1. Introduction
Colorectal cancer (CRC) which comprises cancer of
the colon and/or rectum, is the third most common cancer worldwide,
with more than 1.93 million new cases and 935,173 deaths reported
globally in the year 2020(1). CRC
ranks third in cancer-associated mortality in both males and
females worldwide, and its incidence has uniquely increased in
various high-income countries, including the United States,
Germany, the United Kingdom, Australia, and Canada (2,3).
In the mid-1990s, screening for CRC begun among
average-risk individuals across the population for all adults who
were 50 years of age and above. Since then, the incidence and
subsequently the mortality for ‘late onset CRC (loCRC)’ among
individuals 50 years or older have been decreasing (4). On the contrary, the incidence of CRC
in young adults under 50 years old, defined as early-onset CRC has
dramatically increased since the mid-1990s (2). In this article, the term ‘late-onset
CRC (loCRC)’ will refer to individuals 50 years or older, and
‘early-onset CRC (eoCRC)’ will refer to patients diagnosed with CRC
before the age of 50. Currently, the largest percentage of people
suffering from eoCRC falls within the 40-49 age group, accounting
for approximately one in eight new cases of CRC (5) and comprising an overall 10-12% of
total CRC diagnoses (6).
CRC develops when stem cells resigning at the base
of the colon crypts undergo genetic and epigenetic modifications
which affect oncogenes and tumour suppressor genes, leading to the
transformation of normal stem cells to cancerous stem cells
(7). Through multiple rounds of
clonal expansion of selected cells, colon cells undergo malignant
transformation, resulting in loss of genomic stability (8).
The majority of CRC cases occur sporadically (94%)
with main precursors involving an adenoma or serrated lesion, while
the minority (5%) are the result of inherited predisposition
syndromes, including the Lynch Syndrome and the Familial
Adenomatous Polyposis (FAP) syndromes, and with only 1% being
attributed to chronic inflammatory conditions (9).
Colon tumours are located in the proximal colon,
encompassing the cecum and the ascending and transverse colon, and
in the distal colon, encompassing the descending and sigmoid colon.
Rectal tumours are located in the rectosigmoid junction and rectum.
There is variation in the anatomical dispersion of CRC due to age
and sex; for example, the frequency of cancer diagnosis in the
distal colon and rectum is higher in the younger population group
(adults between 18-49) compared to the frequency reported in the
older population group (adults older than 50 years) (10).
The younger individuals suffering from eoCRC have
differences both in their clinical presentation as well as their
tumour histology when compared to older individuals suffering from
loCRC (11). A higher recurrence
of advanced disease and more invasive features are reported in
younger individuals, signifying the urgent need to understand the
possible aetiologies behind these trends (12,13).
Patients under the age of 50 commonly present with a later stage of
cancer due to a delay in diagnosis (14-16).
One study has supported that the time lapse from the initiation of
symptoms to consultation with a primary care provider may be 6-fold
longer in younger patients than in older patients (17). Young adults may also have greater
odds of a second primary malignancy in the initial 6-11 months
after a CRC diagnosis, compared to older patients who have reduced
rates, possibly due to specific risk factors contributing to the
pathogenesis of eoCRC (18). In
addition to age and family history of CRC, well-documented
modifiable risk factors of eoCRC include obesity, type II diabetes,
unhealthy diet, physical inactivity, and frequent antibiotic use
(19).
Colonoscopy represents a truly preventative CRC
screening measure since it detects adenocarcinoma formation and,
therefore, prevents progression towards malignancy (20). In older individuals, around 65% of
the population at risk is regularly screened, and incidence has
significantly dropped for the age group 50-65 by 0.7% and by 4% in
individuals above 65 years of age from 2007 to 2016(4). On the other hand, the increased
incidence of eoCRC could be attributed to unavailability of
screening, and evidence has shown that approximately 25% of eoCRC
cases could have been averted through awareness of positive family
history being a risk factor and prompt commencement of screening in
high-risk individuals (21).
Evidence is now emerging regarding the drivers of
sporadic onset of eoCRC; yet it remains unclear whether these
individuals present with distinct clinical features and pathways
involved in carcinogenesis. Moreover, it remains to be elucidated
whether certain environmental triggers and sedentary lifestyle are
key contributors to the rising incidence in young adults. Despite
similar treatment options in CRC patients regardless of age,
comprehending the molecular mechanisms of eoCRC will aid in
improving approaches in treatment and prevention (22).
Guidelines with regards to the treatment of eoCRC do
not distinguish among younger and older patients; yet evidence
supports that younger patients may receive more intensive treatment
(23), while clinical outcomes and
response to chemotherapy may be similar across age groups (16).
The aim of this paper is to provide an overview of
our current knowledge on CRC with a focus on epidemiology,
presentation, pathophysiology, and prevention of disease among
young individuals.
2. Methods
The databases used to retrieve articles to be
included in this narrative review were PubMed, EBSCO and Cochrane
Library. We employed the following search terms: ‘colorectal
cancer’, ‘incidence’, ‘early-age onset’, ‘young adults’,
‘sporadic’, ‘mortality’, ‘epidemiology’, ‘developed countries’,
‘risk factors’, ‘obesity’, ‘screening guidelines’. The selected
articles were within the timeframe of 20091 to 2023, and we
exclusively included journal articles published in the English
language. Additional information was retrieved from the American
Cancer Society, WHO and NICE guidelines. The clinical trials were
retrieved from Clinicaltrials.gov using the keywords: ‘gene
mutations’, ‘early age’, ‘genetic analysis’.
3. Epidemiology
Incidence and prevalence
CRC is the third most commonly diagnosed cancer
worldwide among both sexes, accounting for 10% of global cancer
incidence. According to GLOBOCAN 2020, there were 1.15 million new
colon cancer cases, 0.7 million new rectal cancer cases and 50,000
new anal cancer cases in the year 2020 worldwide (1). The age standardized incidence rates
across the world were 23.4 in males and 16.2 in females per 100,000
population in the year 2020 indicating a slight predominance in
males (44% higher incidence in males vs. females) (1).
Globally over 1.93 million new cases of CRC patients
were reported for the year 2020(1), of which 17,930 new cases were
individuals younger than 50 years of age (4). The estimated worldwide incidence of
eoCRC in 2020 revealed that it was the fourth most common cancer
(24). Currently, it is expected
that 1 in 23 men (4.4%) and 1 in 25 women (4.1%) in the US will
have a CRC diagnosis in the course of their lives (4).
In 2019, the average global incidence rate of eoCRC
was 5.7 (per 100,000 person-years), with males having a 6.9
incidence rate (per 100,000 person-years) and females having a 4.6
incidence rate (per 100,000 person-years), also indicating a slight
male predominance in individuals with eoCRC (2.3 higher incidence
rate in males vs. females) (25).
Generally, a rise in incidence has been reported in
low-income and middle-income countries, while it has been declining
or stabilizing in high-income countries that implement screening
for individuals above 50 years of age (26). Contrary to the decline in incidence
patterns from 2014 to 2018, with a decrease of approximately 2% per
year in those aged 50 and older, the incidence of CRC in younger
adults for the same period has risen by 1.5% per year (27). Japan, China and the USA are the top
three countries with the highest incidence of CRC (1). The steepest rises in incidence rates
from a 47-state cancer registry are in non-Hispanic whites in most
states in the USA (3). An increase
in the incidence of CRC in young adults has also been observed in
other high-income countries, including Australia, the United
Kingdom, Canada, and Germany (3).
Age-standardized incidence rates of Hungary, Slovakia and Norway
were the highest in 2020, with rates of 45.3, 43.9 and 41.9 per
100,000 persons, respectively (1).
In the years 2012 to 2016, the incidence rates varied from 30 (per
100,000 persons) in Asia/Pacific Islanders to 45.7 (per 100,000
persons) in non-Hispanic black individuals and 43.3 (per 100,000
persons) in American Indian/Alaskan natives (4). Data from the National Cancer
Institute in the USA indicate that for the time period 2006-2016,
in all five-year age groups from 20-49, the steepest rise was among
the group of 40-49 years of age (23). The National Comprehensive Cancer
Network guidelines estimated that the rate of incidence of colon
and rectal cancers will rise by 90 and 124.2%, respectively, for
patients of 20-34 years of age by the year 2023(28).
Mortality and survival rates
Analogous to the incidence patterns, the mortality
rate of CRC varies in different age groups with steady annual
decline in the elderly and a steady annual increase in those
younger than 50 years, as reported in recent years. According to
data from GLOBOCAN 2020, the highest mortality of CRC in 2020 for
both sexes was in Asia (54.2%), followed by Europe (26.2%), Latin
America and the Caribbean (7.4%), and the lowest in Africa with
4.6% (29).
The CRC mortality rates in the US for the time
period of 2008-2017 decreased by 3% per year in those older than 65
years and by 0.6% per year in those aged 50-64 years. On the
contrary, for individuals younger than 50 years, the mortality rate
increased by 1.3% (4). The global
death rate in eoCRC increased from 1.9 per 100,000 people in 1990
to 2.2 per 100,000 people in 2019, with the highest death rates per
100,000 person-years in Seychelles, Bulgaria and Ukraine (25).
Major advancements in treating CRC, such as the
removal of polyps and improved screening efforts, have contributed
to high five-year survival rates at initial stages of diagnosis.
For the period of 2010-2014, the 5-year net survival in most
countries in western Europe and North America was between 60 and
70%, while in Africa, Asia, South America, and Eastern Europe, it
was less than 50% (30). In the
USA, in the years 2008-2014, the five-year relative survival rates
were 92% for stage I colon cancer, 87% for stage IIA, and 65% for
stage IIB. Surprisingly, the 5-year survival rates were slightly
higher for stage III when compared to stage II, i.e., the survival
rate was 90% for stage IIIA and 72% for stage IIIB, 53% for Stage
IIIC and 12% for stage IV (metastasis) (31). The 5-year relative survival rate
for people diagnosed with colon cancer between 2012 and 2018 was
91% for localized SEER stage, 72% for regional, and 13% for distant
stage. For people diagnosed with rectal cancer in the same time
period, the 5-year relative survival rate was 90% for localized,
74% for regional, and 17% for distant stage (32).
Risk factors
Despite a greater fraction of early CRCs being
hereditary, the majority of CRCs cases are sporadic (4).
Non-modifiable risk factors
The main non-modifiable risk factor for CRC is
family history, as individuals with a first-degree relative with
CRC have a two to four times higher risk of being diagnosed with
the disease before 50 years old and have several affected family
members. Particular under-recognition of inherited colon cancer
conditions like Lynch syndrome and FAP exists, contributing s to
the rise in the incidence rates. For instance, in patients
diagnosed with CRC from 1990 to 2010 under the age of 50, only 27%
undertook genetic screening testing for Lynch Syndrome (5).
Modifiable risk factors
While the mechanisms underlying the established
increase in the incidence of sporadic CRC in young adults remain
unresolved, several modifiable risk factors, namely obesity, type
II diabetes, antibiotic use, diet, and physical activity are
implicated in the pathogenesis of eoCRC (20).
Obesity. Insulin resistance,
hyperinsulinemia, and increased inflammation are associated with
weight gain in early adulthood (at 18 years of age) and an
increased BMI, linking obesity, as well as diabetes and sedentary
lifestyle, to eoCRC (33).
In a study among 85,256 nurses aged 25-42 years, it
was discovered that for every 5-unit rise in Body Mass Index (BMI),
there was a 20% higher risk of early-onset CRC driven sporadically,
and that both weight gain starting from 18 years of age and BMI at
18 promoted this association. Those with a BMI≥30 had almost double
the risk of eoCRC in comparison to those with BMI at
18.5-22.9(33).
Nutrition. A positive association has been
reported between increased CRC incidence and excess consumption of
red and processed meat (34).
Evidence has suggested that antibiotic use, with potential
alterations in the constitution of the gut microbiome, is also
correlated to increased CRC risk. Increased duration of antibiotic
use in age groups 20-39 and 40-59 was significantly related to an
elevated likelihood of colorectal adenoma which is the precursor
for most CRCs (35).
Gut microbiota. Modifications in the gut
microbiome from obesity, antibiotic use, and consumption of red and
processed meats found in Western diet lead to intestinal dysbiosis.
Some bacteria (proteobacteria and fusobacteria) are
pro-tumourigenic and cause alterations in colon integrity, thereby
having a direct effect on inflammatory responses via the production
of toxins, short chain fatty acids, and changes in the composition
of bile acid (34).
4. Disease presentation
The most common symptoms of CRC include rectal
bleeding, abdominal pain, change in bowel habits and anaemia
(36). In addition, haematochezia
and abdominal pain have been reported as the most common symptoms
at presentation in young patients less than 50 years of age when
compared to patients older than 50 (Haematochezia: 28.8% in <50
vs. 23.2% in >50 and Abdominal pain: 41.2% in <50 vs. 27.2%
in >50) (15).
An increased incidence of CRC in younger patients
has been associated with left-sided cancer, which can emerge with a
change in bowel habits because of bowel lumen narrowing. It can
usually manifest with diarrhoea, change in stool form and
subsequently bowel obstruction. Nevertheless, the symptoms are also
typical of other disorders contributing to serious delays in
diagnosis. In comparison to older cohorts, most young patients
suffering from CRC present with a more advanced stage of disease
and poorer tumour differentiation. Abdominal distension, vomiting
and weight loss are indicative of advanced disease and rectal pain
may flag up a bulky tumour with local pelvic invasion. Metastatic
disease has been reported in 61.2% of young patients in comparison
to 44.5% in those above 50, and there may be evident hepatomegaly
or a palpable abdominal mass (37).
5. Diagnosis
Patient and family history
Patient consultations provide the opportunity for
doctors to inquire about their patients' personal and family
history. It is important for doctors to obtain a thorough family
history that spans three generations. Constructing a family
pedigree allows for a visual representation of family cancers, and
this structure can be easily updated as new information becomes
available (38).
In the presence of any possible symptoms, general
practitioners (GPs) should ask if the patient has a family history
of CRC or whether they have a predisposing condition, such as FAP.
Specifically, GPs must collect information regarding the degree of
kinship, the number of relatives diagnosed with CRC, and the age of
affected individuals among those who have biological relatives with
a history of either CRC or colorectal adenomas (39). However, it is important to note
that doctors should be reminded that young adults with eoCRC may
not exhibit evident risk factors, such as family history, which
could guide early diagnosis of the disease (6).
Clinical examination
In cases where colon cancer is suspected, during a
clinical consultation, doctors focus on clinical examination and
laboratory findings. A major contribution to the diagnosis of CRC
in almost one-third of the patients is abdominal examination. A
digital examination is of equal diagnostic importance and more
applicable for rectal compared to colon cancer patients (40).
Non-invasive tests and blood
tests
Primary non-invasive screening tools include guaiac
faecal occult blood tests (gFOBTs) or faecal immunochemical tests
(FITs) (14). A full blood count
is ordered in suspected CRC patients since iron deficiency, with or
without anaemia, is the most common haematological finding in
CRC.
Colonoscopy and biopsy
Colonoscopy assesses tumour location and allows
simultaneous sampling of biopsy and histological evaluation, which
is the gold standard for diagnosing CRC (8). The two predominant premalignant CRC
lesions include serrated lesions and conventional adenomas
(41). The quality of colonoscopy
imaging has improved substantially during the last 20 years with
the use of high-definition white light endoscopy (hWLE), which
encompasses high-resolution video screens (8). Procedural risks associated to
sedation, contraindication of administering bowel preparation due
to the impeding risk of obstruction, operator dependency and its
invasive nature may have contributed towards the alarming increase
in CRC incidence within young adults (42).
CT colonography
CT colonography provides equal sensitivity as
colonoscopy without the need for sedation, thereby allowing an
endoluminal colon view using low-dose CT scanning. Therefore, it
can be used as an alternative method of colonic assessment in
patients who are unsuitable candidates or for whom colonoscopy is
contraindicated. In comparison to double-contrast barium enema, CT
colonography is the test of choice since it is better tolerated and
more effective (41). However,
radiation exposure and costs are important factors for
consideration.
Flexible sigmoidoscopy
Flexible sigmoidoscopy allows visualization of the
rectum and sigmoid colon and is highly sensitive in diagnosing
left-sided CRC neoplasms at low cost and with minimal patient
discomfort (23). Its major
drawback includes a reduced benefit in protecting against
right-sided CRC (41).
CT scan
Once a CRC diagnosis is made, a CT scan of the
chest, pelvis and abdomen is essential for preoperative staging
using oral and intravenous contrast, as well as for the
identification of distant metastases (43).
Staging
CRC is classified using the tumour-node-metastasis
(TNM) classification for staging. T describes the extent of tumour
invasion depth in the various intestinal wall layers, N describes
the number of lymph node involvement, and M describes the presence
of distant metastasis. Stage I is limited to the intestinal wall
and is early-stage cancer without metastasis to the lymph nodes;
stage II is cancer without metastasis to the lymph nodes; stage III
is cancer with lymph node metastasis but no distant metastasis and
stage IV is cancer with presence of distant metastasis (24).
6. Pathophysiology
CRC can either occur sporadically (94%), or due to
inherited mutations (5%) or may be derived due to chronic
inflammatory bowel diseases (IBD) including Ulcerative Colitis or
Crohn's disease (1%) (9).
Sporadic mutations and pathways of
carcinogenesis
Sporadic mutations are associated with three
proposed carcinogenetic pathways which include the chromosomal
instability (CIN), the CpG island methylator phenotype (CIMP) and
the microsatellite instability (MSI) pathways (23).
The CIN pathway
The CIN pathway is the most frequent mutated pathway
in colon cancer corresponding to about 70% loCRC cases and 85% of
eoCRC cases. It is characterised by an imbalance in chromosome
number (aneuploidy) and loss of heterozygosity (22). In this pathway, the adenomatous
polyposis coli (APC) gene, implicated in the
WNT/β-catenin signalling pathway, underdoes loss-of-function
mutations, which trigger abnormal crypt foci formation.
Subsequently, inactivating mutations in tumour suppressor genes
TP53 and activating mutations in proto-oncogenes KRAS
and C-MYC can lead to the transformation of tubular adenomas
into adenocarcinomas. Defects in this pathway are responsible for
85% of eoCRCs, which are associated with an increased rate of
advanced histologic presentation, diagnosis at a later stage, and a
reduced prevalence of CRC in the right colon (20,44).
There are differences in the genetic mutations
reported in loCRC compared to eoCRC. In early onset CIN tumours,
the loss of s chromosomal loci harbouring genes of the FOX
transcription factor family as well as the TJP2 gene, can
disrupt the regulatory control of gene expression, thereby
contributing to carcinogenesis (45). The FOX transcription factor family
comprises of numerous members and each member has a distinct roles
in the regulation of various signalling pathways (e.g. the
WNT/β-catenin pathway) (46). The TJP2 gene codes for the
Tight Junction Protein 2 and plays a crucial role in the formation
and maintenance of tight junctions (47). In CRC, TJP2 is mutated contributing
to disrupted tight junctions and increased permeability, and
potentially promoting carcinogenesis by allowing the escape of
cancerous cells into surrounding tissues (48).
At the same time, early onset CIN tumours gain
chromosomal loci which contain genes for BMPR1A (Bone
Morphogenetic Protein Receptor Type 1A) and
AMPK-(AMP-activated protein kinase) (45). The BMPR1A is gene associated
with the BMP signalling pathway which plays a role in cell growth
and differentiation. Mutations in the BMPR1A gene are linked
to the Juvenile Polyposis Syndrome, a rare autosomal dominant
condition characterized by multiple gastrointestinal hamartomatous
polyps with a high risk of developing CRC (49). The regulatory subunit of AMPK is
indirectly associated with CRC. Dysregulation of AMPK including its
regulatory submit, can impact cellular energy metabolism and
signalling pathways, contributing to carcinogenesis in the colon
(50).
Contrastingly, late-onset CIN tumours lose
chromosomal loci which contain the SMAD4, DCC and
APC genes. The SMAD4 gene is considered a tumour suppressor
gene since it encodes a protein that plays acritical role in the
TGF-β signalling pathway regulating cell growth and apoptosis
(45). The DCC (Deleted in
Colorectal Cancer) gene is also considered as a tumour suppressor
gene and encodes a receptor for the netrin-1 protein and functions
in axon guidance during neural development (51). The APC (Adenomatous
Polyposis Coli) gene regulates the Wnt signalling pathway and it is
also a critical tumour suppressor gene; mutations in this gene are
associated with the development of CRC through the promotion of
uncontrolled cell grown and the formation of precancerous polyps
(45).
The KRAS gene encodes a GTPase that
participates in cell signalling pathways controlling cell growth
and differentiation. KRAS can be considered an oncogene when it
undergoes specific activating mutations that promote uncontrolled
cell growth and contribute to cancer development. Mutations in KRAS
are commonly found in CRC. A higher prevalence of mutations in the
KRAS gene have been found among eoCRC (54%) in comparison to
loCRC (40%) (52). The higher
frequency of KRAS mutations in young individuals compared to older
individuals suggest that these mutations are important in the
development of eoCRC (45).
LINE-1 (Long Interspersed Nuclear Element-1), is a
type of retrotransposon, a repetitive DNA sequence that can
replicate and insert copies of itself at various locations in the
genome (53).
LINE-1 hypomethylation is an epigenetic alteration
i.e., a reduction in the methylation of LINE-1 retrotransposons in
the DNA that is associated with genomic instability and may
contribute to CRC by increasing the potential of genetic mutations
and therefore activation of certain proto-oncogenes.
LINE-1 hypomethylation is an early CRC associated
event; consistently lower levels of LINE-1 hypomethylation are
found in eoCRC compared to loCRC. Moreover, associations between f
LINE-1 hypomethylation with distal tumours and worse prognosis have
been reported. LINE-1 hypomethylation is recognized as an
independent factor for higher mortality, yet it is unclear whether
this is a distinct feature of sporadic eoCRC (54).
The CIMP pathway
The CIMP pathway, corresponding to 30% of the
sporadic CRC cases, involves hypermethylation of CpG islands
contributing to the silencing of genes. Consequently, the promoter
associated CpG-rich regions of tumour suppressor genes undergo
inactivation of transcription leading to cancer development. In
eoCRC, there is a lower number of BRAF mutations and reduced MLH1
promoter hypermethylation, compared to patients with later onset
(55). Typically, CpG island
methylation in colonocytes is an age-related phenomenon, and in
young adults with CRC, this pathway is not usually observed since
they undergo less extensive CpG island methylation (23).
The MSI pathway
The MSI pathway, accounting for 10-15% of the
sporadic CRC cases, involves the disruption of DNA mismatch repair
(MMR) genes that are normally responsible for identifying and
repairing replication errors and proofreading newly synthesized
DNA. This pathway is characterized by accumulation of single
nucleotide mutations and modifications in the lengths of repetitive
microsatellite nucleotide sequences. Tumours of this pathway occur
in two forms of CRC: sporadic cases, where it involves epigenetic
silencing (hypermethylation) of the MLH1 promoter, and
hereditary cases like Lynch syndrome, which include germline
mutations in the MMR gene (56). In comparison to CRC in older
patients, where nearly all MSI cases represent sporadic MSI
tumours, MSI in eoCRC is associated with germinal defects in all
MMR genes (MLH1, MSH2, MSH6,
PMS2) thereby causing various genetic predisposition
syndromes (57).
The carcinogenic pathways are not mutually exclusive
and can sometimes coexist The Microsatellite and Chromosome Stable
(MACS) pathway can exhibit both chromosomal and microsatellite
stability. Banerjea et al reported that the MACS pathway is
most frequently reported in eoCRC patients with MACS tumours had
lower survival than patients with CIN or MSI tumours (58). The MACS pathway is rarely
associated with BRAF mutations, and presents with a
different hypomethylation pattern than MSI and CIN (56).
Genetic predisposition syndromes
The two most common genetic predisposition syndromes
for CRC include the Familial Adenomatous Polyposis (FAP) syndrome
and the Hereditary Nonpolyposis CRC (Lynch syndrome). FAP arises
mostly from the CIN pathway and prompts the formation of hundreds
to thousands of adenomatous polyps during the individuals'
adolescence. If left untreated, these individuals will subsequently
develop CRC at approximately 40 years of age. Lynch syndrome
emerges from the MSI pathway via inherited MMR gene
mutations (56). There is an
increased risk for CRC among young adults at an average age of 44
because of greater undiagnosed Lynch syndrome patients.
Furthermore, there is a high risk of Lynch Syndrome patients
developing extracolonic metastasis including endometrial, small
intestine and ovarian metastasis (59).
Clinical trials
Ongoing clinical trials aim to identify different
mutations occurring in eoCRC and to determine the significance of
these in predicting the treatment outcomes.
One clinical trial (‘Genetic study of young patients
with colorectal cancer’ (NCT00044967)) was conducted to determine
the significance of gene mutations in helping predict the outcome
of treatment in patients who develop stage I, stage II, or stage
III CRC at an early age. The study was completed in 2004 but no
results were posted (60).
Furthermore, another clinical trial (‘Targeted Next-generation
Sequencing Panel for Identification of Germline Mutations in Early
Onset Cancers with Sporadic or Hereditary Presentation (PANEL)’
NCT02664389)), involved the sequencing of 200 selected somatic
cancer genes of eoCRC patients, without genomic alterations in
APC, MLH1, MSH2 and various other genes and
was conducted to identify germline deleterious mutations. The study
was completed in 2017 but no results have been published yet
(61).
Lastly, another clinical trial (‘Young-Onset
Colorectal Cancer’ (NCT02863107)) is being conducted to investigate
the genetic factors that may contribute towards the development of
CRC at a young age. Defining the clinical phenotype of young-onset
vs. later onset CRC and examining germline genetic alterations in
individuals with CRC and families at high risk of cancer may help
identify new cancer genes. The study will be completed in
2030(62).
Differences in the histology of young
vs. older CRC patients
Evidence in the literature supports that CRC in
young adults more commonly occurs in the distal colon and rectum
while older patients more commonly present with proximal tumours.
The histological features which are distinct in young patients with
CRC include a more aggressive pattern like signet-ring cell and
mucinous tumours, in addition to poor differentiation. Furthermore,
invasion of veins and perineuronal regions is more commonly
observed in younger compared to older individuals (59).
7. Prevention
Primary prevention
Primary prevention is designed to prevent a disease
or condition, from occurring and is targeting people not affected
by the disease. Primary prevention includes the measures that
prevent illness onset before the disease process begins. CRC
focuses on encouraging lifestyle modifications including a healthy
diet and increased physical activity which in combination can
prevent obesity, and smoking cessation (63). Evidence from research studies
supports that a higher consumption of dietary fibre or whole
grains, calcium, and dairy and decreased consumption of red and
processed meat and alcohol are associated with reduced CRC risk
(6,64,65).
Health promotion targeting younger populations can
ultimately help shape future lifestyle habits and enhance cancer
prevention behaviours (44). A
number of different strategies have been implemented with the scope
of preventing eoCRC. One approach to effectively reach the target
audience among 30-49 years of age is social media platforms, since
in a national survey 98% of American adults from this age group
have internet access (66).
Organizations established by the government such as National Cancer
Institute have used social media marketing strategies through
multiple platforms and channels, such as blogs and Facebook to
deliver a wide range of health promotion content (67). In countries with low socio-economic
status like Ethiopia, CRC awareness is low (42,40%) and more than a
third of the participants from a cross-sectional study evaluating
CRC awareness in Ethiopia revealed that they received information
related to CRC from mass media followed by social media.
Participants who received information through social media were 2.5
times more likely to have high awareness of CRC than those getting
information from other sources (68). Further evidence in the literature
supports that health promotional campaign launches and access to
peer support groups provide useful means to reach health consumers
since these platforms help with provision of interactions, delivery
of tailored material and enhanced access to health information
(69).
In people with Lynch Syndrome, daily aspirin for
longer than 2 years is recommended to prevent CRC (70). In addition, ongoing research is
conducted to investigate whether primary prevention with aspirin in
average/increased risk patients can reduce the risk of developing
CRC (71). Low-dose aspirin is
also recommended in adults 50 to 59 years of age with CRC who have
a cardiovascular disease risk in the following 10 years and are not
at risk of haemorrhage (72). Use
of NSAIDs (except aspirin) was found to decrease the risk for CRC
in patients 40 years and above with considerable effects mostly at
higher doses, in women, white individuals and cancer at the distal
colon (73).
Secondary prevention
Secondary prevention aims to minimize the impact of
a disease that has already occurred and includes measures leading
to early diagnosis and prompt disease treatment to halt or slow
down its progress. Unlike other cancers, CRC is a slowly
progressive cancer. Therefore, as a form of secondary prevention,
adenomas can be detected and removed during colonoscopy. Early
detection allows diagnosis at an earlier treatable stage and
improves survival (63).
Successful CRC secondary prevention entails
screening (72). Since the
beginning of this century both the incidence and mortality of CRC
for older individuals above 50 have decreased by about one-third
due to screening colonoscopy (3).
In contrast, the incidence and mortality in young adults who do not
undergo screening are rising. For this reason, the American Cancer
Society has decreased recommended age for commencing screening for
CRC in the population from age 50 to 45(74). In 2021, the National Health Service
(NHS) in England reduced the screening age from 60 to 50 years old,
while the screening recommendations by the US Preventive Services
Task Force start at 45 and continue through to 75 years old
(75). Screening using colonoscopy
from ages 45 to 75, instead of 50 to 75, has shown an estimated 6%
increase in years of life gained, with a 17% increase in the number
of colonoscopies performed (76).
First degree relatives of CRC individuals with
documented advanced adenomas are instructed to begin screening on
average 10 years before the youngest age that an affected
first-degree relative was diagnosed or from the age of 40(41). The UK National Screening Committee
recommends screening every 2 years from the age of 50 using FIT
which has shown effectiveness in prevention and decreased
mortality. It remains to be elucidated whether a combination of FIT
screening and bowel scope testing provides additional benefit
compared to FIT screening alone (77).
Strategies to enhance CRC screening
compliance in eoCRC patients
Primary care providers play a key role in the
prevention of CRC and have a major impact on the patients'
compliance to successful screening (78). A successful increase in screening
rates relies on implementing effective systems of decision support,
such as electronic medical record reminders, as well as practices
for screening delivery, like registries in the practices of primary
care providers (79).
Additionally, targeting high-risk patients by informing them about
the significance and availability of routine screening tests could
prevent delays in diagnosing cancer early, thereby improving
patients' survival (80).
FIT-based screening could achieve higher compliance rates when
offered as part of organized screening programs, which include
personal invitation letters with test kits and reminder letters
(81).
In a systematic review of mailed FITs, potent
interventions for improved screening comprised of telephone
reminders with instructions, primary care provider encouragement,
simplified kit tests, and letter notifications of incoming mailed
tests sent in advance (82). In
one study, a targeted mobile app was introduced among women aged
44-70 with the aim of enhancing CRC screening and providing each
individual woman with the screening option best suited for her,
based on her responses to the questions from the mobile app. The
results from this study indicated the willingness of younger women
under 50 to learn about their screening options and make informed
decisions regarding CRC screening (83).
Future prospects look into using genome-wide
association study results of polygenetic risk scores to determine
risk categorisation (84). For
individuals with Lynch syndrome, screening colonoscopy is usually
recommended from the age of 20 to 25 years, while screening
commences at the age of 10-12 years for individuals diagnosed with
FAP syndrome (85). Guidelines
from the British Society of Gastroenterology/Association of
Coloproctology of Great Britain and Ireland (BSG/ACPGBI) recommend
that people with Lynch Syndrome who carry mutations in the
MLH1 and MSH2 gene should undergo colonoscopy from the age
of 25 years of age whereas MSH6 and PMS2 gene
carriers should undergo colonoscopy at the age of 35(86). The American Society for
Gastrointestinal Endoscopy (ASGE) recommends that testing should
begin at the age of 18 to 20 years if FAP is suspected (87).
Public Health England has launched invitation
leaflets explaining CRC screening and colonoscopy to the public,
which are available at screening hubs. Enhancing care and support
in dealing with the effects of CRC and its treatment is the primary
goal of the ‘Never Too Young campaign’, launched after the increase
in CRC incidence in young individuals (88). The Colorectal Cancer Alliance in
the USA uses survey reports from the Never Too Young Task force to
track and learn about the self-reported medical, psychosocial and
quality of life experiences of the young groups and address their
needs and concerns (89).
Tertiary prevention
Tertiary prevention includes efforts to prevent the
of complications in people who have already developed the disease
and in whom disease prevention is no longer possible.
Several lifestyle factors are associated with
improved clinical outcomes in cancer patients. Notably, one study
has reported that incorporating physical activity into the lives of
patients with stage III CRC who are actively receiving active
chemotherapy is correlated with prolonged survival and improved
prognosis (90).
The use of low-dose aspirin has also been observed
to enhance the survival of patients following a CRC diagnosis
emerging as a potential tertiary prevention strategy (91). Moreover, high-dose of vitamin D
supplements have shown promise in improving progression-free life
span in people with metastatic CRC, and as such, there are
opportunities for further exploration of vitamin D in tertiary
prevention (92). The American
Society of Clinical Oncology has initiated efforts to enhance
education, supporting people in understanding the pathophysiology
of weight gain and introduce efficacious dietary habits in cancer
survivors to help reduce obesity which is strongly correlated with
CRC (93).
8. Conclusions
CRC presents a significant public health challenge,
and it is expected that the incidence among young adults under the
age of 50 will continue to rise in the following years. eoCRC
differs from CRC in terms of epidemiology, disease presentation,
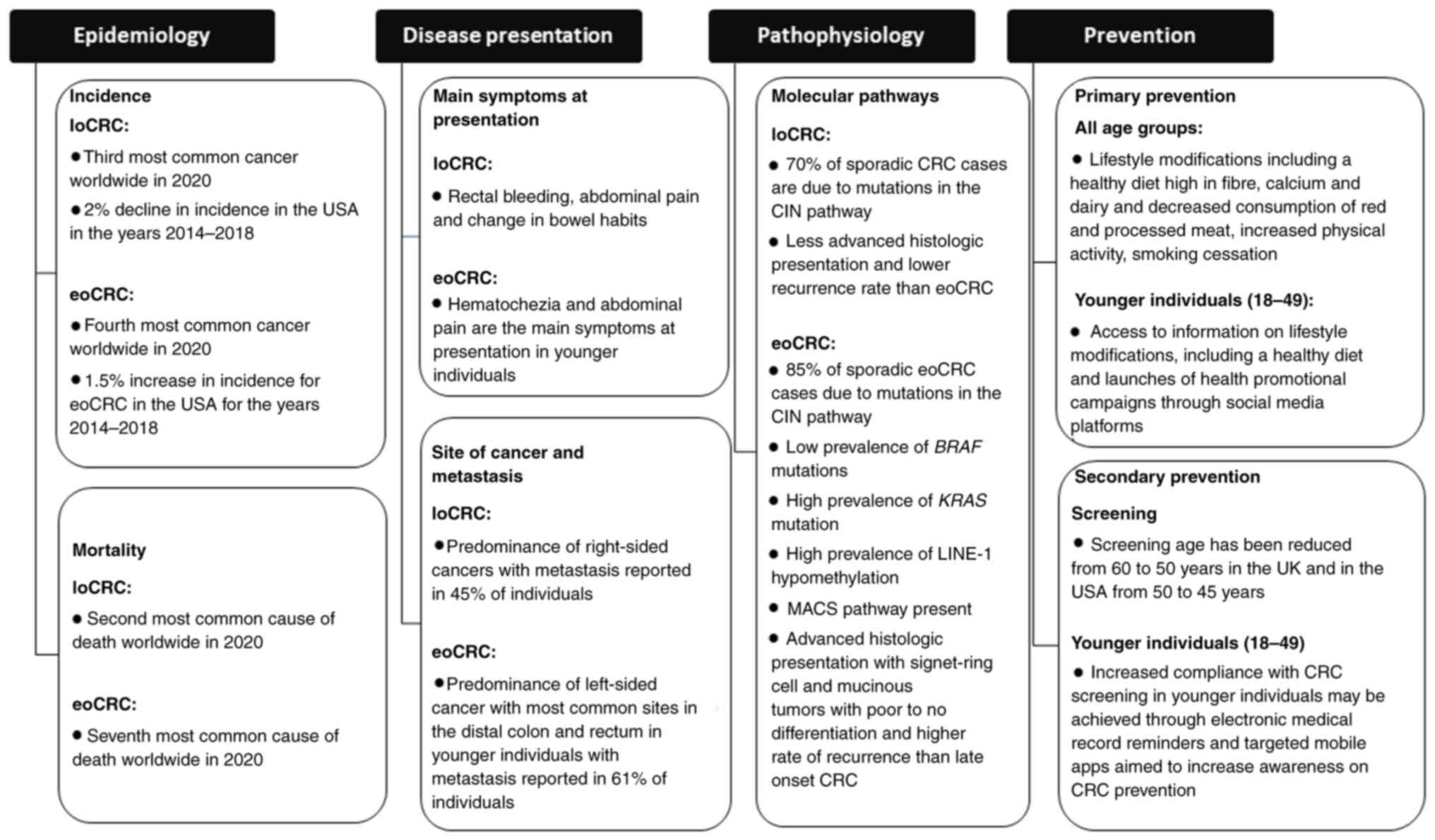
pathophysiology, and prevention (Fig.
1). Analysing emerging trends in incidence and mortality, as
well as identifying of risk factors (environmental, genetic, and
behavioural) associated with CRC across different age groups, are
promising future directions for the field. Genetic screening and
counselling are particularly important due to the common genetic
predisposition associated with CRC. Improving the information
provided by primary care providers and using electronic medical
record reminders, targeted mobile apps and simplified kit tests can
enhance compliance and screening.
In the past few years there has been an increasing
interest in eoCRC (94). Our
narrative review builds on this interest and includes detailed
epidemiological data on age-standardized incidence, prevalence,
mortality, and survival in a wider range of countries with clearer
trends in both loCRC and eoCRC. Different types of diagnostic tools
are being discussed highlighting the advantages and disadvantages
of each with regards to effectiveness, invasiveness, and
sensitivity. The review also provides a brief overview of the
importance of doctors obtaining a good family history from
patients. In terms of prevention strategies, it includes a more
thorough overview of the different levels of prevention and the
various efforts used to raise awareness, strategies to enhance
screening compliance as well as lifestyle changes. In addition,
some clinical trials are included in order to show that indeed
there are different carcinogenetic pathways involved in eoCRC and
that different mutations are involved and may play a role in the
clinical phenotype.
Currently, there is a pressing necessity to
understand the most significant pathophysiological differences
between eoCRC and lo CRC. In the near future, the use of multigene
panels is expected to help identify the full spectrum of germline
mutations and distinct molecular pathways in eoCRC patients
compared to CRC in older patients. Furthermore, prospective cohort
studies that will involve the collection of biospecimens from
stool, saliva, and blood can be used to analyse the tumour
microenvironment and metagenomics to help establish the aetiologies
of eoCRC. The results of such studies will inform the design of
randomized clinical trials with new drugs targeting mutations
specific to eoCRC patients, providing insight into whether young
patients can benefit from more specific treatment options based on
new molecular targets.
In summary, establishing comprehensive and early
risk assessment, targeted screening tools and improved molecular
targets will have a significant impact on reducing the burden of
eoCRC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
VC and CC were involved in the conceptualization of
the current manuscript, and in the literature search and review of
the resources that were used in the current manuscript. Both
authors were involved in the writing and revision of the
manuscript, and read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Constantina Constantinou: ORCID iD:
0000-0001-6167-4023.
References
|
1
|
Xi Y and Xu P: Global colorectal cancer
burden in 2020 and projections to 2040. Transl Oncol.
14(101174)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer Statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegel RL, Torre LA, Soerjomataram I,
Hayes RB, Bray F, Weber TK and Jemal A: Global patterns and trends
in colorectal cancer incidence in young adults. Gut. 68:2179–2185.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Murphy CC, Sandler RS, Sanoff HK, Yang YC,
Lund JL and Baron JA: Decrease in incidence of colorectal cancer
among individuals 50 years or older after recommendations for
population-based screening. Clin Gastroenterol Hepatol.
15:903–909.e6. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stoffel EM and Murphy CC: Epidemiology and
mechanisms of the increasing incidence of colon and rectal cancers
in young adults. Gastroenterology. 158:341–353. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zeki SS, Graham TA and Wright NA: Stem
cells and their implications for colorectal cancer. Nat Rev
Gastroenterol Hepatol. 8:90–100. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kuipers EJ, Grady WM, Lieberman D,
Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ and Watanabe T:
Colorectal cancer. Nat Rev Dis Primers. 1(15065)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
American Cancer Society. Colorectal cancer
facts and figures, 2020-2022. 2022.
|
|
11
|
Glover M, Mansoor E, Panhwar M, Parasa S
and Cooper GS: Epidemiology of colorectal cancer in average risk
adults 20-39 years of age: A population-based national study. Dig
Dis Sci. 64:3602–3609. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Willauer AN, Liu Y, Pereira AAL, Lam M,
Morris JS, Raghav KPS, Morris VK, Menter D, Broaddus R,
Meric-Bernstam F, et al: Clinical and molecular characterization of
early-onset colorectal cancer. Cancer. 125:2002–2010.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fu J, Yang J, Tan Y, Jiang M, Wen F, Huang
Y, Chen H, Yi C, Zheng S and Yuan Y: Young patients (≤35 years old)
with colorectal cancer have worse outcomes due to more advanced
disease: A 30-year retrospective review. Medicine (Baltimore).
93(e135)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
AlZaabi A, AlHarrasi A, AlMusalami A,
AlMahyijari N, Al Hinai K, ALAdawi H and Al-Shamsi HO: Early onset
colorectal cancer: Challenges across the cancer care continuum. Ann
Med Surg (Lond). 82(104453)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen FW, Sundaram V, Chew TA and Ladabaum
U: Advanced-Stage colorectal cancer in persons younger than 50
years not associated with longer duration of symptoms or time to
diagnosis. Clin Gastroenterol Hepatol. 15:728–737.e3.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cercek A, Chatila WK, Yaeger R, Walch H,
Fernandes GDS, Krishnan A, Palmaira L, Maio A, Kemel Y, Srinivasan
P, et al: A comprehensive comparison of early-onset and
average-onset colorectal cancers. J Natl Cancer Inst.
113:1683–1692. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scott EC, Gardner EJ, Masood A, Chuang NT,
Vertino PM and Devine SE: A hot L1 retrotransposon evades somatic
repression and initiates human colorectal cancer. Genome Res.
26:745–755. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tiritilli A and Ko C: Patients with
early-onset colorectal cancer have an increased risk of second
primary malignancy. Dig Dis Sci. 67:1328–1336. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Murphy N, Moreno V, Hughes DJ, Vodicka L,
Vodicka P, Aglago EK, Gunter MJ and Jenab M: Lifestyle and dietary
environmental factors in colorectal cancer susceptibility. Mol
Aspects Med. 69:2–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Venugopal A and Carethers JM: Epidemiology
and biology of early onset colorectal cancer. EXCLI J. 21:162–182.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stanich PP, Pelstring KR, Hampel H and
Pearlman R: A high percentage of early-age onset colorectal cancer
is potentially preventable. Gastroenterology. 160:1850–1852.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Patel SG and Ahnen DJ: Colorectal cancer
in the young. Curr Gastroenterol Rep. 20(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Segev L, Kalady MF, Plesec T, Mor E,
Schtrechman G, Nissan A and Church JM: The location of premalignant
colorectal polyps under age 50: A further rationale for screening
sigmoidoscopy. Int J Colorectal Dis. 35:529–535. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
World Health Organization. International
Agency for Research on Cancer.
|
|
25
|
Gu WJ, Pei JP, Lyu J, Akimoto N, Haruki K,
Ogino S and Zhang CD: The Burden of early-onset colorectal cancer
and its risk factors from 1990 to 2019: A systematic analysis for
the global burden of disease study 2019. Cancers (Basel).
14(3502)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
National Comprehensive Cancer Network.
NCCN clinical practice guidelines in oncology: management of
immunotherapy-related toxicities. 2022.
|
|
29
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000-14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
SEER: An Interactive Website for SEER
Cancer Statistics. Surveillance Research Program. National Cancer
Institute, 2023.
|
|
32
|
American Cancer Society. Cancer Facts
& Figures 2023. American Cancer Society, Atlanta, GA, 2023.
|
|
33
|
Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH,
Song M, He X, Fuchs CS, Ogino S, Willett WC, et al: Association of
obesity with risk of early-onset colorectal cancer among women.
JAMA Oncol. 5:37–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Okita Y, Koi M, Takeda K, Ross R,
Mukherjee B, Koeppe E, Stoffel EM, Galanko JA, McCoy AN, Keku TO,
et al: Fusobacterium nucleatum infection correlates with two types
of microsatellite alterations in colorectal cancer and triggers DNA
damage. Gut Pathog. 12(46)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cao Y, Wu K, Mehta R, Drew DA, Song M,
Lochhead P, Nguyen LH, Izard J, Fuchs CS, Garrett WS, et al:
Long-term use of antibiotics and risk of colorectal adenoma. Gut.
67:672–678. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
BMJ Best Practice. 2022, 2022. Available
from: https://bestpractice.bmj.com/info/.
|
|
37
|
Rho YS, Gilabert M, Polom K, Aladashvili
A, Kopeckova K, Megdanova V, Coleman N, Greally M, Marrelli D,
Roviello F, et al: Comparing clinical characteristics and outcomes
of young-onset and late-onset colorectal cancer: An International
collaborative study. Clin Colorectal Cancer. 16:334–342.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kalady MF and Heald B: Diagnostic approach
to hereditary colorectal cancer syndromes. Clin Colon Rectal Surg.
28:205–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kolligs FT: Diagnostics and epidemiology
of colorectal cancer. Visc Med. 32:158–164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Holtedahl K, Borgquist L, Donker GA,
Buntinx F, Weller D, Campbell C, Månsson J, Hammersley V, Braaten T
and Parajuli R: Symptoms and signs of colorectal cancer, with
differences between proximal and distal colon cancer: A prospective
cohort study of diagnostic accuracy in primary care. BMC Fam Pract.
22(148)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rex DK, Boland CR, Dominitz JA, Giardiello
FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D and Robertson
DJ: Colorectal cancer screening: recommendations for physicians and
patients from the U.S. Multi-Society task force on colorectal
cancer. Am J Gastroenterol. 112:1016–1030. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gupta N, Kupfer SS and Davis AM:
Colorectal cancer screening. JAMA. 321:2022–2023. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vogel JD, Felder SI, Bhama AR, Hawkins AT,
Langenfeld SJ, Shaffer VO, Thorsen AJ, Weiser MR, Chang GJ,
Lightner AL, et al: The American Society of Colon and Rectal
surgeons clinical practice guidelines for the management of colon
cancer. Dis Colon Rectum. 65:148–177. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lassi ZS, Salam RA, Das JK, Wazny K and
Bhutta ZA: An unfinished agenda on adolescent health: Opportunities
for interventions. Semin Perinatol. 39:353–360. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cavestro GM, Mannucci A, Zuppardo RA, Di
Leo M, Stoffel E and Tonon G: Early onset sporadic colorectal
cancer: Worrisome trends and oncogenic features. Dig Liver Dis.
50:521–532. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Laissue P: The forkhead-box family of
transcription factors: Key molecular players in colorectal cancer
pathogenesis. Mol Cancer. 18(5)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kim YJ, Jung YD, Kim TO and Kim HS:
Alu-related transcript of TJP2 gene as a marker for colorectal
cancer. Gene. 524:268–274. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rosner G, Petel-Galil Y, Laish I, Levi Z,
Kariv R, Strul H, Gilad O and Gluck N: Adenomatous polyposis
phenotype in BMPR1A and SMAD4 variant carriers. Clin Transl
Gastroenterol. 13(e00527)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li W, Saud SM, Young MR, Chen G and Hua B:
Targeting AMPK for cancer prevention and treatment. Oncotarget.
6:7365–7378. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Duman-Scheel M: Deleted in colorectal
cancer (DCC) Pathfinding: Axon guidance gene finally turned tumor
suppressor. Curr Drug Targets. 13:1445–1453. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Watson R, Liu TC and Ruzinova MB: High
frequency of KRAS mutation in early onset colorectal
adenocarcinoma: Implications for pathogenesis. Hum Pathol.
56:163–170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Rodić N and Burns KH: Long interspersed
element-1 (LINE-1): Passenger or driver in human neoplasms? PLoS
Genet. 9(e1003402)2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Antelo M, Balaguer F, Shia J, Shen Y, Hur
K, Moreira L, Cuatrecasas M, Bujanda L, Giraldez MD, Takahashi M,
et al: A high degree of LINE-1 hypomethylation is a unique feature
of early-onset colorectal cancer. PLoS One.
7(e45357)2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Perea J, Rueda D, Canal A, Rodríguez Y,
Álvaro E, Osorio I, Alegre C, Rivera B, Martínez J, Benítez J and
Urioste M: Age at onset should be a major criterion for
subclassification of colorectal cancer. J Mol Diagn. 16:116–126.
2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Silla IO, Rueda D, Rodríguez Y, García JL,
de la Vigo C and Perea J: Early-onset colorectal cancer: A separate
subset of colorectal cancer. World J Gastroenterol. 20:17288–17296.
2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Khan SA, Morris M, Idrees K, Gimbel MI,
Rosenberg S, Zeng Z, Li F, Gan G, Shia J, LaQuaglia MP and Paty PB:
Colorectal cancer in the very young: A comparative study of tumor
markers, pathology and survival in early onset and adult onset
patients. J Pediatr Surg. 51:1812–1817. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Banerjea A, Hands RE, Powar MP, Bustin SA
and Dorudi S: Microsatellite and chromosomal stable colorectal
cancers demonstrate poor immunogenicity and early disease
recurrence. Colorectal Dis. 11:601–608. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ballester V, Rashtak S and Boardman L:
Clinical and molecular features of young-onset colorectal cancer.
World J Gastroenterol. 22:1736–1744. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
ClinicalTrials Gov: ‘Genetic Study of
Young Patients with Colorectal Cancer’. NCT00044967, 2004.
|
|
61
|
ClinicalTrials Gov: Targeted
Next-generation Sequencing Panel for Identification of Germline
Mutations in Early Onset Cancers With Sporadic or Hereditary
Presentation (PANEL). NCT02664389, 2017.
|
|
62
|
ClinicalTrials Gov: Young-Onset Colorectal
Cancer. NCT02863107, 2023.
|
|
63
|
Brenner H and Chen C: The colorectal
cancer epidemic: Challenges and opportunities for primary,
secondary and tertiary prevention. Br J Cancer. 119:785–792.
2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Nimptsch K and Wu K: Is timing important?
the role of diet and lifestyle during early life on colorectal
neoplasia. Curr Colorectal Cancer Rep. 14:1–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mehta SS, Arroyave WD, Lunn RM, Park YM,
Boyd WA and Sandler DP: A prospective analysis of red and processed
meat consumption and risk of colorectal cancer in women. Cancer
Epidemiol Biomarkers Prev. 29:141–150. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Pew Research Center: Internet, Science
& Techonology. 2023. Available from: https://www.pewresearch.org/topic/internet-technology/.
|
|
67
|
Miller AS: The zombie apocalypse: The
viral impact of social media marketing on health. Journal of
Consumer Health on the Internet. 17:362–368. 2013.
|
|
68
|
Hamza A, Argaw Z and Gela D: Awareness of
colorectal cancer and associated factors among adult patients in
jimma, south-west ethiopia: An institution-based cross-sectional
study. Cancer Control. 28(10732748211033550)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Dixon HG, Pratt IS, Scully ML, Miller JR,
Patterson C, Hood R and Slevin TJ: Using a mass media campaign to
raise women's awareness of the link between alcohol and cancer:
Cross-sectional pre-intervention and post-intervention evaluation
surveys. BMJ Open. 5(e006511)2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
National Institute for Health and Care
Excellence. Colorectal cancer. 2022.
|
|
71
|
Guo LL, Li YT, Yao J, Wang LS, Chen WW, He
KY, Xiao L and Tang SH: Dairy Consumption and risk of conventional
and serrated precursors of colorectal cancer: A systematic review
and meta-analysis of observational studies. J Oncol.
2021(9948814)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Bibbins-Domingo K: U.S. Preventive
Services Task Force: Aspirin use for the primary prevention of
cardiovascular disease and colorectal cancer: U.S. preventive
services task force recommendation statement. Ann Intern Med.
164:836–845. 2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Tomić T, Domínguez-López S and
Barrios-Rodríguez R: Non-aspirin non-steroidal anti-inflammatory
drugs in prevention of colorectal cancer in people aged 40 or
older: A systematic review and meta-analysis. Cancer Epidemiol.
58:52–62. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Wolf AMD, Fontham ETH, Church TR, Flowers
CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC,
Shih YT, et al: Colorectal cancer screening for average-risk
adults: 2018 guideline update from the American Cancer Society. CA
Cancer J Clin. 68:250–281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
US Preventive Services Task Force.
Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM,
Donahue KE, Doubeni CA, Krist AH, et al: Screening for colorectal
cancer: US preventive services task force recommendation statement.
JAMA. 325:1965–1977. 2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Peterse EFP, Meester RGS, Siegel RL, Chen
JC, Dwyer A, Ahnen DJ, Smith RA, Zauber AG and Lansdorp-Vogelaar I:
The impact of the rising colorectal cancer incidence in young
adults on the optimal age to start screening: Microsimulation
analysis I to inform the American Cancer Society colorectal cancer
screening guideline. Cancer. 124:2964–2973. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Bowel cancer screening: programme
overview. 2023. Available from: https://www.gov.uk/guidance/bowel-cancer-screening-programme-overview.
|
|
78
|
Triantafillidis JK, Vagianos C, Gikas A,
Korontzi M and Papalois A: Screening for colorectal cancer: The
role of the primary care physician. Eur J Gastroenterol Hepatol.
29:e1–e7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Clouston K, Katz A, Martens PJ, Sisler J,
Turner D, Lobchuk M, McClement S and Crow G: CIHR/CCMB Team in
Primary Care Oncology (PCO-NET). Does access to a colorectal cancer
screening website and/or a nurse-managed telephone help line
provided to patients by their family physician increase fecal
occult blood test uptake?: Results from a pragmatic cluster
randomized controlled trial. BMC Cancer. 14(263)2014.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Gonzalez SJ, de Rubb MCM and Levine RS:
Primary and secondary prevention of colorectal cancer: An
evidencebased review. Family Medicine and Community Health.
5:78–84. 2017.
|
|
81
|
van der Vlugt M, Grobbee EJ, Bossuyt PM,
Bongers E, Spijker W, Kuipers EJ, Lansdorp-Vogelaar I, Essink-Bot
ML, Spaander MC and Dekker E: Adherence to colorectal cancer
screening: four rounds of faecal immunochemical test-based
screening. Br J Cancer. 116:44–49. 2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Goodwin BC, Ireland MJ, March S, Myers L,
Crawford-Williams F, Chambers SK, Aitken JF and Dunn J: Strategies
for increasing participation in mail-out colorectal cancer
screening programs: A systematic review and meta-analysis. Syst
Rev. 8(257)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Brittain K, Kamp KJP and Salaysay Z:
Colorectal cancer awareness for women via facebook: A pilot study.
Gastroenterol Nurs. 41:14–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Weigl K, Thomsen H, Balavarca Y, Hellwege
JN, Shrubsole MJ and Brenner H: Genetic risk score is associated
with prevalence of advanced neoplasms in a colorectal cancer
screening population. Gastroenterology. 155:88–98.e10.
2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Syngal S, Brand RE, Church JM, Giardiello
FM, Hampel HL and Burt RW: American College of Gastroenterology.
ACG clinical guideline: Genetic testing and management of
hereditary gastrointestinal cancer syndromes. Am J Gastroenterol.
110:223–262; quiz 263. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Monahan KJ, Bradshaw N, Dolwani S, Desouza
B, Dunlop MG, East JE, Ilyas M, Kaur A, Lalloo F, Latchford A, et
al: Guidelines for the management of hereditary colorectal cancer
from the British Society of Gastroenterology (BSG)/Association of
Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom
Cancer Genetics Group (UKCGG). Gut. 69:411–444. 2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Yang J, Gurudu SR, Koptiuch C, Agrawal D,
Buxbaum JL, Abbas Fehmi SM, Fishman DS, Khashab MA, Jamil LH, Jue
TL, et al: American Society for Gastrointestinal Endoscopy
guideline on the role of endoscopy in familial adenomatous
polyposis syndromes. Gastrointest Endosc. 91:963–982.e2.
2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
BowelCancerUK. 2022. Available from:
https://www.bowelcanceruk.org.uk/.
|
|
89
|
Colorectal Cancer Alliance. 2023.
Available from: https://socialpresskit.com/colorectal-cancer-alliance.
|
|
90
|
Van Blarigan EL, Chan H, Van Loon K,
Kenfield SA, Chan JM, Mitchell E, Zhang L, Paciorek A, Joseph G,
Laffan A, et al: Self-monitoring and reminder text messages to
increase physical activity in colorectal cancer survivors (Smart
Pace): A pilot randomized controlled trial. BMC Cancer.
19(218)2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Frouws MA, Reimers MS, Swets M,
Bastiaannet E, Prinse B, van Eijk R, Lemmens VE, van Herk-Sukel MP,
van Wezel T, Kuppen PJ, et al: The Influence of BRAF and KRAS
mutation status on the association between aspirin use and survival
after colon cancer diagnosis. PLoS One. 12(e0170775)2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ng SC, Shi HY, Hamidi N, Underwood FE,
Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, et
al: Worldwide incidence and prevalence of inflammatory bowel
disease in the 21st century: A systematic review of
population-based studies. Lancet. 390:2769–2778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Ligibel JA, Alfano CM, Hershman DL,
Merrill JK, Basen-Engquist K, Bloomgarden ZT, Demark-Wahnefried W,
Dixon S, Hassink SG, Jakicic JM, et al: American Society of
clinical oncology summit on addressing obesity through
multidisciplinary provider collaboration: Key findings and
recommendations for action. Obesity (Silver Spring). 25 (Suppl
2):S34–S39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Saraiva MR, Rosa I and Claro I:
Early-onset colorectal cancer: A review of current knowledge. World
J Gastroenterol. 29:1289–1303. 2023.PubMed/NCBI View Article : Google Scholar
|