older (Fig. 1). Pancreatic only patients who
identified as Black had lower odds of definitive diagnosis [OR,
0.94 (0.88, 1.00)] compared to White patients. Pancreatic only
patients of 65 years had higher odds of definitive diagnosis [OR,
1.45 (1.34, 1.57)] compared to patients 85 years or older.
Pancreatic only patients who identified as a race other than Black
or White had higher odds of definitive diagnosis [OR, 1.15 (1.05,
1.25)] compared to White patients; higher odds of definitive
diagnosis for those with a Charlson comorbidity score of 0 [OR,
1.90 (1.81, 2.00)] or 1 [OR, 1.25 (1.19, 1.32)] compared to a score
of 2 or higher; and higher odds for definitive diagnosis for
epithelial/unspecified histology compared to all other histology
types [OR, 1.34 (1.28, 1.41)].
Discussion
To our knowledge, this is the first population-based
study focusing on metastatic pancreatic cancer in patients
initially diagnosed with CUP. CUP patients had higher odds of
receiving a definitive pancreatic cancer diagnosis if they
identified as a race other than Black or White. CUP patients had
lower odds of receiving a definitive pancreatic cancer diagnosis if
they were older, had fewer or no comorbidities, and histology
confirmed as epithelial/unspecified. Patients with comorbidities
may receive health services more often than patients without
comorbidities, thus are more likely to come in contact with the
health care system (14). However,
older patients with comorbidities may be unable to complete the
diagnostic workup necessary to make a definitive diagnosis
(15). Characteristics associated
with delay of definitive diagnosis in CUP to a specified primary
site including older age, epithelial/unspecified histology, and
higher comorbid burden of disease correspond with current
scientific literature on CUP patterns of care, namely
population-based studies focusing on patient characteristics and
healthcare utilization (4,16,17),
adherence and diagnostic guidelines (10), and risk factors and clinical
management (18,19).
In patients diagnosed with stage 3 or stage 4
pancreatic cancer only, definitive diagnosis was similar to CUP
patients by race, however, this subpopulation was younger and had
fewer comorbidities overall. Furthermore, the comorbidity score and
whether histology was epithelial/unspecified were not barriers to
definitive diagnosis for the pancreatic cancer only group,
suggesting there are imbalances in delivery of care compared to
patients initially diagnosed with CUP. This is likely due to (a)
the complexity of identifying the primary tumor site in CUP,
whereas in identification of pancreatic cancer, the clinician at
least has a point from which to begin a well-informed diagnostic
process; and (b) poor performance status of the patient with CUP, a
potential confounder this study could not account for.
These findings further elucidate the health
disparities evident in CUP and pancreatic cancer diagnoses.
Scientific literature on cancer health disparities reports higher
incidence of metastatic pancreatic cancer among Black and Latino
patients, as well as lower occurrence of treatment (primarily
surgical intervention), poor access to quality health care, and
higher rates of overall morbidity and mortality (20-22).
An area of future research should focus on the patterns of care
associated with race, ethnicity, and social determinants of health
(to include socioeconomic status) in patients diagnosed with CUP
and pancreatic cancer, especially those in younger age groups given
increased incidence of pancreatic cancer in this population.
While SEER-Medicare data provided a robust sample
size, there are limitations in this study. Our study population was
limited to patients 65 years and older and did not include patients
with private insurance coverage. However, the age range of an
average patient with CUP is 80 years or older and the vast majority
of patients 65 years and older are insured through Medicare
(23). This study only
investigated patients with a final metastatic pancreatic cancer
diagnosis. It is also important to note clinicians may need to
report a definitive diagnosis to justify treatment for insurance
claims. Claims data for administrative and billing purposes might
be inaccurate from a biological or clinical disease
perspective.
There are significant deficiencies in the research
of other CUP-primary site cancers, for example ovarian and lung
cancers, as well as available studies comparing site-specific
therapy and empiric chemotherapy (24). These deficiencies include
limitations in recruitment methodology, study design, heterogeneity
among the CUP classifiers (e.g., epigenetic vs. transcriptomic
profiling), and incomparable therapies (24). An assessment of recently published
CUP literature recommends two comprehensive clinical trial designs
to address these limitations (24). Both designs are amenable to
implementing the latest diagnostics and therapeutic advances to
improve the quality of CUP research and the prognosis of many
patients (24).
This brief report discusses patient characteristics
associated with the definitive diagnosis of metastatic pancreatic
cancer in patients who initially presented with CUP based on a
large and representative population-based cohort. This profile
elucidates that about a quarter of patients who initially present
with CUP receive a definitive pancreatic cancer diagnosis and are
typically older with a higher burden of comorbidities. These
characteristics may contribute to delays in definitive diagnosis,
thereby negatively affecting survival and quality of life. Future
studies should focus on patterns of care and survival outcomes in
patients who initially present with CUP with other primary site
cancers.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Institutes of
Health (grant no. P30 CA042014-30).
Availability of data and materials
The data that support the findings of this study are
available from the National Cancer Institute but restrictions apply
to the availability of these data, which were used under license
for the current study, and so are not publicly available. Data are
however available from the authors upon reasonable request and with
permission of the National Cancer Institute.
Authors' contributions
LLW was involved in study conceptualization, study
design, data management, data programming, formal analysis,
manuscript writing, manuscript review and editing, and funding
acquisition. JSG was involved in study conceptualization, data
management, data programming, manuscript review and editing, and
funding acquisition. LE was involved in study design, and
manuscript review and editing. ML was involved in formal analysis,
and manuscript review and editing. LLW and JSG confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethics approval was waived by the Institutional
Review Board at University of Nevada, Reno in view of the
retrospective nature of the study, citing SEER-Medicare data are
exempt [CFR 46.104(4)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
National Cancer Institute (NCI). Cancer of
unknown primary-health professional version. National Cancer
Institute, 2017. https://www.cancer.gov/types/unknown-primary/hp.
|
|
2
|
Pavlidis N and Pentheroudakis G: Cancer of
unknown primary site. Lancet. 379:1428–1435. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rassy E, Parent P, Lefort F, Boussios S,
Baciarello G and Pavlidis N: New rising entities in cancer of
unknown primary: Is there a real therapeutic benefit? Crit Rev
Oncol Hematol. 147(102882)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schaffer AL, Pearson SA, Dobbins TA, Er
CC, Ward RL and Vajdic CM: Patterns of care and survival after a
cancer of unknown primary (CUP) diagnosis: A population-based
nested cohort study in Australian government department of
Veterans' Affairs clients. Cancer Epidemiol. 39:578–584.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moran S, Martinez-Cardús A, Boussios S and
Esteller M: Precision medicine based on epigenomics: The paradigm
of carcinoma of unknown primary. Nat Rev Clin Oncol. 14:682–694.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
American Cancer Society (ACS). Key
statistics for pancreatic cancer. American Cancer Society, 2020.
https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html#references.
|
|
7
|
Henrikson NB, Aiello Bowles EJ, Blasi PR,
Morrison CC, Nguyen M, Pillarisetty VG and Lin JS: Screening for
pancreatic cancer: Updated evidence report and systematic review
for the US preventive services task force. JAMA. 322:445–454.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khalaf N, El-Serag HB, Abrams HR and
Thrift AP: Burden of pancreatic cancer: From epidemiology to
practice. Clin Gastroenterol Hepatol. 19:876–884. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Q, Zeng L, Chen Y, Lian G, Qian C,
Chen S, Li J and Huang K: Pancreatic cancer epidemiology,
detection, and management. Gastroenterol Res Pract.
2016(8962321)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sève P, Mackey J, Sawyer M, Lesimple T, de
La Fouchardière C, Broussolle C, Dumontet C and Ray-Coquard I:
Impact of clinical practice guidelines on the management for
carcinomas of unknown primary site: A controlled ‘before-after’
study. Bull Cancer. 96:E7–E17. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pentheroudakis G, Stahel R, Hansen H and
Pavlidis N: Heterogeneity in cancer guidelines: Should we eradicate
or tolerate? Ann Oncol. 19:2067–2078. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Smith-Gagen J, Drake CM, White LL and
Pinheiro PS: Extent of diagnostic inquiry among a population-based
cohort of patients with cancer of unknown primary. Cancer Rep Rev.
3:2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Klabunde CN, Warren JL and Legler JM:
Assessing comorbidity using claims data: An overview. Med Care. 40
(8 Suppl):IV26–IV35. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Department of Health Pharmaceutical
Oncology Initiative (DHPOI). The impact of patient age on clinical
decision-making in oncology. London, UK: Department of Health,
2012.
|
|
15
|
Massarweh NN, Park JO, Bruix J, Yeung RS,
Etzioni RB, Symons RG, Baldwin LM and Flum DR: Diagnostic imaging
and biopsy use among elderly medicare beneficiaries with
hepatocellular carcinoma. J Oncol Pract. 7:155–160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schaffer AL, Pearson SA, Perez-Concha O,
Dobbins T, Ward RL, van Leeuwen MT, Rhee JJ, Laaksonen MA, Craigen
G and Vajdic CM: Diagnostic and health service pathways to
diagnosis of cancer-registry notified cancer of unknown primary
site (CUP). PLoS One. 15(e0230373)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jones W, Allardice G, Scott I, Oien K,
Brewster D and Morrison DS: Cancers of unknown primary diagnosed
during hospitalization: A population-based study. BMC Cancer.
17(85)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rassy E and Pavlidis N: The currently
declining incidence of cancer of unknown primary. Cancer Epidemiol.
61:139–141. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pavlidis N, Rassy E and Smith-Gagen J:
Cancer of unknown primary: Incidence rates, risk factors and
survival among adolescents and young adults. Int J Cancer.
146:1490–1498. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Noel M and Fiscella K: Disparities in
pancreatic cancer treatment and outcomes. Health Equity. 3:532–540.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tavakkoli A, Singal AG, Waljee AK,
Elmunzer BJ, Pruitt SL, McKey T, Rubenstein JH, Scheiman JM and
Murphy CC: Racial disparities and trends in pancreatic cancer
incidence and mortality in the United States. Clin Gastroenterol
Hepatol. 18:171–178.e10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou H, Zhang Y, Wei X, Yang K, Tan W, Qiu
Z, Li S, Chen Q, Song Y and Gao S: Racial disparities in pancreatic
neuroendocrine tumors survival: A SEER study. Cancer Med.
6:2745–2756. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Federal Interagency Forum (FIF) on
Aging-Related Statistics. Older Americans: Key indicators of
well-being. U.S. Government Printing Office, 2015.
|
|
24
|
Rassy E, Labaki C, Chebel R, Boussios S,
Smith-Gagen J, Greco FA and Pavlidis N: Systematic review of the
CUP trials characteristics and perspectives for next-generation
studies. Cancer Treat Rev. 107(102407)2022.PubMed/NCBI View Article : Google Scholar
|















