Introduction
Gastric cancer is one of the most common
malignancies of the gastrointestinal system. Every year, 7 million
patients die from cancer, and 1 in 10 patients die of gastric
cancer. Gastric malignancies have the fourth highest incidence
rates and the second highest mortality rates among all cancers. The
incidence rate of gastric cancer is second only to lung cancer in
China and the mortality rate ranks first among various cancers
(1,2). At present, the primary conventional
treatment for gastric cancer remains surgical resection
supplemented by radiotherapy and chemotherapy, and chemotherapy has
gradually become an important method for patients who miss the
opportunity to undergo surgery and comprehensive treatment for
gastric cancer (3). However,
chemotherapy drugs that target and suppress cancer also exert
substantial effects on normal cells, exhibit poor targeting and
killing of tumor cells, and cause different degrees of toxicity;
during the process of treatment, certain patients develop multiple
drug resistance to different chemotherapy drugs and the dose of
chemotherapy drugs must be increased, making the quality of life of
patients decrease and causing patients to refuse chemotherapy or
end treatment due to pain (4,5).
Therefore, finding strategies to avoid or even solve these
problems, reduce the pain experienced by patients and improve
patient quality of life has become an urgent need in cancer
treatment.
The anticancer active peptide is a novel biological
agent with anticancer activities and numerous years of experiments
have confirmed that it has obvious antitumor and tumor suppressor
effects without toxic side effects (6). ACBP exerts a certain inhibitory
effect on the growth of various tumor cells, such as human
leukemia, colorectal cancer and mouse sarcoma cells, and it may
enhance the antitumor effect of lymphocytes and sensitivity to
chemotherapeutic drugs (7,8). It was previously found that ACBP
exhibits antineoplastic activity and inhibits tumor growth in nude
mice with dutch gallbladder carcinoma. It also increased the
chemotherapeutic sensitizing effect and decreased side effects
associated with chemotherapy (9).
Oxaliplatin (OXA) is a commonly used chemotherapeutic drug in the
treatment of gastric cancer, with obvious efficacy compared to
other chemotherapeutic drugs, such as cisplatin and carboplatin,
but its toxic effects are unavoidable, which limits its application
in the treatment of gastric cancer (9).
According to their functions, cytokines may be
divided into interleukins (ILs), interferons (IFNs), tumor necrosis
factors (TNFs), chemokines and growth factors. Cytokines have
various biological functions, including regulating innate immunity,
adaptive immunity, hemopoiesis, cell growth and differentiation, as
well as tissue damage repair. Numerous cytokines promote or
restrict each other in the body, forming a complex cytokine immune
regulation network. IL is a family of cytokines capable of
bidirectional regulating factors of the immune system, mainly
involved in the differentiation and activation of immune cells.
Cytokines, such as IL-6, TNF-α, IL-10 and IFN-γ-induced protein
(IP)10, are key transcription factors involved in controlling the
expression of proinflammatory cytokines and responses to infection
(10). They have important roles
in immune regulation, cell differentiation, cell apoptosis and cell
cycle regulation. IL-1, IL-10, IL-12, family members have a key
role in the differentiation and function of polarized innate and
adaptive lymphoid cells (11).
IL-2, an important immune regulator that is synthesized and
released by lymphocytes by specific antigen stimulation, enhances
the T-cell population by promoting T cells to transition from the
G1 phase to the S phase of the cell cycle. It is currently
suggested that IL-2 has anticancer effects. IL-4 is a cytokine with
various biological functions, including stimulating B cells,
promoting B-cell transformation into plasma cells and inducing
B-cell IgE production, promoting T helper (Th) cells to transform
into Th2 cells and inhibiting the production of Th1 cells.
Growing evidence suggests that IL-8 has pivotal
roles in the pathogenesis of cancer through the modulation of the
tumor immune response or the promotion of angiogenesis (12). IL-15 serves multiple functions,
including dictating the T-cell response, regulating tissue repair
and B-cell homing, modulating inflammation and activating NK cells
(13). IL-17 has a well-recognized
role in immune surveillance at mucosal and barrier surfaces but has
also been increasingly implicated as a driver of immunopathology in
settings of autoimmunity and chronic inflammation (14). IL-1 receptor antagonist (IL-1RA)
can suppress tumors by promoting tumor angiogenesis through
vascular endothelial growth factor (VEGF) production, and a study
indicated that downregulation of IL-1RA is closely related to TNM
staging and survival prognosis (15). IL-9 exerts unprecedented antitumor
immune effects not only by inducing innate and adaptive immune
responses but also by directly promoting the apoptosis of tumor
cells (16). Eotaxin-1/eosinophils
appear to have a role in coronary artery disease independent of
known risk factors (17).
IFN is a special kind of protein or glycoprotein
produced by the body in response to various stimuli (including
viruses). It can regulate innate immunity, activated antiviral
properties and antiproliferative function. Furthermore, IFN-γ is
probably one of the most relevant cytokines for orchestrating the
immune response in vertebrates (18).
Growth factors are a class of peptides that can
affect the growth, differentiation and apoptosis process and
regulate immunity of a variety of cells. They include a wide
variety of substances, including insulin-like growth factor,
epidermal growth factor and transforming growth factor-β. VEGF is a
growth factor with important pro-angiogenic activity, and it exerts
mitogenic and anti-apoptotic effects on endothelial cells,
increases vascular permeability and promotes cell migration
(19). Furthermore, basic
fibroblast growth factor (bFGF) lacks somnogenic activity (14), but it stimulates proliferation and
hyaluronan production. Platelet-derived growth factor-BB (PDGF-BB)
has a role in suppressing proliferation, angiogenesis and
osteogenesis (20).
TNF is a pro-inflammatory cytokine. It has a
pro-inflammatory effect and can induce necrosis of tumor cells. In
addition, it is also involved in the occurrence of fever and
inflammation. Macrophage inflammatory protein (MIP)-1β and TNF-α
are produced by individual unstimulated and
lipopolysaccharide-stimulated human macrophages (21), and elevated circulating levels of
MIP-1β may be associated with a lower risk of rheumatoid arthritis
(RA) (22). Chemokines are
cytokines with chemotactic properties for inflammatory cells and
other cell types. Regulated upon activation, normal T cell
expressed and presumably secreted (RANTES), MCP-1 (also known as
MCAF), IL-8 and IL-13 have fundamental roles in histamine and
serotonin generation and cell function in mast cells (23). MIP-1α is an important chemokine and
a prognostic biomarker in both solid and hematological malignancies
(24). IFN-γ-induced protein 10
(IP-10) participates in the formation of the proinflammatory immune
microenvironment during early pregnancy by regulating the
distribution of immune cells and promoting the production of
proinflammatory cytokines (25).
Specifically targeting the crosstalk between T cells and myeloid
cells through granulocyte-macrophage colony-stimulating factor
(GM-CSF) holds promise for the development of therapeutics to
combat chronic tissue inflammation (26). Therefore, in the present study, the
levels of 25 cytokines in four differentiated gastric cancer cell
lines were measured after treatment with ACBP combined with OXA.
Gaining better insight into the host immune response and inhibition
of gastric cancer cell proliferation may be the basis for the
identification of immunotherapeutic targets, particularly for
severe cases in which ACBP combined with OXA treatment is not
sufficient.
Materials and methods
Cells and materials
The anticancer active peptide used in the present
study, anticancer bioactive peptide (ACBP) is a
low-molecular-weight active substance that was obtained from the
Clinical Medical Research Center, Affiliated Hospital of Inner
Mongolia Medical University (Hohhot, China). The poorly
differentiated human gastric adenocarcinoma cell line of MKN-45,
the highly differentiated human gastric adenocarcinoma cell line
NCI-N87 and the immortalized and non-tumorigenic human gastric
mucosal epithelial cell line GES-1 were purchased from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China).
RPMI-1640 medium and high-glucose DMEM were purchased from Hyclone
(Cytiva). Penicillin, streptomycin, fetal bovine serum and 0.25%
trypsin were from Gibco (Thermo Fisher Scientific, Inc.).
The Bio-Plex ProT Human Cytokine 27-plex Assay (cat.
no. M500KCAFOY; Bio-Rad Laboratories, Inc.) is a multiplex assay
that detects cytokines including IL-1β, IL-1RA, IL-2, IL-4, IL-6,
IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, Eotaxin, bFGF,
GM-CSF, IFN-γ, MCP-1 (MCAF), IP-10, MIP-1α, PDGF-BB, MIP-1β,
RANTES, TNF-α and VEGF. OXA was obtained from Jiangsu Hengrui
Pharmaceutical Co., Ltd.
Cell recovery
MKN-45 cells were cultured in high-glucose DMEM
supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin and
10% fetal bovine serum. N87 cells and GES-1 cells were cultured in
RPMI-1640 medium supplemented with 100 U/ml penicillin, 100 µg/ml
streptomycin and 10% fetal bovine serum. The MKN-45, N87 and GES-1
cells were mixed well and centrifuged at 85 x g at 25˚C for 5 min.
The supernatants were discarded and the cells were resuspended in 1
ml complete medium. Then, the cells were plated in 10-cm dishes,
complete medium was added to a final volume of 10 ml; the cells
were then mixed and placed in a 37˚C incubator with saturated
humidity and 5% CO2.
Cell treatment and cytokine assay
MKN-45, N87 and GES-1 cells were digested,
suspended, counted and seeded in 96-well plates at 5,000 cells per
well. After 24 h, the cells were divided into four groups: OXA
group (11.7 µg/ml), ACBP group (18.8 µg/ml), combined drug group
(5.85 µg/ml OXA + 9.4 µg/ml ACBP) and control group. Cell
supernatants were collected 48 h after treatment and the protein
concentrations were determined. In the present study, the Bio-Plex
ProT Human Cytokine 27-plex Assay was used for determining the
levels of the 25 cytokines, and the expression levels of multiple
cytokines were detected. Cytokines, including IL-1β, IL-1RA, IL-2,
IL-4, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17,
Eotaxin, bFGF, GM-CSF, IFN-γ, MCP-1 (MCAF), IP-10, MIP-1α, PDGF-BB,
MIP-1β, RANTES, TNF-α and VEGF, were diluted to 1X and added to
plates at volumes of 50 µl per well at room temperature for 30 min.
They were washed twice with 100 µl wash buffer and 50 µl of the
collected cell supernatant was added to each well. The plates were
sealed and incubated in the dark at room temperature with shaking
for 30 min. The cells were washed 3 times with 100 µl wash buffer.
Subsequently, 25 µl of 1X antibodies were added per well. The
plates were sealed and incubated in the dark at room temperature
with shaking at room temperature for 30 min. The plates were washed
3 times with 100 µl wash buffer and 50 µl 1X
streptavidin-phycoerythrin was then added to each well. The wells
were sealed, incubated in the dark at room temperature with shaking
at 600 mm/sec for 10 min and washed 3 times with 100 µl wash
buffer. The pellet was resuspended in 125 µl assay buffer, sealed
and incubated in the dark at room temperature with shaking at 600
mm/sec for 30 sec. The biotin-labeled antibody and
streptavidin-phycoerythrin conjugates were part of the assay kit.
After a further wash, the assay buffer was added to wells to
re-suspend the beads, and the fluorescence was measured using an
automatic immunoassay analyzer (Bio-Plex® 200 System;
Bio-Rad Laboratories, Inc.). Finally, the cytokine concentration
was calculated from the standard curve (27).
Statistical analysis
Statistical analyses were performed using SPSS
version 15.0 (SPSS, Inc.). Values are expressed as the mean ±
standard deviation. One-way analysis of variance was used for
multiple-group comparisons and Bonferroni's post-hoc test was used
for subsequent pairwise comparisons. P<0.05 was considered
statistically significant.
Results
Expression of immune regulatory
factors
As indicated in Fig.
1, the levels of IL-2, IL-4, IL-7, IL-10, IFN-γ and GM-CSF in
the supernatants of MKN-45 gastric cancer cells were decreased
after treatment with ACBP compared with the control group
(P<0.01). The IL-12, IFN-γ, GM-CSF, VEGF, IL-10 and IL-13 levels
were decreased in the supernatants of the N87 gastric cancer cell
line after treatment with ACBP compared with the control
(P<0.01). The IL-2, GM-CSF and IFN-γ levels in the supernatants
of GES-1 cells were increased by ACBP treatment compared with the
control (P<0.01). In addition, the levels of IL-2, IL-12, IL-7,
IL-10, IL-13, GM-CSF and IFN-γ and VEGF in the supernatants of
MKN-45 and N87 cells were decreased by ACBP and combination
treatment compared with the control (P<0.01). However, the IL-4,
IL-7, IFN-γ and GM-CSF levels were increased in the supernatants of
GES-1 cells after combination treatment compared with the control
(P<0.01). Furthermore, the IL-2 and IL-13 expression levels were
not significantly different from those in the control group in
GES-1 cells.
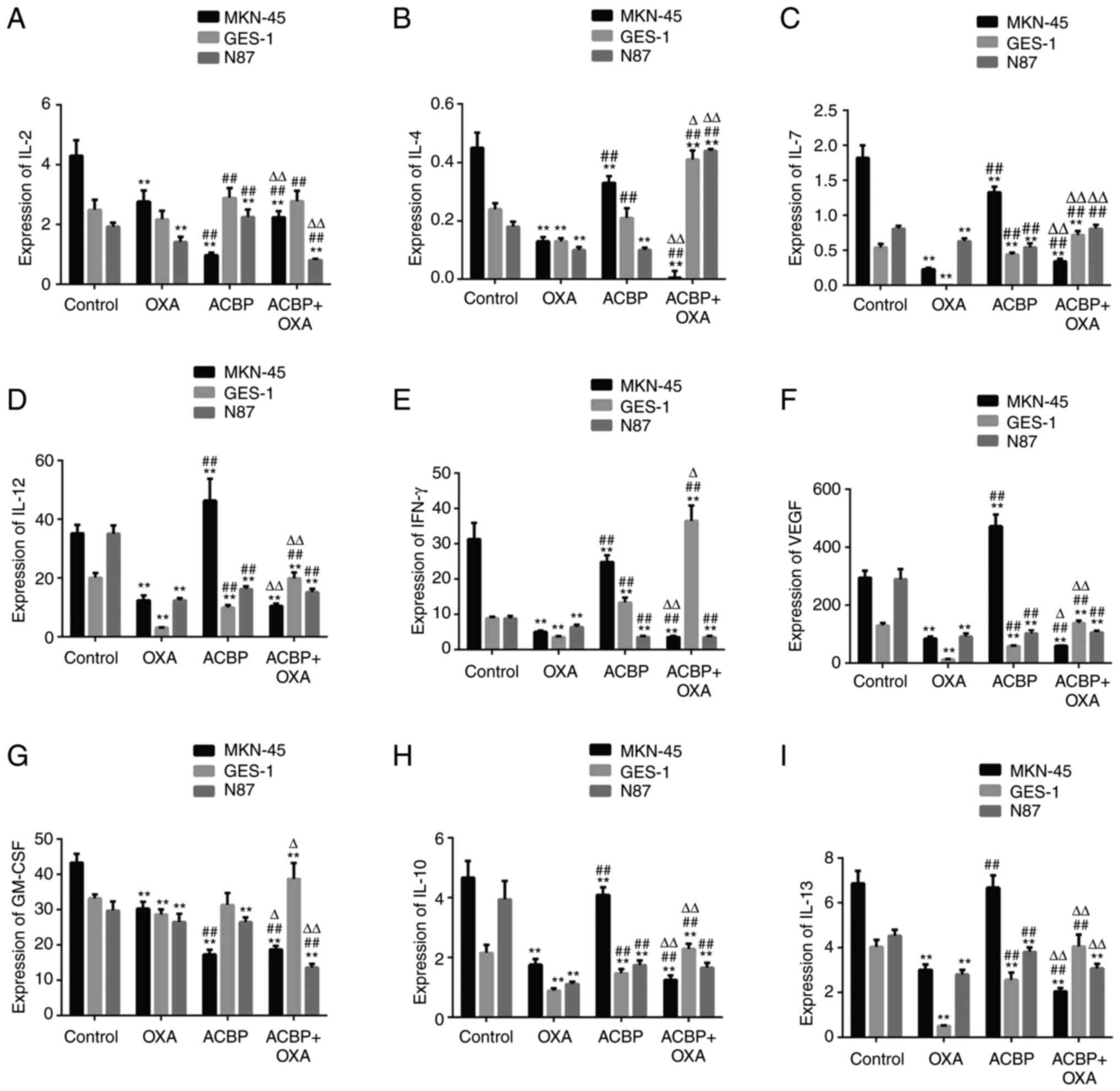 | Figure 1Expression of immune regulatory
factors. The levels of (A) IL-2, (B) IL-4, (C) IL-7, (D) IL-12, (E)
IFN-γ, (F) VEGF, (G) GM-CSF, (H) IL-10, (I) IL-13 in the
supernatants of GES-1, MKN-45 and N87 gastric cancer cells were
decreased after treatment with ACBP or ACBP combined with OXA.
**P<0.01 vs. control group; ##P<0.01
vs. OXA group, ∆P<0.05, ∆∆P<0.01 vs.
ACBP group. GM-CSF, granulocyte-macrophage colony-stimulating
factor; IFN, interferon; IL, interleukin; OXA, oxaliplatin; ACBP,
anticancer bioactive peptide. |
Compared with ACBP, IL-2 and GM-CSF levels were
significantly increased in MKN-45 cells after treatment with ACBP
combined with OXA, while the IL-4, IL-7, IL-10, IL-12, IL-13, IFN-γ
and VEGF levels were significantly decreased (P<0.01). After N87
cells were treated with the combination of ACBP and OXA, IL-4 and
IL-7 levels were increased (P<0.01), IL-2, IL-13 and GM-CSF
levels were decreased (P<0.01, P<0.05) and IL-10, IL-12,
IFN-γ and VEGF levels were not significantly different compared
with the ACBP group. Furthermore, after combination treatment, the
IL-7, IL-10, IL-12, IL-13, IFN-γ, GM-CSF and VEGF levels were
significantly increased (P<0.01 or P<0.05) in the
supernatants of the GES-1 cells compared with the ACBP group.
Compared with OXA, the levels of IL-4, IL-7, IL-10,
IL-12, IL-13, IFN-γ, and VEGF in the supernatants of MKN-45 were
significantly increased (P<0.01) after treatment with ACBP. In
addition, the levels of IL-7 in the supernatants of MKN-45 were
significantly increased, while the levels of IL-2, IL-10, IL-13,
IFN-γ, and GM-CSF and VEGF expression were decreased (P<0.01)
after treatment with ACBP combined with OXA. Furthermore, compared
with OXA, the levels of IL-2, IL-7, IL-10, IL-12, IL-13, IFN-γ,
VEGF and GM-CSF in the supernatants of N87 were significantly
increased or decreased (P<0.01) after treatment with ACBP. The
levels of IL-2, IL-4, IL-7, IL-10, IL-12, IFN-γ, VEGF and GM-CSF in
the supernatants of N87 were significantly increased or decreased
(P<0.01) after treatment with ACBP combined with OXA. Compared
with OXA, the levels of IL-2, IL-4, IL-7, IL-10, IL-12, IL-13,
IFN-γ and VEGF in the supernatants of GES-1 were significantly
increased (P<0.01) after treatment with ACBP or ACBP combined
with OXA, while the levels of GM-CSF showed no significant
difference.
Expression of tumor growth
factors
As presented in Fig.
2, the results showed that the levels of IL-9, IL-15, bFGF and
PDGF-BB in the supernatants of the MKN-45 and N87 gastric cancer
cell lines were significantly decreased after treatment with ACBP
and combination of ACBP and OXA compared with the control group
(P<0.01). The IL-8 levels were significantly increased in the
supernatants of the MKN-45 cells and GES-1 cells (P<0.01) after
ACBP treatment and combination treatment. The IL-1β and IL-15
levels were significantly decreased in N87 cells, and IL-1RA,
IL-1β, IL-8, IL-9, IL-17, bFGF and PDGF-BB levels were
significantly increased in the supernatants of GES-1 cells after
treatment with ACBP and OXA compared with the control group
(P<0.01).
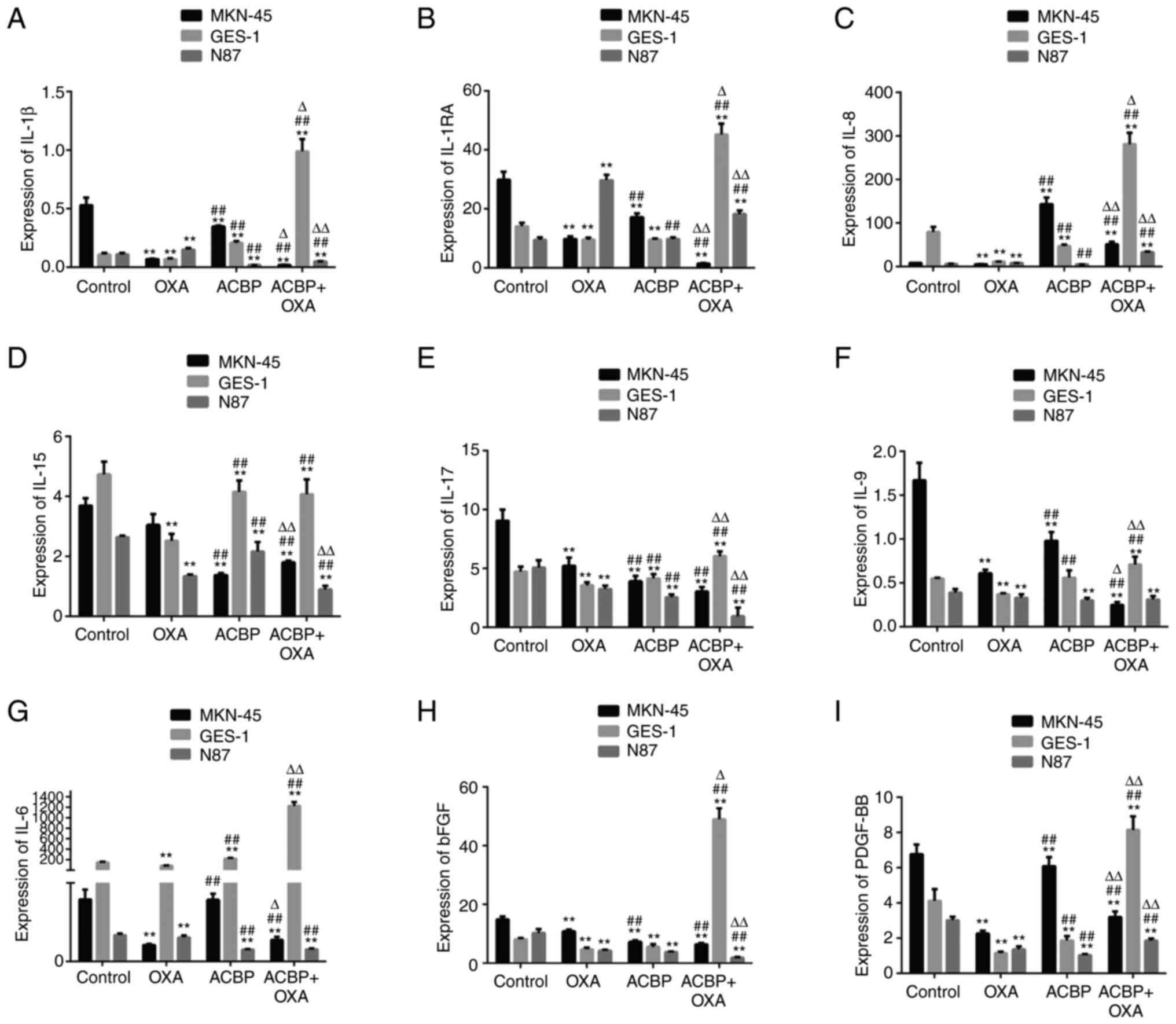 | Figure 2Expression of tumor growth factors.
The levels of (A) IL-1β, (B) IL-1RA, (C) IL-8, (D) IL-15, (E)
IL-17, (F) IL-9, (G) IL-6, (H) basic FGF and (I) PDGF-BB in the
supernatants of GES-1, MKN-45 and N87 gastric cancer cells were
decreased after treatment with ACBP or ACBP combined with OXA.
**P<0.01 vs. control group; ##P<0.01
vs. OXA group, ∆P<0.05, ∆∆P<0.01 vs.
ACBP group. FGF, fibroblast growth factor; IL-1RA, interleukin 1
receptor antagonist; PDGF, platelet-derived growth factor; OXA,
oxaliplatin; ACBP, anticancer bioactive peptide. |
As shown in Fig. 2,
the IL-1RA, IL-1β, IL-6, IL-9 and PDGF-BB levels were significantly
decreased in the supernatants of MKN-45 cells after combination
treatment compared with ACBP-treated cells (P<0.01), while the
levels of IL-17 and bFGF showed no significant difference. After
combination treatment, the IL-1RA, IL-1β, IL-6, IL-8, IL-9, IL-17
and PDGF-BB levels in the supernatants of the GES-1 cells were all
significantly increased (P<0.01 or P<0.05) compared with the
ACBP group. The results showed that the expression of IL-8 in the
culture medium of the gastric cancer cell line MKN-45 was
significantly increased (P<0.01). After treating MKN-45 cells
with the combination of drug treatment, IL-8 was also elevated but
not significantly increased when using the ACBP alone. The
treatment of N87 cell with ACBP showed no significant change in
IL-8 expression (P>0.01), while the expression level of IL-8 was
significantly increased after combination of drug treatment
(P<0.01). Compared with the OXA group, the IL-1β, IL-8, IL-6,
IL-15, IL-17 and PDGF-BB levels in the supernatants of MKN-45,
GES-1 and N87 cells were significantly different after ACBP
treatment (P<0.01). The IL-1RA, IL-1β, IL-8, IL-6, IL-15, IL-17,
bFGF and PDGF-BB levels in the supernatants of MKN-45 cells, GES-1
and N87 after ACBP combined with OXA treatment exhibited
significant differences compared with OXA treatment
(P<0.01).
Expression of chemotactic factors
As indicated in Fig.
3, the levels of MCP-1 (MCAF), Eotaxin, MIP-1α and MIP-1β were
significantly decreased in the supernatants of MKN-45 cells after
treatment with ACBP compared with the control (P<0.01), while
IP-10, TNF-α and RANTES showed no significant difference
(P>0.05). The Eotaxin, IP-10 and RANTES levels were
significantly increased in the supernatants of N87 cells after ACBP
treatment (P<0.01). The MCP-1 (MCAF), MIP-1α and MIP-1β levels
were not significantly different in N87 cell after ACBP treatment.
Furthermore, the MCP-1 (MCAF), MIP-1β, IP-10 and RANTES levels were
significantly decreased, while the MIP-1α, Eotaxin and TNF-α levels
were significantly decreased in the supernatants of GES-1 cells
after ACBP treatment (P<0.01).
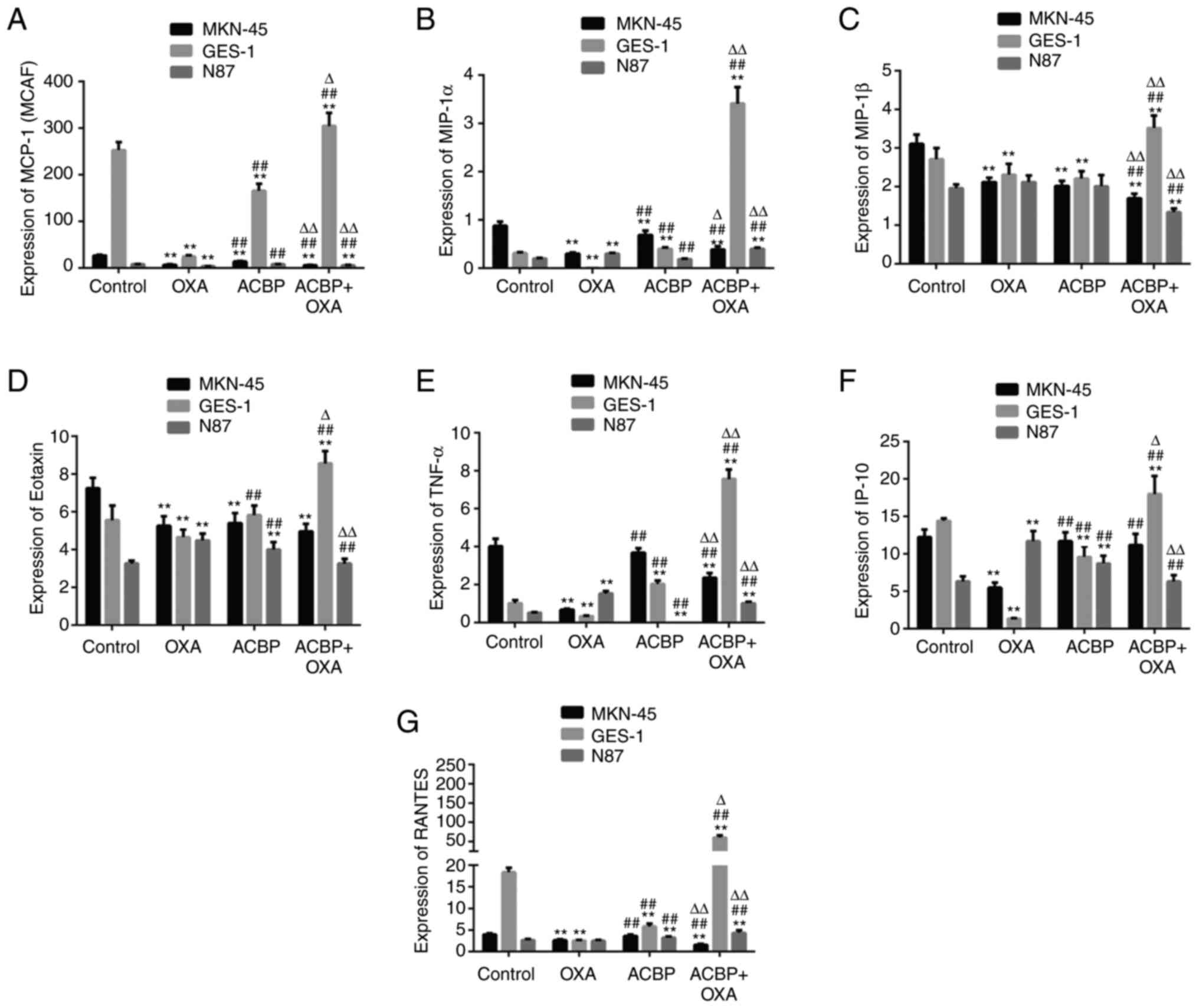 | Figure 3Expression of chemotactic factors.
The levels of (A) MCP-1, (B) MIP-1α, (C) MIP-1β, (D) Eotaxin, (E)
TNF-α, (F) IP-10 and (G) RANTES in the supernatants of GES-1,
MKN-45 and N87 gastric cancer cells were decreased after treatment
with ACBP or ACBP combined with OXA. **P<0.01 vs.
control group; ##P<0.01 vs. OXA group,
∆P<0.05, ∆∆P<0.01 vs. ACBP group. MIP,
macrophage inflammatory protein; OXA, oxaliplatin; ACBP, anticancer
bioactive peptide; MCP, monocyte chemoattractant protein; IP-10,
interferon-γ-induced peptide 10; RANTES, regulated upon activation,
normal T cell expressed and presumably secreted. |
Compared with ACBP, the levels of MCP-1 (MCAF),
MIP-1α, MIP-1β, TNF-α and RANTES were shows significantly
difference in the supernatants of GES-1, MKN-45 and N87 cells after
ACBP combined with OXA treatment (P<0.01). The MCP-1 (MCAF),
MIP-1β, Eotaxin levels were significantly decreased in the
supernatants of MKN-45 and N87 cells after ACBP combined with OXA
treatment (P<0.01). The MIP-1α, TNF-α and RANTES levels were
significantly increased in the supernatants of N87 cells after
combination treatment (P<0.01). However, the levels of IP-10 and
Eotaxin showed no significant difference after combination
treatment in MKN-45 cells.
Compared with OXA, the levels of MCP-1 (MCAF),
MIP-1α, MIP-1β, TNF-α and RANTES were significantly increased
significantly in the supernatants of GES-1 and decreased in the
MKN-45 by ACBP combined with OXA treatment (P<0.01).
Furthermore, the Eotaxin levels in the supernatants of GES-1 were
significantly increased and decreased in the supernatants of N87
cells after combined treatment compared with OXA (P<0.01). The
level of IP-10 in the supernatants of MKN-45 and GES-1 was
significantly increased (P<0.01), while it exhibited no
significant difference in N87 cells after the ACBP and OXA
combination treatment, compared with the OXA group.
Discussion
The anti-inflammatory cytokines are a series of
immunoregulatory molecules that control the proinflammatory
cytokine response. Major anti-inflammatory cytokines include
IL-1RA, IL-4, IL-6, IL-10, IL-11 and IL-13.
IL-2 is an important immune modulator synthesized
and released after activation by lymphocytes by specific antigen
stimulation (28). IL-2 has
antitumor effects and it binds to the IL-2 receptor on the cell
membrane. IL-2, the first cytokine that was molecularly cloned, was
shown to be a T-cell growth factor essential for the proliferation
of T cells and the generation of effector and memory cells
(29). IL-4 is also one of the key
cytokines in tumor immunity. IL-4 causes the body to eliminate
tumors. In different ways, IL-4 leads to weakened T cell-mediated
immune function, promoting tumorigenesis and progression. IL-13 is
a multipotent proinflammatory cytokine with a molecular weight of
~12 kDa that is mainly secreted by Th2 cells. The results of the
present study indicated that the IL-13 levels in MKN-45 cells did
not change significantly after ACBP treatment, but the IL-13 levels
in MKN-45 cells decreased after the combination treatment. The
levels of IL-13 in the N87 gastric cancer cell line were decreased
after ACBP or combination treatment. The levels of IL-13 in GES-1
cells were reduced after ACBP treatment, with no significant change
in the IL-13 levels in GES-1 cells after the combination
treatment.
The IL-4 and IL-13 may have a significant role in
the downregulation of inflammatory processes underlying RA
pathology and beneficially modulate the course of the disease
(30).
IL-6 is a prototypical cytokine for maintaining
homeostasis (31). The IL-6
cooperative factor granulocyte colony-stimulating factor (G-CSF)
can alter neutrophil function before neutrophils enter the tumor
microenvironment. Neutrophils regulated by G-CSF/IL-6 promote tumor
angiogenesis before entering the tumor microenvironment, so IL-6
can promote tumorigenesis and progression (32). IL-10 is an important
immunosuppressor in the process of tumor development. It can exert
its immunosuppressive role by suppressing the functions of
lymphocytes, macrophages, dendritic cells and other immune cells so
that tumors can develop through immune escape (33). The cytokine IL-10 is a key
anti-inflammatory mediator ensuring protection of a host from
over-exuberant responses to pathogens and microbiota, while having
important roles in other settings such as sterile wound healing,
autoimmunity, cancer and homeostasis (34).
IL-7 is a cytokine that is mainly secreted by
thymocytes, bone marrow stromal cells, small intestinal epithelial
cells and skin keratinocytes during the normal development of the
human immune system. IL-7 has an important role in maintaining
human immune system homeostasis (35). In addition, it can induce the
growth and proliferation of hematopoietic cells and hematological
malignant tumor cells (such as leukemia and lymphoma).
As a cytokine and immunological modulatory factor,
IL-12 has a direct antagonistic effect on tumors and has an
important role in the immune response of both primary and secondary
tumors (36). The results of the
present study showed that the levels of IL-12 were significantly
increased after treatment of MKN-45 cells with ACBP, but IL-12
levels decreased after the combination treatment. The anticancer
biological activity and the levels of IL-12 in the supernatant were
decreased in the N87 gastric cancer cell line.
GM-CSF was originally identified as a growth factor
due to its ability to promote the proliferation and differentiation
of bone marrow progenitor cells into granulocytes and macrophages
in vitro (37). It can not
only stimulate the generation of blood cells such as macrophages
but also induce humoral and cellular immunity, thus increasing the
bactericidal activity of effector cells in natural immunity.
GM-CSF, as an immune adjuvant for melanoma vaccines, was more
durable and more effectively enhanced the immune effect of tumor
vaccines relative to other cytokines (IL-4, IL-6, etc.).
Considering the well-characterized antitumor effects of this
cytokine, many clinical trials and immunotherapy approaches have
been designed to enhance IFN-γ-mediated immunity for different
types of cancer (38). The results
of this experiment showed that the GM-CSF and IFN-γ levels were
decreased in MKN-45 and N87 cells after ACBP and OXA combined
treatment.
Cancer development and its response to therapy are
regulated by inflammation, which either promotes or suppresses
tumor progression, potentially displaying opposing effects on
therapeutic outcomes. Chronic inflammation facilitates tumor
progression and treatment resistance, whereas induction of acute
inflammatory reactions often stimulates the maturation of dendritic
cells and antigen presentation, leading to anti-tumor immune
responses (39). IL-1β is a strong
inhibitor of gastric acid secretion that contributes to H.
pylori invasion, leading to more severe gastritis, and it may
have a role in gastric atrophy as well as gastric adenocarcinoma
progression (40). The results of
the present study showed that the levels of IL-1β in the
supernatants of the MKN-45 and N87 gastric cancer cell lines were
decreased. However, the levels in the GES-1 cells were increased
after treatment with the combination of ACBP and OXA. IL-1RA is
able to inhibit inflammation and gastric cancer development
(41). Research has indicated that
stomach-specific expression of human IL-1β in transgenic mice leads
to spontaneous gastric inflammation and cancer that correlate with
early recruitment of myeloid-derived suppressor cells to the
stomach. The same study showed that the levels of IL-1RA were
decreased in the MKN-45 and increased in the N87 gastric cancer
cell lines. Therefore, ACBP may not inhibit the growth of cancer
cells by affecting the expression level of this factor. In the
present study, the levels of IL-1RA in GES-1 cells increased
significantly after treatment with a combination of ACBP and
OXA.
High expression of IL-8 in precancerous lesions and
gastric cancer is not only associated with the occurrence,
development and division of gastric cancer but also related to the
progression of gastric cancer. The expression of IL-8 is 10 times
higher in gastric cancer than in normal tissues and is not related
to the pathological histotype of gastric cancer. Meta-analysis
results provided evidence that the IL-8-251A>T polymorphism is
significantly associated with an increased risk of gastric
carcinogenesis in Asian populations (42). The results of the present study
showed that the levels of IL-8 in the supernatants of the MKN-45
gastric cancer cell line were significantly increased after ACBP
treatment, and these levels were higher than those observed after
the MKN-45 cell line was treated with the drug combination. IL-8
levels were increased in N87 cells treated with the drug
combination, while there were no significant changes after
treatment with ACBP alone. The IL-8 levels in GES-1 cells were
decreased after ACBP treatment and the IL-8 levels were
significantly increased after treatment with the combination.
IL-9 is involved in mediating the association
between tumor cells and nonmalignant inflammatory infiltrating
cells (43). The results of the
present study showed that the levels of IL-9 in the supernatants of
the MKN-45 and N87 gastric cancer cell lines were decreased after
treatment with ACBP alone or the combination. Treatment of GES-1
cells with ACBP caused no significant change in IL-9 expression,
while IL-9 expression increased after the combination treatment of
GES-1 cells.
IL-15 is an important cytokine that promotes the
proliferation, activation and survival of NK and CD8+ T cells
(44). The IL-15 domain activates
NK and CD8+ T cells and is thus used for the treatment of tumors.
The results of the present study showed that the levels of IL-15 in
MKN-45, GES-1 and N87 cells were decreased after ACBP and the
combination treatment.
IL-17 is known as a Th17-cell-derived
proinflammatory cytokine (45).
Increased expression of IL-17 in tumor tissues can have an
oncogenic role by promoting the generation of blood vessels in
tumor tissues (46). How IL-17
inhibits tumors and the specific mechanisms of the antitumor
effects of IL-17 remain to be fully elucidated. It may also promote
the formation of blood vessels in tumor tissues and have a
tumor-promoting role. The results of the present study showed that
the levels of IL-17 were decreased after anticancer bioactive
peptides were administered to MKN-45 and N87 cells, and the levels
of IL-17 in MKN-45 and N87 cells were significantly decreased after
the combination treatment. Treatment of the GES-1 cells decreased
IL-13 expression, while IL-13 expression was increased in GES-1
cells upon combination treatment. bFGF is a pleiotropic growth
factor that promotes growth of mesenchymal and epithelial cells,
and stimulates angiogenesis and neuroprotection (47). bFGF is a polypeptide factor that,
in addition to its pro-proliferative effect on fibroblasts and
vascular endothelial cells, is involved in tumor development,
particularly tumor invasion and metastasis. bFGF promotes the
growth and development of tumor blood vessels and accelerates tumor
growth. Studies have shown that PDGF-BB promotes endothelial cell
proliferation and neovascularization, promoting tumor growth
(48). It has an important role in
promoting the emergence of new lymphatic vessels and the lymphatic
metastasis of tumors. The present results showed that the
expression levels of PDGF-BB were decreased in MKN-45 and M87 cells
after treatment with ACBP combined with OXA. Furthermore, the
levels of bFGF were decreased after the ACBP and the combination
treatment were applied to MKN-45 and N87 cells. The levels of bFGF
were decreased after treatment of the normal gastric cell line
GES-1 with ACBP, whereas the bFGF levels were significantly
increased in GES-1 cells after combination treatment.
Molecular biological studies have confirmed that
Eotaxin-1 can promote epithelial-mesenchymal transition by
activating the downstream MAPK kinase 1, ERK1/2 and transducer and
activator of transcription 3 signaling pathways, thus increasing
the invasion and metastasis abilities of tumor cells (49). The results showed that the
Eotaxin-1 levels in MKN-45 cells were decreased after ACBP or
combination treatment.
Studies have reported that the diagnostic accuracy
of IP-10 is on par with that of IFN-γ (50). IP-10 promotes the production of
IL-17 and IFN-γ and promotes the migration and differentiation of
uterine decidual T cells into Th1 cells and Th17 cells (25). As a chemokine, IP-10 is a protein
whose expression is induced by interferon stimulation, and it may
be inferred that IP-10 secretion is closely related to surgical
trauma. With postoperative injury repair, IP-10 levels gradually
decrease but are still higher than normal levels, suggesting that
IL-8 and IP-10 participate in local inflammatory infiltration and
tissue damage in HCC. MCP-1, also known as C-C-motif chemokine
ligand 2, is a member of the family of CC chemokines (51). Because in vitro-cultured
tumor cells often produce significant amounts of MCP-1, tumor cells
are considered to be the main source of MCP-1. MCP-1 production in
tumors is a consequence of complex interactions between tumor cells
and non-tumor cells (52). MIP-1α
is a member of the chemokine family and was originally determined
to be a soluble factor secreted by activated macrophages (53). MlP-1α has a strong chemotactic
capacity for monocytes, giant warm cells and lymphocytes, and
higher expression of adhesion molecules and additional related
cytokines may inhibit the growth of tumor cells through activation
of the release of lysozyme. The present results showed that the
levels of MlP-1α were decreased in MKN-45 cells after ACBP and
combination drug treatment.
TNF-α, IL-1α and IL-1β have pleiotropic properties.
Both cytokines are now known to be potent inducers of a number of
cell-selective chemotactic cytokines, which belong to a novel
superfamily of structurally related low-molecular-weight proteins.
One of the most prominent members is termed IL-8 and represents a
neutrophil-selective attractant, whereas another one called MCP-1
is a monocyte-selective chemotaxin (54). The expression of chemotactic
cytokines, i.e., MCP-1, MIP-1β, RANTES and TNF-α, is closely linked
with tumor progression and metastasis due to their overexpression
and the subsequent induction of chemoresistance (55). MIP-1β, an inflammatory cytokine,
has a cancer-promoting effect, but high concentrations can induce
tumor apoptosis. The expression of RANTES is significantly enhanced
in numerous malignant tumor tissues (56). RANTES directly acts on tumor cells
through paracrine mechanisms and it promotes tumor development,
enhances the tumor invasion ability, promotes tumor blood vessel
formation, suppresses the cellular immune responses and promotes
tumor growth (55). TNF-α is
mainly secreted by activated macrophages and can also be produced
by tumor cells, and TNF-α exerts direct antitumor effects (57). Inhibition of VEGF has been
confirmed to be an efficacious antiangiogenetic approach for cancer
treatment (58). Its main effect
is to promote neovascularization in the tumor body and the immune
suppression of the body.
The present results showed that the levels of
MIP-1β, RANTES, VEGF and TNF-α in MKN-45 cells were decreased after
treatment with ACBP combined with OXA. However, the use of only one
assay is a limitation of the present study. However, the
anti-cancer effects of these 25 cytokines are challenging to
explain/discuss due to the lack of tests dedicated to proliferation
and cell interactions, which is also a limitation of the present
study. Future studies should include apoptosis, signaling pathway
analysis or the behavior of immune cells (e.g., in co-culture).
In conclusion, in the present study, the levels of
growth factors, immune regulatory factors and chemokines that were
secreted after treatment with ACBP or ACBP+OXA were quantified. An
analysis of immune regulatory factors (IL-2, IL-4, IL-7, IL-10,
IL-12, IL-13, IFN-γ, VEGF and GM-CSF), growth factors (IL-1RA,
IL-1β, IL-8, IL-9, IL-15, IL-17, bFGF and PDGF-BB) and chemokines
[MCP-1 (MCAF), IP-10, Eotaxin, MIP-1α, MIP-1β, TNF-α and RANTES]
was performed and cytokines and growth factors from different
gastric cancer cells that can induce humoral and cellular immunity
and have anticancer biological activity were identified. These
findings support the use of the combination of ACBP and OXA for
gastric cancer treatment to inhibit tumorigenesis and progression
via the cytokines MCP-1, MIP-1β and IL-13. Therefore, the mechanism
by which gastric cancer cell proliferation and neovascularization
are promoted and tumor growth is driven will allow us to understand
and improve clinical outcomes.
Acknowledgements
The authors thank Professor Xiulan Su Inner Mongolia
Medical University (Hohhot, China) for providing the anticancer
active peptide used in the present study.
Funding
Funding: This work was financially supported by the National
Natural Science Foundation of China (grant nos. 22168028, 81660468
and 81960560), the Inner Mongolia Natural Science Fund surface
project (grant no. 2021MS02005), the Inner Mongolia Autonomous
Region Health Science and Technology Plan Project (grant no.
202201274) and the Inner Mongolia Autonomous Region ‘Grassland
Talent’ plan project.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
XLS and XL conceived and designed the study. JQP
conducted experiments and contributed new reagents or analytical
tools. XL analyzed data and wrote the manuscript. XLS and JQP
checked and approved the authenticity of the raw data. All authors
have read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang K, Chen XZ and Hu JK: Factors
associated with recurrence and survival in N0 gastric cancer. Ann
Surg. 266:e10–e11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang F, Yan Y, Cao X, Zhang J, Li Y and
Guo C: Methylation of microRNA-338-5p by EED promotes
METTL3-mediated translation of oncogene CDCP1 in gastric cancer.
Aging (Albany NY). 13:12224–12238. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Billones R, Liwang JK, Butler K, Graves L
and Saligan LN: Dissecting the fatigue experience: A scoping review
of fatigue definitions, dimensions, and measures in non-oncologic
medical conditions. Brain Behav Immun Health.
15(100266)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tian J and Hong JS: Validation of the
Chinese version of multidimensional fatigue Inventory-20 in Chinese
patients with cancer. Support Care Cancer. 20:2379–2383.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li X, Xia L, Ouyang X, Suyila Q, Su L and
Su X: Bioactive peptides sensitize cells to anticancer effects of
oxaliplatin in human colorectal cancer xenografts in nude mice.
Protein Pept Lett. 26:512–522. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Su X, Dong C, Zhang J, Su L, Wang X, Cui H
and Chen Z: Combination therapy of anti-cancer bioactive peptide
with Cisplatin decreases chemotherapy dosing and toxicity to
improve the quality of life in xenograft nude mice bearing human
gastric cancer. Cell Biosci. 4(7)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li X, Gao B and Su X: Anticancer bioactive
peptide combined with docetaxel and its mechanism in the treatment
of breast cancer. Exp Ther Med. 20:1917–1924. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li X, Wu H, Ouyang X, Zhang B and Su X:
New bioactive peptide reduces the toxicity of chemotherapy drugs
and increases drug sensitivity. Oncol Rep. 38:129–140.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang X, Guo J, Wang Y, Xiao Y, Wang L and
Hua S: Expression levels of interferon regulatory factor 5 (IRF5)
and related inflammatory cytokines associated with severity,
prognosis, and causative pathogen in patients with
community-acquired pneumonia. Med Sci Monit. 24:3620–3630.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Garlanda C, Dinarello CA and Mantovani A:
The interleukin-1 family: Back to the future. Immunity.
39:1003–1018. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gao LB, Pan XM, Jia J, Liang WB, Rao L,
Xue H, Zhu Y, Li SL, Lv ML, Deng W, et al: IL-8-251A/T polymorphism
is associated with decreased cancer risk among population-based
studies: Evidence from a meta-analysis. Eur J Cancer. 46:1333–1343.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Patidar M, Yadav N and Dalai SK:
Interleukin 15: A key cytokine for immunotherapy. Cytokine Growth
Factor Rev. 31:49–59. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Abusleme L and Moutsopoulos NM: IL-17:
Overview and role in oral immunity and microbiome. Oral Dis.
23:854–865. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen S, Shen Z, Liu Z, Gao L, Han Z, Yu S
and Kang M: IL-1RA suppresses esophageal cancer cell growth by
blocking IL-1α. J Clin Lab Anal. 33(e22903)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zheng N and Lu Y: Targeting the IL-9
pathway in cancer immunotherapy. Hum Vaccin Immunother.
16:2333–2340. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kalayci M and Gul E: Eotaxin-1 levels in
patients with myocardial infarction. Clin Lab: 68, 2022 doi:
10.7754/Clin.Lab.2021.210806.
|
|
18
|
Pereiro P, Figueras A and Novoa B:
Insights into teleost interferon-gamma biology: An update. Fish
Shellfish Immunol. 90:150–164. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|
|
20
|
Gao SY, Lin RB, Huang SH, Liang YJ, Li X,
Zhang SE, Ouyang DQ, Li K, Zheng GS and Liao GQ: PDGF-BB exhibited
therapeutic effects on rat model of bisphosphonate-related
osteonecrosis of the jaw by enhancing angiogenesis and
osteogenesis. Bone. 44(115117)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Khajvand T, Huang P, Li L, Zhang M, Zhu F,
Xu X, Huang M, Yang C, Lu Y and Zhu Z: Interfacing droplet
microfluidics with antibody barcodes for multiplexed single-cell
protein secretion profiling. Lab Chip. 21:4823–4830.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Qian Y, He Z, Zhao SS, Liu B, Chen Y, Sun
X, Ye D, Jiang X, Zheng H, Wen C, et al: Genetically determined
circulating levels of cytokines and the risk of rheumatoid
arthritis. Front Genet. 13(802464)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Castellani ML, De Lutiis MA, Toniato E,
Conti F, Felaco P, Fulcheri M, Theoharides TC, Caraffa A, Antinolfi
P, Conti P, et al: Impact of RANTES, MCP-1 and IL-8 in mast cells.
J Biol Regul Homeost Agents. 24:1–6. 2010.PubMed/NCBI
|
|
24
|
Ntanasis-Stathopoulos I, Fotiou D and
Terpos E: CCL3 signaling in the tumor microenvironment. Adv Exp Med
Biol. 1231:13–21. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang Y, Huang F, Chai X, Yuan W, Ding H
and Wu X: The role of IP-10 and its receptor CXCR3 in early
pregnancy. Mol Immunol. 140:59–69. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Becher B, Tugues S and Greter M: GM-CSF:
From growth factor to central mediator of tissue inflammation.
Immunity. 45:963–973. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kanda T, Yoshida A, Ogihara K, Minami H,
Yamaguchi N, Ikebuchi Y, Nakao K and Isomoto H: Detection of
cytokine storm in patients with achalasia using ELISA. Biomed Rep.
15(62)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bernstein JM, Ballow M, Xiang S and O'Neil
K: Th1/Th2 cytokine profiles in the nasopharyngeal lymphoid tissues
of children with recurrent otitis media. Ann Otol Rhinol Laryngol.
107:22–27. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Abbas AK, Trotta E, R Simeonov D, Marson A
and Bluestone JA: Revisiting IL-2: Biology and therapeutic
prospects. Sci Immunol. 3(eaat1482)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Iwaszko M, Biały S and Bogunia-Kubik K:
Significance of Interleukin (IL)-4 and IL-13 in Inflammatory
Arthritis. Cells. 10(3000)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tanaka T, Narazaki M and Kishimoto T:
Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol.
10(a028456)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yan B, Wei JJ, Yuan Y, Sun R, Li D, Luo J,
Liao SJ, Zhou YH, Shu Y, Wang Q, et al: IL-6 cooperates with G-CSF
to induce protumor function of neutrophils in bone marrow by
enhancing STAT3 activation. J Immunol. 190:5882–5893.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yaseen MM, Abuharfeil NM, Darmani H and
Daoud A: Mechanisms of immune suppression by myeloid-derived
suppressor cells: The role of interleukin-10 as a key
immunoregulatory cytokine. Open Biol. 10(200111)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Saraiva M, Vieira P and O'Garra A: Biology
and therapeutic potential of interleukin-10. J Exp Med.
217(e20190418)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang C, Kong L, Kim S, Lee S, Oh S, Jo S,
Jang I and Kim TD: The Role of IL-7 and IL-7R in cancer
pathophysiology and immunotherapy. Int J Mol Sci.
23(10412)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nguyen KG, Vrabel MR, Mantooth SM, Hopkins
JJ, Wagner ES, Gabaldon TA and Zaharoff DA: Localized
Interleukin-12 for cancer immunotherapy. Front Immunol.
11(575597)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Achuthan AA, Lee KMC and Hamilton JA:
Targeting GM-CSF in inflammatory and autoimmune disorders. Semin
Immunol. 54(101523)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kursunel MA and Esendagli G: The untold
story of IFN-γ in cancer biology. Cytokine Growth Factor Rev.
31:73–81. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y
and Li Y: Inflammation and tumor progression: Signaling pathways
and targeted intervention. Signal Transduct Target Ther.
6(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Micev M and Cosić-Micev M: Pathology and
pathobiology of the gastric carcinoma. Acta Chir Iugosl. 58:39–52.
2011.PubMed/NCBI View Article : Google Scholar : (In Serbian).
|
|
41
|
Tu S, Bhagat G, Cui G, Takaishi S,
Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl
O, Fox JG and Wang TC: Overexpression of interleukin-1beta induces
gastric inflammation and cancer and mobilizes myeloid-derived
suppressor cells in mice. Cancer Cell. 14:408–419. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cheng D, Hao Y, Zhou W and Ma Y: Positive
association between Interleukin-8-251A>T polymorphism and
susceptibility to gastric carcinogenesis: A meta-analysis. Cancer
Cell Int. 13(100)2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sun PS, Gao ZJ, Fan LX, Liu YF, Chen BH,
Mu SZ and Yan ZQ: The regulatory function of tumor-infiltrating Th9
cells to anti-tumor activity of CD8(+) T cells in patients with
gastric cancer. Zhonghua Zhong Liu Za Zhi. 44:1186–1193.
2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
44
|
Jakobisiak M, Golab J and Lasek W:
Interleukin 15 as a promising candidate for tumor immunotherapy.
Cytokine Growth Factor Rev. 22:99–108. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shi JL, Zheng ZM, Chen M, Shen HH, Li MQ
and Shao J: IL-17: An important pathogenic factor in endometriosis.
Int J Med Sci. 19:769–778. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Meng XY, Zhou CH, Ma J, Jiang C and Ji P:
Expression of interleukin-17 and its clinical significance in
gastric cancer patients. Med Oncol. 29:3024–3028. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Florkiewicz RZ, Ahluwalia A, Sandor Z,
Szabo S and Tarnawski AS: Gastric mucosal injury activates bFGF
gene expression and triggers preferential translation of high
molecular weight bFGF isoforms through CUG-initiated, non-canonical
codons. Biochem Biophys Res Commun. 409:494–499. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xue Y, Lim S, Yang Y, Wang Z, Jensen LD,
Hedlund EM, Andersson P, Sasahara M, Larsson O, Galter D, et al:
PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing
erythropoietin production in stromal cells. Nat Med. 18:100–110.
2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shi L, Wang L and Wang X: Osteopontin
induces Epithelial-to-Mesenchymal transitions in human lung cancer
cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Chest.
149(A332)2016.
|
|
50
|
Ruhwald M, Aabye MG and Ravn P: IP-10
release assays in the diagnosis of tuberculosis infection: Current
status and future directions. Expert Rev Mol Diagn. 12:175–187.
2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Singh S, Anshita D and Ravichandiran V:
MCP-1: Function, regulation, and involvement in disease. Int
Immunopharmacol. 101(107598)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yoshimura T: The chemokine MCP-1 (CCL2) in
the host interaction with cancer: A foe or ally? Cell Mol Immunol.
15:335–345. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hata H: Bone lesions and macrophage
inflammatory protein-1 alpha (MIP-1a) in human multiple myeloma.
Leuk Lymphoma. 46:967–972. 2005.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Schröder JM: Peptides and cytokines. Arch
Dermatol Res. 284 (Suppl 1):S22–S26. 1992.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shim YJ, Kang BH, Choi BK, Park IS and Min
BH: Clusterin induces the secretion of TNF-α and the chemotactic
migration of macrophages. Biochem Biophys Res Commun. 422:200–205.
2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Propst SM, Estell K and Schwiebert LM:
CD40-mediated activation of NF-kappa B in airway epithelial cells.
J Biol Chem. 277:37054–37063. 2002.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wang Y, Bao M, Hou C, Wang Y, Zheng L and
Peng Y: The Role of TNF-α in the pathogenesis of Temporomandibular
disorders. Biol Pharm Bull. 44:1801–1809. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Peng K, Bai Y, Zhu Q, Hu B and Xu Y:
Targeting VEGF-neuropilin interactions: A promising antitumor
strategy. Drug Discov Today. 24:656–664. 2019.PubMed/NCBI View Article : Google Scholar
|

















