Introduction
Cervical cancer ranks as the fourth most frequently
diagnosed cancer and the fourth leading cause of cancer-related
mortality among women globally, with an estimated 604,000 incident
cases and 342,000 deaths worldwide in 2020(1). Notably, the incidence rates exhibit a
significant disproportionality, with low-income countries
experiencing higher rates compared to their high-income
counterparts (18.8 vs. 11.3 per 100,000), a trend mirrored in
mortality rates (12.4 vs. 5.2 per 100,000) (1). In the context of Italy, the annual
calculation of cervical cancer cases stands at 2,400, representing
1.3% of all female tumors, with mortality figures below 500 cases.
Encouragingly, the 5-year survival rate is recorded at 67%
(2). Human papillomavirus (HPV)
stands as the primary risk factor for cervical cancer (3). Supplementary factors contributing to
susceptibility include inadequate sanitation, sexually transmitted
infections such as HIV and Chlamydia trachomatis, tobacco use,
higher parity, and prolonged use of oral contraceptives (4). Notably, significant reductions in
cervical cancer incidence and mortality have been observed across
many countries, a trend attributed not solely to socioeconomic
conditions but also to the decline in persistent HPV infection
(5). Cervical screening has also
played a pivotal role in reducing cancer incidence rates, by
facilitating the detection of precancerous conditions such as
cervical intraepithelial neoplasia (CIN 2) and CIN 3(6). Cervical cancer is widely acknowledged
as being nearly completely preventable owing to the remarkably
efficacious primary prevention provided by the HPV vaccine and the
secondary prevention afforded by screening programs. Regrettably,
the implementation of these preventive measures has not been
uniformly applied across countries or even within them. As of May
2020, less than 30% of low- and middle-income countries (LMICs) had
instituted national HPV vaccination programs, in stark contrast
with over 80% of high-income countries (HICs) (7). According to the World Health
Organization (WHO), screening is advised for women aged between 30
and 49, using either a Papanicolaou test (cervical cytology) every
3-5 years or an HPV test every 5 years. This screening regimen aims
to detect precancerous forms and must be complemented by prompt and
efficient treatment (8,9). In 2018, acknowledging the substantial
global burden of cervical cancer and the growing disparities, the
WHO Director-General issued a call for worldwide efforts toward the
elimination of cervical cancer, defined as achieving an incidence
rate of ≤4 per 100,000 women worldwide. This ambitious goal is to
be pursued through a triple-intervention strategy, encompassing: 1)
vaccinating 90% of all girls by age 15 years, 2) screening 70% of
women twice in the age range of 35 to 45 years, and 3) ensuring the
treatment of at least 90% of all precancerous lesions detected
during screening (10). Despite
concerted efforts to facilitate early diagnosis of lesions, the
presence of invasive tumors continues to pose a significant public
health challenge, frequently being identified at an advanced stage
and carrying a poor prognosis.
This study aims to delineate the pattern of
precancerous lesions and invasive cervical cancer in relation to
age, stage, and country of origin.
Materials and methods
Patients
Data from this population-based cohort study were
obtained from the Reggio Emilia Cancer Registry (RE-CR), approved
by the provincial Ethics Committee of Reggio Emilia (Protocol no.
2014/0019740 of 04/08/2014). The leading information sources of the
RE-CR comprise anatomic pathology reports, hospital discharge
records, and mortality data, integrated with laboratory tests,
diagnostic reports, and information sourced from general
practitioners or by directly consulting medical records.
Encompassing a population of 532,000 inhabitants, the RE-CR is
considered a high-quality CR distinguished by its contemporaneous
data (extending to the end of 2021), a high percentage of
microscopic confirmation (100% for cervical cancer cases), and the
absence of death certificate only (DCO) cases (11).
Clinicopathological data
Cervical cancer cases were identified and classified
according to the International Classification of Diseases for
Oncology, 3rd Edition (ICD-O-3) (12), specifically under the topography
code C53. The study included all cases of cervical infiltrating
cancer diagnosed between 2008 and 2018. Data on the cancer stage,
as per the TNM 7th edition (13),
were obtained by reviewing medical records from hospital
archives.
Statistical analysis
Descriptive analyses were conducted to examine
patients' characteristics, age at diagnosis, and tumor morphology,
stratified by tumor behavior: non infiltrating tumors (CIN 2/3) and
infiltrating tumors. For cases of invasive malignancy, additional
descriptive statistics were computed for tumor stage and T
classification. In addition, specific incidence rates were
calculated for each tumor behavior category using the population of
the Province of Reggio Emilia (recorded on January 1st of each
year) as denominators. To allow for meaningful comparisons,
incidence rates were standardized using the direct method, with the
2013 European Standard Population as a reference. The reported
rates are presented on an annual basis: trends of incidence were
performed for the years 2000-2020. The 5-year relative survival
rate of cancers diagnosed between 2008 and 2018 was determined.
Relative survival estimates overall survival adjusted for
contributing causes of death and is defined as the ratio of the
observed survival proportion in a cohort of cancer patients to the
expected survival proportion in a comparable group of cancer-free
individuals. Relative survival was estimated using the Pohar Perme
method, wherein net survival for a cohort is assessed by weighting
with the inverse of the individual-specific expected survival
probabilities. Additionally, a multivariable Cox proportional
hazard regression model was developed to investigate the
relationship between stage, age, and overall survival, with time
expressed in years. Kaplan-Meier methods were employed to ascertain
time-to-event outcomes, specifically overall survival by age,
stage, and age-adjusted stage. The 95% confidence intervals were
also provided. Finally, trends over time were analyzed by
calculating the annual percent change (APC) in age-standardized
rates using Joinpoint Regression analysis. All analyses were
performed using Stata 16.1 software.
Results
Between 2008 and 2018, a total of 1,752 cases of CIN
2/3 and 152 cases of invasive malignancy of the uterine cervix were
registered (Table I). The mean age
at diagnosis for CIN 2/3 cases was 37.8 years, while for invasive
malignancy it was 56.7 years. Analysis of the distribution by age
group revealed that 64.5% of CIN cases occurred in women under the
age of 40, whereas 82.2% of the infiltrating forms were diagnosed
in women aged 40 and above (Table
I, Fig. 1). Among CIN 2/3
lesions, a striking 98.2% of cases exhibited squamous morphology,
underscoring its predominant nature. In contrast, among the
infiltrating forms, squamous morphology remained prominent,
accounting for over 76.4%, while 16.4% showed adenocarcinoma
morphology, and the remaining 7.2% represented other morphologies.
Regarding stage distribution, the findings indicated that 42.1% of
cases were diagnosed at stage I, followed by 12.5% at stage II,
20.4% at stage III, and 23% at stage IV (Table I). Analysis of T classification
distribution revealed that 46.7% of cases were categorized as T1,
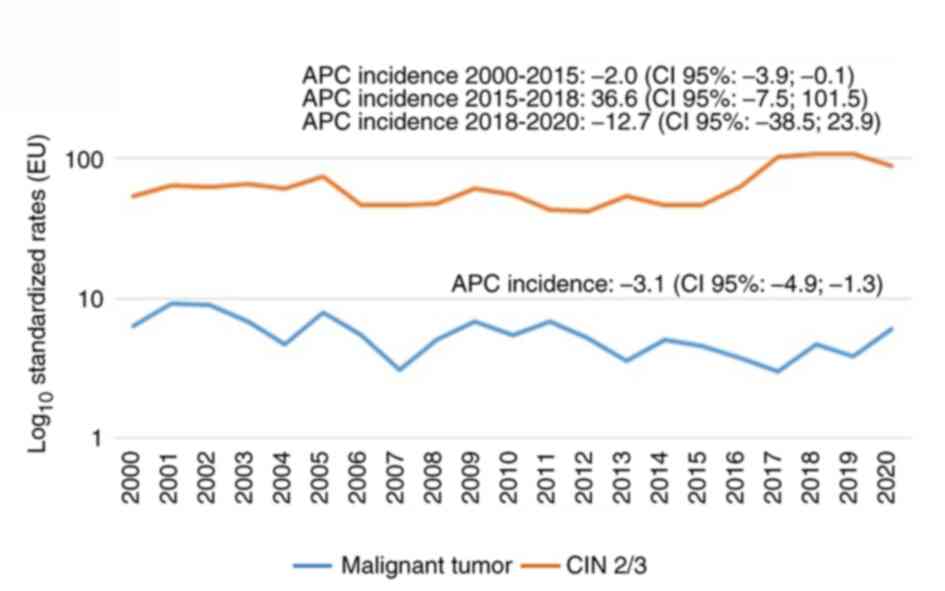
followed by 16.5% as T2, 11.2% as T3, and 15.1% as T4 (Table I). Over the years, the incidence of
infiltrating forms showed a declining trend, decreasing from 6.4 to
4.7 per 100,000 individuals, with an APC of -3.1% (Fig. 2A). Conversely, there has been a
notable increase in the incidence of CIN 2/3 lesions, with an APC
of 36.6% in the period 2015-2018, after decreasing in the following
period 2018-2020 (Fig. 2B). In
terms of stage distribution, among women under 40 years old, the
majority of tumors (55.6%) were diagnosed in stage I, a pattern
that remains consistent among women aged 40-64 (53.9%). On the
other hand, stages III and IV prevail among elderly women,
collectively constituting 86.1% of the entire case series (Table II). The overall 5-year survival
rate stands at 66.6%, exhibiting a robust gradient correlated with
the tumor stage. Specifically, survival rates were 88.2, 82.1,
74.1, and 12.1% for stages I, II, III, and IV, respectively
(Table III). Additionally, age
demonstrated a significant gradient, with survival rates of 84.3,
79.7, and 36.9%, for women under 40 years old, those aged 40-64,
and 65+, respectively. The univariable analysis (Table IV) confirmed an increased risk
associated with stages III (HR 3.3; 95% CI 1.4-7.7) and IV (HR
28.3; 95% CI 12.7-62.7), as well as for women aged 40-64 (HR 6.0;
95% CI 2.9-12.7) and those aged 65+ (HR 119.7; 95% CI 30.1-126.3).
In the multivariable analysis, the excess risk persisted
independently from other confounders for stages III (HR 3.1; 95% CI
1.3-7.3) and IV (HR 19.6; 95% CI 8.2-46.7), as well as exclusively
in women aged 65+ (HR 2.8; 95% CI 1.0-8.1). Furthermore, the impact
associated with stage and age is evident in the Kaplan-Meier
survival estimates by stage (Fig.
3A) and age (Fig. 3B).
However, upon adjusting for age, only stages III and IV exhibited a
worse prognosis (Fig. 3C). Among
the registered precancerous lesions, 19.9% of women were of foreign
origin. However, the percentage increased to 22.4% among the
infiltrating malignant lesions. While there are minor disparities
in the distribution by stage between the early and locally advanced
forms, notable differences emerged for stage III (17.8 vs. 29.4%)
and IV (27.9 vs. 5.9%) (Table
V).
 | Table IDistribution of CIN2/3 and
infiltrating cervical tumors by age, morphology and stage in the
Province of Reggio Emilia, January 2008-December 2018. |
Table I
Distribution of CIN2/3 and
infiltrating cervical tumors by age, morphology and stage in the
Province of Reggio Emilia, January 2008-December 2018.
| | CIN 2/3 (n=1752) | Infiltrating
(n=152) |
|---|
| Parameter | Mean, n | SD (%) | Mean, n | SD (%) |
|---|
| Age at diagnosis,
years | 37.8 | 10.5 | 56.7 | 16.6 |
| Age at diagnosis
(group), years | | | | |
|
<40 | 1130 | 64.5 | 27 | 17.8 |
|
40-64 | 602 | 34.4 | 76 | 50.0 |
|
≥65 | 20 | 1.1 | 49 | 32.2 |
| Morphology | | | | |
|
Squamous
cell neoplasmsa | 1721 | 98.2 | 116 | 76.4 |
|
Adenocarcinomaa | 26 | 1.5 | 25 | 16.4 |
|
Carcinomaa | 5 | 0.3 | - | - |
|
Others | - | - | 11 | 7.2 |
| Stage | | | | |
|
I | - | - | 64 | 42.1 |
|
II | - | - | 19 | 12.5 |
|
III | - | - | 31 | 20.4 |
|
IV | - | - | 35 | 23.0 |
|
Unknown | - | - | 3 | 2.0 |
| T | | | | |
|
T1 | - | - | 71 | 46.7 |
|
T2 | - | - | 25 | 16.5 |
|
T3 | - | - | 17 | 11.2 |
|
T4 | - | - | 23 | 15.1 |
|
Unknown | - | - | 16 | 10.5 |
 | Table IIDistribution of infiltrating cervical
tumors by stage and age in the Province of Reggio Emilia, January
2008-December 2018. |
Table II
Distribution of infiltrating cervical
tumors by stage and age in the Province of Reggio Emilia, January
2008-December 2018.
| | <40 years | 40-64 years | 65+ years |
|---|
| Stage | n | % | n | % | n | % |
|---|
| I | 15 | 55.6 | 41 | 53.9 | 8 | 16.3 |
| II | 3 | 11.1 | 5 | 6.6 | 11 | 22.5 |
| III | 6 | 22.2 | 16 | 21.1 | 9 | 18.4 |
| IV | 3 | 11.1 | 12 | 15.8 | 20 | 40.8 |
| Unknown | 0 | 0.0 | 2 | 2.6 | 1 | 2.0 |
 | Table IIIProvince of Reggio Emilia, January
2008-December 2018: 5-year relative survival overall of
infiltrating cervical tumors, by stage and age. |
Table III
Province of Reggio Emilia, January
2008-December 2018: 5-year relative survival overall of
infiltrating cervical tumors, by stage and age.
| Parameter | % | 95% Confidence
intervals |
|---|
| Overall | 66.6 | 58.1-73.7 |
| Stage | | |
|
I | 88.2 | 76.6-94.2 |
|
II | 82.1 | 53.7-93.9 |
|
III | 74.1 | 53.1-86.8 |
|
IV | 12.1 | 3.7-25.6 |
| Age, years | | |
|
<40 | 84.3 | 63.3-93.8 |
|
40-64 | 79.7 | 68.6-87.3 |
|
65+ | 36.9 | 23.0-50.9 |
 | Table IVCox regression analysis of
infiltrating cervical tumors adjusted for age and stage in the
Province of Reggio Emilia, 2008-2018. |
Table IV
Cox regression analysis of
infiltrating cervical tumors adjusted for age and stage in the
Province of Reggio Emilia, 2008-2018.
| | Univariable
analysis | Multivariable
analysis |
|---|
| Parameter | HR | 95% CI | HR | 95% CI |
|---|
| Stage | | | | |
|
I | 1.0 | Ref. | 1.0 | Ref. |
|
II | 2.1 | 0.7-6.1 | 1.6 | 0.5-5.1 |
|
III | 3.3 | 1.4-7.7 | 3.1 | 1.3-7.3 |
|
IV | 28.3 | 12.7-62.7 | 19.6 | 8.2-46.7 |
| Age group,
years | | | | |
|
<40 | 1.0 | Ref. | 1.0 | Ref. |
|
40-64 | 6.0 | 2.9-12.7 | 1.6 | 0.6-5.1 |
|
65+ | 119.7 | 30.1-126.3 | 2.8 | 1.0-8.1 |
 | Table VNumber and percentage of women with
CIN2/3 and infiltrating cervical tumors in the Province of Reggio
Emilia, January 2008-December 2018. |
Table V
Number and percentage of women with
CIN2/3 and infiltrating cervical tumors in the Province of Reggio
Emilia, January 2008-December 2018.
| | CIN 2/3 | Infiltrating | Total |
|---|
| Population | n | % | n | % | n | % |
|---|
| Italians | 1,403 | 80.1 | 118 | 77.6 | 1,521 | 79.9 |
| Non-Italians | 349 | 19.9 | 34 | 22.4 | 383 | 20.1 |
| Total | 1,752 | 100 | 152 | 100 | 1,904 | 100 |
Discussion
This study aimed to provide a comprehensive overview
of cervical lesions over a ten-year period, examining their
incidence in relation to age, stage, and country of origin. One
significant finding is the remarkably low incidence of infiltrating
forms observed in our series, constituting only 0.8% of all female
tumors. This incidence rate contrasts with the 1.3% reported in
Italy nationally, according to the AIOM report (2), and the 2.5 % reported in Europe
(14). In our study, incidence
trends indicate a decline, with the Age-Standardized Incidence Rate
(ASIR) decreasing from 6.4 to 4.7 per 100,000 individuals (APC
-3.1%). Accordingly, findings from the literature spanning from
1990 to 2019 suggest a general decline in both incidence and
mortality rates, except in East Asia and South Africa, where there
has been a notable increase in the APC among women aged
15-49(15). For instance, in
China, there has been an observed increase in incidence, with the
TSD rising from 11.1 to 16.4 (APC 3.7%), accompanied by a
corresponding increase in mortality from 3.2 to 4.8 (APC 3.6%)
(16). Furthermore, this increase
appears more pronounced among women residing in rural areas
(17). In Canada, a notable
decline in cervical cancer incidence has been documented, with
rates decreasing from 19 to 7 per 100,000 individuals. However, it
is noteworthy that the highest incidence rates are recorded in
advanced stages and among women over 55 years old (HR 1.34)
(18). Similarly, in Taiwan, a
significant drop in incidence rates has been noted with an APC of
-7.2% (19). Our study,
concomitant with the decline in infiltrating forms, showed a
distinct increase in precancerous lesions, which have risen from
120 to 260 cases per year. This increase can be attributed to the
introduction of HPV research, which has enhanced the detection rate
by 40% (20). Notably, this
increase predominantly affects younger women, with 65% of diagnoses
occurring in those under 40 years old. HPV plays a key role in the
development of cervical cancer. The persistence of high-risk
(HR)-HPV infection following conization is widely recognized as a
significant risk factor for CIN 3 recurrence (21), particularly in cases with positive
endocervical margins (22).
Indeed, HR-HPV-negative high-grade cervical dysplasia is associated
with more favorable outcomes compared to patients with documented
HR-HPV infection (23). In
Switzerland, the age-standardized incidence of cervical cancer and
CIN 3 has shown an increase from 2.4 to 3.3/100,000 and from 11.6
to 26.9/100,000, respectively (24). The average age at diagnosis is 56.7
years, slightly higher than findings from an Indian study which
reported an average age of 52.9 years (25). Additionally, an increase in
diagnoses has been seen among young women aged 43 to 49, with no
significant differences noted for those aged 65 and above (26,27).
In developed countries, well-organized cervical cancer screening
programs have been instrumental in achieving a significant
reduction in invasive cancer incidence and mortality rates. These
programs, which typically involve cytological screening combined
with colposcopy and HPV triage, have led to a dramatic decline in
the burden of invasive cancer incidence and mortality (28). However, there persists a glaring
disparity in the cervical cancer burden between high-income
countries (HICs) and low- and middle-income countries (LMICs), with
the highest incidence and mortality rates observed in regions such
as Africa, South Asia, and Latin America (29). While population-based screening
programs have effectively decreased cervical cancer incidence and
mortality in HICs, the same success has not been replicated in
LMICs due to lack of effective organized screening programs. Data
from the Norwegian CR show a clear reduction in mortality, with an
80% drop noted in women aged 26-69 who undergo screening (30). Finally, our study shows that 42% of
infiltrating tumors are diagnosed in stage I. However, the
incidence of metastatic tumors remains significantly high,
accounting for 23% of the entire case series and escalating to 66%
among women aged 65 and above. In our study, the 5-year survival
rate was 66%, with survival rates for the first three stages
hovering around 80% but sharply dropping to 12% for stage IV. Age
exhibits similar trends, with survival rates overlapping for
younger individuals and adults, exceeding 80%, but halving to 44%
for those over 65 years old. After adjusting for age and stage, the
multivariable analysis revealed an excess of risk for elderly
women, and a significant risk persists for both stage III and
particularly stage IV. From the literature we understand that
advanced age is consistently recognized as a prognostic factor for
survival (31), and in the
presence of an advanced stage, the risk can be amplified up to 8
times (32). Data from the
Surveillance, Epidemiology, and End Results (SEER) program confirm
poorer survival outcomes for women 70 and above, attributed
partially to receiving fewer treatments, with a 2.8 times increased
risk of dying (33), a finding
further corroborated by other studies (34). Regarding the relationship between
Italians and non-Italians, among women with CINs, 80% are Italian
and 20% are non-Italian. However, for infiltrating forms, the
proportion of non-Italians rises to 22.4%. Interestingly, there are
no differences observed in the distribution by stage for the early
stages, whereas metastatic forms are more frequently recorded among
Italians compared to non-Italians (28 vs 6%). This discrepancy is
not surprising considering the fact that the average age of
non-Italian patients with infiltrating cancer is 49 years, while
among Italians it is 59 years. A wealth of information exists
concerning cervical cancer screening and its implications. In 206
European countries, the availability of Pap tests stands at 78%,
while HPV screening is accessible in 35% of these nations.
Compliance with screening among women aged 30-49 varies
significantly, ranging from 84% in HICs to 48% in LMICs, and
dropping to 11% in countries with very low income (35). SEER data reveal a higher prevalence
of squamous cell carcinoma in Black Hispanic women, with elevated
mortality rates and lower 5-year survival rates across all subtypes
and stages (36). A Swedish study
showed that immigrants had lower incidence rates of cervical cancer
compared to those residing in their countries of origin but higher
than native Swedes (Relative Risk 1.13) (37). Unfavorable factors associated with
survival include receiving care in low-volume hospitals (38), socioeconomic deprivation (39), and being followed in non-university
or non- (40). Additionally, black
ethnicity, being displaced (41),
and low level of education are risk factors associated with poor
survival (25), as treatment also
plays a crucial role in influencing outcomes (42). Among the strengths of this work, we
highlight the fact that it is population-based data and therefore
there is no selection bias, and it is recent and of good quality.
Unfortunately, the work involves only one center, so in the future
it would be important to repeat the analyses involving other
Italian centers. Another limitation of the study is the lack of
data on HPV vaccination and on the socio-economic status of the
patients, which could have a strong impact on the incidence of
these tumors.
In conclusion, with high adherence to screening and
the availability of HPV vaccination programs, cervical cancer has
become increasingly rare in Western countries. The reduction of
cervical cancer incidence hinges on the widespread dissemination of
the HPV vaccine among the entire at-risk population, coupled with
ensuring screening for timely detection and treatment of high-grade
dysplasia. Healthcare providers should focus on minimizing the
occurrence of advanced-stage cancers by diligently attending to
symptoms, especially in elderly women. It is imperative to conduct
targeted investigations in this age group, which may not be
included in routine screening and vaccination campaigns. By
adopting a comprehensive approach that includes vaccination,
screening, and symptom awareness, we can continue to make strides
in the prevention and early detection of cervical cancer,
ultimately saving lives and improving public health outcomes.
Acknowledgements
Not applicable.
Funding
Funding: This study was partially supported by the Italian
Ministry of Health-Ricerca Corrente Annual Program 2025.
Availability of data and materials
The data generated in the present study are not
publicly available due to ethical and privacy issues to but may be
requested from the corresponding author. Requests for data must be
approved by the Ethics Committee after the presentation of a study
protocol.
Authors' contributions
LM conceptualized the study, performed the
experiments, wrote the original draft, visualized the data and
supervised the study. FMa analyzed the data. IB acquired the data,
reviewed and edited the manuscript, and visualized the data and
supervised the study. FR and VM performed the experiments and
supervised the study. FMo, AN and LA analyzed and interpretated the
data, and supervised the study. VDM conceptualized the study, wrote
the original draft, performed the experiments and managed the
study. All authors have read and agreed to the final version of the
manuscript. LM and FMa confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This population-based cohort study used data from
the Reggio Emilia Cancer Registry, approved by the Provincial
Ethics Committee of Reggio Emilia (approval no. 2014/0019740 of
August 2014). The Ethics Committee authorized, even in the absence
of consent, the processing of personal data, including those
suitable for revealing the state of health of patients who are
deceased or untraceable for the execution of the study.
Patient consent for publication
According to Italian legislation, population-based
cancer registries collect pseudonymized personal data for
surveillance purposes that do not need the collection of explicit
individual consent, without any direct or indirect intervention on
patients, therefore the approval of a research ethics committee was
not required (43).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
AIOM AIRTUM, SIAPEC-IAP: I Cancer numbers
in Italy, Intermedia editore, September 2020.
|
|
3
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thun M, Linet MS, Cerhan JR, Haiman CA and
Schottenfeld D: Cancer Epidemiology and Prevention. 4th edition.
Oxford University Press, pp925-946, 2018.
|
|
5
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36
Cancers in 185 Countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Loopik DL, IntHout J, Ebisch RMF, Melchers
WJG, Massuger LFAG, Siebers AG and Bekkers RLM: The risk of
cervical cancer after cervical intraepithelial neoplasia grade 3: A
population-based cohort study with 80,442 women. Gynecol Oncol.
157:195–201. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Global HPV Vaccine Introduction Overview:
Projected and current national introductions, demonstration/pilot
projects, gender-neutral vaccination programs, and global HPV
vaccine introduction maps (2006-2023). PATH, 2020. Available from:
path.org/resources/global-hpv-vaccine-introduction-overview/.
|
|
8
|
World Health Organization (WHO): ‘Best
buys’ and other recommended interventions for the prevention and
control of non-communicable diseases: Updated Appendix 3 of the
Global Action Plan for the Prevention and Control of Non
communicable Diseases 2013-2020WHO, 2017. Available from:
who.int/ncds/governance/appendix3-update/en/).
Accessed on April 2024.
|
|
9
|
World Health Organization (WHO): WHO
guidelines for the use of thermal ablation for cervical pre-cancer
lesions. WHO, 2019. Available from: Who.int/reproductivehealth/publications/thermal-ablation-for-cervical-pre-cancer-lesions/en/).
Accessed on April 2024.
|
|
10
|
World Health Organization (WHO): WHO
Director-General calls for all countries to take action to help end
the suffering caused by cervical cancer. WHO, 2018. Available from:
who.int/reproductivehealth/call-to-action-elimination-cervical-cancer/en/).
Accessed on April 2024.
|
|
11
|
Mangone L, Borciani E, Michiara M,
Vicentini M, Carrozzi G, Mancuso P, Sacchettini C and Rossi P:
Tumors in the provinces of the Vast Northern Emilia Area: Piacenza,
Parma, Reggio Emilia and Modena: Years 2013-2014. The Cancer
Registries, 2015. https://www.ausl.mo.it/media/interno.pdf?x14278.
Accessed on April, 2024.
|
|
12
|
Fritz A, Percy C, Jack A, Shanmugaratnam
K, Sobin L, Parkin DM and Whelan S: International Classification of
Disease for Oncology. 3rd edition. World Health Organization,
2013.
|
|
13
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumours. 7th edition. UICC,
Wiley-Blackwell, Hoboken, NJ, 2010.
|
|
14
|
ECIS (European Cancer Information System).
https://ecis.jrc.ec.europa.eu/ (accessed
on April 2024).
|
|
15
|
Yang M, Du J, Lu H, Xiang F, Mei H and
Xiao H: Global trends and age-specific incidence and mortality of
cervical cancer from 1990 to 2019: An international comparative
study based on the global burden of disease. BMJ Open.
12(e055470)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yuan M, Zhao X, Wang H, Hu S and Zhao F:
Trend in cervical cancer incidence and mortality rates in China,
2006-2030: A bayesian age-period-cohort modeling study. Cancer
Epidemiol Biomarkers Prev. 32:825–833. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun K, Zheng R, Lei L, Zhang S, Zeng H,
Wang S, Li L, Chen R, Han B, Peng J, et al: Trends in incidence
rates, mortality rates, and age-period-cohort effects of cervical
cancer-China, 2003-2017. China CDC Wkly. 4:1070–1076.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Raveinthiranathan N, Simkin J, Donken R,
Ogilvie G, Smith L, Van Niekerk D, Lee M and Woods RR: Age-specific
trends of invasive cervical cancer incidence in British Columbia,
Canada, 1971-2017. Curr Oncol. 30:7692–7705. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kau YC, Liu FC, Kuo CF, Huang HJ, Li AH,
Hsieh MY and Yu HP: Trend and survival outcome in Taiwan cervical
cancer patients: A population-based study. Medicine (Baltimore).
98(e14848)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rossi PG, Marsili LM, Camilloni L, Iossa
A, Lattanzi A, Sani C, Di Pierro C, Grazzini G, Angeloni C,
Capparucci P, et al: The effect of self-sampled HPV testing on
participation to cervical cancer screening in Italy: A randomised
controlled trial (ISRCTN96071600). Br J Cancer. 104:248–254.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bogani G, DI Donato V, Sopracordevole F,
Ciavattini A, Ghelardi A, Lopez S, Simoncini T, Plotti F, Casarin
J, Serati M, et al: Recurrence rate after loop electrosurgical
excision procedure (LEEP) and laser Conization: A 5-year follow-up
study. Gynecol Oncol. 159:636–641. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Giannini A, Di Donato V, Sopracordevole F,
Ciavattini A, Ghelardi A, Vizza E, D'Oria O, Simoncini T, Plotti F,
Casarin J, et al: Outcomes of high-grade cervical dysplasia with
positive margins and HPV persistence after cervical conization.
Vaccines (Basel). 11(698)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bogani G, Sopracordevole F, Di Donato V,
Ciavattini A, Ghelardi A, Lopez S, Simoncini T, Plotti F, Casarin
J, Serati M, et al: High-risk HPV-positive and -negative high-grade
cervical dysplasia: Analysis of 5-year outcomes. Gynecol Oncol.
161:173–178. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ochs K, Meili G, Diebold J, Arndt V and
Günthert A: Incidence trends of cervical cancer and its
precancerous lesions in women of Central Switzerland from 2000
until 2014. Front Med (Lausanne). 5(58)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sivaranjini K, Oak A, Cheulkar S,
Maheshwari A, Mahantshetty U and Dikshit R: Role of education and
income on disparities of time-to-treatment initiation and its
impact on cervical cancer survival. Indian J Public Health.
67:235–239. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gnade CM, Hill EK, Botkin HE, Hefel AR,
Hansen HE, Sheets KA, Mott SL, Hardy-Fairbanks AJ and Stockdale CK:
Is the age of cervical cancer diagnosis changing over time? J
Gynecol Obstet Hum Reprod. 50(102040)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cooley JJP, Maguire FB, Morris CR,
Parikh-Patel A, Abrahão R, Chen HA and Keegan THM: Cervical cancer
stage at diagnosis and survival among women ≥65 years in
california. Cancer Epidemiol Biomarkers Prev. 32:91–97.
2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hull R, Mbele M, Makhafola T, Hicks C,
Wang SM, Reis RM, Mehrotra R, Mkhize-Kwitshana Z, Kibiki G, Bates
DO and Dlamini Z: Cervical cancer in low and middle-income
countries. Oncol Lett. 20:2058–2074. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Baasland I, Vie GÅ, Romundstad PR and
Lönnberg S: Cervical cancer mortality in Norway according to
screening attendance and age. Acta Obstet Gynecol Scand.
101:952–959. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kassa R, Irene Y, Woldetsadik E, Kidane E,
Higgins M, Dejene T and Wells J: Survival of women with cervical
cancer in East Africa: A systematic review and meta-analysis. J
Obstet Gynaecol. 43(2253308)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mwaliko E, Itsura P, Keter A, De Bacquer
D, Buziba N, Bastiaens H, Jackie A, Obala A, Naanyu V, Gichangi P
and Temmerman M: Survival of cervical cancer patients at Moi
teaching and referral hospital, Eldoret in Western Kenya. BMC
Cancer. 23(1104)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Quinn BA, Deng X, Colton A, Bandyopadhyay
D, Carter JS and Fields EC: Increasing age predicts poor cervical
cancer prognosis with subsequent effect on treatment and overall
survival. Brachytherapy. 18:29–37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Benitez-Restrepo CC, Arias-Ortiz NE and
Arboleda-Ruiz WA: Cervical cancer incidence and patient survival in
Manizales, Colombia, 2008-2012. Rev Peru Med Exp Salud Publica.
37:438–445. 2020.PubMed/NCBI View Article : Google Scholar : (In Spanish,
English).
|
|
35
|
Bruni L, Serrano B, Roura E, Alemany L,
Cowan M, Herrero R, Poljak M, Murillo R, Broutet N, Riley LM and de
Sanjose S: Cervical cancer screening programmes and age-specific
coverage estimates for 202 countries and territories worldwide: A
review and synthetic analysis. Lancet Glob Health. 10:e1115–e1127.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cohen CM, Wentzensen N, Castle PE,
Schiffman M, Zuna R, Arend RC and Clarke MA: Racial and ethnic
disparities in cervical cancer incidence, survival, and mortality
by histologic subtype. J Clin Oncol. 41:1059–1068. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Azerkan F, Zendehdel K, Tillgren P,
Faxelid E and Sparén P: Risk of cervical cancer among immigrants by
age at immigration and follow-up time in Sweden, from 1968 to 2004.
Int J Cancer. 123:2664–2670. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tjioe KC, Miranda-Galvis M, Johnson MS,
Agrawal G, Balas EA and Cortes JE: The interaction between social
determinants of health and cervical cancer survival: A systematic
review. Gynecol Oncol. 181:141–154. 2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Donkers H, Bekkers R and Galaal K:
Systematic review on socioeconomic deprivation and cervical cancer:
Inequalities in survival. J Health Care Poor Underserved.
32:751–766. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hung P, Zahnd WE, Brandt HM, Adams SA,
Wang S and Eberth JM: Cervical cancer treatment initiation and
survival: The role of residential proximity to cancer care. Gynecol
Oncol. 160:219–226. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu G, Yang Z and Wang D: A Bayesian
network predicting survival of cervical cancer patients-Based on
surveillance, epidemiology, and end results. Cancer Sci.
114:1131–1141. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang C, Lester B, Huang L, Sun S and Ko
JJ: Patient, disease, and survival outcomes for stage IB to stage
IV cervical cancer-A population study. Womens Health (Lond).
19(17455057231164551)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Presidente del Consiglio dei Ministri:
Decreto del Presidente del Consiglio dei Ministri, 3/3/2017,41
Identificazione dei sistemi di sorveglianza e dei registri di
mortalità, di tumori e di altre patologie. 17A03142, GU Serie
Generale n.109 del (12-05-2017). Available from: https://www.gazzettaufficiale.it/eli/id/2017/05/12/17A03142/sg.
Accessed on May, 2024.
|
|
44
|
Cox DR: Regression models and life-tables.
Journal of the Royal Statistical Society, Series B. 34:187–220.
1972.
|