Introduction
The occipital artery (OA) is a main artery that
originates from the external carotid artery (ECA) and may be
involved in a number of diseases, such as moyamoya disease, dural
arteriovenous fistula (DAVF), aneurysm, etc. (1). Apart from its involvement in these
diseases, the OA is a crucial artery due to its main anatomical
variations and its usage in intra- and extracranial bypasses;
therefore, the understanding of the anatomy of the OA is of utmost
importance (2).
Previous studies on the OA have been mostly based on
cadavers and catheter-based angiography procedures (3-5).
Studies using computed tomography angiography (CTA) are rarely
reported (6,7). Therefore, the present study was
performed to examine the anatomy of the OA using CTA. Importantly,
the data of the present study were derived from Han Chinese
subjects, a population cohort that has rarely been reported in such
cases.
In addition to providing OA parameters, most
importantly, in the present study, the OA anatomy was compared
between healthy subjects and patients with internal carotid artery
(ICA) stenosis and occlusion to determine whether the OA parameters
differed. This was performed as the OA can become dilated or
thicker than normal in the presence of a DAVF (1). To date, no study has explored this
issue, at least to the best of our knowledge.
Materials and methods
Study participants
An imaging study was performed on Han Chinese
candidates, including healthy subjects and patients with ICA
stenosis and occlusion, who underwent cervical CTA examinations
between January, 2020 and September, 2021 at the First Hospital of
Jilin University, Changchun, China. The healthy subjects underwent
a cervical CTA examination during routine physical
examinations.
Prior to the CTA, the following exclusion criteria
were used: A history of severe or anaphylactic reaction to
iodinated contrast, an inability to cooperate with scanning
protocols, hemodynamic instability, diabetes, the administration of
any anticoagulant medication, renal impairment.
The present study was approved by the Ethics
Committee of the First Hospital of Jilin University (approval no.
2021-533). Written informed consent was obtained from all
participants. The original CTA data were further processed on a GE
Workstation (version 4.7) (GE Healthcare; Cytiva).
CTA imaging protocol
CTA examinations were performed on 256-multidetector
CT scanners (GE Healthcare; Cytiva) at the First Hospital of Jilin
University, utilizing THE test bolus injection technique to acquire
the optimal trigger time (8). The
scanned area ranged from the aortic arch to the frontal sinus. On
the basis of the body weight of the patient, a dose of 2 ml/kg of
nonionic iodinated contrast media (Omnipaque 350; GE Healthcare;
Cytiva) and 20-30 ml saline chaser were injected via an antecubital
vein through an 18-gauge peripheral intravenous line at a flow rate
of 4 to 5 ml/sec using a MedRad Mark V power injector (MedRad,
Inc.) (9).
Inclusion criteria and grouping
First, contrast-enhanced imaging upon CTA of the
participants was clear, and the ECA and OA could be clearly seen
and measured. For the healthy subjects, no ECA or ICA abnormities
were observed. For patients with ICA stenosis and occlusion, the
history of ICA stenosis and occlusion was chronic and progressive
due to arteriosclerosis; in these patients, the ECA was normal and
had no stenosis at the origin. No tumor or vascular diseases, such
as scalp or intramuscular hemangioma (10,11),
arteriovenous malformation (12), or
DAVF (13), were associated with the
OA.
Accordingly, the patients were divided into a mild
and moderate ICA stenosis (ICA stenosis <70%) group, a severe
ICA stenosis (ICA stenosis 70-99%) group and an ICA occlusion group
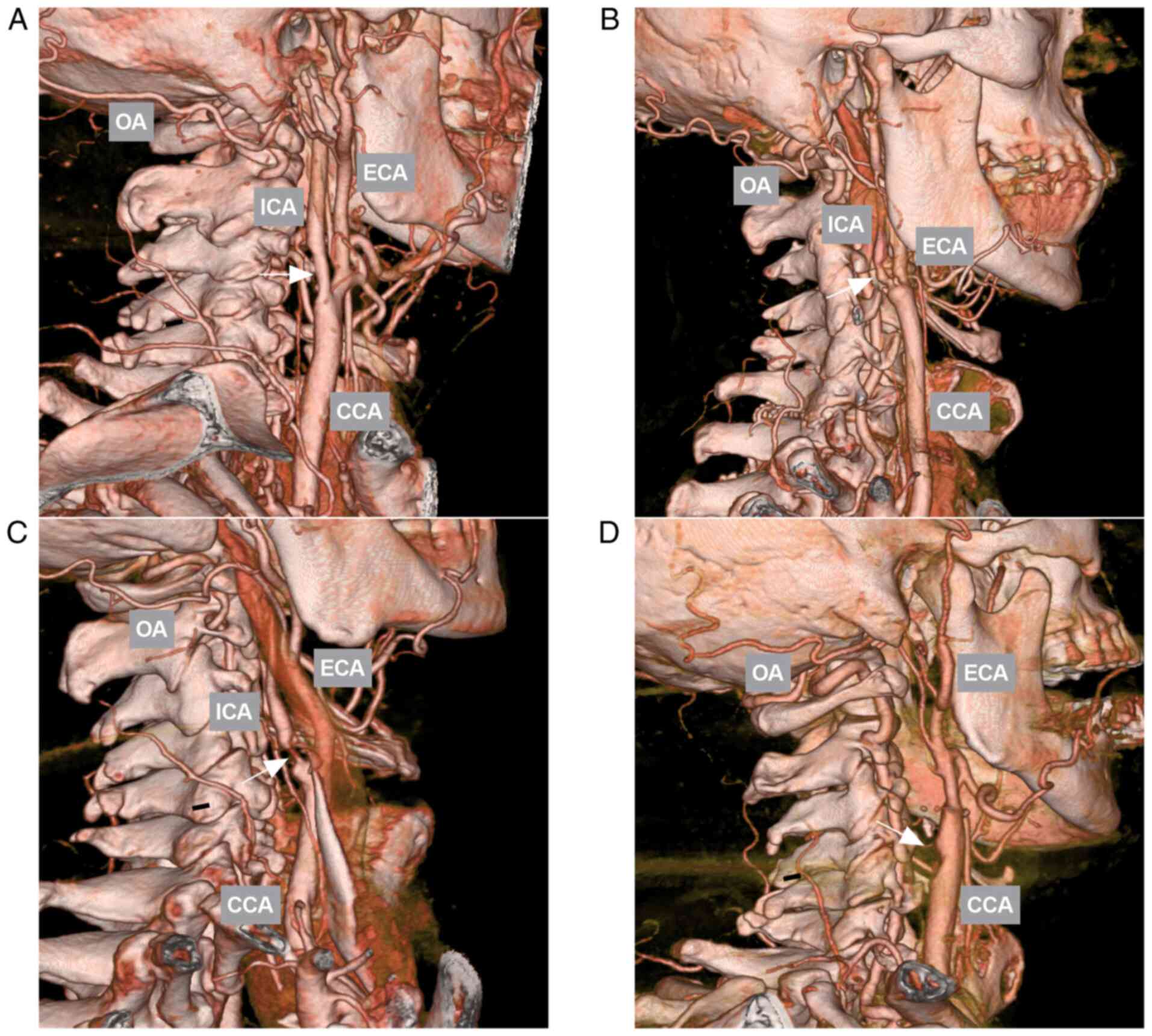
(Fig. 1) (14).
Software and tools used for
post-processing
The raw CTA data were post-processed using the GE
Workstation (version 4.7; GE Healthcare; Cytiva). The raw CTA data
were primarily reconstructed using volume rendering. Structures
that affected the measurements were removed using the cutting tool.
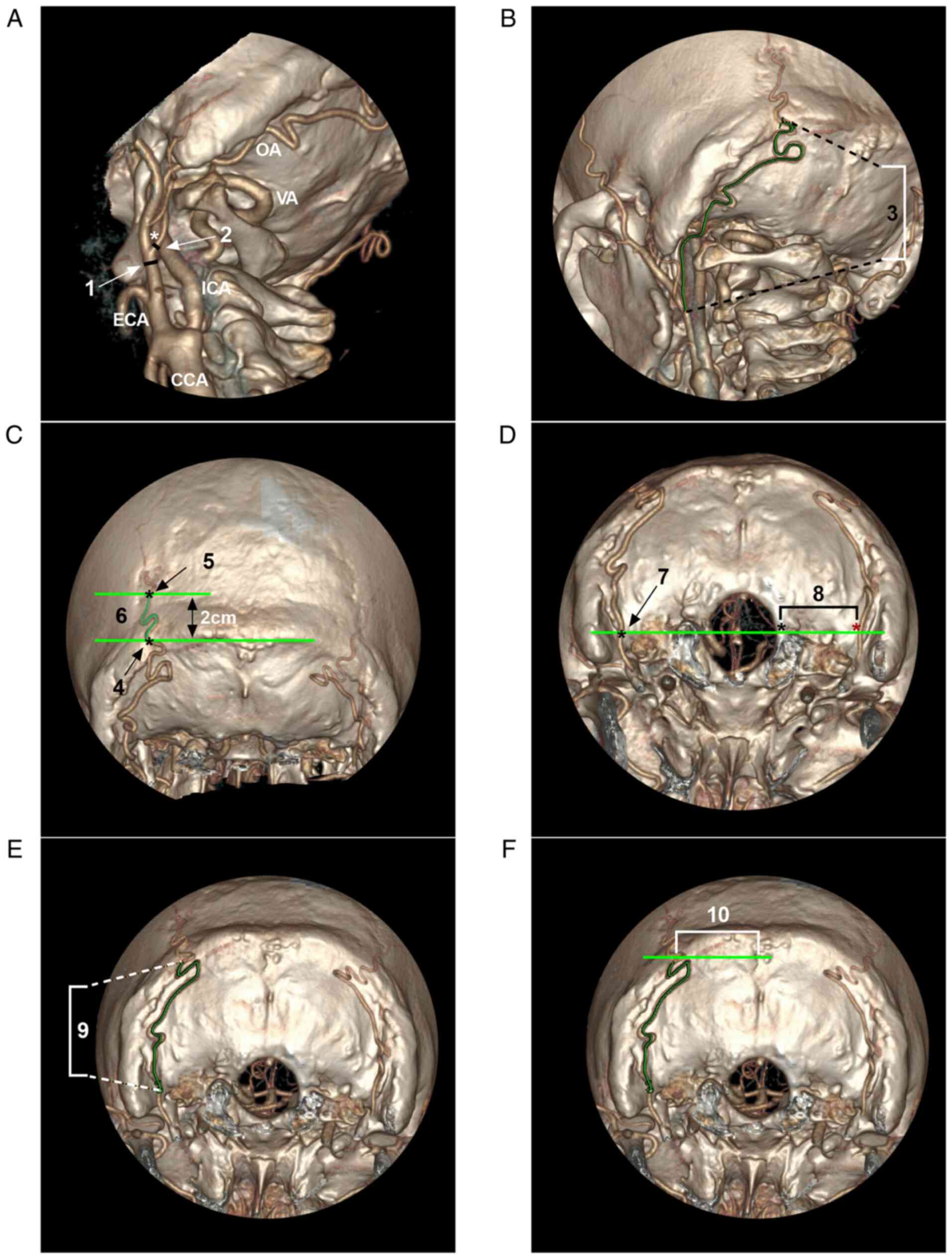
The diameter of the vessel and the distances between structures
were obtained using the ‘measure distance’ tool. The curved length
of a vessel was measured using the two-click AVA tool, and the
curved three-dimensional length could be measured accurately. All
the parameters were measured three times, and the average value was
used for analysis.
Measured parameters Vessel
diameter
The vessel diameters included the ECA diameter at
the OA origin, the OA diameter at its origin, the OA diameter at
the superior nuchal line, the OA diameter 2 cm above the superior
nuchal line and the OA diameter at the midline of the foramen
magnum. When measuring the OA diameter at the superior nuchal line
and OA diameter 2 cm above the superior nuchal line, if the OA was
branched, the thickest portion of the main trunk was measured.
Curve length of the vessel. The curve lengths
included the lengths of the OA from its origin to the superior
nuchal line, between the superior nuchal line and 2 cm above the
superior nuchal line, and from the midline level of the foramen
magnum to the superior nuchal line. When measuring these
parameters, if the OA was branched, the thickest portion of the
main trunk was measured.
Spatial distance. The spatial distances
included the distance from the edge of the foramen magnum to the OA
and the distance from the midline to the OA at the level of the
superior nuchal line. For the distance from the midline to the OA
at the level of the superior nuchal line, if the OA was branched,
the thickest portion of the main trunk was measured. All measured
parameters are illustrated in Fig.
2.
OA variations
A number of variations were recorded, including the
common origin with other ECA branches arising from the ICA and
OA-vertebral artery anastomosis (Fig.
3).
Statistical analysis
Statistical assessments were performed using
GraphPad Prism (version 8.02) software (GraphPad Software, Inc.).
Continuous variables are expressed as the mean ± standard
deviation. A paired t-test was used for the comparison of two
continuous variables. Ordinary one-way ANOVA followed by Tukey's
multiple comparisons test was used for the comparison of multiple
continuous variables. The Chi-squared test was used to compare
count data among multiple groups. A P-value <0.05 was considered
to indicate a statistically significant difference.
Results
General information
A total of 205 Han Chinese participants who met the
inclusion criteria were selected for further investigation. A total
of 50 healthy subjects (100 sides, left and/or right) were selected
as the control group. In total, 155 patients (180 sides) were
selected as the stenosis and occlusion groups, including the mild
and moderate ICA stenosis group (50 sides, left and/or right),
severe ICA stenosis group (80 sides, left and/or right) and ICA
occlusion group (50 sides, left and/or right).
In the control group (50 subjects), the average age
was 60.14±11.56 years (range, 33-88 years), and the ratio of males
to females was 1.94:1 (33/17). In the mild and moderate ICA
stenosis group (38 patients), the average age was 64.26±9.38 years
(range, 37-80), and the ratio of males to females was 2.8:1
(28/10). In the severe ICA stenosis group (71 patients), the
average age was 64.73±8.31 years (range, 33-79 years), and the
ratio of males to females was 1.54:1 (43/28). In the ICA occlusion
group (46 patients), the average age was 62.04±9.361 years (range,
41-80 years), and the ratio of males to females was 1.54:1 (30/16).
The age and sex data, and their comparisons are summarized in
Table I, Table II and Table III.
 | Table IAge data of the study
participants. |
Table I
Age data of the study
participants.
| Group | Range (years) | Mean (years) | P-valuea |
|---|
| Control (50
subjects) | 33-88 | 60.14±11.56 | 0.0527 |
| Mild and moderate ICA
stenosis (38 subjects) | 37-80 | 64.26±9.38 | |
| Severe ICA stenosis
(71 subjects) | 33-79 | 64.73±8.31 | |
| ICA occlusion (46
subjects) | 41-80 | 62.04±9.36 | |
 | Table IISex data of the study
participants. |
Table II
Sex data of the study
participants.
| Group | Male (no. of
participants) | Female (no. of
participants) | P-valuea |
|---|
| Control (50
subjects) | 33 | 17 | 0.5947 |
| Mild and moderate ICA
stenosis (38 subjects) | 28 | 10 | |
| Severe ICA stenosis
(71 subjects) | 43 | 28 | |
| ICA occlusion (46
subjects) | 30 | 16 | |
 | Table IIIMultiple comparisons of the age and
sex data in Tables I and II. |
Table III
Multiple comparisons of the age and
sex data in Tables I and II.
| | P-value |
|---|
| Comparison between
groups | Age | Sex |
|---|
| Control vs. mild and
moderate ICA stenosis | 0.1942 | 0.4388 |
| Control vs. severe
ICA stenosis group | 0.0505 | 0.5423 |
| Control vs. ICA
occlusion group | 0.7672 | 0.9357 |
| Mild and moderate ICA
stenosis vs. severe ICA stenosis group | 0.9950 | 0.1707 |
| Mild and moderate ICA
stenosis vs. ICA occlusion group | 0.7184 | 0.4035 |
| Severe ICA stenosis
group vs. ICA occlusion group | 0.4530 | 0.6117 |
It should be noted that for the participants, their
weight, height and body mass index should be included. However, as
some data were from the out-patient department, these data could
not be provided.
Measured parameters
Among the healthy subjects, the ECA diameter at the
OA origin was 4.37±0.93 mm (range, 2.5-8.7 mm). The OA diameter at
its origin was 1.94±0.33 mm (range, 1.3-2.8 mm). The length of the
OA from its origin to the superior nuchal line was 142.2±19.39 mm
(range, 104.9-185.9 mm). The OA diameter at the superior nuchal
line was 1.68±0.35 mm (range, 0.8-3.0 mm). The OA diameter 2 cm
above the superior nuchal line was 1.14±0.39 mm (range, 0.4-2.3
mm). The length of the OA between the superior nuchal line and 2 cm
above the superior nuchal line was 39.34±9.1 mm (range, 22.6-71.2
mm). The OA diameter at the midline of the foramen magnum was
2.07±0.39 mm (range, 1.5-3.1 mm). The distance from the edge of the
foramen magnum to the OA was 29.88±3.47 mm (range, 24.1-42.1 mm).
The length of the OA from the midline level of the foramen magnum
to the superior nuchal line was 89.82±14.89 mm (range, 56.9-129.5
mm). The distance from the midline to the OA at the level of the
superior nuchal line was 36.09±4.42 mm (range, 25.6-48.7 mm). The
overall data and their comparisons are summarized in detail in
Tables IV and V.
 | Table IVMeasured parameters in the different
groups. |
Table IV
Measured parameters in the different
groups.
| No. | Parameter | Groups | Range (mm) | Mean (mm) |
P-valuea |
|---|
| 1 | ECA diameter at the
OA origin | Control | 2.5-8.7 | 4.37±0.93 | 0.0888 |
| | | Mild and moderate
stenosis | 3.0-5.9 | 4.14±0.61 | |
| | | Severe
stenosis | 2.9-5.7 | 4.09±0.66 | |
| | | Occlusion | 2.1-6 | 4.3±0.86 | |
| 2 | OA diameter at its
origin | Control | 1.3-2.8 | 1.94±0.33 | 0.1260 |
| | | Mild and moderate
stenosis | 1.3-2.5 | 1.89±0.26 | |
| | | Severe
stenosis | 0.8-2.8 | 2.02±0.34 | |
| | | Occlusion | 1.4-3.1 | 2.0±0.31 | |
| 3 | Length of the OA
from its origin to the superior nuchal line | Control | 104.9-185.9 | 142.2±19.39 | 0.1467 |
| | | Mild and moderate
stenosis | 97-211.1 | 147.1±22.3 | |
| | | Severe
stenosis | 89.8-198.6 | 143.1±22.15 | |
| | | Occlusion | 109.1-200.3 | 149.6±19.92 | |
| 4 | OA diameter at the
superior nuchal line | Control | 0.8-3 | 1.68±0.35 | 0.5392 |
| | | Mild and moderate
stenosis | 0.7-2.3 | 1.59±0.39 | |
| | | Severe
stenosis | 0.7-2.7 | 1.63±0.45 | |
| | | Occlusion | 0.9-2.7 | 1.68±0.41 | |
| 5 | OA diameter 2 cm
above the superior nuchal line | Control | 0.4-2.3 | 1.14±0.39 | 0.6269 |
| | | Mild and moderate
stenosis | 0.4-2.2 | 1.07±0.45 | |
| | | Severe
stenosis | 0.4-2.0 | 1.08±0.38 | |
| | | Occlusion | 0.5-2.2 | 1.06±0.39 | |
| 6 | Length of the OA
between the superior nuchal line and 2 cm above the superior nuchal
line | Control | 22.6-71.2 | 39.34±9.10 | 0.0782 |
| | | Mild and moderate
stenosis | 23.9-64.1 | 36.71±7.74 | |
| | | Severe
stenosis | 20.4-61.1 | 36.25±8.55 | |
| | | Occlusion | 22.4-59.1 | 36.83±7.93 | |
| 7 | OA diameter at the
midline of the foramen magnum | Control | 1.5-3.1 | 2.07±0.39 | 0.3562 |
| | | Mild and moderate
stenosis | 1.5-2.8 | 1.97±0.24 | |
| | | Severe
stenosis | 1.4-3.4 | 2.04±0.37 | |
| | | Occlusion | 1.4-2.8 | 2.08±0.36 | |
| 8 | Distance from the
edge of the foramen magnum to the OA | Control | 24.1-42.1 | 29.88±3.47 | 0.8757 |
| | | Mild and moderate
stenosis | 24.6-38.4 | 29.54±3.20 | |
| | | Severe
stenosis | 24.3-36.2 | 29.72±2.64 | |
| | | Occlusion | 23.2-35.9 | 29.49±2.98 | |
| 9 | Length of the OA
from the midline level of the foramen magnum to the superior nuchal
line | Control | 56.9-129.5 | 89.82±14.89 | 0.8427 |
| | | Mild and moderate
stenosis | 54.9-129.9 | 92.25±15.56 | |
| | | Severe
stenosis | 41.3-153.5 | 91.08±19.36 | |
| | | Occlusion | 51.4-136.7 | 91.44±15.33 | |
| 10 | Distance from the
midline to the OA at the level of the superior nuchal line | Control | 25.6-48.7 | 36.09±4.42 | 0.0723 |
| | | Mild and moderate
stenosis | 27.7-45.2 | 35.96±4.16 | |
| | | Severe
stenosis | 19.3-48.3 | 37.75±5.15 | |
| | | Occlusion | 26.1-45.5 | 36.34±4.72 | |
 | Table VMultiple comparisons of the
parameters in the different groups shown in Table IV. |
Table V
Multiple comparisons of the
parameters in the different groups shown in Table IV.
| | P-value |
|---|
| No. | Parameter | Control vs. mild
and moderate ICA stenosis | Control vs. severe
ICA stenosis group | Control vs. ICA
occlusion group | Mild and moderate
ICA stenosis vs. severe ICA stenosis group | Mild and moderate
ICA stenosis vs. ICA occlusion group | Severe ICA stenosis
group vs. ICA occlusion group |
|---|
| 1 | ECA diameter at the
OA origin | 0.3497 | 0.0906 | 0.9637 | 0.9839 | 0.7388 | 0.4445 |
| 2 | OA diameter at its
origin | 0.8024 | 0.4240 | 0.6987 | 0.1438 | 0.3135 | 0.9959 |
| 3 | Length of the OA
from its origin to the superior nuchal line | 0.5281 | 0.9915 | 0.1656 | 0.7133 | 0.9262 | 0.3013 |
| 4 | OA diameter at the
superior nuchal line | 0.5571 | 0.8511 | >0.9999 | 0.9358 | 0.6683 | 0.9078 |
| 5 | OA diameter 2 cm
above the superior nuchal line | 0.7506 | 0.7821 | 0.6997 | 0.9981 | 0.9998 | 0.9940 |
| 6 | Length of the OA
between the superior nuchal line and 2 cm above the superio nuchal
line | 0.2906 | 0.0811 | 0.3340 | 0.9905 | 0.9999 | 0.9809 |
| 7 | OA diameter at the
midline of the foramen magnum | 0.3476 | 0.9360 | 0.9997 | 0.6864 | 0.4262 | 0.9374 |
| 8 | Distance from the
edge of the foramen magnum to the OA | 0.9223 | 0.9877 | 0.8910 | 0.9874 | 0.9999 | 0.9760 |
| 9 | Length of the OA
from the midline level of the foramen magnum to the superior nuchal
line | 0.8301 | 0.9562 | 0.9416 | 0.9796 | 0.9948 | 0.9994 |
| 10 | Distance from the
midline to the OA at the level of the superior nuchal line | 0.9985 | 0.0928 | 0.9909 | 0.1476 | 0.9784 | 0.3350 |
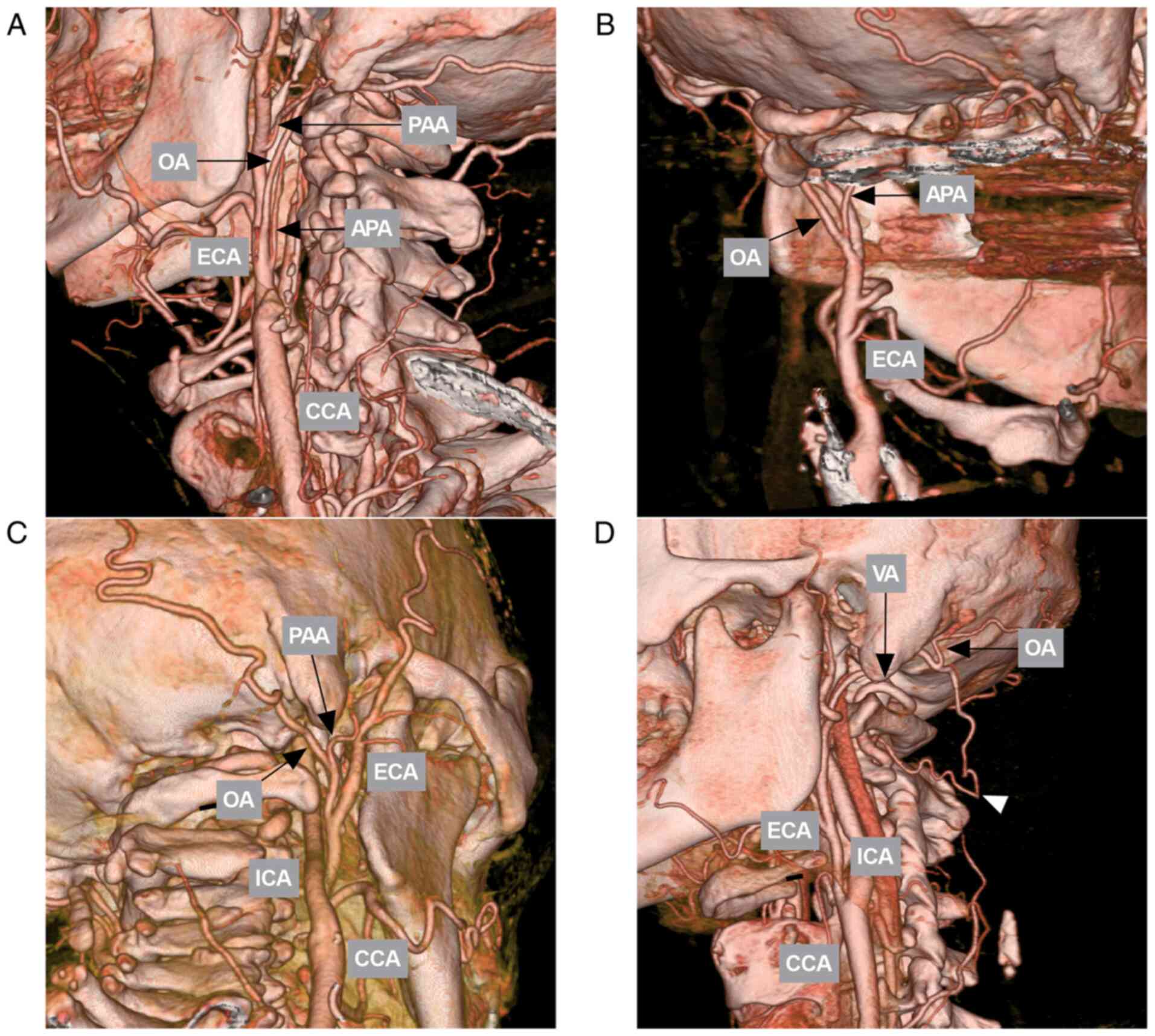
OA variations
A common origin of the OA and the ascending
pharyngeal artery was found on 15.4% of sides (43/280). A common
origin of the OA and the posterior auricular artery was observed on
3.6% of sides (10/280). The OA-vertebral artery was found in 2.1%
of sides (6/280). The OA arising from the ICA was not observed.
Results of the statistical
analysis
There were no significant differences in age, sex,
or anatomical parameters of the OA among the control, mild and
moderate ICA stenosis group, severe ICA stenosis group and ICA
occlusion groups (P>0.05) (Table
I, Table II, Table III, Table IV and Table V). In the control group, no
significant difference in anatomical parameters between the left
and right sides was observed (Table
VI).
 | Table VIMeasured parameters in the control
group. |
Table VI
Measured parameters in the control
group.
| No. | Parameter | Side | Range (mm) | Mean (mm) | P-value |
|---|
| 1 | ECA diameter at the
OA origin | L | 2.8-8.7 | 4.35±0.97 | 0.8079 |
| | | R | 2.5-7.4 | 4.38±0.89 | |
| 2 | OA diameter at its
origin | L | 1.4-2.8 | 1.93±0.34 | 0.5843 |
| | | R | 1.3-2.6 | 1.95±0.32 | |
| 3 | Length of the OA
from its origin to the superior nuchal line | L | 106.9-185.9 | 143.6±18.06 | 0.3137 |
| | | R | 104.9-178.5 | 140.8±20.73 | |
| 4 | OA diameter at the
superior nuchal line | L | 0.8-3.0 | 1.69±0.37 | 0.7572 |
| | | R | 0.9-2.5 | 1.67±0.33 | |
| 5 | OA diameter 2 cm
above the superior nuchal line | L | 0.5-2.3 | 1.16±0.38 | 0.3075 |
| | | R | 0.4-2.2 | 1.12±0.40 | |
| 6 | Length of the OA
between the superior nuchal line and 2 cm above the superior nuchal
line | L | 22.6-64.4 | 40.09±9.32 | 0.4840 |
| | | R | 22.8-71.2 | 38.62±8.91 | |
| 7 | OA diameter at the
midline of the foramen magnum | L | 1.5-3.0 | 2.06±0.38 | 0.8261 |
| | | R | 1.6-3.1 | 2.08±0.40 | |
| 8 | Distance from the
edge of the foramen magnum to the OA | L | 24.1-42.1 | 30.29±3.69 | 0.1594 |
| | | R | 24.7-40.0 | 29.46±3.22 | |
| 9 | Length of the OA
from the midline level of the foramen magnum to the superior nuchal
line | L | 56.9-126.5 | 89.28±16.21 | 0.5473 |
| | | R | 65.0-129.5 | 90.36±13.59 | |
| 10 | Distance from the
midline to the OA at the level of the superior nuchal line | L | 27.6-47 | 36.78±4.24 | 0.0957 |
| | | R | 25.6-48.7 | 35.44±4.54 | |
Discussion
The OA is a crucial structure (1), and is often be used as a donor graft
for cerebral revascularization (15). This vessel may also be involved in
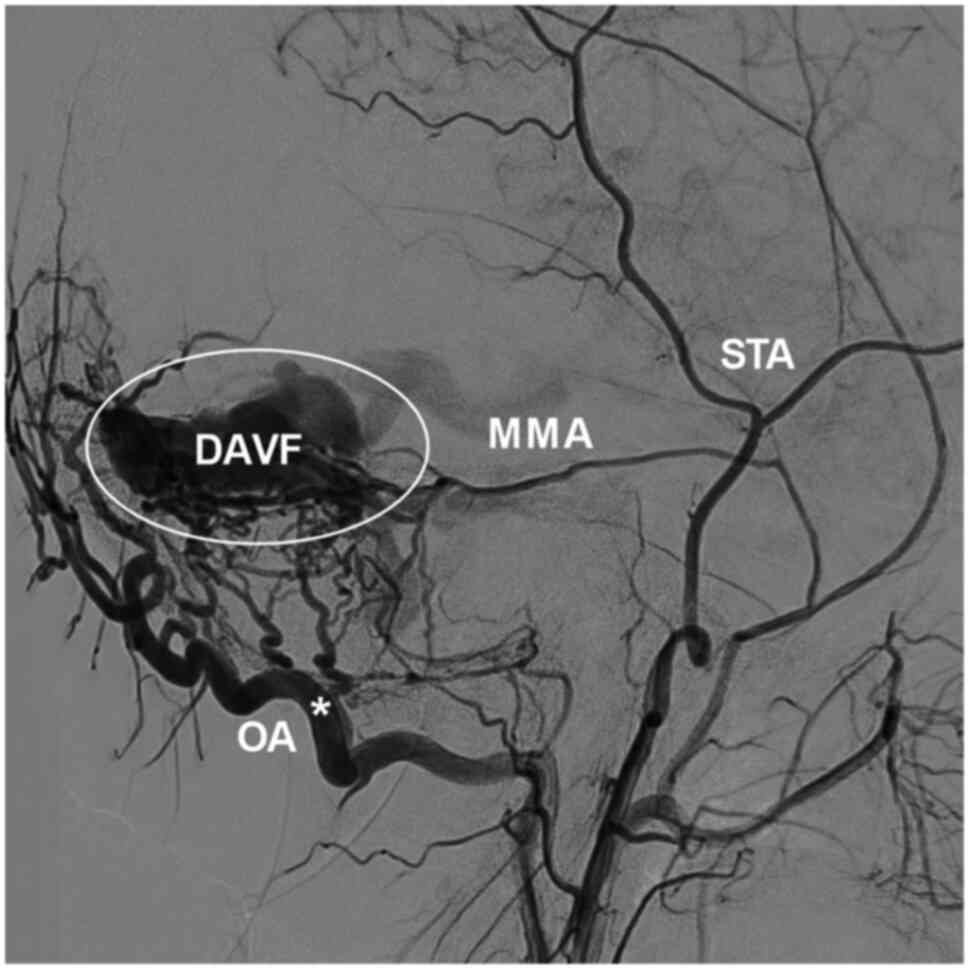
high-flow arteriovenous shunts, functioning as an accomplice, such
as in cases of DAVF where the OA is dilated and hyperplastic
(Fig. 4) (1). In cases of stenosis and occlusion of
the ICA, the blood flow through the ECA is entirely from the common
carotid artery (16). In these
cases, it is unclear whether the OA becomes thicker or longer. If
the OA becomes thicker or longer, performing a bypass between the
suboccipital segment of the OA and intracranial artery may help
treat brain hypoperfusion.
In the present study, CTA data were collected from a
control group with a normal ICA and patients with stenosis and
occlusion of the ICA. The analysis of the OA diameter did not
reveal any differences among the different locations and OA lengths
among all groups, indicating that the redistribution of blood flow
due to severe stenosis or occlusion of the ICA was insufficient in
changing the anatomic characteristics of the OA. In addition, these
OA data are valuable, as there are no previous studies that have
provided CTA data on the OA in Han Chinese population, at least to
the best of our knowledge.
The OA is a large artery that has been reported to
provide a mean blood flow of 15 to 80 ml/min when used for
posterior fossa bypass (17). In a
previous microanatomical study by Alvernia et al (18), the outer diameter of the OA at the
origin varied from 2.2-2.9 mm.
In the present study, in healthy subjects, the OA
diameter at its origin was 1.94 mm, which is smaller than that in
the study by Alvernia et al (18); this may be due to the fact that the
OA diameter in the present study was the inner diameter.
Additionally, in the present study, in healthy subjects, the ECA
diameter at the OA origin was measured to be 4.37 mm, which is much
larger than that of the OA. An inner diameter of 1.94 mm is
sufficient for endovascular treatment through the OA, easily
allowing double 0.017' microcatheters to pass through (19).
The OA branches from the ECA; in rare cases, the OA
can arise from the ICA (20). In the
present study, the latter was not found, perhaps due to the small
sample size. However, a common trunk of the OA with other branches
of the ECA or OA anastomosis with the vertebral artery were found;
a common origin with the ascending pharyngeal artery was observed
in 15.4% of sides, and a common origin with the posterior auricular
artery in 3.6% of sides. In the study by Hayashi et al
(21), the incidence of the origin
of the ascending pharyngeal artery from the OA was 19%, which was
similar to that presented herein. These variations are important;
for instance, during endovascular treatment via the OA, care must
be taken to avoid damaging the ascending pharyngeal artery and
posterior auricular artery. The OA-vertebral artery anastomosis was
in 2.1% of sides in the present study. Anastomosis is important,
and when the ICA or vertebral artery is occluded, the OA can serve
as an important path of collateral circulation.
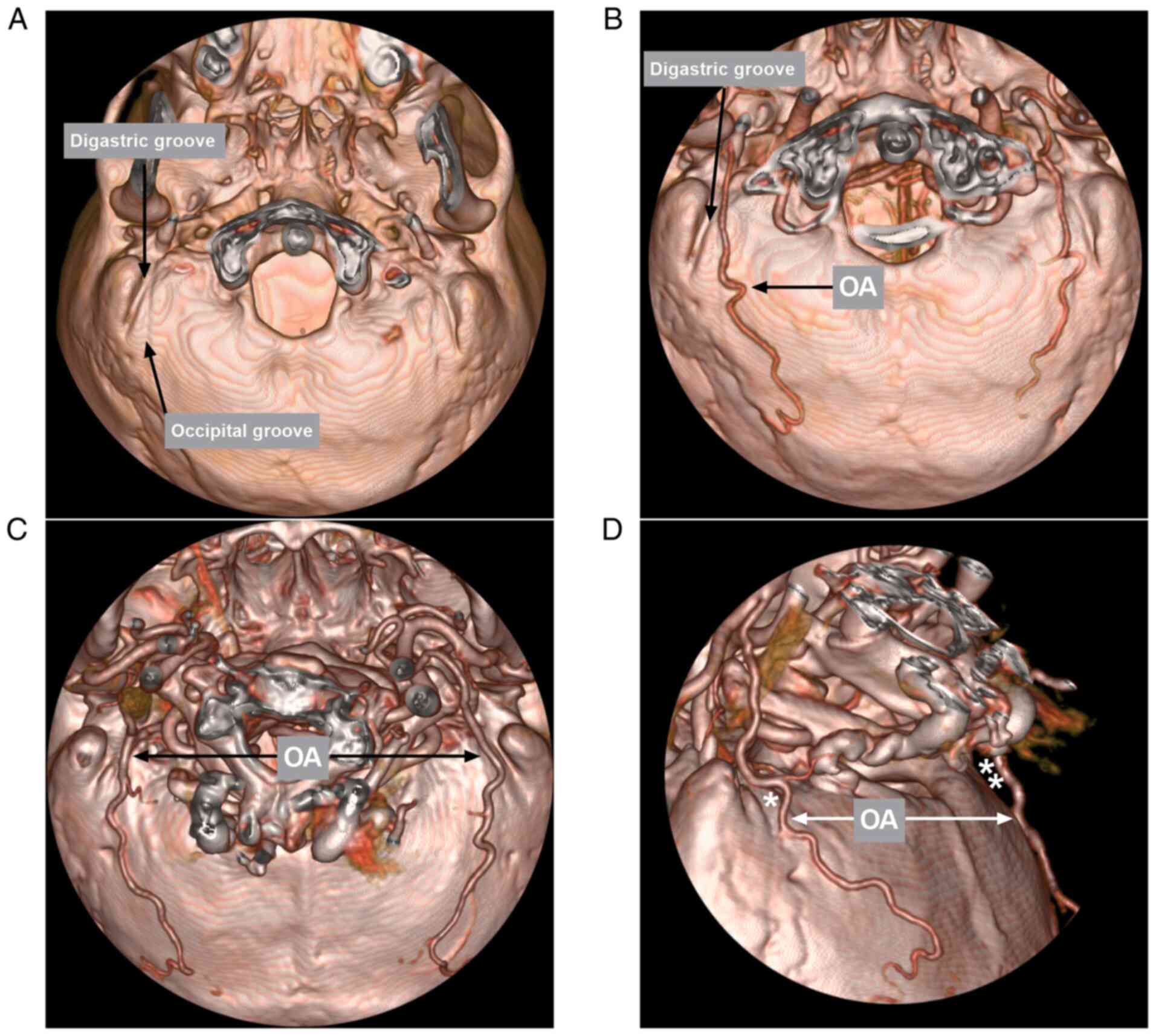
After leaving the ECA, the OA then runs in the
occipital groove of the temporal bone, medial to the digastric
groove (Fig. 5) (22). The digastric groove is an important
landmark; a number of studies have measured the diameter of the OA
at the level of the digastric groove. For instance, in a previous
microanatomical study by Matsuo et al (3), the diameter of the OA at the level of
the digastric groove was 2.1 mm. In another microanatomical study
by Kawashima et al (23), the
diameter of the OA was 2.05 mm at its exit from the digastric
groove. The present study measured the OA diameter at the midline
of the foramen magnum, which corresponds to the digastric groove;
the diameter in healthy subjects was 2.07 mm; thus the results of
the present study were similar to those of the previous
aforementioned studies. Furthermore, the distance from the edge of
the foramen magnum to the OA was measured, which was 29.88 mm,
providing the spatial position of the OA at the level.
For a bypass between the OA and intracranial artery,
the OA should be dissected from its distal end, and the dissection
should extend to the level of the mastoid process after the
digastric groove (4). In a previous
microsurgical study by Kawashima et al (23), the mean length of the OA from the
exit of the digastric groove to the level of the superior nuchal
line was 81.9 mm. In the present study, the length in the healthy
subjects was 89.82 mm. This difference may be derived from the fact
that the study by Kawashima et al (23) was a microsurgical study, and the
dissection could not reach the digastric groove; however, the
present study used CTA, avoiding muscle disturbances. Nossek et
al (24) recommend that a total
length of 10-12 cm be dissected to reach the anastomosis site to
avoid any tension in the region of the anastomotic sutures. In the
present study, the length of the OA between the superior nuchal
line and 2 cm above the superior nuchal line in healthy subjects
was 39.34 mm, and the total length from the digastric groove was
12.92 cm (89.82 plus 39.34 mm), which was a sufficient length.
The OA crossed upwards over the superior nuchal
line; however, its diameter did not decrease significantly at this
point. In the study by Kawashima et al (23), the mean diameter of the OA was 2.01
mm at the level of the superior nuchal line. In the present study,
this value in healthy subjects was 1.68 mm, which is smaller than
that in the aforementioned study. The reason for this difference
may be that the parameter measured for the peripheral OA on CTA was
the inner diameter, which is expected to be smaller than the values
obtained in microanatomical studies. In addition, due to the poor
filling of peripheral vessels, the measured parameters were not
accurate (25).
For surgical purposes, it is important to consider
that the diameter of the main trunk of the OA remains >1.0 mm.
In the study by Alvernia et al (18), the diameter of the OA was still
>1.0 mm at 50 mm above the superior nuchal line. In the present
study, ot was found that this measurement became inaccurate 20 mm
above the superior nuchal line o CTA; thus, the OA diameter at this
point was measured only in healthy subjects and a value of 1.14 mm
was obtained. For an OA bypass, an accurate understanding of the
orientation is crucial, and previous studies have demonstrated that
the OA travels 3.0-4.5 cm on the superior nuchal line lateral to
the inion (3,4). In the present study, this parameter in
healthy subjects was measured to be 36.09 mm, in agreement with the
aforementioned studies (3,4).
In conclusion, the presents study reported data on
the OA diameter in Han Chinese patients. In addition, it was
demonstrated that the stenosis and occlusion of the ICA did not
significantly alter the anatomy of the OA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY designed the study and drafted the manuscript. TL
collected the data. TL and JY confirm the authenticity of all the
raw data. JY and TL revised the manuscript. Both authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Hospital of Jilin University (Approval no.
2021-533). Written informed consent was obtained from the
participants.
Patient consent for publication
The participants or their parents/guardians provided
consent and agreed to have their data (shown in the figures)
published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guo Y, Chen H, Chen X and Yu J: Clinical
importance of the occipital artery in vascular lesions: A review of
the literature. Neuroradiol J. 32:366–375. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guo Y, Song Y, Hou K and Yu J:
Intracranial fusiform and circumferential aneurysms of the main
trunk: Therapeutic dilemmas and prospects. Front Neurol.
12(679134)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matsuo S, Komune N, Akiyama O, Amano T and
Nakamizo A: Surgical anatomy of the donor arteries for
extracranial-intracranial bypass surgery: An anatomic and
radiologic study. World Neurosurg. 136:e447–e459. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ateş O, Ahmed AS, Niemann D and Başkaya
MK: The occipital artery for posterior circulation bypass:
Microsurgical anatomy. Neurosurg Focus. 24(E9)2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lasjaunias P, Théron J and Moret J: The
occipital artery. Anatomy-normal arteriographic
aspects-embryological significance. Neuroradiology. 15:31–37.
1978.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hou K, Li Q, Xu K, Xu B and Yu J:
Anatomical features of the superficial temporal artery in
hemorrhagic moyamoya disease based on CT angiography. Exp Ther Med.
19:2143–2148. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ji T, Hou K, Li C and Yu J: Imaging
features of internal maxillary artery and extracranial middle
meningeal artery and their relationships on head CTA. Neuroradiol
J. 34:629–641. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen Y, Xue HD, Liu W, Sun H, Wang X, Su
BY, Duo C, Ming WD, De J, Ji B, et al: Dual-energy computed
tomographic angiography of head and neck arteries with different
contrast material doses in second generation dual-source computed
tomography system. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 32:628–633.
2010.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
9
|
Chen G, Xue Y, Wei J and Duan Q: The
undiagnosed potential clinically significant incidental findings of
neck CTA: A large retrospective single-center study. Medicine
(Baltimore). 99(e22440)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen H, Xu B, Wang G, Guo Y, Hou K and Yu
J: Posterior occipital intramuscular hemangioma mimicking
arteriovenous malformation: Case report. Medicine (Baltimore).
98(e14678)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yuan Y, Xu B, Guo Y, Xu K, Luo Q and Yu J:
Surgical excision of large scalp hemangiomatosis: A case report and
literature review. Int J Clin Exp Me. 9:6853–6861. 2016.
|
|
12
|
Oishi H, Yoshida K, Tange Y, Tsuji O and
Sonobe M: Treatment of a scalp arteriovenous malformation by a
combination of embolization and surgical removal. Interv
Neuroradiol. 8:293–297. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Su H, Xu K, Wang Y and Yu J: Is the middle
meningeal artery the optimal path for dural arteriovenous fistula
embolization? Front Neurol. 12(675355)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Barnett HJ, Taylor DW, Eliasziw M, Fox AJ,
Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC,
Sackett DL, et al: Benefit of carotid endarterectomy in patients
with symptomatic moderate or severe stenosis. North American
symptomatic carotid endarterectomy trial collaborators. N Engl J
Med. 339:1415–1425. 1998.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Verbraeken B, Aboukais R, Voormolen M,
Boogaarts HD, Leclerc X, Lejeune JP and Menovsky T: Extreme lateral
supracerebellar infratentorial approach (ELSCIT) for occipital
artery-to-posterior cerebral artery bypass: Results in 3 cases.
World Neurosurg. 152:214–220. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu B, Li C, Guo Y, Xu K, Yang Y and Yu J:
Current understanding of chronic total occlusion of the internal
carotid artery. Biomed Rep. 8:117–125. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Khodadad G: Short- and long-term results
of microvascular anastomosis in the vertebrobasilar system, a
critical analysis. Neurol Res. 3:33–65. 1981.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Alvernia JE, Fraser K and Lanzino G: The
occipital artery: A microanatomical study. Neurosurgery. 58 (Suppl
1):ONS114–ONS122. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kelly ME, Gonugunta V, Woo HH, Turner R IV
and Fiorella D: Double-balloon trapping technique for embolization
of a large wide-necked superior cerebellar artery aneurysm: Case
report. Neurosurgery. 63 (Suppl 2):S291–S292. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Demirbas AT, Demirtas I, Sonmez Topcu F,
Karasu S and Ayyildiz B: A rare case report: Bilateral occipital
artery arising from the vertebral artery. Surg Radiol Anat.
43:1901–1904. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hayashi N, Hori E, Ohtani Y, Ohtani O,
Kuwayama N and Endo S: Surgical anatomy of the cervical carotid
artery for carotid endarterectomy. Neurol Med Chir (Tokyo).
45:25–30. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Benet A, Tabani H, Ding X, Burkhardt JK,
Rodriguez Rubio R, Tayebi Meybodi A, Nisson P, Kola O, Gandhi S,
Yousef S and Lawton MT: The transperiosteal ‘inside-out’ occipital
artery harvesting technique. J Neurosurg. 130:207–212.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kawashima M, Rhoton AL Jr, Tanriover N,
Ulm AJ, Yasuda A and Fujii K: Microsurgical anatomy of cerebral
revascularization. Part II: Posterior circulation. J Neurosurg.
102:132–147. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nossek E, Chalif DJ and Dehdashti AR: How
I do it: Occipital artery to posterior inferior cerebellar artery
bypass. Acta Neurochir (Wien). 156:971–975. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Naito H, Naka H, Kobayashi M, Kanaya Y,
Naito K, Kurashige T, Tokinobu H and Matsumoto M: Prevalences of
peripheral arterial disease diagnosed by computed tomography
angiography in patients with acute ischemic stroke. J Stroke
Cerebrovasc Dis. 25:1128–1134. 2016.PubMed/NCBI View Article : Google Scholar
|