Introduction
With the development and increased interest in
cancer immunotherapy, immune checkpoint inhibitors (ICIs),
including targeting programmed cell death-1 (PD-1), PD-1 ligand 1
(PD-L1) and the checkpoint T lymphocyte-associated protein 4
(CTLA-4), have emerged as a novel therapeutic strategy in certain
types of cancer. However, the majority of patients showed no
response to ICI therapies and numerous responders eventually
developed resistance (1,2). Several biomarkers, including
tumor-infiltrating lymphocytes (TILs), tumor mutational burden
(TMB) and microsatellite instability (MSI), have been used to
select potential responders to ICI therapy. However, these
biomarkers were solely focused on tumor features and did not
reflect the systemic immune status of patients (3). Therefore, exploring simpler,
available patient characteristics, such as body mass index (BMI) or
body composition, seems feasible to assess the association with
outcomes and response to ICI therapy.
In recent years, the efficacy of ICIs in obese
populations with cancer has drawn increased interest from
researchers. According to statistics from the World Health
Organization (WHO) for 2020, the proportion of overweight and obese
individuals older than 18 years within the world population
accounted for 39 and 13%, respectively (4). Epidemiological studies have
established a strong association between obesity and multiple
cancer types. Obesity was determined to be a risk factor for the
incidence and progression of certain cancer types (5). While previous research has focused
predominantly on the effects of obesity on altered endocrine
factors, growth factors and signaling pathways, little is known
about its impact on cancer immunotherapy (5). As the number of overweight and obese
individuals continues to rise, the influence of obesity on cancer
treatment efficacy should not be ignored.
The BMI commonly measures obesity as a marker for
the nutritional state (6).
Previous clinical studies have indicated that an increased BMI is
associated with improved survival of patients with cancer receiving
immunotherapy (7-9).
For instance, obesity improved the progression-free survival (PFS)
and overall survival (OS) of patients with metastatic melanoma who
received targeted therapy, immunotherapy or chemotherapy (8). By contrast, another study on
metastatic melanoma reported no association between obesity and
outcome (10). Whether obesity is
a predictive factor regarding survival of patients receiving
immunotherapy needs to be further studied. Previous meta-analyses
have explored the impact of the BMI on the outcomes of ICI
treatment for patients with cancer, but the date of their
literature search included only studies published up to 2021
(11-14).
Thus, based on the latest literature, the present study aimed to
evaluate the predictive value of the BMI in patients with cancer
receiving immunotherapy.
As the BMI is calculated from the whole-body weight,
it is not the most suitable measure for evaluating obesity
(6). Recently, imaging-measured
adipose distribution has been investigated to estimate the
influence of obesity on the efficacy of ICI therapy. It was
reported that a higher fat distribution is associated with improved
survival of patients with cancer rather than the BMI (15-17).
However, the results appeared to be inconsistent due to the
different methods used to evaluate the body's composition. Thus,
the potential association between adipose distribution and clinical
outcomes in patients with cancer treated with immunotherapy remains
controversial. Therefore, another objective of the present study
was to explore the association between survival and different types
of fat in patients treated with immunotherapy.
Materials and methods
Search strategy
A systematic literature search was conducted using
the PubMed (https://pubmed.ncbi.nlm.nih.gov/), MEDLINE (https://www.medline.eu/), EMBASE (https://www.embase.com/) and Cochrane Library
(https://www.cochranelibrary.com/)
databases for entries of studies published between January 2017 and
July 2022, with no language restrictions. The main keywords for the
literature search included ‘cancer’, ‘tumor’, ‘oncology’,
‘neoplasm’, ‘body mass index’, ‘BMI’, ‘obesity’, ‘overweight’,
‘weight’, ‘mass’, ‘body composition’, ‘body fat distribution’,
‘adiposity’, ‘fat’, ‘PD-1’, ‘PD-L1’, ‘CTLA-4’, ‘nivolumab’,
‘pembrolizumab’, ‘atezolizumab’, ‘avelumab’, ‘durvalumab’,
‘ipilimumab’, ‘tremelimumab’ and ‘immune checkpoint inhibitor’. Any
studies missed by the electronic search were manually searched from
references of included studies and relevant systematic reviews. The
protocol of the current meta-analysis was registered in the
International Prospective Register of Systematic Reviews database
(https://www.crd.york.ac.uk/PROSPERO/;
accession no. CRD42022344713).
Selection criteria
Two investigators, LXY and WC, independently
searched and selected articles for eligibility. If there were any
disagreements, all authors jointly re-evaluated these studies.
Full-text articles of clinical studies were screened. The inclusion
criteria for the meta-analysis were as follows: i) Patients had
been diagnosed with cancer and treated with ICIs; ii) based on the
BMI, patients were categorized into normal (BMI, 18.5-24.9
kg/m2), overweight (BMI, 25.0-29.9 kg/m2) and
obese (BMI, ≥30 kg/m2) or into two groups (BMI <25
kg/m2 and BMI ≥25 kg/m2); iii) the survival
outcomes included PFS and OS; and iv) associations between the BMI
and OS or PFS were analyzed using Cox proportional hazards
regression models and were reported as hazard ratio (HR) and 95%
confidence interval (CI). The inclusion criteria for systematic
reviews were as follows: i) Studies focused on adipose tissue
distribution; ii) body fat was measured by computed tomography
(CT); and iii) associations between adiposity and patient survival
with cancer immunotherapy were evaluated.
Data extraction
Two authors (GH and FS) independently reviewed and
extracted data from the included studies. Any discrepancy was
resolved through discussion with all authors. The following data
were extracted from each of the included studies in the
meta-analysis: i) Name of first author, year of publication,
country, sample size, percentage of male patients and study type;
ii) cancer type and ICI drugs; iii) BMI cut-off value; and iv) OS
and PFS.
Article quality evaluation
The quality of the included studies on the BMI was
evaluated using the Newcastle-Ottawa scale (18). Quality was assessed according to
the following inclusion criteria: i) representativeness of the
exposed; ii) selection of the non-exposed; iii) ascertainment of
exposure; iv) demonstration that outcome of interest was not
present at the start; v) study controls for age and sex; vi) study
controls for any additional factors (chemoradiotherapy, curative
resection and drug resistance); vii) assessment of outcome; viii)
follow-up time of >36 months; and ix) adequacy of follow-up of
cohorts.
Sensitivity and publication bias
Contour-enhanced meta-analysis funnel plots were
used to distinguish publication bias from other asymmetry causes.
Publication bias was assessed using Begg's and Egger's tests.
Sensitivity analysis was conducted by excluding one study at a
time.
Statistical analysis
OS and PFS were used to evaluate the clinical
outcomes of ICI treatment. The association between BMI and ICI
efficacy in patients with cancer was measured by the hazard ratio
(HR) with 95% CI. Statistical heterogeneity among the included
studies was evaluated using the I2 statistic.
I2 values of <40, 40-60, 60-75 and >75% were
considered to indicate low, moderate, substantial and considerable
heterogeneity, respectively. I2>40% or P<0.1 was
considered to indicate statistical heterogeneity. A random-effects
model was applied to calculate the summary HR and 95% CI. All
analyses were performed with the meta package of R 4.0.5-win
software (https://rstudio.com/products/rstudio/). A two-sided
P<0.05 was considered to indicate a statistically significant
difference.
Results
Study selection
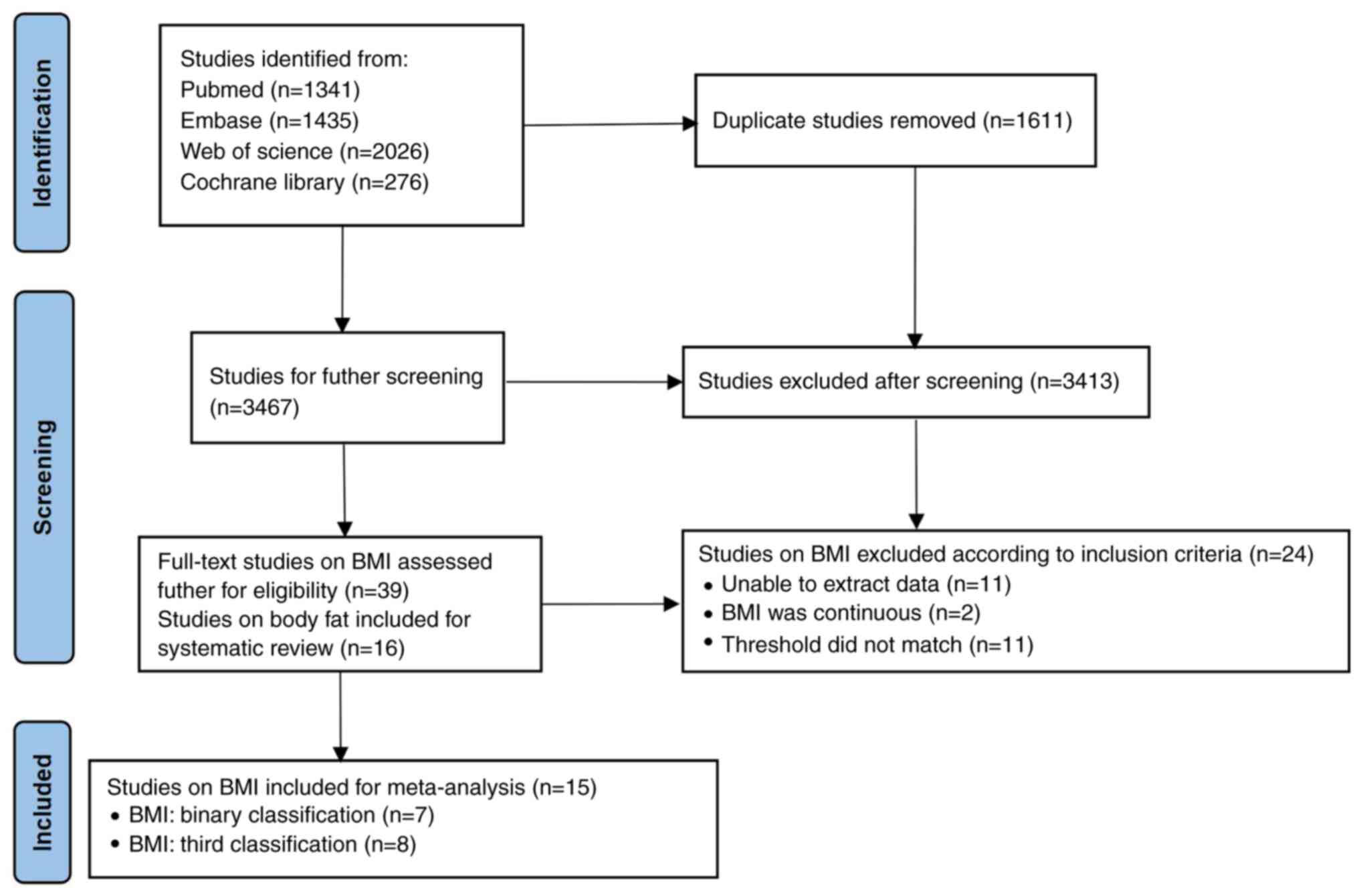
A total of 5,078 studies were retrieved through the
initial literature search, with 3,467 studies remaining after
removing duplicates. Next, 3,413 articles were excluded through
reviewing titles and abstracts. The remaining 54 studies were
reviewed and screened according to the present inclusion and
exclusion criteria. Finally, 15 studies reporting on the BMI
(7-10,15,16,19-27)
were included in the current meta-analysis (Fig. 1). The association between body fat
and survival was not suitable for meta-analysis. Therefore, 16
studies reporting on body fat (15-17,24,27-38)
were included in the systematic review and descriptively summarized
in one table.
Characteristics of studies included in
the meta-analysis
General information on the included studies
reporting on the BMI is presented in Table I. All analyses were published
between 2017 and 2022, of which 12 studies were retrospective and 3
studies were prospective. A total of 5,205 male and 3,105 female
patients were included in the meta-analysis. The patients in the
meta-analysis were from the USA, Canada, Italy, France, Israel,
Spain, Australia and Japan. Melanoma was the most commonly reported
cancer type. All enrolled patients were at an advanced or
metastatic stage. Since the cut-off values for the BMI in the
selected studies were not consistent, 8 studies that stratified the
patients based on the BMI value into normal weight (18.5-24.9
kg/m2), overweight (25.0-29.9 kg/m2) and
obese (≥30 kg/m2) groups were included, as well as 7
studies that divided the patients by their BMI value into BMI
<25 kg/m2 and BMI ≥25 kg/m2 groups. With
regard to the types of ICIs used, 6 studies used anti-PD-1/PD-L1
monotherapy, 1 study utilized anti-CTLA-4 monotherapy and 8 studies
used anti-PD-1/PD-L1 monotherapy or anti-CTLA-4 monotherapy or
their combination. The quality of the included studies was assessed
with the Newcastle-Ottawa scale, which revealed high or moderate
quality of evidence in the included studies (Fig. S1).
 | Table IBaseline characteristics of included
studies including the BMI. |
Table I
Baseline characteristics of included
studies including the BMI.
| Author, year | Sample size | Country | Study design | Males, n (%) | Cancer type | Treatment | BMI cut-off
value | Outcomes | (Refs.) |
|---|
| Yoo, 2022 | 1,840 | USA | Prospective
cohort | 1,059(57) | Pancancer | Anti-PD-1/PD-L1,
anti-CTLA4 Anti-CTLA4+anti-PD-1/PD-L1 | 18.5-24.9; 25-29.9;
≥30 | PFS; OS | (9) |
| Tateishi, 2021 | 324 | Japan | Retrospective
cohort | 235(72) | NSCLC |
Anti-PD-1/PD-L1 | ≥25; <25 | PFS | (26) |
| Esposito, 2021 | 153 | Italy | Retrospective
cohort | 62(40) | Multiple |
Anti-PD-1/PD-L1 | ≥25; <25 | PFS; OS | (25) |
| Collet, 2021 | 272 | France | Retrospective
cohort | 174(63) | Multiple | Anti-PD-1/PD-L1,
anti-CTLA4, anti-CTLA4+anti-PD-1/PD-L1 | ≥25; <25 | OS | (21) |
| Martini i),
2021 | 70 | USA | Retrospective
cohort | 49(70) | UC |
Anti-PD-1/PD-L1 | ≥25; <25 | PFS; OS | (16) |
| Martini ii),
2021 | 79 | USA | Retrospective
cohort | 58(73) | RCC |
Anti-PD-1/PD-L1 | ≥25; <25 | PFS; OS | (15) |
| Ahmed, 2021 | 297 | USA | Prospective
cohort | 177(59) | Multiple | Anti-PD-1/PD-L1,
anti-CTLA4, anti-CTLA4+anti-PD-1/PD-L1 | ≥25; <25 | PFS; OS | (20) |
| Young, 2020 | 287 | USA | Retrospective
cohort | 184(64) | Melanoma | Anti-PD-1/PD-L1,
anti-CTLA4, anti-CTLA4+anti-PD-1/PD-L1 | 18.5-24.9; 25-29.9;
≥30 | PFS; OS | (27) |
| Di Filippo,
2020 | 1,214 | French | Retrospective
cohort | 738(61) | Melanoma | Anti-PD-1,
anti-CTLA4 Anti-PD-1+anti-CTLA-4 | 18.5-24.9; 25-29.9;
≥30 | PFS; OS | (10) |
| Labadie, 2019 | 90 | USA, Canada,
Spain | Retrospective
cohort | 65(72) | RCC |
Anti-PD-1/PD-L1 | 18.5-24.9; 25-29.9;
≥30 | OS | (7) |
| Kichenadasse,
2019 | 1,434 | USA, Australia | Retrospective
cohort | 890(62) | NSCLC |
Anti-PD-1/PD-L1 | 18.5-24.9; 25-29.9;
≥30 | PFS; OS | (19) |
| Donnelly, 2019 | 423 | USA | Retrospective
cohort | 267(63) | Melanoma | Anti-PD-1,
anti-CTLA-4 Anti-PD-1+anti-CTLA-4 | 18.5-24.9; 25-29.9;
≥30 | PFS; OS | (24) |
| De Giorgi,
2019 | 313 | Italy | Prospective
cohort | 235(75) | RCC |
Anti-PD-1/PD-L1 | ≥25; <25 | OS | (23) |
| Cortellini,
2019 | 976 | Italy | Retrospective
cohort | 663(67) | Multiple |
Anti-PD-1/PD-L1 | 18.5-24.9; 25-29.9;
≥30 | PFS; OS | (22) |
| McQuade, 2018 | 538 | USA, Australia | Retrospective
cohort | 349(64) | Melanoma | Anti-PD-1/PD-L1
Anti-CTLA-4 | 18.5-24.9; 25-29.9;
≥30 | PFS; OS | (8) |
Association between BMI and OS in
patients with cancer receiving immunotherapy
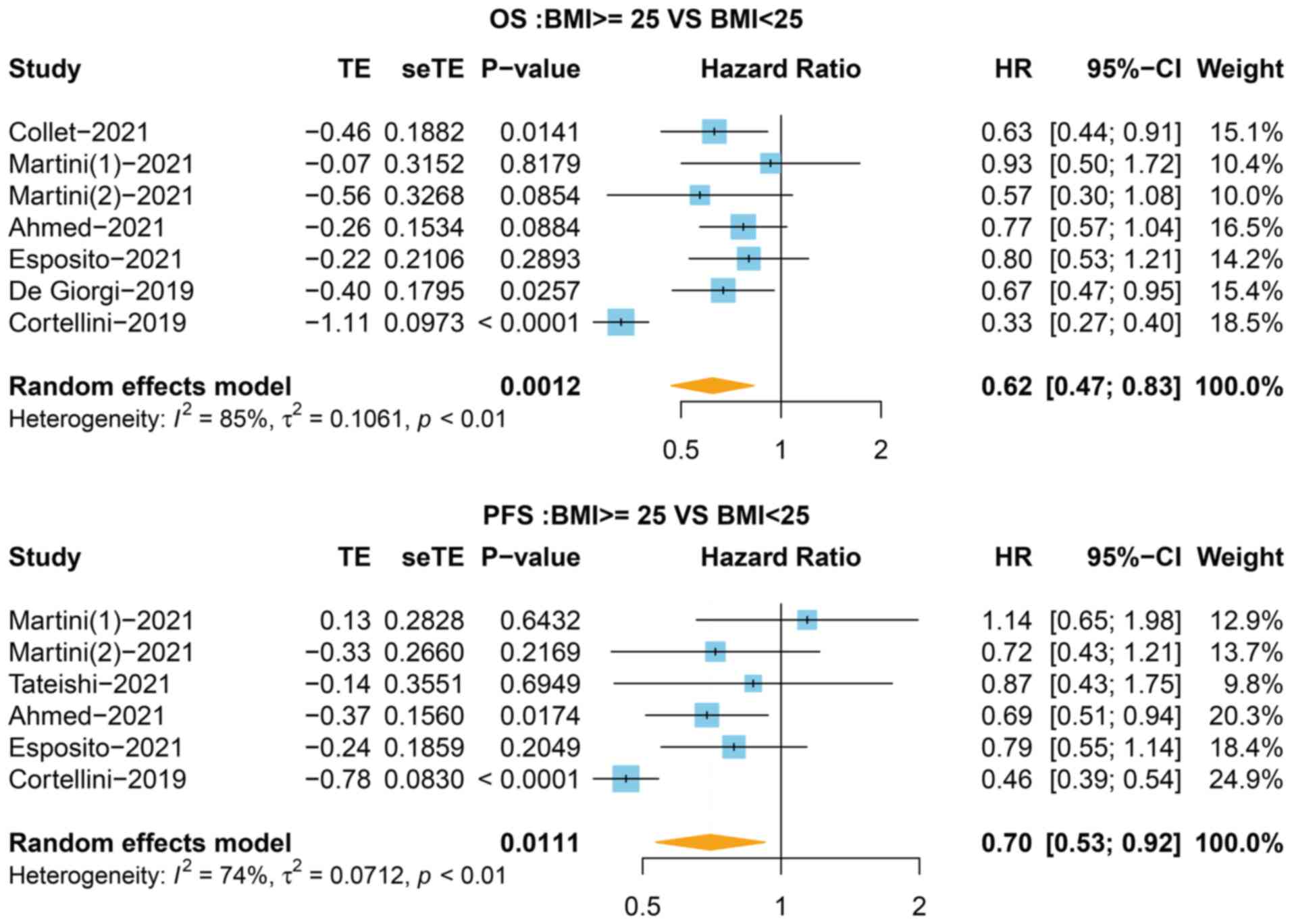
To evaluate the association between BMI and
survival, the HR for OS in 7 studies was first analyzed,
stratifying the BMI value into <25 and ≥25 kg/m2
groups. As shown in Fig. 2,
patients with a BMI ≥25 kg/m2 exhibited increased OS
compared with the BMI <25 kg/m2 group (HR=0.62, 95%
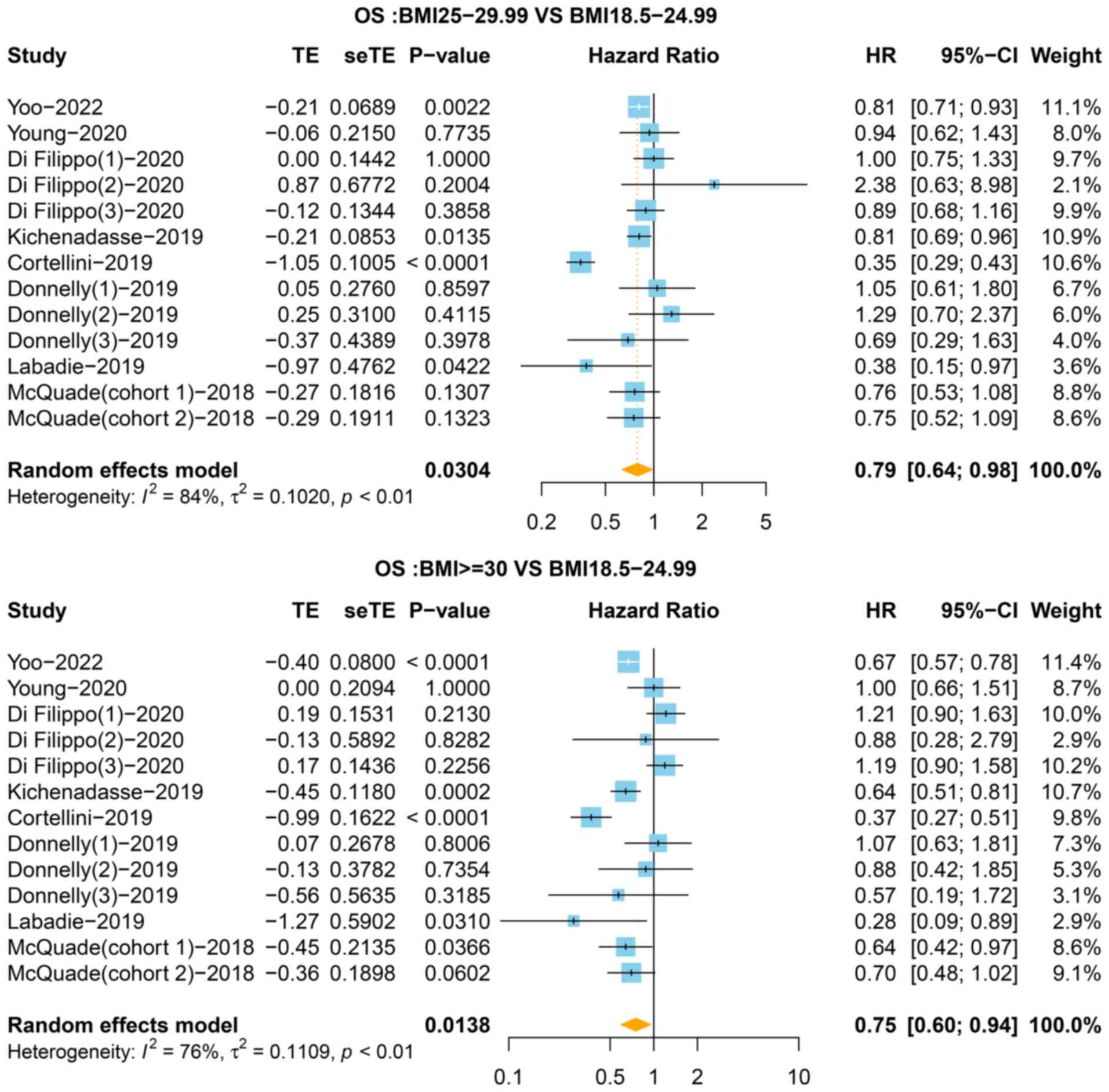
CI=0.47-0.83, P=0.001, I2=85%). Next, the OS of
overweight and obese patients was compared with that of the normal
group. The results of the pooled analysis showed that improved OS
was observed in the overweight (HR=0.79, 95% CI=0.64-0.98, P=0.030,
I2=84%) and obese (HR=0.75, 95% CI=0.60-0.94, P=0.014,
I2=76%) groups compared with the normal group (Fig. 3). The heterogeneity test indicated
that there was heterogeneity among the studies in terms of OS.
Thus, a sensitivity analysis was performed to assess the impact of
a single study on the overall outcomes, which revealed that the
results were stable (Figs. S2 and
S3).
Association between BMI and PFS in
patients with cancer receiving immunotherapy
In total, 6 of the 9 studies that stratified the BMI
value into <25 and ≥25 kg/m2 groups reported the HR
for PFS. As shown in Fig. 2, the
BMI ≥25 kg/m2 group was associated with improved PFS
(HR=0.70, 95% CI=0.53-0.92, P=0.011) with a high level of
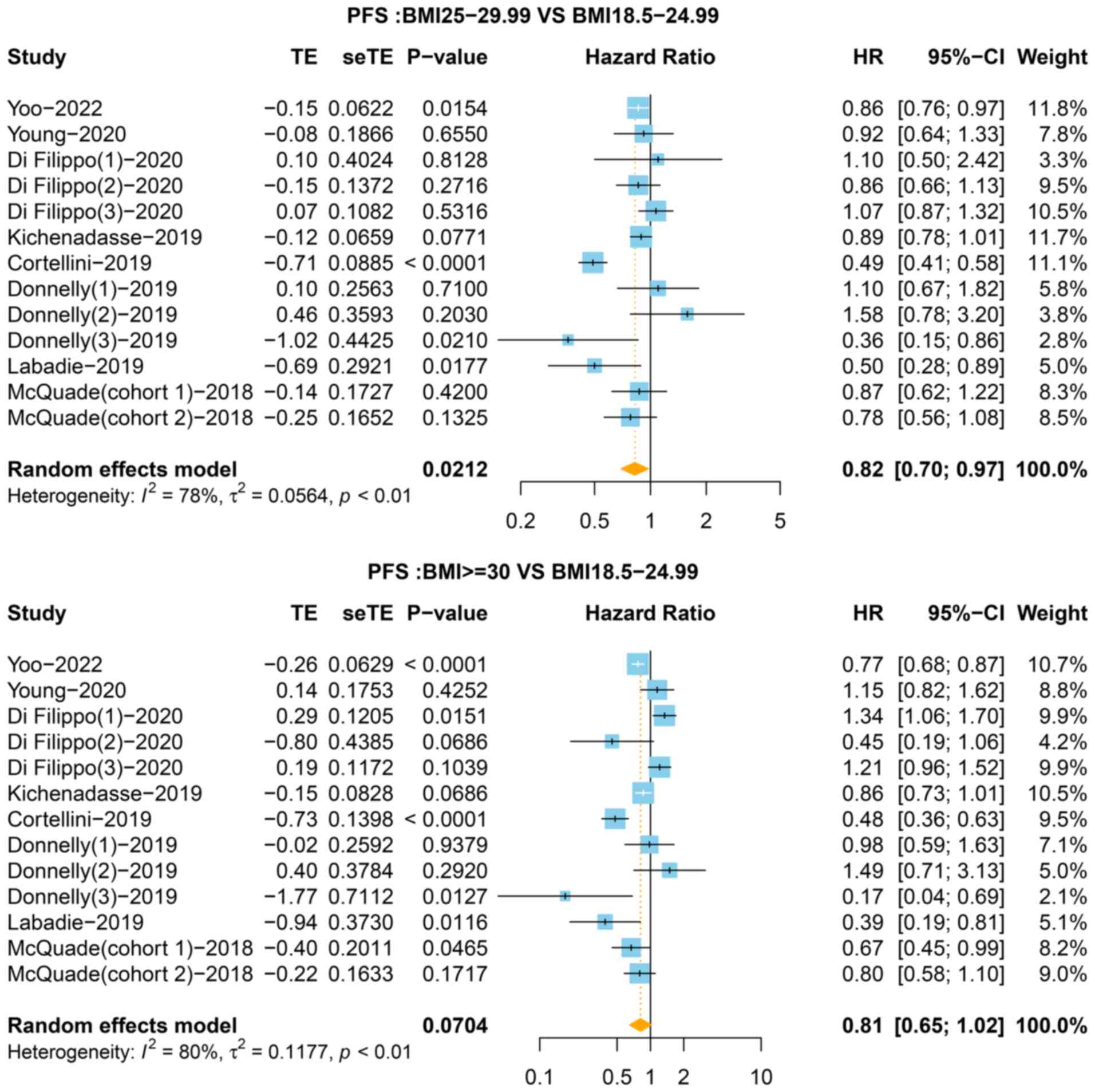
heterogeneity (I2=74%). In the third classification,
compared with the normal group, the pooled HR for PFS was 0.82 (95%
CI=0.70-0.97, P=0.021, I2=78%) for the overweight group
and 0.81 (95% CI: 0.65-1.02, P=0.070, I2=80%) for the
obese group (Fig. 4). The
sensitivity analysis showed that no single study significantly
changed the pooled results (Figs.
S2 and S4). As presented in
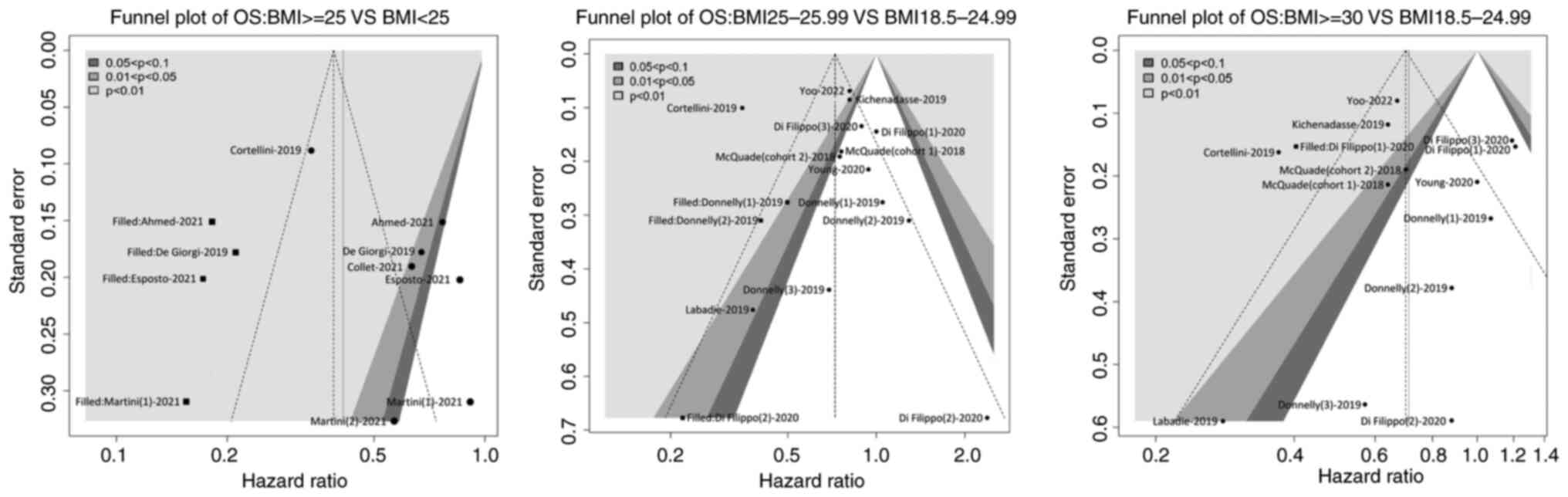
Fig. 5, funnel plots showed no
significant publication bias in the present meta-analysis.
Characteristics of studies involving
body fat and immunotherapy
A total of 16 studies that were published from 2019
to 2022 involving 1,888 patients focused on body fat analysis and
were included in the present study. Among them, males accounted for
61.5% of patients. These studies were performed in Asia, North
America and Europe. In total, 5 studies were conducted on patients
with non-small cell lung cancer (NSCLC) (29-31,34,35);
3 on patients with melanoma (27,33,37);
3 on patients with multiple cancer types (25,28,32);
2 on patients with renal cancer (15,38);
1 on patients with breast cancer (36); 1 on patients with liver cancer
(17); and 1 on patients with
urothelial cancer (16). The
average patient age and immunotherapy drugs were similar in all the
studies. Most studies adopted baseline abdominal CT images in the
middle of the third lumbar vertebra (mid-L3). Subcutaneous,
intermuscular, intramuscular and visceral fat were measured using
different segmentation methods. The majority of studies used the
Hounsfield unit (HU) value to quantify adipose tissue (-29 to +150
HU for skeletal muscle; -190 to -30 HU for subcutaneous and
intermuscular fat; and -150 to -50 HU for visceral fat). Further
details are presented in Table
II.
 | Table IIBaseline characteristics of studies
involving body fat and immunotherapy. |
Table II
Baseline characteristics of studies
involving body fat and immunotherapy.
| Author, year | Country | Sample size | Cancer type | ICIs treatment | Body composition
analysis method | Adipose parameters
and cut-offs | Outcomes | (Refs.) |
|---|
| Martini, 2019 | USA | 90 | Multiple |
Anti-PD-1/PD-L1 | CT at L3; -190 to
-30 HU for subcutaneous and intermuscular fat and -150 to -50 HU
for visceral fat | SFI, IFI and VFI
Identify the cut-off values for SFI and IFI by a recursive
partitioning and regression trees method and set 3-level risk
stratification (low, intermediate and high risk) | Low-risk group (SFI
≥73) had a significantly longer OS (HR=0.20; 95% CI: 0.09-0.46) and
PFS (HR=0.38; 95% CI: 0.20-0.72) compared with patients at
intermediate risk (SFI<73 and IFI<3.4) and poor risk
(SFI<73 and IFI≥3.4) | (28) |
| Popinat, 2019 | France | 55 | NSCLC | Nivolumab | PET-CT at L3;
measured by 3D automatic software | FBM, VFM and SCFM
SCFM (kg/m2): 5.0; VFM (kg/m2): 1.38; FBM
(kg/m2): 5.7 | In univariate
analysis using continuous values, SCFM (HR=0.75, P=0.003) and FBM
(HR=0.80, P=0.004) were significant prognostic factors | (29) |
| Magri, 2019 | Israel | 46 | NSCLC | Nivolumab | CT at L3; -190 to
-30 HU for subcutaneous and intramuscular adipose tissue and -150
to -50 HU for visceral adipose tissue | FFMI and FMI
continuous variable | In univariate
analysis, only PS, albumin and weight change were found to be
statistically significantly correlated with OS | (30) |
| Minami, 2019 | Japan | 74 | NSCLC |
Anti-PD-1/PD-L1 | CT at L3; the
skeletal muscle and adipose tissue areas were investigated by
SYNAPSE VIN CENT software | IMAC, VSR and VFA
IMAC (cm2/m2): 6.36 (men), 3.92 (women) VSR:
1.33 (men), 0.93 (women) VFA (cm2): 100 | According to
multivariate analyses, IMAC was a significant prognostic factor for
OS (HR=0.43, P=0.0496), but not for PFS | (31) |
| Young, 2020 | USA | 287 | Melanoma | Ipillimumab+
nivolumab Pembrolizumab Nivolumab Atezolizumab | CT at L3; -150 to
-50 HU for visceral fat | TATI Use
tertiles | In the univariate
analyses, there were no statistically significant associations
between TATI (assessed by tertiles) and PFS or OS | (27) |
| Crombé, 2020 | France | 117 | Multiple | Anti-PD-1/PD-L1
Anti-PD-L1+ anti-CTLA4 | CT at L3; -190 to
-30 HU for fat | SATI, VATI and TATI
Use absolute change in SATI, VATI or TATI from 1st day of ICI
treatment to CT1. Tertiles assessed on whole population were used
for dichotomization | Δt-SATI was
correlated with PFS (HR=2.82, P=0.0004) | (32) |
| Martini, 2021 | USA | 70 | Urothelial
carcinoma | Pembrolizumab
Atezolizumab | CT at L3; -190 to
-30 HU for subcutaneous and intermuscular fat, -150 to -50 HU for
visceral fat | SFI, VFI and IFI
The optimal cutoff point was identified that maximizes the
separation between the two groups (high vs. low) via a
bias-adjusted log-rank test | High VFI was
significantly associated with improved PFS (HR=1.76; P=0.040) and
showed a trend toward longer OS (HR=1.82; P=0.055). High SFI was
significantly associated with prolonged OS (HR=1.99; P=0.043) but
had no significant association with PFS (P=0.477) | (16) |
| Esposito, 2021 | Italy | 153 | Multiple |
Anti-PD-1/PD-L1 | CT at L2-L3; SAT
and VAT were measured by applying predefined image display settings
(window width: -195 to -45 HU; center: -120 HU) | SFA, VFA and TFA
Use tertiles | Univariate analysis
did not show differences in OS according to VFA (1st versus 2nd
tertile: HR=1.09, 95% CI 0.70-1.69, P=0.70; 1st vs. 3rd tertile:
HR=0.78, 95% CI 0.49-1.24, P=0.29), SFA (1st vs. 2nd tertile:
HR=1.00, 95% CI 0.64-1.57, P=0.99; 1st vs. 3rd tertile: HR=0.82,
95% CI 0.52-1.29, P=0.39), TFA (1st vs. 2nd tertile: HR=0.70, 95%
CI 0.44-1.10, P=0.12; 1st vs. 3rd tertile: HR=0.75, 95% CI
0.48-1.18, P=0.21). However, a higher VFA/SFA ratio (1st and 2nd
tertile vs. 3rd tertile) had increased OS (HR=0.88, 95% CI
0.78-1.00, P=0.047) | (25) |
| Faron, 2021 | Germany | 107 | Melanoma | Anti-PD-1
Anti-PD-1+ anti-CTLA4 | CT at L3/4; a deep
learning model for automated body composition analysis was used for
tissue segmentation | VAI and SAI The
cohort was binarized according to median SMI, VAI and SAI based on
gender-specific cutoffs | No significant
differences in 3-year mortality regarding adipose tissue
compartments were observed (low vs. high VAI, 26 vs. 30%, P=0.48;
low vs. high SAI, 26 vs. 30%, P=0.731) | (33) |
| Baldessari,
2021 | Italy | 44 | NSCLC | Pembrolizumab | CT at L3; -180 to
-30 HU for visceral fat | SFI and VFR
continuous variable | In univariate
analysis, SFI and VFR were not correlated with survival | (34) |
| Degens, 2021 | Netherlands | 80 | NSCLC | Nivolumab | CT at L1; -190 to
-30 HU for subcutaneous and intermuscular fat, -150 to -50 HU for
visceral fat | VAT and SAT The
loss of VAT and SAT from baseline to week 6 | Loss of body weight
of >2% at week 6 was an independent predictor for poor OS
(HR=2.39, 95% CI: 1.51-3.79, P<0.001). In patients with >2%
body weight loss between baseline and week 6, a significant loss of
VAT (P=0.047) and SAT (P=0.042) was observed, compared with
patients with weight maintenance | (35) |
| Martini, 2021 | USA | 79 | mRCC | Anti-PD-1 | CT at L3; -190 to
-30 HU for subcutaneous and intermuscular fat, -150 to -50 HU for
visceral fat | SFI, IFI, VFI and
TFI Each fat index was characterized as high vs. low for each
variable at gender-specific optimal cuts using OS as the primary
outcome through a bias-adjusted log-rank test searching
algorithm | Low TFI had
significantly shorter OS (HR=2.72, CI: 1.43-5.17, P=0.002), PFS
(HR=1.91, CI: 1.09-3.35, P=0.025). Low SFI also had significantly
shorter OS (HR=2.06, CI: 1.10-3.85, P=0.024), PFS (HR=2.02, CI:
1.21-3.37, P=0.007). Low VFR was significantly associated with
shorter PFS (HR=1.94; CI: 1.18-3.21, P=0.01) but had no significant
association with OS (P=0.199) | (15) |
| Xiao, 2022 | China | 172 | Primary liver
cancer |
Anti-PD-1/PD-L1 | CT at L3; -190 to
-30 HU for subcutaneous and inter-muscular fat, -150 to -50 HU for
visceral fat | VATI, SATI, TATI
and VSR The Youden Index was used to identify the optimal cut-off
values | OS of patients with
a high VATI was better (HR=0.30; 95% CI: 0.15-0.59; P=0.001) than
that of patients with a low VATI; however, no difference was noted
in PFS. The OS of patients with a high SATI and of those with a
high TATI was better than that of patients with a low SATI
(HR=0.31; 95% CI: 0.17-0.58; P<0.001) and a low TATI (HR=0.31;
95% CI: 0.17-0.57; P<0.001); however, no difference was observed
in PFS. There were no statistically significant associations
between VSR and OS or PFS | (17) |
| Palleschi,
2022 | Italy | 43 | HER2 positive
metastatic breast cancer | Pertuzumab
Trastuzumab | CT at L3; -190 to
-30 HU for subcutaneous and inter-muscular fat,-150 to -50 HU for
visceral fat | SFI, VFI and TAFTI
SFI (cm2/m2): 82.97; VFI
(cm2/m2): 37.1; TAFTI
(cm2/m2): 118.82 | High SFI and TAFTI
were significantly associated with improved PFS (HR=2.04, P=0.047;
HR=2.17, P=0.03). VFI was not associated with PFS | (36) |
| Lee, 2022 | Korea | 266 | Melanoma | Pembrolizumab
Nivolumab | CT at L3; image
segmentation was completed by using a commercially available deep
learning-based software | SFI and VFI SFI
(cm2/m2): 46; VFI
(cm2/m2): 25 | OS was
significantly longer in patients with high VFI (mean OS, 49.1
months; 95%CI: 44.4-53.8 months), compared with patients with low
VFI (mean OS, 38.0 months; 95% CI: 31.1-44.8 months) (log-rank
P<0.001). SFI was not associated with OS. PFS was not
significantly different between the subgroups stratified by SFI or
VFI | (37) |
| Ged, 2022 | USA | 205 | Metastatic clear
cell renal carcinoma | Anti-PD-1/PD-L1
Anti-PD-1+ Anti-CTLA4 Anti-PD-1+ Anti-PD-L1 | CT at L3; -150 to
-50 HU for visceral fat | VATI and SATI Low
VATI: Males, <55 cm2/m2; females, <33
cm2/m2; low SATI: Males, <57
cm2/m2; females, <88
cm2/m2 | VATI and SATI were
not associated with survival | (38) |
Association between body fat and
outcomes in patients with cancer receiving immunotherapy
Due to the different parameters and statistical
methods, the findings were not consistent. As presented in Table II, in NSCLC, 2 studies showed that
subcutaneous adipose tissue (SAT) was associated with prognosis.
Popinat et al (29)
reported that low subcutaneous fat mass was significantly
associated with poor survival (HR=0.75, P=0.006). Degens et
al (35) showed that loss of
SAT at week 6 of treatment with nivolumab was a significant poor
prognostic factor for survival. A total of 4 studies assessed the
association between visceral adipose tissue (VAT) and survival
(29,31,34,35),
but only one of them reported that VAT loss at week 6 of treatment
with nivolumab was associated with poor OS (35). Out of 3 studies, 1 study indicated
that low body adipose mass was significantly associated with poor
survival (HR=0.80, P=0.004) (29).
Only 1 study explored the correlation between intramuscular fat and
prognosis in NSCLC (31). In
addition, 3 studies reported no association between skeletal muscle
and survival (30,34,35).
These studies indicated that increased body fat, rather than
skeletal muscle was associated with improved survival in patients
with NSCLC receiving ICI therapy.
In melanoma, SAT was not associated with survival
(33). However, increased VAT or
total adipose tissue (TAT) predicted favorable survival in patients
treated with ICIs (27,37). In renal cell carcinoma (RCC), 1
article showed that low subcutaneous fat index (SFI), low visceral
fat index (VFI) or low total fat index (TFI) were associated with
significantly inferior survival in metastatic RCC (15). Of note, another article reported no
association between body fat and survival in metastatic clear cell
RCC (38).
In breast cancer, only 1 study found that a high
quantity of subcutaneous or total abdominal fat tissue was a poor
prognostic factor in patients receiving
trastuzumab/pertuzumab-based first-line treatment for human
epidermal growth factor receptor 2 (HER2)-positive metastatic
breast cancer (36). Of note,
visceral fat was not associated with outcome.
In urothelial carcinoma, only 1 article reported
that increased SFI and VFI, and decreased intermuscular fat index
(IFI) were associated with improved outcomes in patients treated
with immunotherapy (16). In liver
cancer, increased VAT and TAT were associated with improved
survival rates in patients treated with ICIs (17).
Regarding multiple cancer types, 3 studies presented
different results. Esposito et al (25) showed that neither subcutaneous fat
area (SFA), visceral fat area (VFA) or total fat area influenced
patient survival. However, a higher VFA/SFA ratio led to increased
OS in patients treated with ICIs. Martini et al (28) reported that increased SFI and
decreased IFI were associated with prolonged survival in patients
with cancer treated with immunotherapy. Crombé et al
(32) determined that changes in
the subcutaneous adipose tissue index from the first day of
patients' treatment to 2 months later was associated with survival,
while none of the baseline fat parameters were associated with PFS
in metastatic cancer patients treated with ICIs.
Discussion
Although obesity has been considered a significant
risk factor for developing several types of cancer, it appears to
have a contradictory protective effect in patients with cancer
treated with targeted therapy, chemotherapy and ICIs (8,39).
Thus, this ‘obesity paradox’ has propelled a reconsideration of
whether defining obesity by the BMI is correct. It is widely known
that obesity is characterized by large fat accumulation. Due to the
method of BMI calculation, it cannot correctly distinguish
different types of fat (visceral, subcutaneous, intermuscular or
intramuscular). Therefore, the present study investigated the
association between adiposity and clinical outcomes using the BMI
and fat indices in patients with cancer subjected to ICI
treatments.
The association between different BMI groups and the
OS or PFS of patients with cancer treated with ICIs was first
investigated. Through systematic literature screening, the current
meta-analysis included 15 eligible studies containing 8,310
patients aimed to assess the impact of the BMI on the efficacy of
ICIs. The results revealed that overweight and obese patients with
cancer treated with ICIs exhibited improved OS and PFS. According
to the weight characteristics of each population, the association
between different comparative models of BMI categories and survival
was examined in different countries. For instance, several Japanese
studies set the cutoff values for the BMI as 18.5 or 20
kg/m2, and it was found that a low BMI was a negative
predictive factor in patients with NSCLC or melanoma (40,41).
In a Chinese study, the cutoff value for the BMI was 24.0
kg/m2. This study showed that a high BMI was associated
with improved OS and PFS in patients treated with PD-1 inhibitors
(42). Furthermore, Wang et
al (43) observed a marked
improvement in the clinical outcomes of obese (BMI ≥30
kg/m2) vs. nonobese (BMI <30 kg/m2)
patients in a cohort of 250 US patients treated with PD-L1
inhibitors for a variety of cancer types. All of these studies
concluded that the BMI could be a predictive factor of
immunotherapy outcomes.
Besides the BMI classification, subgroup analyses
based on sex, cancer type, study region and type of ICI have also
assessed the efficacy of immunotherapy. Meta-analyses showed that a
high BMI was associated with a lower risk of mortality after ICI
treatment in multiple cancer types, including NSCLC and melanoma
(11,12). By contrast, no consistent results
were obtained from these meta-analyses regarding RCC (11-14).
When stratifying by sex, the results of the analysis conducted by
Xu et al (12) suggested
that male and female patients with a high BMI (≥25
kg/m2) who were treated with ICIs exhibited similar
survival. However, according to the findings of Chen et al
(11), an improvement in OS was
observed in male patients with a higher BMI. In addition, the study
revealed an association between the survival of patients and
treatment with anti-PD1/PD-L1 but not with anti-CTLA-4 therapy. No
association was observed between BMI and the survival of American
patients (11). The difference in
results may be attributed to the absence of a homogeneous cutoff
value for the BMI. Therefore, a more standard cutoff value
definition was required to stratify by the BMI and reduce
heterogeneity between studies. Subgroup analyses based on sex,
cancer type, study region and type of ICI should also be conducted
to evaluate the influence of the BMI on patient survival after
immunotherapy. If all the raw data from the included studies could
be obtained, it may be possible to set the optimal cutoff for the
BMI using statistical analysis, such as receiver operating
characteristic curve analysis.
Tumor heterogeneity has been recognized to be
associated with clinical outcomes for ICIs, such PD-L1 protein
expression, TILs, TMB and MSI (3).
The difference may affect the influence of the BMI on the prognosis
of patients with cancer treated with ICIs. In addition, different
treatment procedures and regimens for various cancer types may be
another factor affecting the relationship between the BMI and
cancer survival. Future additional studies are needed to explore
the effect of the BMI on the outcomes of different therapy methods
for patients with cancer.
The complex body composition cannot be accurately
reflected by the BMI alone. It has been reported that a subset of
obese patients (BMI ≥30 kg/m2) with a healthy
distribution of fat mass and normal inflammatory profile displayed
a decreased risk for diseases related to obesity, such as cancer
and cardiovascular diseases (44).
Another study showed no influence of the BMI on the outcomes of ICI
treatment in patients with RCC, while a high body fat mass was a
favorable factor for immunotherapy (15). Thus, the prognostic implication of
the bodily composition may be more important than that of the BMI
in obese patients with cancer treated with ICIs.
Fat composition measurement is mainly based on the
calculation of visceral and subcutaneous adipose tissues. Body fat
is typically measured via the visceral/subcutaneous adipose area
(cm2), area divided by height squared
(cm2/m2) or other methods. TAT is generally
considered the sum of subcutaneous and visceral adipose tissues.
The adipose area can be measured by surface area (cm2)
at the third lumbar landmark using a single cross-sectional CT
image (45). In the present study,
it was observed that the association of imaging-measured visceral,
subcutaneous and total adiposity with survival was not consistent
in patients with cancer receiving immunotherapy. For patients with
NSCLC, RCC and urothelial cancer, increased subcutaneous adiposity
was reported to be associated with improved survival (15,16,29,35).
Similarly, high visceral adiposity was also associated with an
increased survival rate in patients with NSCLC, melanoma, RCC,
liver cancer and urothelial cancer (15-17,35,37).
In addition, total adiposity was also a favorable factor in
patients with NSCLC, melanoma, RCC, breast cancer and liver cancer
(15,17,27,29,36).
These studies suggested that a high fat distribution may be a good
predictor of immunotherapy survival outcome. However, a
meta-analysis of the aforementioned studies was not performed due
to the inconsistent cutoff values in adipose metrics and the small
number of studies on certain cancer types.
The current study confirmed an association between
improved survival and high BMI or increased
subcutaneous/visceral/total adiposity in patients with cancer
receiving immunotherapy. A retrospective study on RCC found that
patients with a higher SFI, VFI or TFI showed improved survival,
while no influence of the BMI on survival outcomes of immunotherapy
was observed (15). Another study
on patients with HER2-positive metastatic breast cancer reported no
association between BMI and cancer patient survival, but found an
association between low SFI or TAFI and better outcomes (36). Based on the currently available
data, it may be speculated that the body fat distribution may be
strongly associated with the survival of patients with cancer
subjected to immunotherapy.
Recently, the underlying mechanisms behind the
positive association between obesity and immunotherapy have been
explored. Adipose tissue, as an endocrine organ, influences the
homeostasis of the immune system by releasing pro-inflammatory
hormones such as leptin, tumor necrosis factor (TNF)-α and
interleukin (IL)-6(46). A high
level of leptin in obese patients may result in increased
expression of PD-1 and dysfunction of CD8+ T cells,
which leads to a more pronounced response to immunotherapy
(43). In addition, it was
previously found that increased leptin secreted from adipose
tissues may cause upregulation of PD-1 receptors on T cells through
signal transduction and activator of transcription 3(47). Elevated PD-1 expression is
associated with increased T-cell exhaustion, which may explain why
targeted PD-1 therapy may improve survival outcomes in obese
populations (48). Obesity induces
chronic low-grade inflammation, which is accompanied by innate and
adaptive immune suppression and immune aging acceleration (49). For instance, obesity-associated
hyperinsulinemia reduced T regulatory cells, thus inhibiting IL-10
and TNF-α production via the AKT/mTOR signaling pathway (50). Natural killer cells, which are
responsible for innate immunity and anticancer functions, have been
shown to be impaired in obese patients (51). Furthermore, obesity may lead to an
imbalance in the ratio of M1/M2 macrophages, thus resulting in an
upregulation of M1 ‘pro-inflammatory’ macrophages and a
downregulation of M2 ‘anti-inflammatory’ macrophages (52). The above factors caused
exacerbation of the chronic inflammatory state. This evidence
suggests that alterations of the anti-tumor immune function in
obese patients may explain the favorable outcomes of cancer
immunotherapy.
Different adipose tissues have various regulatory
roles in the body's immune microenvironment and metabolism, which
may impact cancer survival. In the present study, one of the
articles included reported that increased VAT, but not SAT,
predicted favorable survival in patients with liver cancer treated
with ICIs (17). A possible
explanation for this is that visceral fat may increase a range of
inhibitory immune checkpoints on the surface of T cells, including
T-cell immunoglobulin and ITIM domain, adenosine A2a receptor,
PD-L2 and CD160, which may be beneficial for ICIs to control
anticancer immunity (53). A total
of 2 studies reviewed in the present study showed that subcutaneous
fat was significantly associated with survival, while there was no
association between visceral fat and survival (34,38).
A potential explanation is that high subcutaneous fat indicates a
better nutritional status and it resists the energy consumption
caused by tumors. Another reason may be that subcutaneous fat may
induce the expression of PD-1 on T cells by secreting leptin,
thereby improving the response to immunotherapy (43,54).
Further studies are needed to explore the mechanisms of different
types of body fat affecting the survival outcomes of
immunotherapy.
Several limitations in the current meta-analysis
need to be considered. First, certain confounding risk factors
across studies, such as age, sex, cancer type and immunotherapy
regimen may have increased the heterogeneity among studies.
Furthermore, the HR provided in certain studies was not available;
thus, these studies were excluded to improve the accuracy of the
results. In addition, since certain studies did not stratify the
BMI cutoff value according to the WHO, only studies categorizing
patients based on the BMI into three groups (normal, overweight and
obese) or into two groups (BMI <25 kg/m2 and BMI ≥25
kg/m2) were included, which may have resulted in certain
selection bias. Finally, due to the different parameters and
statistical methods, the association between body fat and survival
was not quantitatively determined by any meta-analysis.
In conclusion, the study of body fat composition as
a predictive marker in cancer immunotherapy is an area of
compelling interest. Clinical CT scans may provide precise
estimates of adipose tissue beyond the BMI for predicting the
effectiveness of immunotherapy. Identifying the accurate
quantification ability and cutoff values of different indicators of
adipose tissue is a challenging endeavor, but it is likely to
improve the current understanding of the effects of obesity on
cancer patient survival. Body composition evaluation is an
effective method for predicting the efficacy of cancer
immunotherapy. Defining the biological mechanisms linking obesity
and efficacy of immunotherapy will provide guidance for obese
patients receiving cancer immunotherapy.
Supplementary Material
Quality assessment of the included
studies using the Newcastle-Ottawa scale. «, the study satisfied
the item; -, the study did not satisfy the item; RE,
representativeness of the exposed; SNE, selection of the
non-exposed; AE, ascertainment of exposure; DON, demonstration that
outcome of interest was not present at start; SC, study controls
for age, sex; SCA, study controls for any additional factors
(chemoradiotherapy, curative resection and drug resistance); AO,
assessment of outcome; FUL, follow-up time >36 months; AFU,
adequacy of follow-up of cohorts.
Sensitivity analysis of the
association between BMI (<25 and ≥25 kg/m2 groups)
and survival in patients with cancer receiving immunotherapy. BMI,
body mass index; IV, inverse variance; PFS, progression-free
survival; OS, overall survival.
Sensitivity analysis of the
association between BMI (overweight, obese and normal groups) and
OS in patients with cancer receiving immunotherapy. BMI, body mass
index; IV, inverse variance; OS, overall survival.
Sensitivity analysis of the
association between BMI (overweight, obese and normal groups) and
PFS in patients with cancer receiving immunotherapy. BMI, body mass
index; IV, inverse variance; PFS, progression-free survival.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XYL, SF and CW performed data collection and
meta-analysis. HG and LQY completed the systematic review. XGS and
DPL contributed to the conception and design of the study. HG and
DPL checked and confirmed the authenticity of the raw data. All
authors revised the manuscript and read and approved the final
version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ribas A and Wolchok J: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gibney GT, Weiner LM and Atkins MB:
Predictive biomarkers for checkpoint inhibitor-based immunotherapy.
Lancet Oncol. 17:e542–e551. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Friedenreich CM, Ryder-Burbidge C and
McNeil J: Physical activity, obesity and sedentary behavior in
cancer etiology: Epidemiologic evidence and biologic mechanisms.
Mol Oncol. 15:790–800. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Avgerinos KI, Spyrou N, Mantzoros CS and
Dalamaga M: Obesity and cancer risk: Emerging biological mechanisms
and perspectives. Metabolism. 92:121–135. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Caan BJ, Feliciano EM and Kroenke CH: The
importance of body composition in explaining the overweight paradox
in cancer-counterpoint. Cancer Res. 78:1906–1912. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Labadie BW, Liu P, Bao R, Crist M,
Fernandes R, Ferreira L, Graupner S, Poklepovic AS, Duran I, Vareki
SM, et al: BMI, irAE, and gene expression signatures associate with
resistance to immune-checkpoint inhibition and outcomes in renal
cell carcinoma. J Transl Med. 17(386)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McQuade JL, Daniel CR, Hess KR, Mak C,
Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al:
Association of body-mass index and outcomes in patients with
metastatic melanoma treated with targeted therapy, immunotherapy,
or chemotherapy: A retrospective, multicohort analysis. Lancet
Oncol. 19:310–322. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yoo SK, Chowell D, Valero C, Morris LGT
and Chan TA: Outcomes among patients with or without obesity and
with cancer following treatment with immune checkpoint blockade.
JAMA Netw Open. 5(e220448)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Di Filippo Y, Dalle S, Mortier L, Dereure
O, Dalac S, Dutriaux C, Leccia MT, Legoupil D, Saiag P,
Brunet-Possenti F, et al: Relevance of body mass index as a
predictor of systemic therapy outcomes in metastatic melanoma:
Analysis of the MelBase French cohort data(✩). Ann Oncol.
32:542–551. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen H, Wang D, Zhong Q, Tao Y, Zhou Y and
Shi Y: Pretreatment body mass index and clinical outcomes in cancer
patients following immune checkpoint inhibitors: A systematic
review and meta-analysis. Cancer Immunol Immunother. 69:2413–2424.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu H, Cao D, He A and Ge W: The prognostic
role of obesity is independent of sex in cancer patients treated
with immune checkpoint inhibitors: A pooled analysis of 4090 cancer
patients. Int Immunopharmacol. 74(105745)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
An Y, Wu Z, Wang N, Yang Z, Li Y, Xu B and
Sun M: Association between body mass index and survival outcomes
for cancer patients treated with immune checkpoint inhibitors: A
systematic review and meta-analysis. J Transl Med.
18(235)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nie R, Chen GM, Wang Y, Yuan SQ, Zhou J,
Duan J, Liu WW, Chen S, Cai MY and Li YF: Association between body
mass index and survival outcomes in patients treated with immune
checkpoint inhibitors: Meta-analyses of individual patient data. J
Immunother Cancer. 44:371–375. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Martini DJ, Olsen TA, Goyal S, Liu Y,
Evans ST, Magod B, Brown JT, Yantorni L, Russler GA, Caulfield S,
et al: Body composition variables as radiographic biomarkers of
clinical outcomes in metastatic renal cell carcinoma patients
receiving immune checkpoint inhibitors. Front Oncol.
11(707050)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Martini DJ, Shabto JM, Goyal S, Liu Y,
Olsen TA, Evans ST, Magod BL, Ravindranathan D, Brown JT, Yantorni
L, et al: Body composition as an independent predictive and
prognostic biomarker in advanced urothelial carcinoma patients
treated with immune checkpoint inhibitors. Oncologist.
26:1017–1025. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xiao LS, Li RN, Cui H, Hong C, Huang CY,
Li QM, Hu CY, Dong ZY, Zhu HB and Liu L: Use of computed
tomography-derived body composition to determine the prognosis of
patients with primary liver cancer treated with immune checkpoint
inhibitors: a retrospective cohort study. BMC Cancer.
22(737)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kichenadasse G, Miners JO, Mangoni AA,
Rowland A, Hopkins AM and Sorich MJ: Association between body mass
index and overall survival with immune checkpoint inhibitor therapy
for advanced non-small cell lung cancer. JAMA Oncol. 6:512–518.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ahmed M, von Itzstein MS, Sheffield T,
Khan S, Fattah F, Park JY, Popat V, Saltarski JM, Gloria-McCutchen
Y, Hsiehchen D, et al: Association between body mass index, dosing
strategy, and efficacy of immune checkpoint inhibitors. J
Immunother Cancer. 9(e002349)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Collet L, Delrieu L, Bouhamama A, Crochet
H, Swalduz A, Nerot A, Marchal T, Chabaud S and Heudel PE:
Association between body mass index and survival outcome in
metastatic cancer patients treated by immunotherapy: Analysis of a
French retrospective cohort. Cancers (Basel).
13(2200)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cortellini A, Bersanelli M, Buti S,
Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di
Marino P, et al: A multicenter study of body mass index in cancer
patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors:
When overweight becomes favorable. J Immunother Cancer.
7(57)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
De Giorgi U, Procopio G, Giannarelli D,
Sabbatini R, Bearz A, Buti S, Basso U, Mitterer M, Ortega C, Bidoli
P, et al: Association of systemic inflammation index and body mass
index with survival in patients with renal cell cancer treated with
nivolumab. Clin Cancer Res. 25:3839–3846. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Donnelly D, Bajaj S, Yu J, Hsu M, Balar A,
Pavlick A, Weber J, Osman I and Zhong J: The complex relationship
between body mass index and response to immune checkpoint
inhibition in metastatic melanoma patients. J Immunother Cancer.
7(222)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Esposito A, Marra A, Bagnardi V, Frassoni
S, Morganti S, Viale G, Zagami P, Varano GM, Buccimazza G, Orsi F,
et al: Body mass index, adiposity and tumour infiltrating
lymphocytes as prognostic biomarkers in patients treated with
immunotherapy: A multi-parametric analysis. Eur J Cancer.
145:197–209. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tateishi A, Horinouchi H, Yoshida T,
Masuda K, Jo H, Shinno Y, Okuma Y, Goto Y, Yamamoto N and Ohe Y:
Correlation between body mass index and efficacy of anti-PD-1
inhibitor in patients with non-small cell lung cancer. Respir
Investig. 60:234–240. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Young AC, Quach HT, Song H, Davis EJ,
Moslehi JJ, Ye F, Williams GR and Johnson DB: Impact of body
composition on outcomes from anti-PD1 +/- anti-CTLA-4 treatment in
melanoma. J Immunother Cancer. 8(e000821)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Martini D, Kline M, Liu Y, Shabto J,
Williams M, Khan A, Lewis C, Collins H, Akce M, Kissick H, et al:
Adiposity may predict survival in patients with advanced stage
cancer treated with immunotherapy in phase 1 clinical trials.
Cancer. 126:575–582. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Popinat G, Cousse S, Goldfarb L, Becker S,
Gardin I, Salaün M, Thureau S, Vera P, Guisier F and Decazes P:
Sub-cutaneous Fat Mass measured on multislice computed tomography
of pretreatment PET/CT is a prognostic factor of stage IV non-small
cell lung cancer treated by nivolumab. Oncoimmunology.
8(e1580128)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Magri V, Gottfried T, Di Segni M, Urban D,
Peled M, Daher S, Stoff R, Bar J and Onn A: Correlation of body
composition by computerized tomography and metabolic parameters
with survival of nivolumab-treated lung cancer patients. Cancer
Manag Res. 11:8201–8207. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Minami S, Ihara S, Tanaka T and Komuta K:
Sarcopenia and visceral adiposity did not affect efficacy of
immune-checkpoint inhibitor monotherapy for pretreated patients
with advanced non-small cell lung cancer. World J Oncol. 11:9–22.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Crombe A, Kind M, Toulmonde M, Italiano A
and Cousin S: Impact of CT-based body composition parameters at
baseline, their early changes and response in metastatic cancer
patients treated with immune checkpoint inhibitors. Eur J Radiol.
133(109340)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Faron A, Opheys NS, Nowak S, Sprinkart AM,
Isaak A, Theis M, Mesropyan N, Endler C, Sirokay J, Pieper CC, et
al: Deep learning-based body composition analysis predicts outcome
in melanoma patients treated with immune checkpoint inhibitors.
Diagnostics (Basel). 11(2314)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Baldessari C, Pecchi A, Marcheselli R,
Guaitoli G, Bonacini R, Valoriani F, Torricelli P, Reverberi L,
Menozzi R, Pugliese G, et al: Body composition and inflammation
impact in non-small-cell lung cancer patients treated by first-line
immunotherapy. Immunotherapy. 13:1501–1519. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Degens J, Dingemans AC, Willemsen ACH,
Gietema HA, Hurkmans DP, Aerts JG, Hendriks LEL and Schols A: The
prognostic value of weight and body composition changes in patients
with non-small-cell lung cancer treated with nivolumab. J Cachexia
Sarcopenia Muscle. 12:657–664. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Palleschi M, Iamurri AP, Scarpi E,
Mariotti M, Maltoni R, Mannozzi F, Barone D, Paganelli G, Casi M,
Giampalma E, et al: Computed tomography based analyses of body mass
composition in HER2 positive metastatic breast cancer patients
undergoing first line treatment with pertuzumab and trastuzumab.
Sci Rep. 12(3385)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee JH, Hyung S, Lee J and Choi SH:
Visceral adiposity and systemic inflammation in the obesity paradox
in patients with unresectable or metastatic melanoma undergoing
immune checkpoint inhibitor therapy: A retrospective cohort study.
J Immunother Cancer. 10(e005226)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ged Y, Sanchez A, Patil S, Knezevic A,
Stein E, Petruzella S, Weiss K, Duzgol C, Chaim J, Akin O, et al:
Associations between pretreatment body composition features and
clinical outcomes among patients with metastatic clear cell renal
cell carcinoma treated with immune checkpoint blockade. Clin Cancer
Res. 28:5180–5189. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dahlberg SE, Schiller JH, Bonomi PB,
Sandler AB, Brahmer JR, Ramalingam SS and Johnson DH: Body mass
index and its association with clinical outcomes for advanced
non-small-cell lung cancer patients enrolled on Eastern cooperative
oncology group clinical trials. J Thorac Oncol. 8:1121–1127.
2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Katayama Y, Shimamoto T, Yamada T, Takeda
T, Yamada T, Shiotsu S, Chihara Y, Hiranuma O, Iwasaku M, Kaneko Y,
et al: Retrospective efficacy analysis of immune checkpoint
inhibitor rechallenge in patients with non-small cell lung cancer.
J Clin Med. 9(102)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kondo T, Nomura M, Otsuka A, Nonomura Y,
Kaku Y, Matsumoto S and Muto M: Predicting marker for early
progression in unresectable melanoma treated with nivolumab. Int J
Clin Oncol. 24:323–327. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Qi Y, Liao D, Fu X, Gao Q and Zhang Y:
Elevated platelet-to-lymphocyte corresponds with poor outcome in
patients with advanced cancer receiving anti-PD-1 therapy. Int
Immunopharmacol. 74(105707)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang Z, Aguilar EG, Luna JI, Dunai C,
Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, et
al: Paradoxical effects of obesity on T cell function during tumor
progression and PD-1 checkpoint blockade. Nat Med. 25:141–151.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kovarik M, Hronek M and Zadak Z:
Clinically relevant determinants of body composition, function and
nutritional status as mortality predictors in lung cancer patients.
Lung Cancer. 84:1–6. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mourtzakis M, Prado CM, Lieffers JR,
Reiman T, McCargar LJ and Baracos VE: A practical and precise
approach to quantification of body composition in cancer patients
using computed tomography images acquired during routine care. Appl
Physiol Nutr Metab. 33:997–1006. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mojibi Y, Seif F, Mojibi N, Aghamajidi A,
Mohsenzadegan M and Torang HA: Efficacy of immunotherapy in obese
patients with cancer. Immunopharmacol Immunotoxicol. 44:471–483.
2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bu LL, Yu GT, Wu L, Mao L, Deng WW, Liu
JF, Kulkarni AB, Zhang WF, Zhang L and Sun ZJ: STAT3 induces
immunosuppression by upregulating PD-1/PD-L1 in HNSCC. J Dent Res.
96:1027–1034. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Woodall MJ, Neumann S, Campbell K,
Pattison ST and Young SL: The effects of obesity on anti-cancer
immunity and cancer immunotherapy. Cancers (Basel).
12(1230)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Aguilar EG and Murphy WJ: Obesity induced
T cell dysfunction and implications for cancer immunotherapy. Curr
Opin Immunol. 51:181–186. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Han JM, Patterson SJ, Speck M, Ehses JA
and Levings MK: Insulin inhibits IL-10-mediated regulatory T cell
function: implications for obesity. J Immunol. 192:623–629.
2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bahr I, Jahn J, Zipprich A, Pahlow I,
Spielmann J and Kielstein H: Impaired natural killer cell subset
phenotypes in human obesity. Immunol Res. 66:234–244.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kraakman MJ, Murphy AJ, Jandeleit-Dahm K
and Kammoun HL: Macrophage polarization in obesity and type 2
diabetes: Weighing down our understanding of macrophage function?
Front Immunol. 5(470)2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Davern M, Bracken-Clarke D, Donlon NE,
Sheppard AD, O'Connell F, Heeran AB, Majcher K, Conroy MJ, Mylod E,
Butler C, et al: Visceral adipose tissue secretome from early and
late-stage oesophageal cancer patients differentially affects
effector and regulatory T cells. J Cancer Res Clin Oncol.
149:6583–6599. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Porter SA, Massaro JM, Hoffmann U, Vasan
RS, O'Donnel CJ and Fox CS: Abdominal subcutaneous adipose tissue:
A protective fat depot? Diabetes Care. 32:1068–1075.
2009.PubMed/NCBI View Article : Google Scholar
|