A total of 19 Wnt and 10 frizzled (FZD) proteins
have been identified in mammalian cells (3). These proteins activate Wnt signaling
when the receptor binds to its ligand. At least three Wnt pathways
have been identified: Classical Wnt/β-catenin pathway and two
non-classical Wnt/planar cell polarity (PCP) and
Wnt/Ca2+ pathways.
Classical Wnt signaling, known as
β-catenin-dependent signaling, has been extensively studied
(4-7).
This pathway comprises three primary components: Cell membrane
proteins, degradation complexes and β-catenin. Cell membrane
proteins include Wnt ligands (Wnt1, Wnt2, Wnt3a and Wnt8), seven
transmembrane receptors (FZD), auxiliary receptors and low-density
lipoprotein receptor-related proteins 5/6 (LRP5/6). The degradation
complex is primarily composed of glycogen synthase kinase 3β
(GSK-3β), adenomatous polyposis coli (APC), casein kinase 1α (CK1α)
and scaffolding protein axin (4).
GSK-3β is a serine/threonine protein kinase that phosphorylates
residues Thr41, Ser33 and Ser37 on β-catenin. APC increases the
affinity of other components of the complex to β-catenin, whereas
CK1α is a tyrosine kinase that phosphorylates Thr45 on β-catenin.
Furthermore, axin serves as a scaffolding protein that keeps the
degradation complex tightly bound and stable. β-catenin is a member
of the connexin family. Activation of the Wnt/β-catenin signaling
pathway also involves transduction of Wnt signaling in the cell
membrane, maintenance of β-catenin stability in the cytoplasm, and
activation of Wnt-associated target genes in the nucleus (5). When Wnt ligands are absent from the
cell surface, most β-catenin located at the cell membrane junctions
forms a complex with epithelial-type calcium adhesion protein
(E-cadherin) and α-catenin to regulate the cytoskeleton and
maintain intercellular adhesion. A small amount of unbound
β-catenin in the free state is ubiquitinated by the degradation
complex via amino-terminal phosphorylation and recognized by the E3
ubiquitin ligase β-transducin repeat-containing protein, which
eventually leads to its degradation by the proteasome. The
cytoplasm contains low levels of β-catenin in the free state.
Therefore, it cannot enter the nucleus to initiate transcription of
T cell factor/lymphoid enhancer factor (TCF/LEF), blocking the
expression of downstream target genes (6). This blockage inactivates the Wnt
pathway. In the presence of extracellular Wnt ligands, Wnt proteins
bind to FZD and LRP5/6 to activate disheveled (DVL) proteins in the
cytoplasm. Activated DVL inhibits GSK-3β in the degradation
complex. Inactive GSK-3β cannot phosphorylate β-catenin, which
gradually accumulates in the cytoplasm. When β-catenin reaches a
certain level, it is transferred to the nucleus and initiates the
transcription of c-Myc, cyclin D1, Dickkopf-associated protein 1,
matrix metalloproteinase (MMP)-7, axin 2 and other downstream
target genes by binding to TCF/LEF in the nucleus, leading to
abnormal cell proliferation and resistance to apoptosis, thereby
inducing tumor formation (7).
Tumors are induced during this process.
By contrast with activation of the classical
Wnt/β-catenin pathway, activation of non-classical Wnt signaling is
not dependent on β-catenin. Activation of the Wnt/PCP pathway is
initiated by binding of cell-secreted Wnt ligand proteins to the
cell membrane receptor FZD and co-receptors receptor-like tyrosine
kinase and receptor tyrosine-kinase-like orphan receptor. These
co-receptors control activity of small GTPases and regulate
cytoskeletal remodeling (8). The
binding of Wnt proteins to FZD recruits DVL to the cell membrane
for activation (8). DVL activates
DVL-associated activator of morphogenesis 1. This process is
followed by activation of ρGTPase, which further activates myosin
and ρ-associated kinase, thereby altering actin and cytoskeletal
rearrangement in the presence of activated Rac GTPase. Activated
Rac stimulates c-Jun amino-terminal kinase activation, leading to
downstream target gene expression (9). Moreover, Wnt/Ca2+
signaling is activated when Wnt binds to FZD, recruiting DVL to the
cell membrane via guanine nucleotide-binding proteins. This
activates phospholipase C and calmodulin-dependent kinase II,
causing increased intracellular calcium ion release and further
regulating downstream signaling pathways (10).
Wnt signaling plays an important role in the
development of many types of tumors, including non-small cell lung
cancer (NSCLC). Smoking is a key risk factor for lung cancer and
cigarette smoke can activate Wnt signaling (11). In a mouse lung cancer model with
KRAS mutations, activation of the β-catenin pathway accelerates
growth of lung cancer tumors (12). β-catenin, a key component of the
classical Wnt/β-catenin pathway, is often aberrantly expressed in
lung cancer (13). β-catenin
levels in Wnt1-positive NSCLC are higher than those in
Wnt1-negative NSCLC (14).
Odd-skipped related 1 (OSR1) decreases Wnt signaling activity by
inhibiting β-catenin expression in lung cancer OSR1-overexpressing
H1299 cells (15). Furthermore,
immunohistochemical staining shows that Wnt1 and Wnt5a are highly
expressed in NSCLC. Overexpression of Wnt1 is causes more
aggressive NSCLC by inducing expression of survivin (16), whereas Wnt7a is considerably
decreased in NSCLC cell lines and lung tumors. Contrastingly, Wnt7a
interacts directly with the Wnt receptor FZD9(17). The total DVL expression is high in
NSCLC cells but negative in normal bronchial and alveolar
epithelial cells, suggesting DVL could promote the progression of
NSCLC (18). Pygopus2, a
downstream functional protein in Wnt/β-catenin signaling, is more
elevated in the nucleus of NSCLC compared with normal lung tissues
(19). In addition to lung cancer,
β-catenin abnormalities are found in some digestive system cancers,
such as liver, gastric and colorectal cancer (20-22).
The gene catenin beta 1 (CTNNB1), which encodes β-catenin, is
commonly mutated in hepatocellular carcinoma (23), whereas CTNNB1, TCF7L2 and APC are
mutated in gastric cancer (24).
Similarly, APC is mutated in colorectal cancer (22). Overexpression of Wnt11 may
antagonize classical Wnt signaling by phosphorylating β-catenin in
human hepatocellular carcinoma cells (25). However, activation or inhibition of
Wnt signaling in hepatocellular carcinoma depends on the
differentiation status of hepatocellular carcinoma cells. Classical
and non-classical Wnt signaling serve complementary roles, with
classical signaling inducing tumors and non-classical signaling
promoting tumor progression (26).
Overexpression of Wnt and β-catenin nuclear translocation are
observed in gastric cancer (27).
The localization of β-catenin from the cell membrane to the
cytoplasm and nucleus has also been observed during colorectal
cancer development (28).
Similarly, Wnt10a and Wnt6 mRNA are detected in gastric cancer cell
lines. Furthermore, upregulation of Wnt10a expression activates
Wnt/β-catenin/TCF signaling, which is involved in gastric
carcinogenesis (29). The
expression of runt-related transcription factor 1 (RUNX1) is
upregulated in colorectal cancer tissue. RUNX1 directly interacts
with β-catenin to activate Wnt/β-catenin signaling (30). Wnt signaling is also aberrant in
tumors common in female patients, such as breast and ovarian
cancers (31,32). One study used microarray analysis
to compare molecular changes in Wnt signaling in triple-negative
breast cancer (TNBC) and non-TNBC (33). FZD7, LRP6 and TCF7 are
overexpressed in TNBC. In addition, classical Wnt signaling
associated with TCF7 is essential for breast carcinogenesis
(33). Yoshioka et al
(34) examined all Wnt ligands in
malignant ovarian tumors and normal ovarian tissue and found high
expression of Wnt7a and Wnt7b and low expression of Wnt3 and Wnt4.
Additionally, Wnt1, Wnt5a, and Frizzled-1 levels are markedly
higher in ovarian cancer than in normal ovaries (35).
Tumors comprise a heterogeneous population of tumor
cells, a small group of which are CSCs. Similar to normal SCs, CSCs
have a self-renewal capacity and differentiation potential, two
properties that make tumor cell populations heterogeneous. CSCs
have high oncogenic potential and serve a major role in tumor
initiation, metastasis, drug resistance and tumor recurrence
(36). Wnt signaling maintains
stemness in CSCs (37-39).
SOX2 participates in various stages of embryonic
development by activating Wnt signaling and maintaining CSC
stemness. Colon cancer-associated transcript 1 (CCAT1) elevates the
expression of SOX2 and activates Wnt signaling in A549 and H460
lung cancer cells. However, the self-renewal capacity of lung CSCs
is lost when microRNA (miR)-Let-7c binds CCAT1(40). In NSCLC cell lines,
nuclear-enriched abundant transcript 1 may activate the Wnt pathway
and promote the CSC phenotype by inhibiting epigallocatechin
gallate-upregulated copper transporter 1(41). Octamer binding transcription factor
4 (OCT-4) is a lung cancer surface marker of SCs whose expression
is regulated by Wnt signaling. When cisplatin-resistant human lung
adenocarcinoma A549/DDP cells are stimulated with lithium chloride,
an inhibitor of GSK-3β, expression of Wnt signaling target genes
Cyclin D1 and OCT-4 is upregulated. Moreover, the proliferation,
clonogenic ability, migration and drug resistance of A549/DDP cells
is enhanced (42).
Wnt signaling is also involved in the maintenance of
gastric cancer stemness. Stable overexpression of Wnt1 increases
proliferation and tumor sphere formation in the human gastric
adenocarcinoma cell line AGS. Additionally, AGS cells express the
CSC surface markers OCT-4 and CD44. Activation of
Wnt1 accelerates gastric CSC proliferation, suggesting that Wnt
signaling contributes to self-renewal of gastric CSCs (37). Human epidermal growth factor 2
(HER2)-overexpressing gastric cancer cells induce increased
stemness by regulating Wnt/β-catenin signaling (43). Placental growth factor (PlGF) is
associated with gastric carcinogenesis. Thus, knockdown of PlGF
expression induces apoptosis through Wnt signaling in gastric CSCs
(44). Ring finger protein 43 is a
member of the E3 ubiquitin ligase family and was originally
identified in SCs. It attenuates the stemness of gastric CSC-like
cells via Wnt/β-catenin signaling (45). The expression of bromodomain and
extra-terminal domain protein is frequently upregulated in gastric
cancer tissue and also promotes the stemness of gastric cancer
cells by activating Wnt/β-catenin signaling (46).
Colorectal carcinogenesis and disease progression
are caused by progressive accumulation of genetic mutations. APC or
β-catenin mutations activate Wnt/β-catenin signaling and initiate
tumor formation. This suggests that Wnt signaling serve a central
role in the regulation of colorectal CSCs (47-49).
Markers on the surface of colon CSCs include CD44, CD133, CD24,
CD29, CD26, CD166, leucine-rich repeat-containing G-protein-coupled
receptor 5 (Lgr5) and aldehyde dehydrogenase 1 (ALDH1) (50). ALDH1B1 is a member of the ALDH1
family that is highly expressed in colon cancer cells. It can
activate Wnt/β-catenin signaling and may be involved in
tumorigenesis of colon CSCs (51).
Higher β-catenin expression levels induce the expansion of Lgr5(+)
cells in colonic crypts and the formation of crypts (52). The transcription factor GATA6 is a
key regulator of Wnt signaling in colorectal cancer. It directly
drives Lgr5 expression in adenoma SCs. Moreover, GATA6 achieves CSC
self-renewal by competing with β-catenin/TCF4 to bind the distal
regulatory region of the bone morphogenetic protein locus (38). Homeobox A5 abrogates the
self-renewal properties of CSC and blocks tumor growth and
metastasis by inhibiting Wnt signaling in colon cancer (53).
Wnt/β-catenin signaling contributes to the
maintenance of breast CSC stemness. B cell lymphoma factor 11A
(BCL11A) is overexpressed in TNBC cells and participates in
tumorigenesis and invasion (54).
The high expression of this transcription factor causes SC-like
characteristics and maintains stemness in breast CSCs by activating
Wnt/β-catenin signaling (39).
Similarly, Lgr4 is frequently overexpressed in BC and is associated
with poor prognosis. Lgr4 regulates Wnt/β-catenin signaling by
mediating breast CSC maintenance (55). The expression of calmodulin 11
(CDH11), a type II calmodulin and mesenchymal protein marker, is
positively correlated with β-catenin and Wnt2 in breast cancer
(56). When CDH11 is inhibited, it
may suppress the mammary CSC-like phenotype by regulating the
Wnt/β-catenin pathway (56).
The surface markers of ovarian CSCs include CD24,
CD44, CD117, CD133, ALDH, SOX2, OCT-4, NANOG and epithelial cell
adhesion molecule, also known as CD326, a single channel type I
membrane glycoprotein. Increased expression of these markers
enables ovarian CSCs to become sphere-forming in vitro and
tumorigenic in vivo, promoting development of epithelial
ovarian cancer (EOC). This makes these cells more resistant to
drugs and produces tumor progenitor cells that lead to tumor
progression, metastasis and recurrence (57). Mounting evidence demonstrates
Wnt/β-catenin signaling involvement in the acquisition of stemness
in ovarian cancer cells (57-59).
In one study, ALDH1A1 was overexpressed in cultured ovarian cancer
spheres in vitro and was directly associated with key
components of β-catenin signaling. This suggests that
β-catenin-regulated ALDH1A1 maintains the sphere-forming ability of
ovarian cancer cells (58).
Another study confirmed that miR-1207 overexpression increases
ovarian CSC-like properties in vitro and in vivo. The
effects of miR-1207 are caused by Wnt/β-catenin signaling
activation via inhibition of negative regulators of this pathway,
such as secreted Frizzled-related protein 1 (SFRP1), axin 2,
β-catenin inhibitor and TCF4(59).
Metastasis is a characteristic of advanced cancer
and a major challenge in cancer treatment. Epithelial-mesenchymal
transition (EMT) refers to loss of intercellular adhesion and
acquisition of mesenchymal cell characteristics by epithelial
cells. This enhances cancer cell invasion and metastasis (60). Activation of Wnt/β-catenin
signaling can increase expression of adhesion molecule suppressors
by reducing E-cadherin and increasing Snail, Slug, Twist, zinc
finger E-box-binding homeobox (ZEB)1 and ZEB2 expression (61). Several molecules, such as forkhead
box protein P3 (FOXP3), long non-coding RNA (lncRNA) JPX and WD
repeat-containing protein 74 (WDR74) contribute to lung cancer
metastasis via Wnt signaling (62-64).
In previous in vitro and in vivo studies, FOXP3
promoted lung tumor growth and metastasis via FOX3-mediated
Wnt/β-catenin signaling activation (62,65).
Some biomolecules, such as serpin family H member 1 (SERPINH1),
lncRNA miR-4435-2HG and LINC01606, cyclin G2 and Zic family member
1 contribute to EMT and invasive metastasis of gastric cancer via
Wnt/β-catenin signaling (66-70).
SERPINH1 is a member of the serine protease inhibitor H subfamily.
Furthermore, expression of Wnt/β-catenin signaling proteins
β-catenin, Wnt2, GSK-3β, Snail, Slug and Twist is downregulated in
the SERPINH1-silenced gastric cell line SGC-7901. This suggests
that SERPINH1 regulates gastric cancer progression via
Wnt/β-catenin signaling (66).
Tumor metastasis in female patients is associated with
Wnt/β-catenin signaling abnormalities. Overexpression of SFRP
attenuates Wnt signaling in cervical cancer CaSki cells and
increases E-cadherin expression by repressing Slug, Twist, and
Snail (71). By contrast,
cysteine-rich intestinal protein 1 activates Wnt/β catenin
signaling and promotes cervical cancer cell migration and invasion
by increasing expression of c-Myc, cyclin D1 and cytoplasmic
β-catenin (72). The early
dissemination and metastasis of HER2(+) breast cancer depends on
non-classical Wnt (Wnt5a, Wnt5b and Wnt11) signaling (73). In addition, Wnt/β-catenin signaling
is involved in remodeling of the EOC extracellular matrix, a
MMP-mediated process. MMP-2 expression is upregulated in ovarian
cancer and promotes cancer cell invasion and metastasis (74).
Chemoradiotherapy resistance often leads to tumor
treatment failure. The causes of chemoradiotherapy resistance are
complex and associated with tumor heterogeneity, drug
efflux/inactivation and survival pathway activation (75). Wnt signaling can enhance tumor
resistance to chemotherapeutic agents or radiotherapy. Furthermore,
inhibitors of Wnt signaling can reverse this resistance and restore
treatment sensitivity (76,77).
Cancer cells expressing Wnt1 resist drug-induced
apoptosis. Moreover, Wnt/β-catenin signaling induces transcription
of drug resistance factors such as multidrug resistance 1 (MDR-1)
that is a membrane glycoprotein encoded by the MDR gene, survivin
and livin (76). Platinum-based
chemotherapy is the first-line treatment option for advanced NSCLC.
However, acquired cisplatin resistance is prevalent in patients
with NSCLC (78). One study
reported that cytoplasmic inhibition of GSK-3β activates
Wnt/β-catenin signaling and upregulates survivin expression,
leading to cisplatin resistance in NSCLC (79). In another study, c-Myc, a
downstream target gene of β-catenin, regulated A549/DDP resistance
to cisplatin (80). Examination of
β-catenin expression in NSCLC cell line PC9 and gefitinib-resistant
cell line PC9/AB (2) revealed
increased nuclear translocation of β-catenin in PC9/AB (2) compared with PC9. In addition,
expression of certain components of β-catenin signaling
(phosphorylated-GSK-3β, DVL1, c-Myc, c-JUN) increases (81). GDK-100017, a 2,3,6-trisubstituted
quinoxaline derivative, inhibits Wnt/β-catenin signaling, blocks
β-catenin-TCF/LEF interactions and increases sensitivity of
A549/Wnt2 cells to radiotherapy (82). FZD8 is a member of the frizzled Wnt
ligand-receptor family. Disruption of FZD8 increases the
sensitivity of lung cancer cells to the chemotherapeutic drug
paclitaxel (83).
Several molecules are involved in resistance to
chemoradiotherapy in gastric cancer. Caveolin-1 (Cav-1) increases
cisplatin resistance in gastric cancer cells by activating Wnt
signaling (84). Similarly, DOCK6,
a guanine nucleotide exchange factor, promotes radiotherapy
resistance in gastric cancer by regulating Wnt signaling (85). ICG-001, an inhibitor of β-catenin,
reduces the chemoresistance of gastric cancer cells by binding to
CREB-binding protein (CBP) and interfering with its interaction
with β-catenin, thereby inhibiting Wnt signaling (86). Cheng et al (87) investigated the mechanisms
underlying regulation of cisplatin resistance by homologous
cassette gene transcript antisense RNA (HOTAIR) in gastric cancer
cells. Low HOTAIR expression attenuates cisplatin resistance in
gastric cancer cells by inhibiting Wnt signaling. The long
noncoding RNA FAM83H-antisense RNA 1 silencing also increases the
chemosensitivity of gastric cancer cells via Wnt signaling
(88). Similarly, basic leucine
zipper ATF-like transcription factor 2, a member of the type I
activator protein-1 family, reverses multidrug resistance in
gastric cancer cells by inactivating Wnt signaling (89).
Wnt signaling plays a key role in chemoradiotherapy
resistance in BC. The MDR1 gene encodes permeability glycoprotein,
a transmembrane transporter glycoprotein that is a member of the
ATP-binding cassette (ABC) transporter protein superfamily. This
protein superfamily mediates drug efflux and is associated with
tumor drug resistance. Pygo2 expression is upregulated in
drug-resistant BC cells and activates MDR1 via Wnt
signaling, thereby mediating chemoresistance in BC (90). The expression of the membrane
transporter protein Cav-1 is upregulated in BC chemoresistance.
Cav-1 promotes drug resistance in breast CSCs via β-catenin/ABCG2
signaling (91). Activation of
classical and non-classical Wnt signaling pathways is detected in
the tamoxifen-resistant estrogen receptor (ER)(+) breast cancer
cell line MCF7. Furthermore, Wnt3a increases tamoxifen resistance
in MCF7 cells (92). Follistatin
like protein 1, an extracellular matrix glycoprotein, is associated
with regulation of cellular signaling pathways. Its expression is
considerably upregulated in drug-resistant BC cells. Moreover, this
gene can act through integrin β3-induced activation of Wnt
signaling (93). Similarly,
lncAFAP1-AS1 can induce radiotherapy resistance in TNBC via Wnt
signaling (94).
In addition to its involvement in BC resistance,
abnormal ABCG2 expression is associated with drug resistance in
ovarian cancer. The SC-associated receptor tyrosine kinase c-kit
promotes ovarian cancer drug resistance via the Wnt/β-catenin/ABCG2
signaling axis. Low c-kit expression increases ovarian cancer cell
sensitivity to chemotherapeutic agents such as cisplatin and
paclitaxel (95). One study showed
that chemoresistance in high-grade plasma ovarian cancer is
associated with Wnt signaling activation. In addition, the
sensitization of ovarian cancer-initiating cells to cisplatin is
restored by a Wnt signaling inhibitor (96). Human copper transporter 1 is a
transmembrane transporter that allows copper and cisplatin to enter
cells through the membrane barrier. Wnt/β-catenin signaling
inhibits expression of this protein in cisplatin-resistant EOC
cells (97). MMP-10 is highly
expressed in cancer stem-like/carcinoma-initiating cells in EOC and
is associated with platinum resistance. It acts by inhibiting Wnt5a
activation during Wnt signaling (98).
Several studies have shown that Wnt signaling is
associated with chemoradiotherapy resistance in cervical cancer
(99-102).
Therefore, β-catenin nuclear expression can be used as a predictive
marker of chemoradiotherapy resistance in cervical squamous
carcinoma (99). Fat mass and
obesity-associated protein, an N6-methyladenine demethylase with
upregulated mRNA expression in cervical squamous carcinoma tissue,
enhances radiotherapy resistance by regulating β-catenin (100). One study showed that
chemotherapeutic drugs activate Wnt/β-catenin signaling in a
eukaryotic translation initiation factor 4 E (eIF4E)-dependent
manner. This suggests that eIF4E/β-catenin signaling serves a
positive regulatory role in chemoresistance in cervical cancer
(101). Similarly, LGR5 acts as a
cancer-promoting factor by activating Wnt signaling in cervical
cancer. Thus, high LGR5 expression in cervical cancer cells
promotes cisplatin resistance (102).
The tumor microenvironment (TME) consists of immune
cells, peripheral blood vessels, fibroblasts, signaling molecules
and extracellular matrix (103).
The overexpression of immune checkpoint molecules in the TME serves
a key role in tumor immune escape and progression. Tumor
immunotherapy is a novel approach for treating tumors and it
activates or reactivates tumor immune circuits (104). Several immune checkpoint
inhibitors (ICIs), such as ibritumomab, nabumab, pembrolizumab and
atezumab, have been approved for cancer therapy. Ibritumomab is an
anti-cytotoxic T lymphocyte-associated protein 4 antibody
(anti-CTLA4), whereas nabumab and pembrolizumab are anti-programmed
death receptor 1 antibodies (anti-PD-1). By contrast, atezumab is
an anti-PD ligand 1 antibody (anti-PD-L1). Anti-PD-1/PD-L1
antibodies have clinical utility in 15 types of cancer (lung
cancer, cervical cancer, gastric cancer, etc.). However, most
patients with advanced cancers do not derive clinical benefits from
these agents (105). This
suggests that immunosuppressive mechanisms in the TME may limit the
efficacy of ICIs (105).
Growing evidence demonstrates that Wnt signaling
blocks all steps of the tumor immune cycle, including tumor antigen
release and presentation, T cell initiation, activation and
infiltration and clearance of tumor cells (106,107). The first step in the tumor immune
cycle is processing of tumor antigens by dendritic cells (DCs) for
presentation to effector T cells. Wnt signaling regulates
maturation and activity of these DCs. One study on lung
adenocarcinoma found that Wnt1 causes transcriptional silencing of
CC/CXC chemokines, T cell rejection and cross-tolerance in
classical DCs. Furthermore, Wnt1 target gene expression is
upregulated in classical DCs within tumors and downregulated when
Wnt1 is silenced through enhanced T cell toxicity (108). Another study revealed that Wnt5a
suppresses CD14 (+/low) monocyte-derived myeloid DC
production and promotes
CD14+/++CD16+ monocyte production
(109). CD8+ T cells
are the primary effector cells in the tumor immune cycle and can be
activated by DCs and costimulatory molecules that infiltrate the
tumor site to kill cancer cells (110). However, tumor cells evade immune
clearance and reject or inactivate CD8+ T cells to
prevent CD8+ T cell infiltration during tumor
progression (111). Therefore,
Wnt signaling is essential for T cell differentiation,
polarization, effector function and migration (112). Tumor-infiltrating T cells
substantially overexpress Wnt3a and β-catenin, leading to
dysfunction and memory T cell depletion (113). In addition, Wnt-mediated
β-catenin/TCF1 activation inhibits naïve T cell and terminal
differentiation of effector CD8+ T cells (113). Helper T (Th) cells mainly
contribute to CD8+ T cell antitumor responses by
releasing cytokines. Wnt signaling also regulates Th cell
development and function (114)
by suppressing Th cells and impairing antitumor immunity. In
colorectal cancer, β-catenin is activated and attenuates
CD4+ T antitumor immunity by suppressing interferon γ
and elevating IL-17a expression (115). Autoimmune
encephalomyelitis-induced endothelial Wnt signaling limits
CD4+ T cell infiltration, which is restored when
signaling is suppressed (116).
These findings demonstrate that Wnt signaling serves a
non-negligible role in immune cell function. Therefore, the
influence of this pathway warrants consideration in tumor
immunotherapy, especially when efficacy is poor.
Numerous studies have confirmed involvement of Wnt
signaling in onset, progression, metastasis and drug resistance of
various cancers (75,117,118). Moreover, strategies targeting
this pathway for cancer treatment are gaining attention (75,118). Preclinical research has revealed
four approaches that target Wnt signaling: i) Blocking
ligand-receptor interactions, ii) blocking FZD/LRP5/6 signaling
[porcupine (PORCN) inhibitors], iii) promoting β-catenin
degradation (tankyrase (TNKS) enzymes or inhibitors) and iv)
blocking β-catenin-TCF interactions (β-catenin inhibitors)
(119).
Different tumors express specific Wnt ligands.
Therefore, blocking specific Wnt ligand-receptor interactions can
inhibit tumor cell proliferation (120,121). In one study, addition of
anti-Wnt1 monoclonal antibodies to human NSCLC, BC, mesothelioma
and sarcoma cell lines led to apoptosis. In addition, the
antibodies inhibited tumor growth in vivo (122). Another study showed that Wnt2
inhibitors decrease clone formation and transplanted tumor volume
in NSCLC cell lines (123). After
transferring interfering RNA of Wnt5a into the human lung squamous
carcinoma cell line H157 and human lung adenocarcinoma cell line
A549, the proliferative capacity of both cell lines was decreased
(124). The recombinant fusion
protein ipafricept (known as OMP-54F28) is formed by fusing the
cysteine-rich structural domain of FZD8 with the structural domain
of immunoglobulin Fc, which blocks Wnt signaling by binding to Wnt
ligands. Preclinical studies have shown that OMP-54F28 slows tumor
growth and has a synergistic effect when combined with
chemotherapeutic agents (125,126). This human monoclonal antibody
interacts with five FZD receptors to block classical Wnt signaling
and clinical trials have shown that it has good tolerability
(125,127).
PORCN is a membrane-bound O-acetyltransferase that
modifies Wnt proteins via palmitoylation; only such modified Wnt
proteins can be secreted outside the cellular membrane to activate
Wnt signaling by interacting with its co-receptors LRP5/6 and FZD
(128). LGK974 is a
small-molecule PORCN inhibitor that blocks Wnt signaling and
induces tumor regression in MMTV-Wnt1 mice. In addition, LGK974
considerably attenuates clone formation in human head and neck
cancer cell line HN30(129).
ETC-159 is another PORCN inhibitor that blocks secretion and
activation of Wnt proteins. Preclinical studies have shown that
ETC-159 is highly effective in treating mouse-transplanted tumors
with R-spondin translocations in patients with colon cancer
(127,130). In another preclinical study,
combination of the PORCN inhibitor RX004 and anti-PD-1 enhanced
antitumor immune effects (131).
PORCN inhibitors have shown therapeutic potential in colorectal,
pancreatic, hepatocellular and head and neck tumors. To date, no
PORCN inhibitors have entered clinical use; only LGK974, ETC159,
CGX1321 and RXC004 have been investigated in phase I clinical
trials (132-135).
End-anchored polymerase (TNKS) is a member of the
poly ADP-ribose polymerase (PARP) family, which includes two
isoforms, TNKS1 (PARP5a) and TNKS2 (PARP5b). These isoforms
regulate classical Wnt signaling via poly ADP-ribosylated axin
proteins. TNKS inhibitors promote β-catenin degradation by
increasing axin levels (120).
Treatment of the NSCLC cell line A549 with XAV939 inhibits cell
proliferation and migratory capacity. Furthermore, it decreases
TNKS, β-catenin and c-Myc protein levels (136). This TNKS inhibitor also decreases
proliferative capacity of the SCLC cell line H446 by inhibiting Wnt
signaling (137). The combination
of XAV939 and chemotherapeutic agent paclitaxel induces apoptosis
and inhibits Wnt signaling in BC cells. In addition, this treatment
suppresses EMT and angiogenesis. Similarly, combined XAV939 and
low-dose (20 nM) paclitaxel results in comparable therapeutic
effects in BC cell lines compared with high-dose (200 nM)
paclitaxel alone (138).
NVP-TNKS656, another TNKS inhibitor, decreases β-catenin protein
expression in the nucleus of colorectal cancer cells when combined
with PI3K or AKT inhibitors, thereby reversing resistance to PI3K
or AKT inhibitors and inhibiting tumor growth (139). Moreover, the TNKS inhibitor
G007-LK has a sensitizing effect on anti-PD-1 antitumor therapy
(140).
An effective way of targeting the classical Wnt
signaling pathway is to block the interaction of β-catenin with
downstream transcription factors (120,121). TCF4 is a member of the TCF/LEF
family and binds to β-catenin to initiate target gene transcription
when Wnt signaling is activated. Inhibitors of β-catenin-TCF4
interactions include PKF115-584, CGP049090, PKF222-815, PKF118-744,
PKF118-310, ZTM000990, iCRT3/5/14, NC043, LF3 and
UU-T02/03(141). PKF115-584
inhibits β-catenin transcription and proliferation in the
adrenocortical tumor cell line H295R in a dose-dependent manner
(142). Similarly, CGP049090 and
PKF115-584 effectively kill chronic lymphocytic leukemia cells
(143). Three inhibitors,
PKF118-310, PKF115-584 and CGP049090, downregulate the expression
of TCF4/β-catenin target genes c-Myc, cyclin D1 and survivin
in hepatocellular carcinoma. These inhibitors also induce apoptosis
and cell cycle arrest and inhibit the growth of transplanted tumors
in mice (144).
The dysregulation of classical and non-classical Wnt
signaling pathways in tumors has been extensively studied in recent
years (117,118,132-134).
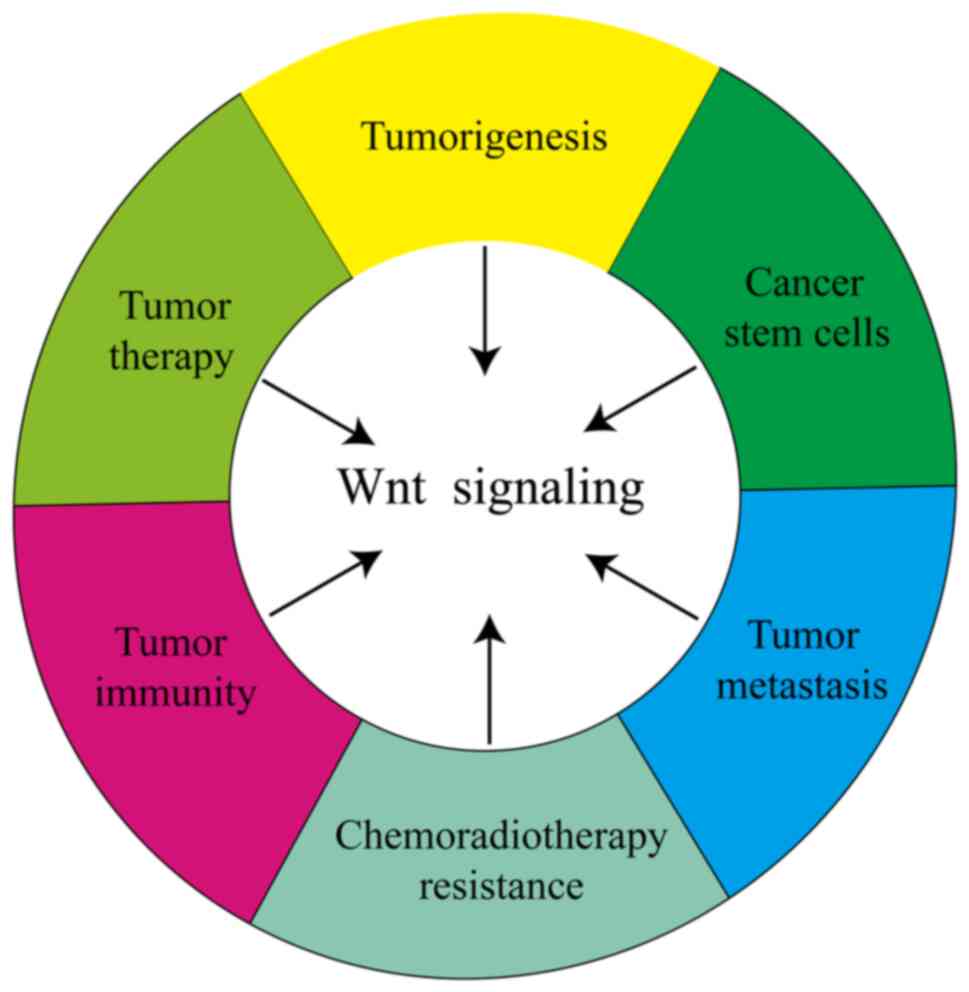
The present review provides an overview of the role of Wnt
signaling in tumorigenesis, progression, metastasis, CSCs,
chemoradiotherapy resistance and antitumor immunity as well as
inhibitors targeting Wnt signaling. Wnt signaling is increasingly
recognized as an anticancer therapeutic target and several studies
have demonstrated the effectiveness of Wnt signaling inhibitors
alone or in combination with other chemotherapeutic agents and ICIs
in antitumor therapy (118,132-134,138).
Furthermore, some Wnt signaling inhibitors (LGK974, ETC159,
CGX1321, and RXC004) have been tested in phase I clinical trials
(132,134,145,146). However, Wnt signaling serves an
important role in physiological processes and the possible side
effects after blockade are not well understood. Therefore,
pharmacological effects and mechanisms underlying Wnt signaling and
its inhibitors for early clinical application warrant further
study. An in-depth understanding of these processes may improve
prognosis in patients with cancer.
Not applicable.
Funding: The present study was supported by the Medical
Scientific Research Project in Xiangtan City (grant. no.
2022-xtyx-16) and Guiding Science and Technology Program of
Xiangtan City (grant. no. ZP-ZDJH2022006).
Not applicable.
HW and MJ designed the review and edited the
manuscript. HW and LZ wrote the manuscript. HW, CH and HL collected
and analyzed data. Data authentication is not applicable. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109.
1982.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Johnson ML and Rajamannan N: Diseases of
Wnt signaling. Rev Endocr Metab Disord. 7:41–49. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Janda CY, Dang LT, You C, Chang J, de Lau
W, Zhong ZA, Yan KS, Marecic O, Siepe D, Li X, et al: Surrogate Wnt
agonists that phenocopy canonical Wnt and β-catenin signalling.
Nature. 545:234–237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van Kappel EC and Maurice MM: Molecular
regulation and pharmacological targeting of the β-catenin
destruction complex. Br J Pharmacol. 174:4575–4588. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang P, Yan R, Zhang X, Wang L, Ke X and
Qu Y: Activating Wnt/β-catenin signaling pathway for disease
therapy: Challenges and opportunities. Pharmacol Ther. 196:79–90.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang D, Zhang Q, Li F, Wang C and Yang C:
β-TrCP-mediated ubiquitination and degradation of Dlg5 regulates
hepatocellular carcinoma cell proliferation. Cancer Cell Int.
19(298)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lin CH, Ji T, Chen CF and Hoang BH: Wnt
signaling in osteosarcoma. Adv Exp Med Biol. 804:33–45.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kohn AD and Moon RT: Wnt and calcium
signaling: Beta-catenin-independent pathways. Cell Calcium.
38:439–446. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Topol L, Jiang X, Choi H, Garrett-Beal L,
Carolan PJ and Yang Y: Wnt-5a inhibits the canonical Wnt pathway by
promoting GSK-3-independent beta-catenin degradation. J Cell Biol.
162:899–908. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lemjabbar-Alaoui H, Dasari V, Sidhu SS,
Mengistab A, Finkbeiner W, Gallup M and Basbaum C: Wnt and Hedgehog
are critical mediators of cigarette smoke-induced lung cancer. PLoS
One. 1(e93)2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pacheco-Pinedo EC, Durham AC, Stewart KM,
Goss AM, Lu MM, Demayo FJ and Morrisey EE: Wnt/β-catenin signaling
accelerates mouse lung tumorigenesis by imposing an embryonic
distal progenitor phenotype on lung epithelium. J Clin Invest.
121:1935–1945. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kren L, Hermanová M, Goncharuk VN, Kaur P,
Ross JS, Pavlovský Z and Dvorák K: Downregulation of plasma
membrane expression/cytoplasmic accumulation of beta-catenin
predicts shortened survival in non-small cell lung cancer. A
clinicopathologic study of 100 cases. Cesk Patol. 39:17–20.
2003.PubMed/NCBI
|
|
14
|
Huang CL, Liu D, Ishikawa S, Nakashima T,
Nakashima N, Yokomise H, Kadota K and Ueno M: Wnt1 overexpression
promotes tumour progression in non-small cell lung cancer. Eur J
Cancer. 44:2680–2688. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Y, Lei L, Zheng YW, Zhang L, Li ZH,
Shen HY, Jiang GY, Zhang XP, Wang EH and Xu HT: Odd-skipped related
1 inhibits lung cancer proliferation and invasion by reducing Wnt
signaling through the suppression of SOX9 and β-catenin. Cancer
Sci. 109:1799–1810. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nakashima N, Huang CL, Liu D, Ueno M and
Yokomise H: Intratumoral Wnt1 expression affects survivin gene
expression in non-small cell lung cancer. Int J Oncol. 37:687–694.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Winn RA, Marek L, Han SY, Rodriguez K,
Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, et
al: Restoration of Wnt-7a expression reverses non-small cell lung
cancer cellular transformation through frizzled-9-mediated growth
inhibition and promotion of cell differentiation. J Biol Chem.
280:19625–19634. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wei Q, Zhao Y, Yang ZQ, Dong QZ, Dong XJ,
Han Y, Zhao C and Wang EH: Dishevelled family proteins are
expressed in non-small cell lung cancer and function differentially
on tumor progression. Lung Cancer. 62:181–192. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Y, Dong QZ, Wang S, Fang CQ, Miao Y,
Wang L, Li MZ and Wang EH: Abnormal expression of Pygopus 2
correlates with a malignant phenotype in human lung cancer. BMC
Cancer. 13(346)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Khalaf AM, Fuentes D, Morshid AI, Burke
MR, Kaseb AO, Hassan M, Hazle JD and Elsayes KM: Role of
Wnt/β-catenin signaling in hepatocellular carcinoma, pathogenesis,
and clinical significance. J Hepatocell Carcinoma. 5:61–73.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Flanagan DJ and Vincan E: Wnt signaling in
cancer: Not a binary On:Off switch. Cancer Res. 79:5901–5906.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Christie M, Jorissen RN, Mouradov D,
Sakthianandeswaren A, Li S, Day F, Tsui C, Lipton L, Desai J, Jones
IT, et al: Different APC genotypes in proximal and distal sporadic
colorectal cancers suggest distinct WNT/β-catenin signalling
thresholds for tumourigenesis. Oncogene. 32:4675–4682.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Russell JO and Monga SP: Wnt/β-catenin
signaling in liver development, homeostasis, and pathobiology. Annu
Rev Pathol. 13:351–378. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Molaei F, Forghanifard MM, Fahim Y and
Abbaszadegan MR: Molecular signaling in tumorigenesis of gastric
cancer. Iran Biomed J. 22:217–230. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Toyama T, Lee HC, Koga H, Wands JR and Kim
M: Noncanonical Wnt11 inhibits hepatocellular carcinoma cell
proliferation and migration. Mol Cancer Res. 8:254–265.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yuzugullu H, Benhaj K, Ozturk N, Senturk
S, Celik E, Toylu A, Tasdemir N, Yilmaz M, Erdal E, Akcali KC, et
al: Canonical Wnt signaling is antagonized by noncanonical Wnt5a in
hepatocellular carcinoma cells. Mol Cancer. 8(90)2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cheng XX, Wang ZC, Chen XY, Sun Y, Kong
QY, Liu J and Li H: Correlation of Wnt-2 expression and
beta-catenin intracellular accumulation in Chinese gastric cancers:
relevance with tumour dissemination. Cancer Lett. 223:339–347.
2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bhattacharya I, Barman N, Maiti M and
Sarkar R: Assessment of beta-catenin expression by
immunohistochemistry in colorectal neoplasms and its role as an
additional prognostic marker in colorectal adenocarcinoma. Med
Pharm Rep. 92:246–252. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kirikoshi H, Sekihara H and Katoh M:
Up-regulation of WNT10A by tumor necrosis factor alpha and
Helicobacter pylori in gastric cancer. Int J Oncol. 19:533–536.
2001.PubMed/NCBI
|
|
30
|
Li Q, Lai Q, He C, Fang Y, Yan Q, Zhang Y,
Wang X, Gu C, Wang Y, Ye L, et al: RUNX1 promotes tumour metastasis
by activating the Wnt/β-catenin signalling pathway and EMT in
colorectal cancer. J Exp Clin Cancer Res. 38(334)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu X, Zhang M, Xu F and Jiang S: Wnt
signaling in breast cancer: Biological mechanisms, challenges and
opportunities. Mol Cancer. 19(165)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu R, Zhai Y, Fearon ER and Cho KR:
Diverse mechanisms of beta-catenin deregulation in ovarian
endometrioid adenocarcinomas. Cancer Res. 61:8247–8255.
2001.PubMed/NCBI
|
|
33
|
Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan
YC, Deng X, Chen L, Kim CCH, Lau S, et al: FZD7 has a critical role
in cell proliferation in triple negative breast cancer. Oncogene.
30:4437–4446. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yoshioka S, King ML, Ran S, Okuda H,
MacLean JA II, McAsey ME, Sugino N, Brard L, Watabe K and Hayashi
K: WNT7A regulates tumor growth and progression in ovarian cancer
through the WNT/β-catenin pathway. Mol Cancer Res. 10:469–482.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Badiglian Filho L, Oshima CT, De Oliveira
Lima F, De Oliveira Costa H, De Sousa Damião R, Gomes TS and
Gonçalves WJ: Canonical and noncanonical Wnt pathway: A comparison
among normal ovary, benign ovarian tumor and ovarian cancer. Oncol
Rep. 21:313–320. 2009.PubMed/NCBI
|
|
36
|
Ahmed N, Abubaker K and Findlay JK:
Ovarian cancer stem cells: Molecular concepts and relevance as
therapeutic targets. Mol Aspects Med. 39:110–125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang
J, Wang H, Tang B, Zhang Q, Yu X, et al: Roles of Wnt/β-catenin
signaling in the gastric cancer stem cells proliferation and
salinomycin treatment. Cell Death Dis. 5(e1039)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Whissell G, Montagni E, Martinelli P,
Hernando-Momblona X, Sevillano M, Jung P, Cortina C, Calon A, Abuli
A, Castells A, et al: The transcription factor GATA6 enables
self-renewal of colon adenoma stem cells by repressing BMP gene
expression. Nat Cell Biol. 16:695–707. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu L, Pan R, Zhou D, Ye G and Tan W:
BCL11A enhances stemness and promotes progression by activating
Wnt/β-catenin signaling in breast cancer. Cancer Manag Res.
11:2997–3007. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chaudhary S, Islam Z, Mishra V, Rawat S,
Ashraf GM and Kolatkar PR: Sox2: A regulatory factor in
tumorigenesis and metastasis. Curr Protein Pept Sci. 20:495–504.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lin S, Zhen Y, Guan Y and Yi H: Roles of
Wnt/β-catenin signaling pathway regulatory long non-coding RNAs in
the pathogenesis of non-small cell lung cancer. Cancer Manag Res.
12:4181–4191. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/beta-catenin signaling regulates cancer stem cells in lung
cancer A549 cells. Biochem Biophys Res Commun. 392:373–379.
2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jung DH, Bae YJ, Kim JH, Shin YK and Jeung
HC: HER2 regulates cancer stem cell activities via the Wnt
signaling pathway in gastric cancer cells. Oncology. 97:311–318.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Akrami H, Mehdizadeh K, Moradi B,
Borzabadi Farahani D, Mansouri K and Ghalib Ibraheem Alnajar S:
PlGF knockdown induced apoptosis through Wnt signaling pathway in
gastric cancer stem cells. J Cell Biochem. 120:3268–3276.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gao Y, Cai A, Xi H, Li J, Xu W, Zhang Y,
Zhang K, Cui J, Wu X, Wei B and Chen L: Ring finger protein 43
associates with gastric cancer progression and attenuates the
stemness of gastric cancer stem-like cells via the Wnt-β/catenin
signaling pathway. Stem Cell Res Ther. 8(98)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Song H, Shi L, Xu Y, Xu T, Fan R, Cao M,
Xu W and Song J: BRD4 promotes the stemness of gastric cancer cells
via attenuating miR-216a-3p-mediated inhibition of Wnt/β-catenin
signaling. Eur J Pharmacol. 852:189–197. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhao H, Han R, Wang Z, Xian J and Bai X:
Colorectal cancer stem cells and targeted agents. Pharmaceutics.
15(2763)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
D'Antonio L, Fieni C, Ciummo SL, Vespa S,
Lotti L, Sorrentino C and Di Carlo E: Inactivation of
interleukin-30 in colon cancer stem cells via CRISPR/Cas9 genome
editing inhibits their oncogenicity and improves host survival. J
Immunother Cancer. 11(e006056)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liao W, Zhang L, Chen X, Xiang J, Zheng Q,
Chen N, Zhao M, Zhang G, Xiao X, Zhou G, et al: Targeting cancer
stem cells and signalling pathways through phytochemicals: A
promising approach against colorectal cancer. Phytomedicine.
108(154524)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hatano Y, Fukuda S, Hisamatsu K, Hirata A,
Hara A and Tomita H: Multifaceted interpretation of colon cancer
stem cells. Int J Mol Sci. 18(1446)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Singh S, Arcaroli J, Chen Y, Thompson DC,
Messersmith W, Jimeno A and Vasiliou V: ALDH1B1 is crucial for
colon tumorigenesis by modulating Wnt/β-catenin, notch and PI3K/Akt
signaling pathways. PLoS One. 10(e0121648)2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hirata A, Utikal J, Yamashita S, Aoki H,
Watanabe A, Yamamoto T, Okano H, Bardeesy N, Kunisada T, Ushijima
T, et al: Dose-dependent roles for canonical Wnt signalling in de
novo crypt formation and cell cycle properties of the colonic
epithelium. Development. 140:66–75. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ordóñez-Morán P, Dafflon C, Imajo M,
Nishida E and Huelsken J: HOXA5 counteracts stem cell traits by
inhibiting wnt signaling in colorectal cancer. Cancer Cell.
28:815–829. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Khaled WT, Choon Lee S, Stingl J, Chen X,
Raza Ali H, Rueda OM, Hadi F, Wang J, Yu Y, Chin SF, et al: BCL11A
is a triple-negative breast cancer gene with critical functions in
stem and progenitor cells. Nat Commun. 6(5987)2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yue Z, Yuan Z, Zeng L, Wang Y, Lai L, Li
J, Sun P, Xue X, Qi J, Yang Z, et al: LGR4 modulates breast cancer
initiation, metastasis, and cancer stem cells. FASEB J.
32:2422–2437. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Satriyo PB, Bamodu OA, Chen JH, Aryandono
T, Haryana SM, Yeh CT and Chao TY: Cadherin 11 inhibition
downregulates β-catenin, deactivates the canonical WNT signalling
pathway and suppresses the cancer stem cell-like phenotype of
triple negative breast cancer. J Clin Med. 8(148)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Nguyen VHL, Hough R, Bernaudo S and Peng
C: Wnt/β-catenin signalling in ovarian cancer: Insights into its
hyperactivation and function in tumorigenesis. J Ovarian Res.
12(122)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Condello S, Morgan CA, Nagdas S, Cao L,
Turek J, Hurley TD and Matei D: β-Catenin-regulated ALDH1A1 is a
target in ovarian cancer spheroids. Oncogene. 34:2297–2308.
2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wu G, Liu A, Zhu J, Lei F, Wu S, Zhang X,
Ye L, Cao L and He S: MiR-1207 overexpression promotes cancer stem
cell-like traits in ovarian cancer by activating the Wnt/β-catenin
signaling pathway. Oncotarget. 6:28882–28894. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Rapp J, Jaromi L, Kvell K, Miskei G and
Pongracz JE: WNT signaling-lung cancer is no exception. Respir Res.
18(167)2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yang S, Liu Y, Li MY, Ng CSH, Yang SL,
Wang S, Zou C, Dong Y, Du J, Long X, et al: FOXP3 promotes tumor
growth and metastasis by activating Wnt/β-catenin signaling pathway
and EMT in non-small cell lung cancer. Mol Cancer.
16(124)2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Pan J, Fang S, Tian H, Zhou C, Zhao X,
Tian H, He J, Shen W, Meng X, Jin X and Gong Z: lncRNA
JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis
of lung cancer by activating Wnt/β-catenin signaling. Mol Cancer.
19(9)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Li Y, Chen F, Shen W, Li B, Xiang R, Qu L,
Zhang C, Li G, Xie H, Katanaev VL and Jia L: WDR74 induces nuclear
β-catenin accumulation and activates Wnt-responsive genes to
promote lung cancer growth and metastasis. Cancer Lett.
471:103–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Qi H, Wang S, Wu J, Yang S, Gray S, Ng
CSH, Du J, Underwood MJ, Li MY and Chen GG: EGFR-AS1/HIF2A
regulates the expression of FOXP3 to impact the cancer stemness of
smoking-related non-small cell lung cancer. Ther Adv Med Oncol.
11(1758835919855228)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Tian S, Peng P, Li J, Deng H, Zhan N, Zeng
Z and Dong W: SERPINH1 regulates EMT and gastric cancer metastasis
via the Wnt/β-catenin signaling pathway. Aging (Albany NY).
12:3574–3593. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wang H, Wu M, Lu Y, He K, Cai X, Yu X, Lu
J and Teng L: LncRNA MIR4435-2HG targets desmoplakin and promotes
growth and metastasis of gastric cancer by activating Wnt/β-catenin
signaling. Aging (Albany NY). 11:6657–6673. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Luo Y, Tan W, Jia W, Liu Z, Ye P, Fu Z, Lu
F, Xiang W, Tang L, Yao L, et al: The long non-coding RNA LINC01606
contributes to the metastasis and invasion of human gastric cancer
and is associated with Wnt/β-catenin signaling. Int J Biochem Cell
Biol. 103:125–134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gao J, Zhao C, Liu Q, Hou X, Li S, Xing X,
Yang C and Luo Y: Cyclin G2 suppresses Wnt/β-catenin signaling and
inhibits gastric cancer cell growth and migration through Dapper1.
J Exp Clin Cancer Res. 37(317)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ge Q, Hu Y, He J, Chen F, Wu L, Tu X, Qi
Y, Zhang Z, Xue M, Chen S, et al: Zic1 suppresses gastric cancer
metastasis by regulating Wnt/β-catenin signaling and
epithelial-mesenchymal transition. FASEB J. 34:2161–2172.
2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Chung MT, Lai HC, Sytwu HK, Yan MD, Shih
YL, Chang CC, Yu MH, Liu HS, Chu DW and Lin YW: SFRP1 and SFRP2
suppress the transformation and invasion abilities of cervical
cancer cells through Wnt signal pathway. Gynecol Oncol.
112:646–653. 2009.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zhang LZ, Huang LY, Huang AL, Liu JX and
Yang F: CRIP1 promotes cell migration, invasion and
epithelial-mesenchymal transition of cervical cancer by activating
the Wnt/β-catenin signaling pathway. Life Sci. 207:420–427.
2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Harper KL, Sosa MS, Entenberg D, Hosseini
H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis
RJ, et al: Mechanism of early dissemination and metastasis in
Her2+ mammary cancer. Nature. 540:588–592.
2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kenny HA and Lengyel E: MMP-2 functions as
an early response protein in ovarian cancer metastasis. Cell Cycle.
8:683–688. 2009.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst.
106(djt356)2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zhong Z and Virshup DM: Wnt signaling and
drug resistance in cancer. Mol Pharmacol. 97:72–89. 2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Geng P, Zhao J, Li Q, Wang X, Qin W, Wang
T, Shi X, Liu X, Chen J, Qiu H and Xu G: Z-Ligustilide combined
with cisplatin reduces PLPP1-mediated phospholipid synthesis to
impair cisplatin resistance in lung cancer. Int J Mol Sci.
24(17046)2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Gao Y, Liu Z, Zhang X, He J, Pan Y, Hao F,
Xie L, Li Q, Qiu X and Wang E: Inhibition of cytoplasmic GSK-3β
increases cisplatin resistance through activation of Wnt/β-catenin
signaling in A549/DDP cells. Cancer Lett. 336:231–239.
2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Xie C, Pan Y, Hao F, Gao Y, Liu Z, Zhang
X, Xie L, Jiang G, Li Q and Wang E: C-Myc participates in
β-catenin-mediated drug resistance in A549/DDP lung adenocarcinoma
cells. APMIS. 122:1251–1258. 2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Fang X, Gu P, Zhou C, Liang A, Ren S, Liu
F, Zeng Y, Wu Y, Zhao Y, Huang B, et al: β-Catenin overexpression
is associated with gefitinib resistance in non-small cell lung
cancer cells. Pulm Pharmacol Ther. 28:41–48. 2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Lee SB, Gong YD, Park YI and Dong MS:
2,3,6-Trisubstituted quinoxaline derivative, a small molecule
inhibitor of the Wnt/beta-catenin signaling pathway, suppresses
cell proliferation and enhances radiosensitivity in A549/Wnt2
cells. Biochem Biophys Res Commun. 431:746–752. 2013.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Wang HQ, Xu ML, Ma J, Zhang Y and Xie CH:
Frizzled-8 as a putative therapeutic target in human lung cancer.
Biochem Biophys Res Commun. 417:62–66. 2012.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wang X, Lu B, Dai C, Fu Y, Hao K, Zhao B,
Chen Z and Fu L: Caveolin-1 promotes chemoresistance of gastric
cancer cells to cisplatin by activating WNT/β-catenin pathway.
Front Oncol. 10(46)2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Chi HC, Tsai CY, Wang CS, Yang HY, Lo CH,
Wang WJ, Lee KF, Lai LY, Hong JH, Chang YF, et al: DOCK6 promotes
chemo- and radioresistance of gastric cancer by modulating
WNT/β-catenin signaling and cancer stem cell traits. Oncogene.
39:5933–5949. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Liu Y, Chen H, Zheng P, Zheng Y, Luo Q,
Xie G, Ma Y and Shen L: ICG-001 suppresses growth of gastric cancer
cells and reduces chemoresistance of cancer stem cell-like
population. J Exp Clin Cancer Res. 36(125)2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Cheng C, Qin Y, Zhi Q, Wang J and Qin C:
Knockdown of long non-coding RNA HOTAIR inhibits cisplatin
resistance of gastric cancer cells through inhibiting the PI3K/Akt
and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int
J Biol Macromol. 107:2620–2629. 2018.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wang B, Guan G and Zhao D: Silence of
FAM83H-AS1 promotes chemosensitivity of gastric cancer through
Wnt/β-catenin signaling pathway. Biomed Pharmacother.
125(109961)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Yang W, Wu B, Ma N, Wang Y, Guo J, Zhu J
and Zhao S: BATF2 reverses multidrug resistance of human gastric
cancer cells by suppressing Wnt/β-catenin signaling. In Vitro Cell
Dev Biol Anim. 55:445–452. 2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Zhang ZM, Wu JF, Luo QC, Liu QF, Wu QW, Ye
GD, She HQ and Li BA: Pygo2 activates MDR1 expression and mediates
chemoresistance in breast cancer via the Wnt/β-catenin pathway.
Oncogene. 35:4787–4797. 2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Wang Z, Wang N, Li W, Liu P, Chen Q, Situ
H, Zhong S, Guo L, Lin Y, Shen J and Chen J: Caveolin-1 mediates
chemoresistance in breast cancer stem cells via β-catenin/ABCG2
signaling pathway. Carcinogenesis. 35:2346–2356. 2014.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Loh YN, Hedditch EL, Baker LA, Jary E,
Ward RL and Ford CE: The Wnt signalling pathway is upregulated in
an in vitro model of acquired tamoxifen resistant breast cancer.
BMC Cancer. 13(174)2013.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Cheng S, Huang Y, Lou C, He Y, Zhang Y and
Zhang Q: FSTL1 enhances chemoresistance and maintains stemness in
breast cancer cells via integrin β3/Wnt signaling under miR-137
regulation. Cancer Biol Ther. 20:328–337. 2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Bi Z, Li Q, Dinglin X, Xu Y, You K, Hong
H, Hu Q, Zhang W, Li C, Tan Y, et al: Nanoparticles (NPs)-meditated
LncRNA AFAP1-AS1 silencing to block Wnt/β-catenin signaling pathway
for synergistic reversal of radioresistance and effective cancer
radiotherapy. Adv Sci (Weinh). 7(2000915)2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Chau WK, Ip CK, Mak ASC, Lai HC and Wong
AST: c-Kit mediates chemoresistance and tumor-initiating capacity
of ovarian cancer cells through activation of
Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene.
32:2767–2781. 2013.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Nagaraj AB, Joseph P, Kovalenko O, Singh
S, Armstrong A, Redline R, Resnick K, Zanotti K, Waggoner S and
DiFeo A: Critical role of Wnt/β-catenin signaling in driving
epithelial ovarian cancer platinum resistance. Oncotarget.
6:23720–23734. 2015.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Chiu WT, Huang YF, Tsai HY, Chen CC, Chang
CH, Huang SC, Hsu KF and Chou CY: FOXM1 confers to
epithelial-mesenchymal transition, stemness and chemoresistance in
epithelial ovarian carcinoma cells. Oncotarget. 6:2349–2365.
2015.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Mariya T, Hirohashi Y, Torigoe T, Tabuchi
Y, Asano T, Saijo H, Kuroda T, Yasuda K, Mizuuchi M, Saito T and
Sato N: Matrix metalloproteinase-10 regulates stemness of ovarian
cancer stem-like cells by activation of canonical Wnt signaling and
can be a target of chemotherapy-resistant ovarian cancer.
Oncotarget. 7:26806–26822. 2016.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Zhang Y, Liu B, Zhao Q, Hou T and Huang X:
Nuclear localizaiton of β-catenin is associated with poor survival
and chemo-/radioresistance in human cervical squamous cell cancer.
Int J Clin Exp Pathol. 7:3908–3917. 2014.PubMed/NCBI
|
|
100
|
Zhou S, Bai ZL, Xia D, Zhao ZJ, Zhao R and
Wang YY: FTO regulates the chemo-radiotherapy resistance of
cervical squamous cell carcinoma (CSCC) by targeting β-catenin
through mRNA demethylation. Mol Carcinog. 57:590–597.
2018.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Xu H, Wang Z, Xu L, Mo G, Duan G, Wang Y,
Sun Z and Chen H: Targeting the eIF4E/β-catenin axis sensitizes
cervical carcinoma squamous cells to chemotherapy. Am J Transl Res.
9:1203–1212. 2017.PubMed/NCBI
|
|
102
|
Cao HZ, Liu XF, Yang WT, Chen Q and Zheng
PS: LGR5 promotes cancer stem cell traits and chemoresistance in
cervical cancer. Cell Death Dis. 8(e3039)2017.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Joyce JA and Fearon DT: T cell exclusion,
immune privilege, and the tumor microenvironment. Science.
348:74–80. 2015.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10.
2013.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Pai SG, Carneiro BA, Mota JM, Costa R,
Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK and Giles FJ:
Wnt/beta-catenin pathway: Modulating anticancer immune response. J
Hematol Oncol. 10(101)2017.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Ganesh S, Shui X, Craig KP, Park J, Wang
W, Brown BD and Abrams MT: RNAi-mediated β-catenin inhibition
promotes T cell infiltration and antitumor activity in combination
with immune checkpoint blockade. Mol Ther. 26:2567–2579.
2018.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Luke JJ, Bao R, Sweis RF, Spranger S and
Gajewski TF: WNT/β-catenin pathway activation correlates with
immune exclusion across human cancers. Clin Cancer Res.
25:3074–3083. 2019.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Kerdidani D, Chouvardas P, Arjo AR,
Giopanou I, Ntaliarda G, Guo YA, Tsikitis M, Kazamias G, Potaris K,
Stathopoulos GT, et al: Wnt1 silences chemokine genes in dendritic
cells and induces adaptive immune resistance in lung
adenocarcinoma. Nat Commun. 10(1405)2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Bergenfelz C, Janols H, Wullt M, Jirström
K, Bredberg A and Leandersson K: Wnt5a inhibits human
monocyte-derived myeloid dendritic cell generation. Scand J
Immunol. 78:194–204. 2013.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Diamond MS, Kinder M, Matsushita H,
Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM,
Kalinke U, et al: Type I interferon is selectively required by
dendritic cells for immune rejection of tumors. J Exp Med.
208:1989–2003. 2011.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Gattinoni L, Ji Y and Restifo NP:
Wnt/beta-catenin signaling in T-cell immunity and cancer
immunotherapy. Clin Cancer Res. 16:4695–4701. 2010.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Schinzari V, Timperi E, Pecora G, Palmucci
F, Gallerano D, Grimaldi A, Covino DA, Guglielmo N, Melandro F,
Manzi E, et al: Wnt3a/β-catenin signaling conditions
differentiation of partially exhausted T-effector cells in human
cancers. Cancer Immunol Res. 6:941–952. 2018.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Wang B, Tian T, Kalland KH, Ke X and Qu Y:
Targeting Wnt/β-catenin signaling for cancer immunotherapy. Trends
Pharmacol Sci. 39:648–658. 2018.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Sun X, Liu S, Wang D, Zhang Y, Li W, Guo
Y, Zhang H and Suo J: Colorectal cancer cells suppress CD4+ T cells
immunity through canonical Wnt signaling. Oncotarget.
8:15168–15181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Lengfeld JE, Lutz SE, Smith JR, Diaconu C,
Scott C, Kofman SB, Choi C, Walsh CM, Raine CS, Agalliu I and
Agalliu D: Endothelial Wnt/β-catenin signaling reduces immune cell
infiltration in multiple sclerosis. Proc Natl Acad Sci USA.
114:E1168–E1177. 2017.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Xu P, Xi Y, Kim JW, Zhu J, Zhang M, Xu M,
Ren S, Yang D, Ma X and Xie W: Sulfation of chondroitin and bile
acids converges to antagonize Wnt/β-catenin signaling and inhibit
APC deficiency-induced gut tumorigenesis. Acta Pharm Sin B.
14:1241–1256. 2024.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Hussain T, Alafnan A, Almazni IA, Helmi N,
Moin A, Baeissa HM, Awadelkareem AM, Elkhalifa AO, Bakhsh T,
Alzahrani A, et al: Aloe-emodin exhibits growth-suppressive effects
on androgen-independent human prostate cancer DU145 cells via
inhibiting the Wnt/β-catenin signaling pathway: An in vitro and in
silico study. Front Pharmacol. 14(1325184)2024.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Suryawanshi A, Hussein MS, Prasad PD and
Manicassamy S: Wnt signaling cascade in dendritic cells and
regulation of anti-tumor immunity. Front Immunol.
11(122)2020.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Haseeb M, Pirzada RH, Ain QU and Choi S:
Wnt signaling in the regulation of immune cell and cancer
therapeutics. Cells. 8(1380)2019.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017.PubMed/NCBI View Article : Google Scholar
|
|
122
|
He B, You L, Uematsu K, Xu Z, Lee AY,
Matsangou M, McCormick F and Jablons DM: A monoclonal antibody
against Wnt-1 induces apoptosis in human cancer cells. Neoplasia.
6:7–14. 2004.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Bravo DT, Yang YL, Kuchenbecker K, Hung
MS, Xu Z, Jablons DM and You L: Frizzled-8 receptor is activated by
the Wnt-2 ligand in non-small cell lung cancer. BMC Cancer.
13(316)2013.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Huang Y, Liu G, Zhang B, Xu G, Xiong W and
Yang H: Wnt-5a regulates proliferation in lung cancer cells. Oncol
Rep. 23:177–181. 2010.PubMed/NCBI
|
|
125
|
Dotan E, Cardin DB, Lenz HJ, Messersmith
W, O'Neil B, Cohen SJ, Denlinger CS, Shahda S, Astsaturov I, Kapoun
AM, et al: Phase Ib study of Wnt inhibitor ipafricept with
gemcitabine and nab-paclitaxel in patients with previously
untreated stage IV pancreatic cancer. Clin Cancer Res.
26:5348–5357. 2020.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Le PN, McDermott JD and Jimeno A:
Targeting the Wnt pathway in human cancers: Therapeutic targeting
with a focus on OMP-54F28. Pharmacol Ther. 146:1–11.
2015.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Taciak B, Pruszynska I, Kiraga L, Bialasek
M and Krol M: Wnt signaling pathway in development and cancer. J
Physiol Pharmacol. 69:185–196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Suryawanshi A, Tadagavadi RK, Swafford D
and Manicassamy S: Modulation of inflammatory responses by
Wnt/β-catenin signaling in dendritic cells: A novel immunotherapy
target for autoimmunity and cancer. Front Immunol.
7(460)2016.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang
T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al: Targeting
Wnt-driven cancer through the inhibition of Porcupine by LGK974.
Proc Natl Acad Sci USA. 110:20224–20229. 2013.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Jimeno A, Gordon M, Chugh R, Messersmith
W, Mendelson D, Dupont J, Stagg R, Kapoun AM, Xu L, Uttamsingh S,
et al: A first-in-human phase I study of the anticancer stem cell
agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in
patients with advanced solid tumors. Clin Cancer Res. 23:7490–7497.
2017.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Bhamra I, Armer R, Bingham M, Eagle C,
Cook A, Phillips C and Woodcock S: Abstract 3764: Porcupine
inhibitor RXC004 enhances immune response in pre-clinical models of
cancer. Cancer Res. 78 (Suppl 13)(S3764)2018.
|
|
132
|
Tabernero J, Van Cutsem E, Garralda E, Tai
D, De Braud F, Geva R, van Bussel MTJ, Fiorella Dotti K, Elez E, de
Miguel MJ, et al: A phase Ib/II study of WNT974 + encorafenib +
cetuximab in patients with BRAF V600E-mutant KRAS wild-type
metastatic colorectal cancer. Oncologist. 28:230–238.
2023.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Goswami VG and Patel BD: Recent updates on
Wnt signaling modulators: A patent review (2014-2020). Expert Opin
Ther Pat. 31:1009–1043. 2021.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Shah K, Panchal S and Patel B: Porcupine
inhibitors: Novel and emerging anti-cancer therapeutics targeting
the Wnt signaling pathway. Pharmacol Res.
167(105532)2021.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Jiang X, Hao HX, Growney JD, Woolfenden S,
Bottiglio C, Ng N, Lu B, Hsieh MH, Bagdasarian L, Meyer R, et al:
Inactivating mutations of RNF43 confer Wnt dependency in pancreatic
ductal adenocarcinoma. Proc Natl Acad Sci USA. 110:12649–12654.
2013.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Li C, Zheng X, Han Y, Lv Y, Lan F and Zhao
J: XAV939 inhibits the proliferation and migration of lung
adenocarcinoma A549 cells through the WNT pathway. Oncol Lett.
15:8973–8982. 2018.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Pan F, Shen F, Yang L, Zhang L, Guo W and
Tian J: Inhibitory effects of XAV939 on the proliferation of
small-cell lung cancer H446 cells and Wnt/β-catenin signaling
pathway in vitro. Oncol Lett. 16:1953–1958. 2018.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Shetti D, Zhang B, Fan C, Mo C and Lee BH:
Low dose of paclitaxel combined with XAV939 attenuates metastasis,
angiogenesis and growth in breast cancer by suppressing Wnt
signaling. Cells. 8(892)2019.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Arqués O, Chicote I, Puig I, Tenbaum SP,
Argilés G, Dienstmann R, Fernández N, Caratù G, Matito J,
Silberschmidt D, et al: Tankyrase inhibition blocks Wnt/β-catenin
pathway and reverts resistance to PI3K and AKT inhibitors in the
treatment of colorectal cancer. Clin Cancer Res. 22:644–656.
2016.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Waaler J, Mygland L, Tveita A, Strand MF,
Solberg NT, Olsen PA, Aizenshtadt A, Fauskanger M, Lund K, Brinch
SA, et al: Tankyrase inhibition sensitizes melanoma to PD-1 immune
checkpoint blockade in syngeneic mouse models. Commun Biol.
3(196)2020.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Zhang X, Wang L and Qu Y: Targeting the
β-catenin signaling for cancer therapy. Pharmacol Res.
160(104794)2020.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Doghman M, Cazareth J and Lalli E: The T
cell factor/beta-catenin antagonist PKF115-584 inhibits
proliferation of adrenocortical carcinoma cells. J Clin Endocrinol
Metab. 93:3222–3225. 2008.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Gandhirajan RK, Staib PA, Minke K, Gehrke
I, Plickert G, Schlösser A, Schmitt EK, Hallek M and Kreuzer KA:
Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling
induces apoptosis in chronic lymphocytic leukemia cells in vitro
and in vivo. Neoplasia. 12:326–335. 2010.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Wei W, Chua MS, Grepper S and So S: Small
molecule antagonists of Tcf4/beta-catenin complex inhibit the
growth of HCC cells in vitro and in vivo. Int J Cancer.
126:2426–2436. 2010.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Rodon J, Argilés G, Connolly RM,
Vaishampayan U, de Jonge M, Garralda E, Giannakis M, Smith DC,
Dobson JR, McLaughlin ME, et al: Phase 1 study of single-agent
WNT974, a first-in-class Porcupine inhibitor, in patients with
advanced solid tumours. Br J Cancer. 125:28–37. 2021.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Phillips C, Bhamra I, Eagle C, Flanagan E,
Armer R, Jones CD, Bingham M, Calcraft P, Edmenson Cook A, Thompson
B and Woodcock SA: The Wnt pathway inhibitor RXC004 blocks tumor
growth and reverses immune evasion in Wnt ligand-dependent cancer
models. Cancer Res Commun. 2:914–928. 2022.PubMed/NCBI View Article : Google Scholar
|