Introduction
The global incidence of urothelial carcinoma is
steadily rising, notably occurring in individuals as young as 55
years old (1). Recent advances in
treatment have resulted in improved survival rates, allowing a
significant proportion of patients to thrive for numerous years,
even in cases of metastatic disease (1).
Enfortumab vedotin (EV) is an innovative
antibody-drug conjugate (ADC) designed to target nectin-4, a highly
expressed adhesion protein in urothelial carcinoma. It employs a
precise binding mechanism with tumor cells, leading to cell death
upon internalization of monomethyl auristatin E (MMAE), a potent
microtubule-disrupting agent (2).
This targeted approach holds promise for patients with metastatic
urothelial carcinoma with limited treatment options, especially
those with disease progression after platinum-containing
chemotherapy and programmed cell death protein 1 (PD-1) or
programmed death-ligand 1 inhibitor treatment. Notably, a landmark
study by Powles et al (2)
reported a 30% lower risk of death in EV patients compared with
that of patients receiving chemotherapy during an 11-month median
follow-up.
However, despite these promising results, the
present case illustrates the critical need for vigilance in
addressing rare yet life-threatening complications of this
treatment.
The present 56-year-old patient with metastatic
urothelial carcinoma treated with EV developed two critical
conditions. First, the patient developed refractory insulin
resistance after receiving two doses of EV, manifesting as
ketoacidosis despite elevated insulin and C-peptide secretion
levels. Subsequently, the patient exhibited hemophagocytic
lymphohistiocytosis (HLH), with this hyperinflammatory syndrome
ultimately leading to a fatal septic shock.
The present case emphasizes some critical care
aspects associated with EV treatment in metastatic urothelial
carcinoma, highlighting the need for early recognition,
understanding and management of such severe complications.
Case report
The present report describes the case of a
56-year-old male patient of Middle Eastern descent with metastatic
urothelial carcinoma. The patient had no significant medical
history except for previous tobacco use, and his family history was
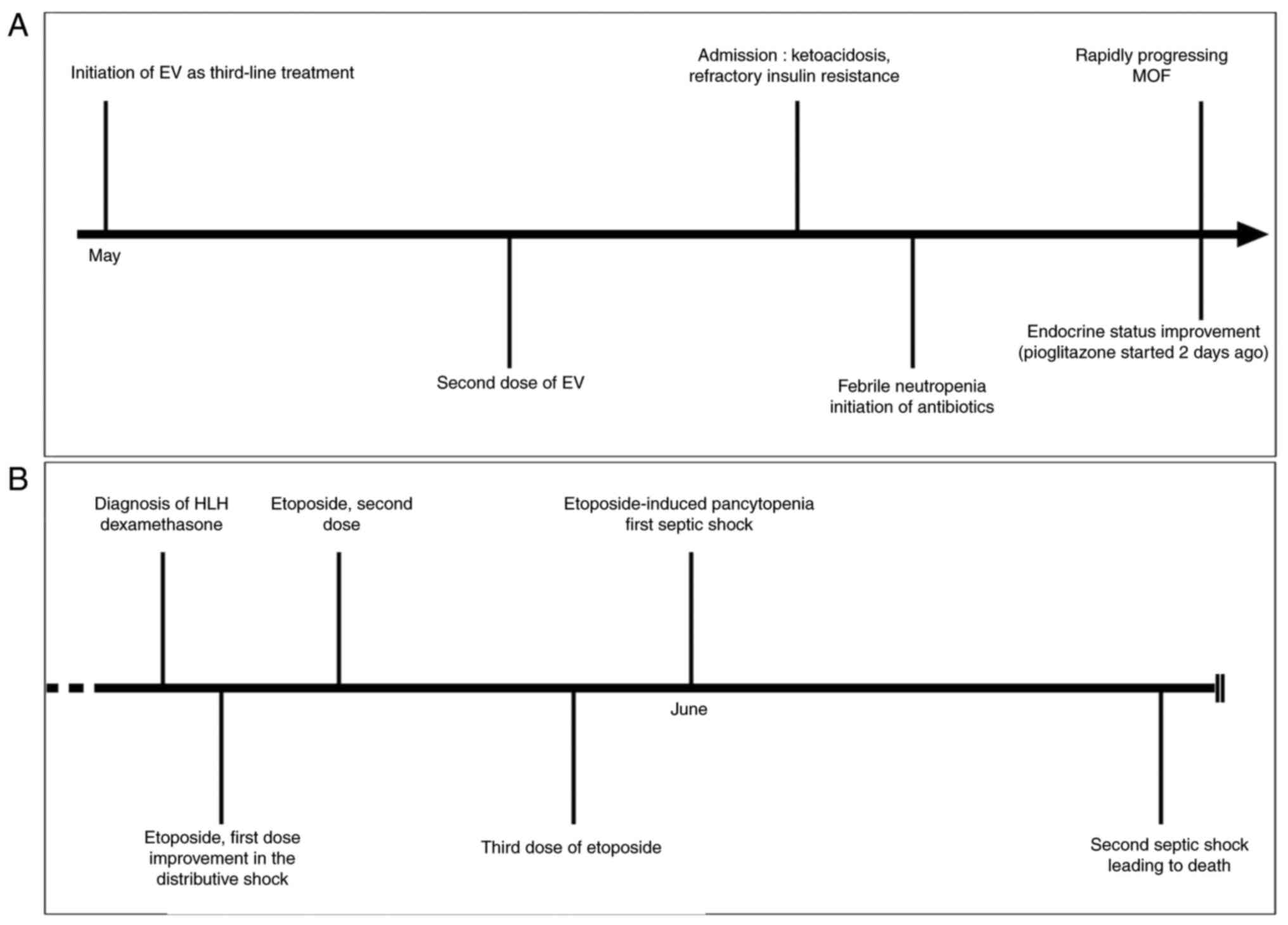
unremarkable. Table I and Fig. 1 provide a chronological summary of
events related to the medical care of the patient.
 | Table IChronological events summary. |
Table I
Chronological events summary.
| Date | Event |
|---|
| Oct 2021 | Initial staging:
T2N1M1 urothelial carcinoma with lung metastases. |
| Oct 2021-Jan
2022 | First-line
chemotherapy: Cisplatin and gemcitabine. |
| Aug 2022-Apr
2023 | Second-line
treatment: Pembrolizumab. Complete remission achieved. |
| May 2023 | Recurrence of lung
metastasis. |
| May 2023 | Initiation of EV as
third-line treatment. |
| May 2023 | Second dose of EV,
seven days after the first dose. |
| May 2023; 1st
hospitalization day, 5 days following EV | Hospitalization due
to refractory insulin resistance and ketoacidosis. Initiation of
intra venous insulin therapy, up to 800 units per day, showing
minimal response. |
| 3rd hospitalization
day | Fever, neutropenia
and increased anemia. Treatment with Piperacillin-Tazobactam
initiated without response ; upgraded with vancomycin, meropenem,
and voriconazole on Jun 2. |
| 7th hospitalization
day | Positive response to
EV treatment assessed on thoraco-abdominal scan, 18 days after the
first dose of EV. |
| 6-9th days of
hospitalization | Treatment with
pioglitazone. Significant improvement in endocrine status on Jun 3.
Discontinuation due to ALFT. |
| 8-11th days of
hospitalization | Rapidly progressing
multiple organ failure, bicytopenia, and distributive shock. |
| 11th hospitalization
day | Diagnosis of HLH.
Initiation of dexamethasone. |
| 12th hospitalization
day | First dose of
etoposide. |
| | Improvement in the
distributive shock following dexamethasone. |
| 14th hospitalization
day | Second dose of
etoposide. |
| 18th hospitalization
day | Third dose of
etoposide. |
| 20th hospitalization
day | Septic shock
following etoposide-induced pancytopenia. |
| 28th hospitalization
day | Second septic shock,
leading to patient's demise. |
The patient had previously undergone treatments with
cisplatin/gemcitabine and pembrolizumab, a PD-1 inhibitor (Table I). Subsequently, the patient
received EV, an ADC targeting nectin-4, at a dose of 1.25 mg/kg,
commencing on May 2023.
Just 5 days after receiving the second dose of EV,
the patient was admitted to CHU Saint-Pierre, a tertiary hospital
in Brussels, Belgium, presenting with symptoms of decreased
appetite, vomiting and severe hyperglycemia with ketoacidosis
(Fig. 1A). Despite intensive
intravenous insulin therapy, ketoacidosis remained refractory.
Elevated insulin and C-peptide levels indicated peripheral insulin
resistance without an insulin production deficiency by pancreatic β
cells. Markedly high glucagon levels were also detected, possibly
compensatory for the reduced glucose uptake by cells (Table II). Notably, the patient had no
history of diabetes prior to presentation. Furthermore, no
autoantibodies suggestive of autoimmune diabetes were detected.
 | Table IIPertinent laboratory values upon
hospital admission and at the time of HLH diagnosis. |
Table II
Pertinent laboratory values upon
hospital admission and at the time of HLH diagnosis.
| Parameter | Values at
admission | HLH diagnosis | Normal values |
|---|
| Hemoglobin, g/dl | 11.3 | 7.1 | 13-18 |
| White blood cell,
x103/µl | 5.64 | 1.14 | 3.50-11 |
| Neutrophils,
x103/µl | 4.18 | 0.19 | 1.50-6.70 |
| Platelet count,
x103/µl | 239 | 146 | 150-440 |
| Prothrombin time,
sec | 11.4 | 15 | 9.9-11.8 |
| Activated cephalin
time, sec | 20.7 | 41.6 | 21.6-28.7 |
| D-dimer, ng/ml | N/A | 2,964 | 0-500 |
| Fibrinogen,
mg/dl | 486 | 250 | 150-400 |
| Creatinine,
mg/dl | 1.55 | 4.56 | 0.7-1.20 |
| AST, UI/l | 43 | 252 | <40 |
| ALT, UI/l | 55 | 123 | <41 |
| Glucose, mg/dl | 403 | 133 | 70-100 |
| Hemoglobin A1c,
% | 7.5 | N/A | 4-6 |
| Insulin,
pmol/l | 5,939 | N/A | 17.8-173 |
| C-peptide,
nmol/l | 4.850 | N/A | 0.370-1.470 |
| Glucagon, ng/l | 853.8 | N/A | 120-208 |
| CRP, mg/l | 37 | 445 | <5 |
| Ferritin, µg/l | N/A | 3,525 | 30-300 |
| Triglycerides,
mg/dl | N/A | 1,870 | <175 |
| Soluble CD25,
pg/ml | N/A | 16,032 | 458-1,997 |
| Diabetes
autoantibodies | | | |
|
Anti-insulin | Negative | N/A | |
|
Anti-IA2 | Negative | N/A | |
|
Anti-GAD65 | Negative | N/A | |
|
Anti-islet
cell | Negative | N/A | |
|
Anti-ZnT8 | Negative | N/A | |
Despite aggressive insulin therapy, the extreme
insulin resistance of the patient showed minimal response. However,
on the eighth day of hospitalization, the endocrine status began to
noticeably improve. It remains uncertain whether this improvement
was primarily due to the passage of time or the initiation of
treatment with pioglitazone, a potent insulin sensitizer from the
thiazolidinedione family, 2 days earlier (Fig. 1A).
Despite the early discontinuation of pioglitazone
following an alteration in liver function tests, there was no
significant recurrence of ketoacidosis. This observation suggests
that the improvement in the endocrine status of the patient may
have been due to the resolution of an initial insult.
On the third day of hospitalization, the patient
developed neutropenia, increased anemia and fever, and exhibited an
inflammatory pattern on laboratory tests. Adequate antibiotic
treatment was initiated to address febrile neutropenia. However,
the infectious source remained elusive, with no microorganisms
identified through tests of microbiological samples and no clear
infection site.
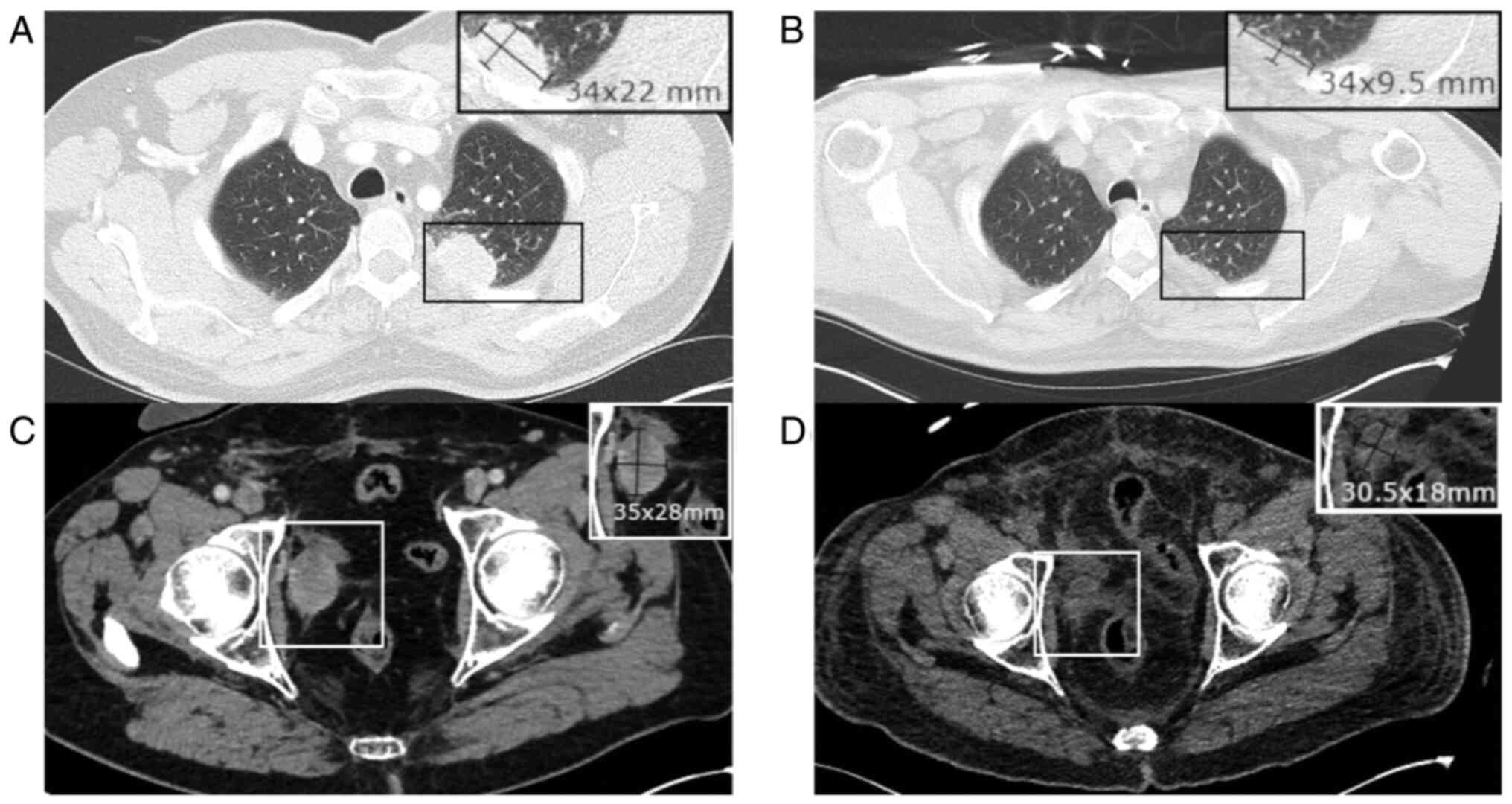
A thoraco-abdominal scan ruled out infection but
revealed marked regression in known pelvic adenopathies and
pulmonary metastases compared with a previous scan before EV
treatment initiation (Fig. 2).
This robust response to EV treatment supported its therapeutic
effectiveness.
On the eighth day of hospitalization, the condition
of the patient worsened, leading to multiple organ failures,
including acute renal failure requiring renal replacement therapy,
liver impairment, coagulopathy, and elevated ferritin and
triglyceride levels. Despite treatment with antibiotics, a marked
inflammatory response persisted, indicated by CRP levels exceeding
400 mg/l. The next day, the patient developed moderate acute
respiratory distress syndrome necessitating mechanical ventilation
and a distributive shock. The rapid deterioration raised concerns
about an underlying hyperinflammatory condition.
With multiple organ failure, persistent fever,
bicytopenia and mild hepatomegaly, the probability of reactive HLH
(reHLH) was assessed using the HScore proposed by Fardet et
al (3) (probability of reHLH,
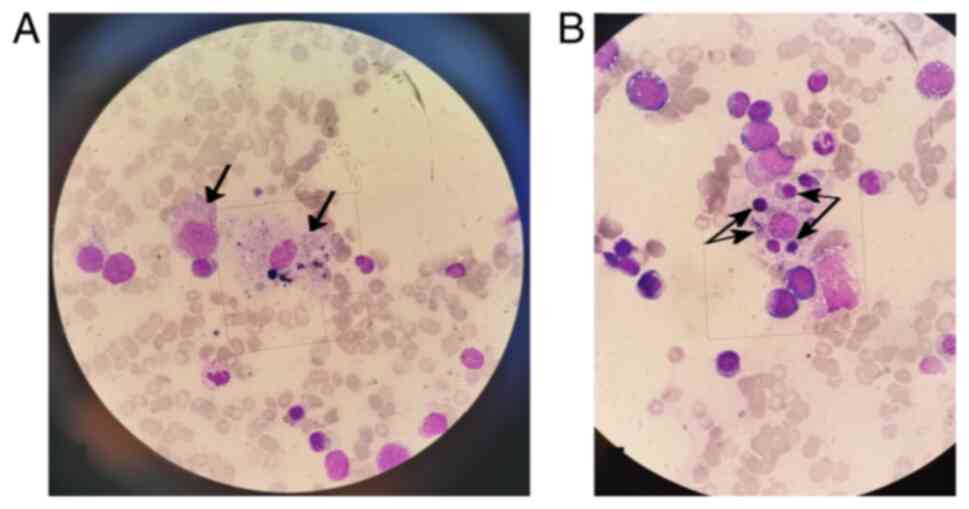
93-96%; HScore, 212/250). On the eleventh day of hospitalization, a
bone marrow puncture strongly supported the HLH diagnosis (Fig. 3), and immunological testing
confirmed elevated soluble CD25 levels (Table II).
Treatment for HLH was promptly initiated following
the HLH-94 protocol, utilizing dexamethasone (10 mg/m2)
and etoposide, with dose adjustments for liver function. This
approach markedly improved distributive shock, reducing vasopressor
requirements and resolving fever (Fig.
1B).
Despite extensive sampling, no microbiological
evidence of an infective trigger for HLH was found. However, herpes
simplex virus 1 (HSV-1) was detected via PCR in bronchoalveolar
lavage (BAL), leading to intravenous acyclovir treatment and
standard prophylactic measures for immunocompromised patients.
The hospitalization course subsequently focused on
managing HLH complications, such as distributive shock,
coagulopathy and pancytopenia-related bleeding. Although it
exacerbated pancytopenia, including severe thrombocytopenia (grade
4), etoposide treatment was initially pursued due to the persistent
inflammatory state of the patient. It was halted following a first
septic shock on day 20 of hospitalization, continuing with
dexamethasone alone. A second septic shock occurred on day 28 of
hospitalization, ultimately resulting in a fatal outcome (Fig. 1B).
Discussion
ICIs, such as pembrolizumab, are associated with
glycemic disorders, including ICI-related diabetes mellitus
(4). Typically, patients present
with ketoacidosis, accompanied by low or undetectable C-peptide
levels, ~20 weeks post-treatment initiation (5). This is attributed to PD-1 pathway
blockade, triggering T cell-mediated autoimmunity against
pancreatic islet cells (6).
Autoantibodies related to type 1 diabetes are frequently detected
(4,5).
By contrast, the current patient presented with a
distinct phenotype >40 weeks post-ICI treatment. The patient
presented with ketoacidosis, markedly elevated C-peptide and
insulin levels, and negative autoantibodies (Table II), suggesting severe insulin
resistance.
The prescribing information of the Food and Drug
Administration (FDA) for pembrolizumab recommends monitoring for
hyperglycemia (7). However, there
is no consensus on the precise techniques and timing for adequate
monitoring.
The experience of the present patient contradicts
the expectation of adverse effects associated with ICI treatment.
Despite irregular monitoring of blood glucose levels during
treatment, no significant dysglycemia was observed.
This, along with the late occurrence of symptoms
after ICI therapy (>40 weeks) and the distinct phenotype of
insulin resistance, suggests that ketoacidosis may not be
attributable to pembrolizumab.
By contrast, the present case suggested a potential
association between EV therapy and insulin resistance. In a phase 3
study, 6.4% of patients treated with EV experienced hyperglycemia,
consistent with phase 2 findings (incidence, ~10%; median onset,
0.5 months post-treatment) (2,8).
Notably, grade 3 hyperglycemia occurred, with 1 case of fatal
metabolic acidosis. FDA data underscore that while hyperglycemia is
not among the most common adverse events, it is predominant among
events graded ≥3(9).
Reports and abstracts have outlined cases of
ketoacidosis following EV therapy (10-14),
suggesting an association with type 2 diabetes, which remains
unclear in the present case due to the absence of a known history
of diabetes. Notably, the hemoglobin A1C (HbA1c) level of the
patient at admission was 7.5%, which differed from normal
measurements taken 2 months prior. The reliability of this value is
questionable in such a specific clinical context. It is recognized
that HbA1c levels may be influenced under various severe
conditions, especially when erythrocyte turnover is affected
(15). Additionally, HbA1c levels
are slightly overestimated in Middle Eastern individuals (16), while typical type 2 diabetic
patients who develop ketoacidosis usually have a history of
long-standing, poorly controlled diabetes and HbA1c levels >10%
(17). Thus, diabetic ketoacidosis
seems unlikely in the present case.
Ketoacidosis presentations similar to those seen
with EV were also observed with another MMAE-containing drug,
brentuximab vedotin, used in Hodgkin's lymphoma (18,19).
Other drugs within this family, as revealed in phase I studies,
were associated with a notable proportion of patients encountering
grade ≥3 hyperglycemia (20,21).
These findings raise questions about the mechanism
behind the role of MMAE in severe hyperglycemia and insulin
resistance. MMAE, a highly cytotoxic drug, binds to and disrupts
the microtubule network, suppressing mitosis (22). When used in ADCs (MMAE-ADC), its
targeted delivery reduces overall toxicity. Previous meta-analyses
have identified anemia, neutropenia and peripheral neuropathy as
consistent adverse events, indicating that MMAE-ADC still carries
systemic toxicity (23,24). Of note, no hyperglycemia, insulin
resistance or ketoacidosis was reported.
Peripheral neuropathy, the primary non-hematologic
toxicity of MMAE-ADC, is considered to occur due to a nonspecific
uptake of the ADC, leading to interference with axonal transport
(22).
As for insulin resistance associated with EV and
potentially other MMAE-containing drug therapies, reports are
scarce, and the mechanism is unknown. A nonspecific uptake of the
ADC by non-targeted tissues, as proposed in peripheral neuropathy
cases (22), could be
hypothesized.
FDA warnings have highlighted diabetic ketoacidosis
and hyperglycemia (25). However,
little is known about insulin resistance as in the present case,
characterized by elevated insulin and C-peptide secretion levels, a
mechanism differing from diabetic ketoacidosis.
Due to the limited number of reports at present, it
is difficult to identify suitable patients. Attention must remain
on diabetic patients. Monitoring of blood glucose levels and
increased vigilance in all patients receiving EV therapy (and
possibly other MMAE-containing drug therapies) might be essential
to understand and prevent the association with insulin
resistance.
HLH is a life-threatening hyperinflammatory syndrome
characterized by uncontrolled immune cell activation, excessive
cytokine production and widespread tissue damage, necessitating
early identification of the underlying trigger (26).
Determining the trigger for HLH is challenging,
given potential causes such as infections, malignancies and drug
reactions (26). In our patient,
despite considering an infectious trigger, no specific infection
was confirmed at the time of HLH diagnosis, and microbiological
samples did not identify any causative microorganism. Although
HSV-1 was detected by a PCR test in BAL, its clinical relevance
remains uncertain, as its pathogenic role in this context has not
been conclusively demonstrated (27).
Malignancies commonly trigger reHLH, with solid
tumors representing only 3% of malignancies associated with reHLH
(26). To the best of our
knowledge, urothelial cancer has not been implicated among solid
tumors associated with reHLH, making it unlikely to be the primary
trigger. It is also noteworthy that the patient's known lesions
were regressing at the time of reHLH. Conversely, observational
data suggest that reHLH is commonly associated with progressive
diseases or occurs at the time of diagnosis, although not
exclusively (28). Of note,
certain medications commonly used for urothelial cancer, such as
Bacille Calmette-Guérin (29) and
ICIs (30,31), have been linked to HLH. There have
been no reported associations of HLH with EV.
ReHLH has also recently been associated with ICIs. A
proposed mechanism implies T-cell activation through PD-1 pathway
blockade, promoting macrophage activation, immune cell activation
and excessive cytokine production (30,31).
Pembrolizumab-associated HLH is unlikely in the present case, as
HLH typically occurs during pembrolizumab administration (median,
3.5 cycles) and within a few weeks after the last infusion (median,
14 days) (31). On the contrary,
the present patient experienced HLH >8 weeks after completion of
ICI treatment.
These elements make it challenging to definitively
attribute the development of HLH to a specific drug, including EV.
While HLH did occur in the present case, its connection with EV
remains uncertain.
Managing rare and severe complications of EV
treatment is challenging. Prompt recognition of refractory insulin
resistance is crucial. Insulin-sensitizing agents such as
pioglitazone may be considered, with careful monitoring for side
effects, such as fluid retention and abnormal liver function
tests.
Regarding HLH, vigilance is advised, especially in
cases with an atypical hyperinflammatory state. Clinical signs such
as persistent fever, hepatosplenomegaly and cytopenias should raise
suspicion for HLH. Early diagnosis and immunosuppressive therapy
are essential. However, the exact relationship between HLH and EV
treatment remains unclear, as shown in the present case.
Immunosuppression-related complications often characterize the
clinical course of these patients.
As EV usage expands in urothelial carcinoma
treatment, vigilant monitoring and reporting of rare complications,
such as refractory insulin resistance, are crucial. Comprehensive
research on adverse events is vital for improved patient care and
therapy safety.
In conclusion, the present case report highlights
refractory insulin resistance and ketoacidosis, followed by reHLH,
in the context of EV therapy. The limited literature on these
complications demonstrates the need for further research to improve
the understanding of the underlying mechanisms. With growing
evidence of the efficacy of EV and evolving survival rates in
urothelial carcinoma, healthcare professionals must remain vigilant
for potential adverse effects, ensuring early recognition and
optimal patient care.
Acknowledgements
The authors would like to thank Dr Mirvate Harb
(Laboratoire Hospitalier Universitaire de Bruxelles, Brussels,
Belgium), Dr Ayoub Mokhtari and Dr Gilles Boumaza (CHU
Saint-Pierre, Brussels, Belgium) for their assistance with images
and findings.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
PR was involved in investigation, data curation,
conceptualization, writing of the original draft, review and
editing, and final approval. LDKN contributed to investigation,
conceptualization, review and editing, validation, and final
approval. PR and LDKN confirm the authenticity of all the raw data.
PB, JK and AH participated in the acquisition and interpretation of
data, in reviewing the work critically for intellectual content,
and in final approval. HA also provided supervision. All authors
agree to be accountable for all aspects of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent for the collection of data and
publication of this report was obtained from the patient's next of
kin following his passing.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID iDs
Pierre Rossignon: https://orcid.org/0009-0006-9391-9914
Le Diep Kieu Nguyen: https://orcid.org/0009-0004-6576-4594
Antoine Herpain: https://orcid.org/0000-0002-0981-0208
References
|
1
|
Saginala K and Barsouk A, Aluru JS, Rawla
P, Padala SA and Barsouk A: Epidemiology of bladder cancer. Med Sci
(Basel). 8(15)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Powles T, Rosenberg JE, Sonpavde GP,
Loriot Y, Durán I, Lee JL, Matsubara N, Vulsteke C, Castellano D,
Wu C, et al: Enfortumab vedotin in previously treated advanced
urothelial carcinoma. N Engl J Med. 384:1125–1135. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fardet L, Galicier L, Lambotte O, Marzac
C, Aumont C, Chahwan D, Coppo P and Hejblum G: Development and
validation of the HScore, a score for the diagnosis of reactive
hemophagocytic syndrome. Arthritis Rheumatol. 66:2613–2620.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Marchand L, Disse E, Dalle S, Reffet S,
Vouillarmet J, Fabien N, Thivolet C and Cugnet-Anceau C: The
multifaceted nature of diabetes mellitus induced by checkpoint
inhibitors. Acta Diabetol. 56:1239–1245. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kotwal A, Haddox C, Block M and Kudva YC:
Immune checkpoint inhibitors: An emerging cause of
insulin-dependent diabetes. BMJ Open Diabetes Res Care.
7(e000591)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mourad D, Azar NS, Eid AA and Azar ST:
Immune checkpoint inhibitor-induced diabetes mellitus: Potential
role of T cells in the underlying mechanism. Int J Mol Sci.
22(2093)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
U.S. Food and Drug Administration: Full
prescribing information: KEYTRUDA. U.S. Food and Drug
Administration, Silver Spring, MD, 2014.
|
|
8
|
Yu EY, Petrylak DP, O'Donnell PH, Lee JL,
van der Heijden MS, Loriot Y, Stein MN, Necchi A, Kojima T,
Harrison MR, et al: Enfortumab vedotin after PD-1 or PD-L1
inhibitors in cisplatin-ineligible patients with advanced
urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2
trial. Lancet Oncol. 22:872–882. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chang E, Weinstock C, Zhang L, Charlab R,
Dorff SE, Gong Y, Hsu V, Li F, Ricks TK, Song P, et al: FDA
approval summary: Enfortumab vedotin for locally advanced or
metastatic urothelial carcinoma. Clin Cancer Res. 27:922–927.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kwok B, Bhatt A, Wise WJ and Gibson CD:
Refractory diabetic ketoacidosis related to Enfortumab vedotin
monotherapy for metastatic urothelial carcinoma. Am J Respir Crit
Care Med. 201(A1736)2020.
|
|
11
|
Iftikhar H and Khoury C: Enfortumab
vedotin-induced diabetic ketoacidosis and AKI: A case report.
Kidney Int Rep. 1 (2 Suppl):S8–S9. 2022.
|
|
12
|
Patel J: Enfortumab vedotin induced
hyperglycemia and diabetic ketoacidosis. Am J Respir Crit Care Med.
207(A5298)2023.
|
|
13
|
Elahi A, Duron J and Porter T: Intractable
diabetic ketoacidosis with Enfortumab vedotin. Diabetes. 72 (Suppl
1):1851–PUB. 2023.
|
|
14
|
Sato T, Suzuki H, Asashima Y and Sone H:
Enfortumab Vedotin-induced hyperglycemia and ileal conduit
reconstruction-induced metabolic acidosis. JCEM Case Rep.
1(luad092)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bonora E and Tuomilehto J: The pros and
cons of diagnosing diabetes with A1C. Diabetes Care. 34 (Suppl
2):S184–S190. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Treister-Goltzman Y, Liberty IF and Peleg
R: Ethnicity affects A1C levels in patients with diagnosed type 2
diabetes in Southern Israel. Diabetes Spectr. 37:86–94.
2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu B, Bu L, Zhang M, Gusdon AM, Zheng L,
Rampersad S, Li J and Qu S: HbA1c as a screening tool for ketosis
in patients with type 2 diabetes mellitus. Sci Rep.
23(39687)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chiang JM, Lai AR, Anderson M and
Rushakoff RJ: Severe insulin resistance with diabetic ketoacidosis
after Brentuximab treatment. AACE Clin Case Rep. 6:e98–e100.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Thakkar K, Khurana S, Sun Y and Hembree
TN: Diabetic ketoacidosis and profound insulin resistance from
Brentuximab vedotin. Cureus. 15(e35804)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McHugh D, Eisenberger M, Heath EI, Bruce
J, Danila DC, Rathkopf DE, Feldman J, Slovin SF, Anand B, Chu R, et
al: A phase I study of the antibody drug conjugate ASG-5ME, an
SLC44A4-targeting antibody carrying auristatin E, in metastatic
castration-resistant prostate cancer. Invest New Drugs.
37:1052–1060. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Danila DC, Szmulewitz RZ, Vaishampayan U,
Higano CS, Baron AD, Gilbert HN, Brunstein F, Milojic-Blair M, Wang
B, Kabbarah O, et al: Phase I study of DSTP3086S, an antibody-drug
conjugate targeting six-transmembrane epithelial antigen of
prostate 1, in metastatic castration-resistant prostate cancer. J
Clin Oncol. 37:3518–3527. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Best RL, LaPointe NE, Azarenko O, Miller
H, Genualdi C, Chih S, Shen BQ, Jordan MA, Wilson L, Feinstein SC
and Stagg NJ: Microtubule and tubulin binding and regulation of
microtubule dynamics by the antibody drug conjugate (ADC) payload,
monomethyl auristatin E (MMAE): Mechanistic insights into MMAE ADC
peripheral neuropathy. Toxicol Appl Pharmacol.
15(115534)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Masters JC, Nickens DJ, Xuan D, Shazer RL
and Amantea M: Clinical toxicity of antibody drug conjugates: A
meta-analysis of payloads. Invest New Drugs. 36:121–135.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li J, Shen G, Liu Z, Liu Y, Wang M, Zhao
F, Ren D, Xie Q, Li Z, Liu Z, et al: Treatment-related adverse
events of antibody-drug conjugates in clinical trials: A systematic
review and meta-analysis. Cancer Innov. 15:346–375. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
U.S. Food and Drug Administration: Full
prescribing information: PADCEV. U.S. Food and Drug Administration,
Silver Spring, MD, 2019.
|
|
26
|
La Rosée P, Horne A, Hines M, von Bahr
Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J,
Girschikofsky M, Jordan MB, et al: Recommendations for the
management of hemophagocytic lymphohistiocytosis in adults. Blood.
133:2465–2477. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Saugel B, Jakobus J, Huber W, Hoffmann D,
Holzapfel K, Protzer U, Schmid RM and Umgelter A: Herpes simplex
virus in bronchoalveolar lavage fluid of medical intensive care
unit patients: Association with lung injury and outcome. J Crit
Care. 32:138–144. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Machaczka M, Vaktnäs J, Klimkowska M and
Hägglund H: Malignancy-associated hemophagocytic
lymphohistiocytosis in adults: A retrospective population-based
analysis from a single center. Leuk Lymphoma. 52:613–619.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ramos-Casals M, Brito-Zerón P,
López-Guillermo A, Khamashta MA and Bosch X: Adult haemophagocytic
syndrome. Lancet. 26:1503–1516. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Malissen N, Lacotte J, Du-Thanh A,
Gaudy-Marqueste C, Guillot B and Grob JJ: Macrophage activation
syndrome: A new complication of checkpoint inhibitors. Eur J
Cancer. 77:88–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang C, Sun W, Li Z, Wu T and Fang W:
Clinical characteristics, treatment, and management of
pembrolizumab induced hemophagocytic lymphohistiocytosis. Invest
New Drugs. 41:834–841. 2023.PubMed/NCBI View Article : Google Scholar
|