Soft tissue sarcoma (STS) is a rare and
heterogeneous tumour with an incidence of STS is fewer than 6 cases
per 100,000 people (1,2). The prognostic factors for STS are
well-known and include tumor size, grade, and age (3-5).
The standard treatment is surgical tumor resection with a wide
margin (6). This implies the
removal of the tumor in a single specimen with a rim of normal
tissue around it. Perioperative radiotherapy or chemotherapy may be
considered for patients with high-grade STS (6). Radiotherapy should be delivered at a
total dose of 50 Gy in 1.8-2 Gy fractions in the preoperative
setting. In the postoperative setting, doses of up to 66 Gy are
administered, depending on clinical presentation, such as age,
tumor site, and surgical margins (6). High-grade, large, and deep-seated STS
is considered high-risk, and perioperative chemotherapy using
doxorubicin and ifosfamide could be a treatment option for of STS
(7). However, even after radical
treatment of primary STS, as many as 50% of these patients
experience local recurrence or distant metastasis (8,9).
Patients receiving systemic chemotherapy for widely metastatic or
locally advanced diseases are unsuitable for surgery or
radiotherapy. Doxorubicin-based chemotherapy is commonly used as
first-line chemotherapy (9,10).
Pazopanib, trabectedin, and eribulin have been administered since
2012. However, the outcome for metastatic patients remains poor,
with a median reported overall survival of 14-20 months (9). Therefore, easy, well-known, and
low-cost markers may help to identify a high risk of tumor relapse.
Most physicians are familiar with C-reactive protein (CRP) as an
inflammatory marker. CRP level is a useful predictor of poor
survival in patients with several types of cancer. Herein, we aimed
to clarify the role of CRP level in predicting clinical outcomes in
patients with STS.
Virchow observed the infiltration of leucocytes in
malignant tissues and proposed the site of chronic inflammation as
the origin of cancer in 1863(11).
For the first time, they proposed a relationship between
inflammation and carcinogenesis. Some tumors develop at the site of
chronic inflammation, and some induce an inflammatory
microenvironment in the tumor (12). The inflammatory component is
present in the microenvironment of tumor cells, which contain white
blood cells, macrophages with cytokines, and chemokines as
principal mediators of inflammation. The inflammatory
microenvironment plays a critical role in tumor progression
(13,14). Lymphocytes are the most important
type of peripheral blood cells involved in cancer cells
proliferation, migration, and invasion (15,16).
Inflammatory cytokines and chemokines, such as IL-6 and tumor
necrosis factor (TNF), which are produced by tumor cells or
tumor-associated leucocytes and platelets, may contribute directly
to tumor progression (13,14). Because of chronic inflammation at
tumor sites, IL-6 is produced by various cell types, including
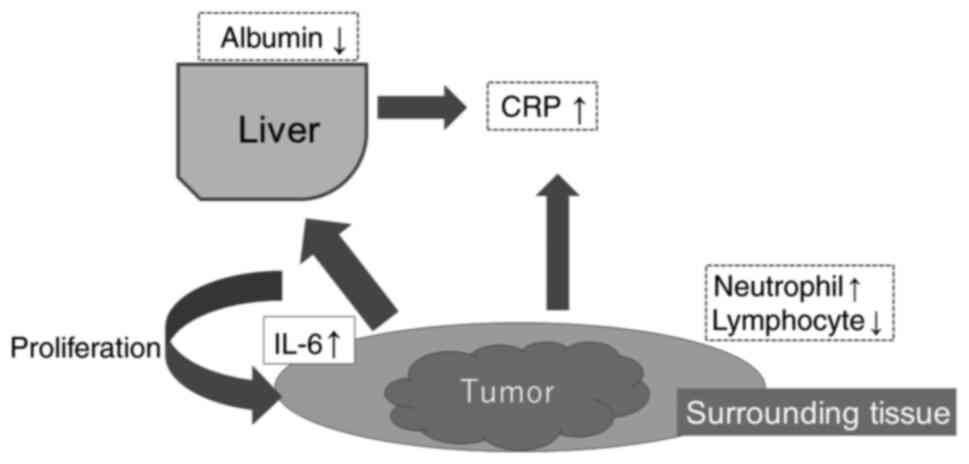
cancer cells and cancer-associated fibroblasts (17). IL-6 also induces CRP production in
hepatocytes (Fig. 1) (18). Nakamura et al (19) found a relationship between IL-6 and
IL-6 receptor (IL-6R) expression in tumor tissues and survival in
86 patients with STSs. Patients exhibiting high expression of both
IL-6 and IL-6R in tumors have poor survival. In contrast, patients
with low tumor expression of both IL-6 and IL-6R had better
survival. They also demonstrated the relationship between serum
IL-6 and CRP levels and the expression of IL-6 in tissues. Fu et
al (20) reported that
positive expression of IL-6 and IL-6R in renal cell cancer was
significantly associated with poor survival in multivariate
analysis. The circumstances around the tumor may reflect systemic
inflammatory conditions. Hagi et al (21) found that serum IL-6 levels could be
useful for differentiating benign soft tissue tumors from STS in 99
patients. Serum IL-6 levels (median: 9.04 pg/ml) in 59 patients
with STS were statistically higher than those (3.31 pg/ml) in 40
patients with benign soft tissue tumors. CRP, hemoglobin levels,
and tumor grade were strongly correlated with serum IL-6 levels. In
the multivariate analysis, they also found that serum IL-6 levels
were associated with tumor-related death in 59 patients. Rutkowski
et al (22) showed that
increased serum levels of IL-6 were observed in 61% of STS
patients. Serum IL-6 levels are correlated with tumor size and
grade (22). The production of CRP
in hepatocytes is primarily induced at the transcriptional level
following the elevation of circulating IL-6. In renal and
esophageal cancers, the immunohistochemical expression of CRP in
tumor samples was a prognostic indicator. Cancer cells may increase
the production of inflammatory proteins, which may explain their
high CRP levels (23,24). However, there are no reports of STS
cells.
Preoperative elevated CRP levels are strongly
associated with oncological events and poor survival in many types
of cancers, such as renal cell, colorectal, lung, gastrointestinal,
prostate, and esophageal cancer (31-37).
In 2012, Nakamura et al (38) reported the relationship between CRP
and STS and first showed the predictive value of CRP for event-free
survival (EFS) in a multivariate analysis. In total, 102 Japanese
patients with primary STS were included in this study. Normal CRP
levels at the hospital were < 0.3 mg/dl. Fourteen (32%) of the
44 patients with grade 3 STS, according to the French Federation of
Cancer Centers Sarcoma Group (FNCLCC) grading system (39), had elevated CRP levels. Nakamura
et al (40) investigated
332 UK patients with high-grade (FNCLCC grades 2 and 3) STS, and
45.8% of the patients had elevated CRP levels. The normal serum CRP
level was < 10 mg/l. CRP elevation was associated with a larger
tumor size and advanced clinical stage. In multivariate analysis,
they first reported that pre-treatment CRP levels were a poor
prognostic factor for disease-specific survival (DSS) and local
control in patients with STS. In the last 10 years (38,40-47),
the value of CRP for predicting clinical outcomes has been
supported by several studies (Table
I).
CRP elevation is an independent predictor of
survival, EFS, and local recurrence-free survival in STS patients.
The cut-off level varied from 0.14 to 1.0 mg/dl (10 mg/l). Many
studies included all types of STS histology, but two studies
included only one histology. Panotopoulos et al (43) analyzed 85 Austrian patients with
liposarcoma (LPS). Patients with other sub-histologies (e.g.
de-differentiated LPS) had more than triple the mean CRP level than
patients with well-differentiated LPS (1.58 vs. 0.55 mg/dl,
P=0.005). This study identified preoperative CRP (cut-off
value=0.87 mg/dl) and alkaline phosphatase (ALP) levels as novel
independent predictors of DSS in patients with LPS. Sambri et
al (44) included 126 Italian
patients with high-grade myxofibrosarcoma (MFS). In multivariate
analysis, tumor size and grade, preoperative CRP values (cut-off
value=0.5 mg/dl) and neutrophil-to-lymphocyte ratio (NLR, cut-off
value=3.5) were confirmed to be independent factors for predicting
DSS. Yanagisawa retrospectively compared the relationship between
CRP levels and survival in patients with and without neoadjuvant
radiotherapy (45). They measured
CRP levels before upfront surgery and neoadjuvant radiotherapy in
49 Japanese patients with STS. Neoadjuvant radiotherapy is
associated with increased CRP levels. However, there was no
difference in overall survival (OS) between high (>0.5 mg/dl)
and low CRP levels among 49 patients receiving neoadjuvant
radiotherapy. In multivariate analysis, CRP was an independent
predictor of OS in 49 patients who underwent upfront surgery, while
CRP was not associated with survival in 49 patients receiving
neoadjuvant radiotherapy. They hypothesized that neoadjuvant
radiotherapy might impact the inflammatory microenvironment around
tumor cells differently than upfront surgery and alter the
interaction of inflammatory markers with the outcome (45,48,49).
Although many studies have evaluated CRP levels before initial
treatment, Sato et al (46)
evaluated CRP levels before treatment with pazopanib in patients
with advanced STS. They analyzed prospectively collected data from
141 Japanese patients with recurrent or metastatic non-round cell
STS who began pazopanib treatment. Multivariate analysis indicated
that pre-treatment NLR (cut off value=3.0), LPS histology, primary
extremity site, Eastern Cooperative Oncology Group (ECOG)
performance status and CRP levels (cut off value=0.3 mg/dl) were
independent predictors of predicting OS. More than half of the
patients (52%) had elevated CRP levels. Nakamura et al
(47) also observed elevated CRP
levels in 20 (42.6%) of 47 patients with metastasis at initial
presentation, indicating that CRP was related to tumor
aggressiveness and progression. In summary, CRP may be a useful
maker for predicting oncological outcome in STS. However, as a
limitation, the heterogeneity of histology and treatment were
included in previous studies. Further studies should be necessary
as prospective studies for evaluating the validation.
Some studies have demonstrated the utility of a
combination of CRP levels and other serum markers (Table II). The Glasgow prognostic score
(GPS), modified GPS (mGPS), and high-sensitivity mGPS (HS-mGPS)
have been shown to predict oncological outcomes in several types of
cancers, including STS (50-52).
A combination of CRP levels and hypoalbuminemia was applied to
these scoring systems. Albumin is the most abundant circulatory
protein, and serum albumin levels vary according to the degree of
catabolism during normal homeostasis and in the presence of disease
(53). Various causes of
hypoalbuminemia have been described in patients with cancer. The
most important cause is increased catabolism and following cachexia
(54). Since 2015, GPS, mGPS, and
HS-mGPS have been shown to provide additional prognostic
information in patients with STS (55-62).
Appropriate GPS may depend on the type of cancer. Recently, Spence
et al (55) reported 493
STS patients using clinical databases from six collaborating
hospitals in three countries. Multivariant Cox regression analysis
demonstrated an elevated mGPS was significantly associated with
reduced overall survival (HR 1.8 (95% CI 1.1 to 2.9); P=0.007).
Therefore, mGPS may be an appropriate tool for predicting survival
in STS. Further studies using GPS and HS-mGPS must be considered in
multicenter or international institutions.
Other studies reported the utility of a combination
of CRP level and absolute lymphocyte count (ALC) in patients with
STS (63,64). Peripheral lymphocytes play a
critical role in host cell-mediated cytotoxic immunity against
tumors by inducing cytotoxic cell death and inhibiting tumor cell
proliferation and migration. High lymphocytic infiltration into the
tumor stroma has been reported to be associated with better
survival and superior response to systemic therapy (65). The combination of CRP levels and
ALC is considered a surrogate marker of immunity and inflammation
in patients with cancer. The lymphocyte-CRP ratio (LCR), the
reciprocal of CLR, has been reported as a poor prognostic marker in
several types of cancer (66-68).
In 2022, two studies were published in the field of STS using LCR
or CLR (63,64). Matsui et al (64) reviewed 113 patients with
retroperitoneal STS. Multivariate analysis showed that elevated CLR
and de-differentiated LPS were associated with poor overall
survival in all retroperitoneal STS cases (64). Interestingly, in de-differentiated
LPS, patients with high preoperative CLR, whose postoperative CLR
was normalized, demonstrated a favorable survival rate similar to
those with low preoperative CLR. Nakamura et al (63) analyzed 132 patients with STS and
found that LCR might be a prognostic factor for predicting
oncological events. However, on Receiver operating characteristic
analysis, there was no significant difference in predicting DSS in
the area under the curve (AUC) between CRP level and LCR. However,
the utility of LCR or CLR for predicting survival in STS were not
validated in multicenter international studies. Finally, Nakamura
et al (69) confirmed
whether the combined use of CRP level and NLR before treatment
predicted DSS in adult patients with STS. In addition to the role
of lymphocytes in the tumor microenvironment (65), neutrophils release mediators to
provide a stimulating microenvironment that allows for more
aggressive tumor behavior by sustaining cell proliferation and
facilitating genomic instability (70). Therefore, NLR has also been
reported to be a prognostic factor for predicting survival in
cancer patients, including STS (55,71,72).
Especially, Spence et al (55) also analyzed 493 STS patients from
six collaborating hospitals in three countries and showed an
elevated NLR (>4) was significantly associated with reduced
overall survival (HR 1.5 (95% CI 1.0 to 2.3); P=0.029) in
multivariate analysis. Although there is no definitive ratio of NLR
for predicting survival, the subgroup of patients with a high NLR
and elevated CRP level is at high risk of oncological events and
may represent a study population for a new adjuvant therapy trial
in the future.
We reviewed the role of CRP in STS. CRP is a
surrogate marker of the cancer-related inflammation. CRP and its
combined use may be useful tools for predicting oncological
outcomes. A new aggressive strategy is necessary to improve future
outcomes in patients with elevated CRP levels.
Not applicable.
Funding: No funding was received.
All data generated or analyzed during this study are
included in this published article.
TN conceived and designed the study. TN, TH and KA
acquired data. TN, TH, KA and AS analyzed and interpreted the data.
TN drafted the manuscript. TH created tables and figures. KA and AS
edited and reviewed the manuscript. AS was responsible for funding
acquisition. Data authentication is not applicable. All authors
have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bourcier K, Le Cense A, Tselikas L, Adam
J, Mir L, Honore C and de Baere T: Basic knowledge in soft tissue
sarcoma. Cardiovasc Intervent Radiol. 42:1255–1261. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pisters PW, Leung DH, Woodruff J, Shi W
and Brennan MF: Analysis of prognostic factors in 1,041 patients
with localized soft tissue sarcomas of the extremities. J Clin
Oncol. 14:1679–1689. 1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Callegaro D, Miceli R, Bonvalot S,
Ferguson P, Strauss DC, Levy A, Griffin A, Hayes AJ, Stacchiotti S,
Pechoux CL, et al: Development and external validation of two
nomograms to predict overall survival and occurrence of distant
metastases in adults after surgical resection of localised
soft-tissue sarcomas of the extremities: A retrospective analysis.
Lancet Oncol. 17:671–680. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
De Sanctis R, Zelic R and Santoro A:
Nomograms predicting local and distant recurrence and
diease-specific mortality for R0/R1 soft tissue sarcomas of the
extremities. Front Oncol. 12(941896)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gronchi A, Miah AB, Dei Tos AP, Abecassis
N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, et
al: ESMO guidelines committee, EURACAN and GENTURIS. Soft tissue
and visceral sarcomas: ESMO-EURACAN-GENTURIS clinical practice
guidelines for diagnosis, treatment, and follow-up. Ann Oncol.
32:1348–1365. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tanaka K, Mizusawa J, Naka N, Kawai A,
Katagiri H, Hiruma T, Matsumoto Y, Tsuchiya H, Nakayama R, Hatano
H, et al: Ten-year follow-up results of perioperative chemotherapy
with doxorubicin and ifosfamide for high-grade soft tissue sarcoma
of the extremities: Japan clinical oncology group study of
JCOG0304. BMC Cancer. 19(890)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nakamura T, Asanuma K, Takao M, Yamanaka
T, Koike H, Chen-Yoshikawa TF, Tsukushi S, Kuroda H, Kozawa E, Sano
M, et al: Clinical outcome in soft tissue sarcoma patients with
lung metastasis who received metastasectomy and/or radiofrequency
ablation: Tokai Musculoskeletal oncology consortium study. Cancer
Manag Res. 13:8473–8480. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kawamoto T, Hara H, Morishita M, Fukase N,
Kawakami Y, Takemori T, Fujiwara S, Kitayama K, Yahiro S, Miyamoto
T, et al: Prognostic influence of the treatment approach for
pulmonary metastasis in patients with soft tissue sarcoma. Clin Exp
Metastasis. 37:509–517. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Smrke A, Wang Y and Simmons C: Update on
systemic therapy for advanced soft-tissue sarcoma. Curr Oncol.
27:25–33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Colotta JF, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hart PC, Rajab IM, Alebraheem M and
Potempa LA: C-reactive protein and cancer-diagnostic and
therapeutic insights. Front Immunol. 11(595835)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu ES, Oduyebo T, Cobb LP, Cholakian D,
Kong X, Fader AN, Levinson KL, Tanner EJ III, Stone RL, Piotrowski
A, et al: Lymphopenia and its association with survival in patients
with locally advanced cervical cancer. Gynecol Oncol. 140:76–82.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matsuyama Y, Nakamura T, Yoshida K,
Nakamura K, Hagi T, Asanuma K and Sudo A: Role the prognostic
nutritional index in patients with soft tissue sarcoma. In Vivo.
35:2349–2355. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kitamura H, Ohno Y, Toyoshima Y, Ohtake J,
Homma S, Kawamura H, Takahashi N and Taketomi A:
Interleukin-6/STAT3 signaling as a promising target to improve the
efficacy of cancer immunotherapy. Cancer Sci. 108:1947–1952.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Castell JV, Gómez-Lechón MJ, David M,
Fabra R, Trullenque R and Heinrich PC: Acute-phase response of
human hepatocytes; regulation of acute-phase protein synthesis by
interleukin-6. Hepatology. 12:1179–1186. 1990.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nakamura K, Nakamura T, Iino T, Hagi T,
Kita K, Asanuma K and Sudo A: Expression of interleukin-6 and the
interleukin-6 receptor predicts the clinical outcome of patients
with soft tissue sarcomas. Cancers (Basel). 12(585)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fu Q, Chang Y, An H, Fu H, Zhu Y, Xu L,
Zhang W and Xu J: Prognostic value of interleukin-6 and
interleukin-6 receptor in organ-confined clear-cell renal cell
carcinoma: A 5-year conditional cancer-specific survival analysis.
Br J Cancer. 113:1581–1589. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hagi T, Nakamura T, Iino T, Matsubara T,
Asanuma K, Matsumine A and Sudo A: The diagnostic and prognostic
value of interleukin-6 in patients with soft tissue sarcomas. Sci
Rep. 7(9640)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rutkowski P, Kaminska J, Kowalska M, Ruka
W and Steffen J: Cytokine serum levels in soft tissue sarcoma
patients: Correlations with clinic-pathological features and
prognosis. Int J Cancer. 100:463–471. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Can C, Acikalin MF, Ozen A and Dundar E:
Prognostic impact of intratumoral C-reactive protein expression in
patients with clear cell renal cell carcinoma. Urol Int.
92:270–275. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nazoe T, Korenaga D, Futatsugi M, Saeki H,
Maehara Y and Sugimachi K: Immunohistochemical expression of
C-reactive protein in squamous cell carcinoma of the
esophagus-significance as a tumor maker. Cancer Lett. 192:89–95.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bruno F, Arrigoni F, Mariani S, Splendiani
A, Cesare ED, Masciocchi C and Barile A: Advanced magnetic
resonance imaging (MRI) of soft tissue tumors: Techniques and
applications. Radiol Med. 124:243–252. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Costa FM, Martins PH, Canella C and Lopes
FPPL: Mutliparametric MR imaging of soft tissue tumors and
psudotumors. Magn Reson Imaging Clin N Am. 26:543–558.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Beaman FD, Jelinek JS and Priebat DA:
Current imaging and therapy of malignant soft tissue tumors and
tumor-like lesions. Semin Musculoskelet Radiol. 17:168–176.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakamura T, Matsumine A, Iino T, Matsubara
T, Asanuma K, Uchida A and Sudo A: Role of high- sensitivity
C-reactive protein in the differentiation of benign and malignant
soft tissue tumors. Anticancer Res. 34:933–936. 2014.PubMed/NCBI
|
|
29
|
Ariizumi T, Kawashima H, Ogose A, Sasaki
T, Hotta T, Hatano H, Morita T and Endo N: The diagnostic and
prognostic value of hematological and chemical abnormalities in
soft tissue sarcomas: A comparative study in patients with benign
and malignant soft tissue tumors. Ann Clin Lab Sci. 48:11–17.
2018.PubMed/NCBI
|
|
30
|
Fujibuchi T, Miyawaki J, Kidani T, Imai H
and Miura H: Prediction of soft tissue sarcoma from clinical
characteristics and laboratory data. Cancers (Basel).
12(679)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yano Y, Ohno T, Komura K, Fukuokaya W,
Uchimoto T, Adachi T, Hirasawa Y, Hashimoto T, Yoshizawa A,
Yamazaki S, et al: Serum C-reactive protein level predicts overall
survival for clear cell and non clear cell renal cell carcinoma
treated with lpilimumab plus nivolmab. Cancers (Basel).
14(5659)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Patel SH, Derweesh IH, Saito K, Patil D,
Meagher MF, Bindayi A, Eldefrawy A, Patel DN, Nasseri R, Yasuda Y,
et al: Preoperative elevation of C-reactive protein is a predictor
for adverse oncologic survival outcomes for renal cell carcinoma:
Analysis from the international market consortium renal cancer
(INMARC). Clin Genitourin Cancer. 19:e206–e215. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ghuman S, Van Hemelrijck M, Garmo H,
Holmberg L, Malmström H, Lambe M, Hammar N, Walldius G, Jungner I
and Wulaningsih W: Serum inflammatory markers and colorectal cancer
risk and survival. Br J Cancer. 116:1358–1365. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Okada S, Shimomura M, Tsunezuka H,
Teramukai S, Ishihara S, Shimada J and Inoue M: Prognostic
significance of preoperative C-reactive protein in resected
non-small cell lung cancer. Semin Thorac Cardiovasc Surg.
32:1046–1055. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lu J, Xu BB, Xue Z, Xie JW, Zheng CH,
Huang CM and Li P: Prioperative CRP: A novel inflammation-based
classification in gastric cancer for recurrence and chemotherapy
benefit. Cancer Med. 10:34–44. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liao DW, Hu X, Wang Y, Yang ZQ and Li X:
C-reactive protein is a predictor of prognosis of prostate cancer:
A systematic review and meta-analysis. Ann Clin Lab Sci.
50:161–171. 2020.PubMed/NCBI
|
|
37
|
Ishibashi Y, Tsujimoto H, Hiraki S, Kumano
I, Yaguchi Y, Horiguchi H, Nomura S, Ito N, Shinto E, Aosasa S, et
al: Prognostic value of preoperative systemic inflammatory measures
in patients with esophageal cancer. Ann Surg Oncol. 25:3288–3299.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nakamura T, Matsumine A, Matsubara T,
Asanuma K, Uchida A and Sudo A: Clinical significance of
pretreatment C-reactive protein level in soft tissue sarcoma.
Cancer. 118:1055–1061. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guillou L, Coindre JM, Bonichon F, Nguyen
BB, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V,
Leroux A, et al: Comparative study of the national cancer institute
and french federation of cancer centers sarcoma group grading
systems in a population of 410 adult patients with soft tissue
sarcoma. J Clin Oncol. 15:350–362. 1997.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nakamura T, Grimer R, Gaston C, Francis M,
Charman J, Graunt P, Uchida A, Sudo A and Jeys L: The value of
C-reactive protein and comorbidity in predicting survival of
patients with high grade soft tissue sarcoma. Eur J Cancer.
49:377–385. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Szkandera J, Gerger A, Liegl-Atzwanger B,
Absenger G, Stotz M, Samonigg H, Maurer-Ertl W, Stojakovic T,
Ploner F, Leithner A and Pichler M: Validation of the prognostic
relevance of C-reactive protein levels in soft tissue sarcoma
patients. Br J Cancer. 109:2316–2322. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Choi ES, Kim HS and Han I: Elevated
preoperative systemic inflammatory markers predict poor outcome in
localized soft tissue sarcoma. Ann Surg Oncol. 21:778–785.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Panotopoulos J, Posch F, Alici B, Funovics
P, Stihsen C, Amann G, Brodowicz T, Windhager R and Ay C:
Hemoglobin, alkalic phoshatase, and C-reactive protein predict the
outcome in patients with liposarcoma. J Orthop Res. 33:765–770.
2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sambri A, Zucchini R, Giannini C, Cevolani
L, Fiore M, Spinnato P, Bianchi G, Donati DM and De Paolis M:
Syetemic inflammation is associated with oncological outcome in
patients with high-grade myxofibrosarcoma of the extremities: A
retrospective analysis. Oncol Res Treat. 43:531–538.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yanagisawa M, Gingrich AA, Judge S, Li CS,
Wang N, Thorpe SW, Kirane AR, Bold RJ, Monjazeb AM and Canter RJ:
Serum C-reactive protein and neutrophil/lymphocyte ratio after
neoadjuvant radiotherapy in soft tissue sarcoma. Anticancer Res.
38:1491–1497. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sato Y, Nakano K, Wang X, Fukuda N,
Urasaki T, Ohmoto A, Hayashi N, Yunokawa M, Ono M, Tomomatsu J, et
al: Pre-treatment neutrophil-to-lymphocyte ratio (NLR) as a
predictive marker of pazopanib treatment for soft-tissue sarcoma.
Cancers (Basel). 13(6266)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nakamura T, Katagiri H, Shido Y, Hamada S,
Yamada K, Nagano A, Yamada S, Tsukushi S, Ishimura D, Matsumine A,
et al: Analysis of factors for predicting survival in soft-tissue
sarcoma with metastatic disease at initial presentation. Anticancer
Res. 37:3137–3141. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Galon J, Angell HK, Bedognetti D and
Marincola FM: The continuum of cancer immunosurveillance:
Prognostic, predictive, and mechanistic signatures. Immunity.
39:11–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Demaria S and Formenti SC: Radiation as an
immunological adjuvant: Current evidence on dose and fractionation.
Front Oncol. 2(153)2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
McMillan DC, Crozier JE, Canna K, Angerson
WJ and McArdle CS: Evaluation of an inflammation-based prognostic
score (GPS) in patients undergoing resection for colon and rectal
cancer. Int J Colorectal Dis. 22:881–886. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wu TH, Tsai YT, Chen KY, Yap WK and Luan
CW: Utility of high-sensitivity modified Glasgow prognostic score
in cancer prognosis: A systematic review and meta-analysis. Int J
Mol Sci. 24(1318)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kimura S, D’ Andrea D, Soria F, Foerster
B, Abufaraj M, Vartolomei MD, Iwata T, Karakiewicz PI, Rink M, Gust
KM, et al: Prognostic value of modified Glasgow prognostic score in
non-muscle-invasive bladder cancer. Urol Oncol. 37:179.e19–179.e28.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Levitt DG and Levitt MD: Human serum
albumin homeostasis: a new look at the roles of synthesis,
catabolism, renal and gastrointestinal excretion, and the clinical
value of serum albumin measurements. Int J Gen Med. 9:229–255.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gatta A, Verardo A and Bolognesi M:
Hypoalbuminemia. Intern Emerg Med. 7 (Suppl 3):S193–S199.
2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Spence S, Doonan J, Farhan-Alanie OM, Chan
CD, Tong D, Cho HS, Sahu MA, Traub F and Gupta S: The MPGS Study
Group. Does the modified Glasgow prognostic score aid in the
management of patients undergoing surgery for a soft-tissue
sarcoma?: An international multicentre study. Bone Joint J.
104-B:168–176. 2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Nakamura T, Matsumine A, Asanuma K,
Matsubara T and Sudo A: The value of the high-sensitivity modified
Glasgow prognostic score in predicting the survival of patients
with a soft-tissue sarcoma. Bone Joint J. 97-B:847–852.
2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Tsuda Y, Ogura K, Kobayashi E, Hiruma T,
Iwata S, Asano N, Kawai A, Chuman H, Ishii T, Morioka H, et al:
Impact of geriatric factors on surgical and prognostic outcomes in
elderly patients with soft-tissue sarcoma. Jpn J Clin Oncol.
47:422–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Jiang SS, Jiang L, Weng DS, Li YF, Pan QZ,
Zhao JJ, Tang Y, Zhou ZW and Xia JC: Immunization-based scores as
independent prognostic predictors in soft tissue sarcoma patients.
J Cancer. 8:606–616. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Aggerholm-Pedersen N, Maretty-Kongstad K,
Keller J and Safwat A: Serum biomarkers as prognostic factors for
metastatic sarcoma. Clin Oncol (R Coll Radiol). 31:242–249.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hou T, Guo T, Nie R, Hong D, Zhou Z, Zhang
X and Liang Y: The prognostic role of the preoperative systemic
immune-inflammation index and high-sensitivity modified Glasgow
prognostic score in patients after radical operation for soft
tissue sarcoma. Eur J Surg Oncol. 46:1496–1502. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Mahyudin F, Edward M, Basuki MH, Basrewan
Y, Hernugrahanto KD and Wahyudiputra AG: Analysis of prognostic
factors in soft tissue sarcoma: Cancer registry from a single
tertiary hospital in Indonesia. Ann Med Surg (Lond). 57:257–263.
2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Nakamura T, Asanuma K, Hagi T and Sudo A:
Modified Glasgow prognostic score is better for predicting
oncological outcome in patients with soft tissue sarcoma, compared
to High-sensitivity modified Glasgow prognostic score. J Inflamm
Res. 15:3891–3899. 2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Nakamura T, Hagi T, Asanuma K and Sudo A:
Is lymphocyte C-reactive protein ratio useful for predicting
survival in patients with non-metastatic soft tissue sarcoma?
Cancers (Basel). 14(5214)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Matsui Y, Matsuda A, Maejima A, Shinoda Y,
Nakamura E, Komiyama M and Fujimoto H: The clinical significance of
perioperative inflammatory index as a prognostic factor for
patients with retroperitoneal soft tissue sarcoma. Int J Clin
Oncol. 27:1093–1100. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Fontana R, Bregni M, Cipponi A, Raccosta
L, Rainelli C, Maggioni D, Lunghi F, Ciceri FL, Mukenge S, Doglioni
C, et al: Peripheral blood lymphocytes genetically modified to
express the self/tumor antigen mage-a3 induce antitumor immune
responses in cancer patients. Blood. 113:1651–1660. 2009.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Nishi M, Shimada M, Tokunaga T,
Higashijima J, Yoshikawa K, Kashihara H, Takasu C, Ishikawa D, Wada
Y, Eto S and Yoshimoto T: Lymphocyte to C-reactive protein ratio
predicts long-term outcomes for patients with lower rectal cancer.
World J Surg Oncol. 19(201)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Yugawa K, Maeda T, Kinjo N, Kawata K,
Ikeda S, Edahiro K, Edagawa M, Omine T, Kometani T, Yamaguchi S, et
al: Prognostic impact of lymphocyte-C-reactive protein ratio in
patients who underwent surgical resection for hepatocellular
carcinoma. J Gastrointest Surg. 26:104–112. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Okugawa Y, Toiyama Y, Yamamoto A,
Shigemori T, Ichikawa T, Yin C, Suzuki A, Fujikawa H, Yasuda H,
Hiro J, et al: Lymphocyte to C-reactive protein ratio and score are
clinically feasible nutrition-inflammation markers of outcome in
patients with gastric cancer. Clin Nutr. 39:1209–1217.
2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Nakamura T, Matsumine A, Matsubara T,
Asanuma K, Uchida A and Sudo A: The combined use of the
neutrophil-lymphocyte ratio and C-reactive protein level as
prognostic predictors in adult patients with soft tissue sarcoma. J
Surg Oncol. 108:481–485. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Powell DR and Huttenlocher A: Neutrophils
in the tumor microenvironment. Trends Immunol. 37:41–52.
2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Idowu OK, Ding Q, Taktak AFG, Chandrasekar
CR and Yin Q: Clinical implication of pretreatment nuetrophil to
lymphocyte ratio in soft tissue sarcoma. Biomarkers. 17:539–544.
2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Cupp MA, Cariolou M, Tzoulaki I, Aune D,
Evangelou E and Berlanga-Taylor AJ: nuetrophil to lymphocyte ratio
and cancer prognosis: An umbrella review of systematic reviews and
meta-analyses of observational studies. BMC Med.
18(360)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hagi T, Nakamura T, Kita K, Iino T,
Asanuma K and Sudo A: Anti-tumour effect of tocilizumab for
osteosarcoma cell lines. Bone Joint Res. 9:821–826. 2020.PubMed/NCBI View Article : Google Scholar
|