1. Introduction
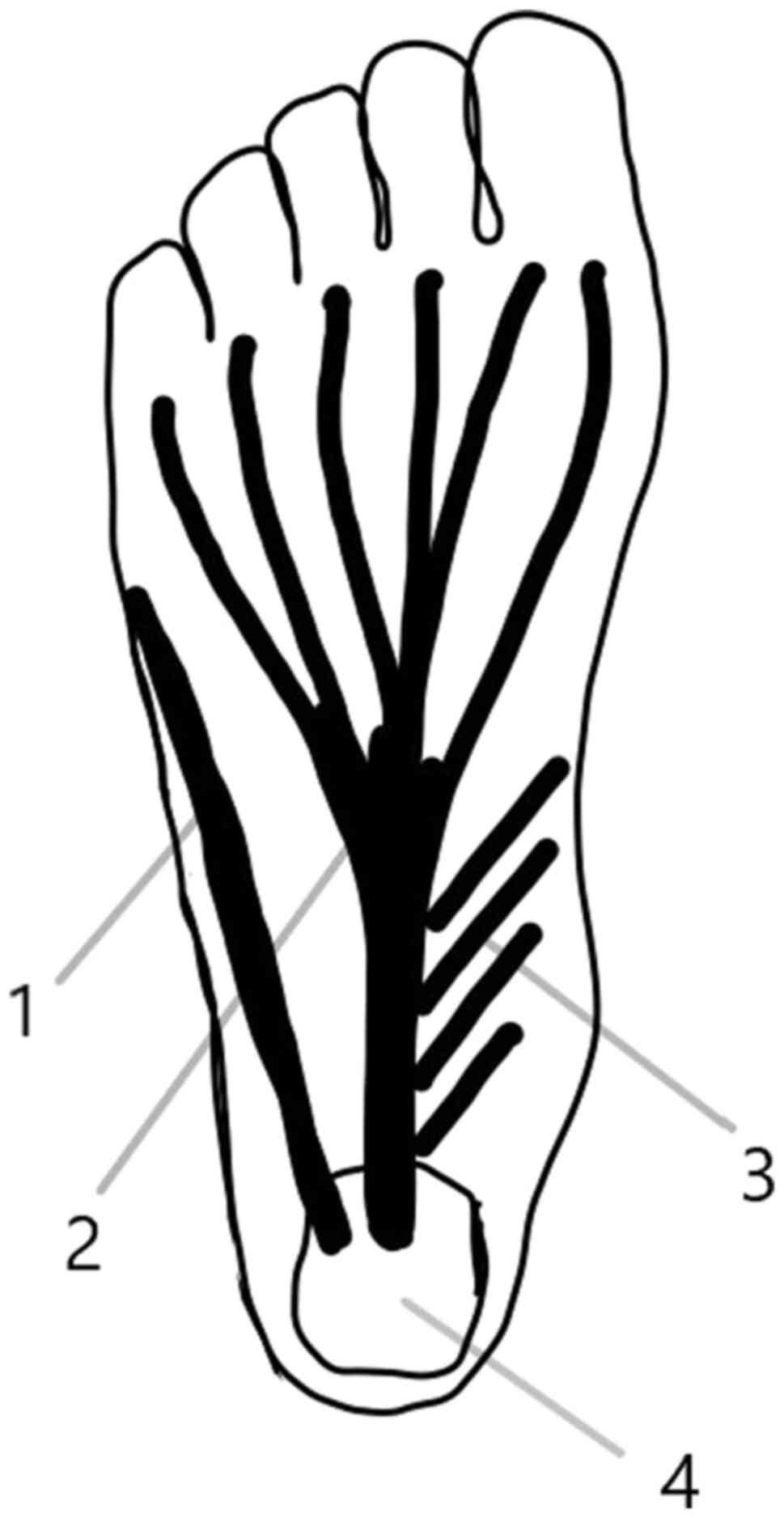
Plantar fascia, plantar aponeurosis or plantar
ligament is a triangular structure covering the sole of the foot.
It originates from the medial tubercle of the calcaneus and it
spreads in five bands or processes for the five toes. The bands
appear at the level of the metatarsal head and divide at the level
of metatarsophalangeal joint into two fascicles, superficial and
profound. The superficial fascicle attaches to the skin and the
deep fascicle divides into two slips to fuse with the flexor
tendons of the toes and with the transverse metatarsal ligament
(1) (Fig.
1).
Plantar fasciitis is the most frequent cause of heel
pain and it challenges the physician as it has a chronic or
relapsing course, interferes with walking and running. It also
requires a number of interventions and patient compliance may
diminish over time.
Although the term ‘fasciitis’ is suggestive of
inflammation, the main process is a degenerative one, with no
inflammatory cells. The terminology may be in fact fasciosis or
fasciopathy, in the same category with tendinosis or tendinopathy.
The main alteration is fibroblastic hypertrophy, disorganized
collagen and vascular hyperplasia with zones of avascularity. The
structural modification is triggered by repetitive microtrauma due
to excessive stretching (2).
2. Treatments
Natural evolution indicates that plantar fasciitis
is a self-limiting condition, and after 6 to 18 months, the pain
and limitations disappear spontaneously. This long duration is,
however, a burden and impediment to the quality of life of
patients.
Complete rest may reduce pain; however, this is not
always feasible, since walking is necessary for daily activities.
Relative rest is recommended for athletes or active adults, in the
form of changing the type of physical activity. A number of
treatment strategies for plantar fasciitis are available, and a
list of these is presented in Table
I.
 | Table ITreatment strategies for plantar
fasciitis. |
Table I
Treatment strategies for plantar
fasciitis.
| Treatment
strategy | Comments |
|---|
| Rest |
Complete/relative |
| Shoe alteration | Replacing old
shoes |
| | Midsole features |
| | Foot stability
control |
| Arch tapping | |
| Orthotics |
Prefabricated/customized |
| | Night splints |
| Physiotherapy | Cryotherapy |
| | Iontophoresis |
| | (acetic acid,
corticosteroids) |
| Physical
exercise | Stretching |
| | Eccentric
strengthening |
| Medication | Analgesics
(acetaminophen, opioids) |
| | Non-steroidal
anti-inflammatory drugs |
| ESWT | Radial/focused |
| Corticosteroids | Local injections |
| Autologous blood | Autologous blood
injection |
| derivates | Platelet rich plasma
(PRP) |
| Prolotherapy | Hypertonic
dextrose |
Shoe alteration and orthotics
Shoe alteration is an important issue to discuss
with the patients. Evaluating and replacing worn-out shoes is the
first step, particularly for athletes. Researchers have underlined
that running shoes lose their shock absorption properties over time
(3). Shoes with thick and
well-cushioned midsoles may offer pain relief for individuals which
have to stand or walk for long periods of time. When overpronation
is the cause of pain, shoes have to be modified to increase foot
stability and to control motion through a number of
characteristics: A semi-curved outer sole shape, slip last or
combination last (a particular shape of the insole wider at the toe
box than at the heel). The toe box should be sufficiently wide and
the sole should provide forefoot flexibility. The amount of
stability or motion control of a shoe depends on the pronation
control required. For underpronation, shoes are designed to
increase flexibility both in the rear foot and in the forefoot,
with a curved outer sole shape and a slip last, cushion midsole and
accommodative heel pad to enhance shock absorption (4).
Arch tapping provides initial short-term pain
relief; it may be used prior to each sport session, as it loses its
effect after 24 min. It is advised to be followed by other
therapeutic interventions. Continuous taping can also lead to skin
lesions. Tapping acts through mechanical support, which is
disputable, and through proprioceptive mechanism acting on
cutaneous, fascial and muscular receptors. It is most suitable for
the under pronated foot, to improve flexibility (5).
Foot orthoses, prefabricated or customized, are
prescribed for two reasons: Mechanical and proprioceptive
corrections. Mechanistically, they assist in shock absorption,
correct overpronation and increase the stability. The
proprioceptive strategy is based on the stimulation of plantar
receptors to modify the postural control (6).
Over-the-counter arch supports are used by patients
with mild pes planus, as they offer highly variable support
depending on the material used. They are preferred by adolescents
whose feet grow rapidly (6).
Custom orthotics require a plaster cast of the foot
in neutral, followed by modeling of an insert, with the aim of
correcting the overpronation and the metatarsal head motion,
particularly the first metatarsal head. Heel cups are used to
decrease the impact on the calcaneus, elevating the heel on a soft
cushion and reducing tension on the plantar fascia (6).
Researchers agree that foot orthoses provide pain
relief for a period of 3 to 12 months. However, there is debate on
which type works more efficiently, the prefabricated or custom-made
insert (6). There is no modification
of gait pattern or postural control, at least after 9 weeks of
wearing (7). It is important to
stress the necessity to wear foot orthoses both indoors and
outdoors.
Night splints are designed to maintain the ankle in
90˚ of dorsiflexion, to passively stretch the fascia and triceps -
Achilles complex and to counterbalance the naturally position of
relaxed plantar flexion during night sleep. They promote the
healing of the fascia in the elongated position, decreasing the
tension during the first steps taken in the morning. There are a
number of types of night splints, varying from a prefabricated
support to a custom made one. For a better tolerance, anterior
night splints have been designed for the purpose of covering a
smaller skin surface than the posterior splints and to allow
walking. They have been reported to relieve pain in a significant
number of patients, although their side-effects may reduce the
compliance: Skin pressure and mild nocturnal discomfort. Combining
physical exercise with night splints leads to better results as
regards pain and local function (8).
Physiotherapy
Ice or other cryotherapy procedures exert a marked
analgesic effect, and are used for short periods of time (10 to 20
min).
Iontophoresis, a non-invasive method, uses a
low-voltage galvanic current to introduce active agents through the
skin, up to a depth of 20 mm, as corticosteroids or acetic acid.
Some researchers have stressed the importance of acetic acid
iontophoresis, as the local chronic process produces excessive
calcium ions, which, as calcium carbonate, form spurs. Acetic acid
iontophoresis delivers the negatively charged acetate ions that
combine with calcium positive ions from local processes, forming
calcium acetate which dissolves into the local blood stream and is
removed (9).
Treatment prescription consists of six alternate-day
sessions, with a total daily dose of 40-mA/min, the intensity of
the galvanic current up to 4 mA, according to skin sensitivity,
using 4 ml acetic acid. Acetic acid (5%) is delivered under the
negative electrode (cathode) placed on the plantar aspect of the
calcaneus, on the most painful spot; the positive electrode is
placed on the posterior aspect of the calf (9).
Corticosteroid iontophoresis (4% dexamethasone)
exerts a less prominent pain-reducing effect on plantar fasciitis
than acetic acid, although better than the placebo (10).
Physical exercise includes the stretching of the
plantar fascia and triceps - Achilles complex. Usually, patients
perform the stretching under medical surveillance, two or three
times a week for 6 to 12 weeks. However, this may be associated
with an economic burden and is a time-consuming activity. In
addition, certain specific situations (as the actual pandemic) may
reduce the mobility of individuals and, consequently, reduces
treatment compliance and participation. Static and dynamic
stretching may be adapted for home-based use and remote medical
surveillance may be initiated (11).
Medication
Simple analgesics and non-steroidal
anti-inflammatory drugs (NSAIDs) are efficient for short periods of
time, particularly during the acute phase. In the chronic phase
however, the use of NSAIDS is controversial. NSAIDs mainly function
by inhibiting prostaglandin synthesis, whereas the production of
other inflammatory mediators (leukotrienes, cytokines and platelet
activating factor) remains unaltered. The addition of a simple
analgesic drug (such as acetaminophen) may provide short-term
relief. It has been demonstrated that primary care physicians
prescribe NSAIDS more often, while pain specialists are more likely
to recommend opioids. Combinations between acetaminophen and
opioids (tramadol or codeine) are often prescribed (4,11,12).
NSAIDs should be used in combination with other
conservative therapies (rest, physical agents, exercise, etc.), as
isolated utilization has failed to reduce consistent pain and
disability (13).
The side-effects of NSAIDs should also be taken into
consideration, particularly during chronic administration. These
side-effects include gastritis, peptic ulcers, esophagitis,
gastrointestinal bleeding, interstitial nephritis, sodium and water
retention, thrombocytopenia, as well as central nervous system and
hepatic complications.
Extracorporeal shock wave therapy
(ESWT)
ESWT and corticosteroid injections are considered
second-line therapies. ESWT is a non-invasive procedure using
mechanical shock waves to alter pain receptors and to promote local
healing through microtrauma. The use of ESWT in the treatment of
musculoskeletal conditions has increased. According to the energy
delivered, ESWT is defined as low energy or high energy, with a
cut-off value of 0.12 mJ/mm2 (14).
Two forms of therapy, radial shock wave (RSW) and
focused shock therapy (FSW) have become available, with the radial
form having a dispersing effect on a large area. Studies have
evaluated both ESWT in general and the specific forms of ESWT and
have found these to be more effective than sham treatment on pain
reduction. RSW seems to have a superior effect (9,13,15).
Moreover, some researchers have integrated both forms of shock
waves, focused and radial, each one with 2,000 pulses (0.2
mJ/mm2) in the same session, to achieve pain reduction
(16,17).
The ideal candidate for this type of treatment is
the patient with chronic or recalcitrant plantar fasciitis, with an
evolution of at least 6 months and a lack of response to
conservative modalities. There is no unique protocol as regards the
energy level, number of impulses and sessions. Generally, there are
3 to 5 weekly sessions, each with 1,000-2,000 pulses, both on the
maximum tenderness area and on the whole fascia. Researchers agree
on pain reduction on the short- (3 weeks) and long-term (6 months)
and an improvement on walking performance (gait speed, cadence and
distance) (18). For acute cases,
with an evolution of up to 1 month, no clinical improvement has
been observed following ESWT (19).
The side-effects of this type of treatment are minor
and transient and include: Post procedural pain, warm or burning
sensations, numbness, tingling, petechiae and ecchymosis; these are
intensity-related reactions. The risk of local bleeding is
increased in patients with coagulopathies and on anticoagulant
therapy (20).
Corticosteroids
Corticosteroids are beneficial in the early stages
of the condition; however, they are associated with multiple risks.
The injection is performed using the palpation method or ultrasound
guidance, via the plantar, posterior or medial approach.
Long-acting corticosteroids, i.e., dexamethasone and betamethasone,
and intermediate-acting corticosteroids, i.e., methylprednisolone,
prednisolone and triamcinolone are used in different regimens, with
no evidence of the superiority of one substance over the other
(21).
In chronic, recalcitrant cases, this therapy is
effective in the reduction of heel pain and plantar fascia
thickness, as proven by ultrasound evaluation (22,23). As
the procedure is painful, some researchers have proposed a
posterior tibial nerve block prior to corticosteroid injection, to
reduce the level of pain experienced during the plantar injection
(23).
The risks associated with corticosteroid use are fat
pad atrophy, rupture of the plantar fascia, pain, local bleeding or
bruising, infection, skin atrophy and osteomyelitis of the
calcaneus (24).
Autologous blood derivatives
Autologous blood derivatives and prolotherapy may be
considered third-line therapy. Autologous blood injection (ABI) and
platelet rich plasma (PRP) are novel therapeutic procedures in
muscle, tendon and ligament pathology, with wide-spread use,
dedicated mainly to recalcitrant or chronic cases, with the failure
of conservative treatments. ABI uses a small amount (2-4 ml) of
patient blood to dispose it into the target tissue. PRP is a
platelet concentrate obtained following the centrifugation of
patient blood. There is no standardized method to obtain PRP; thus,
the end-product may vary in platelet and leucocyte concentration
between studies (25).
Both therapies create a local inflammatory
condition, supply the tissue with growth factors and promote
healing. Some researchers use one single entry point to dispose the
product around the plantar fascia or after dry needling, e.g.,
multiple fascia penetration to produce small mechanical injuries.
Other physicians use a number of entry points, according to the
maximum tenderness sites (25).
One injection of ABI has been found to reduce heel
pain between 40 and 84% in the first 2 months following the
procedure, with a consistent result after 1 year A transient pain
following the procedure (2-3 days) has been reported in ~30% of
cases, which subsides with the use of analgesics. No fascial
rupture has been noted (26,27).
PRP administration may be unique or on a regimen of
three weekly injections, with heel pain resolution at 12 months in
64% of patients. The main side-effect is post-procedural pain,
reported by the majority of patients (79%), with an intensity of
8.1/10 on the visual analogue scale (VAS) scale and which subsides
within 2 h (27).
Anesthesia may be provided locally, by means of a
spray or as a regional posterior tibial nerve block. Post-injection
care varies from total immobilization for a short period of time to
relative rest, with avoiding running or jumping, with a gradual
return to normal physical activity over a period of 3 weeks
(27). Injections can be
administered blindly, although a number of physicians prefer
sonographic guidance.
Plantar fascia sonography is useful to document the
structural changes following ABI or PRP treatments. As previously
demonstrated, after 3 months, the echogenicity of the fascia
normalizes in 88% of patients and the thickness decreases, without
significant difference between the two. Sonographic and pain
evolution are not associated with each other (28,29).
ABI has been found to be superior to conservative
treatment and comparable with corticosteroid injection, while the
pain resolution lasts longer (30).
When comparing PRP to a saline injection, both therapies result in
pain reduction, with a significantly better result for PRP. The
fact that simple saline can lead to symptom reduction may be
explained by the needling effect, another technique which deserves
attention (31,32).
For plantar fasciitis, PRP was compared with
corticosteroids and offered the comparable pain relief in the
short- (2-4 weeks) and intermediate-term (4-8 weeks); however,
better results were observed at long-term (over 24 weeks) (33).
Prolotherapy
Prolotherapy is receiving increasing attention in
musculoskeletal conditions. Hypertonic glucose may contribute to
local healing, through the osmotic rupture of local cells, with
subsequent release of growth factors and healing (34).
Researchers use 2-4 ml dextrose, 15-20%, right into
the fascia via a medial approach, preferably under ultrasound
guidance. Lidocaine may be added for local anesthesia. The
frequency of administration varies from three injections every 3
weeks to two weekly injections. With such a large variety of
administration schedules, all studies have noted an improvement in
pain and functionality in the short- (6 weeks) and long-term (12
months). The results were comparable with those of radial ESWT
(32-35).
3. Conclusions and future perspectives
The chronic or relapsing clinical course of plantar
fasciitis challenges physicians to study and standardize the
therapeutic approaches. The first-line of treatment should include
rest, shoe modification, orthoses and physiotherapy. The addition
of analgesic medication is a current practice. ESWT and
corticosteroids are prescribed as a second-line therapy. New
therapies have also emerged, as autologous blood derivatives and
prolotherapy, with ongoing research stressing their benefits.
Further studies are however required to determine the effectiveness
and efficiency of these therapies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
The authors DP, SCB and AMI were all involved in the
conception and documentation, and in the writing and reviewing of
the present review article. The authors SCB and AMI confirm the
authenticity of the all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Draghi F, Gitto S, Bortolotto C, Draghi AG
and Ori Belometti G: Imaging of plantar fascia disorders: Findings
on plain radiography, ultrasound and magnetic resonance imaging.
Insights Imaging. 8:69–78. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Monteagudo M, de Albornoz PM, Gutierrez B,
Tabuenca J and Álvarez I: Plantar fasciopathy: A current concepts
review. EFORT Open Rev. 3:485–493. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cornwall MW and McPoil TG: Can runners
perceive changes in heel cushioning as the shoe ages with increased
mileage? Int J Sports Phys Ther. 12:616–624. 2017.PubMed/NCBI
|
|
4
|
Lim AT, How CH and Tan B: Management of
plantar fasciitis in the outpatient setting. Singapore Med J.
57:168–170; quiz 171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Podolsky R and Kalichman L: Taping for
plantar fasciitis. J Back Musculoskeletal Rehabil. 28:1–6.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Anderson J and Stanek J: Effect of foot
orthoses as treatment for plantar fasciitis or heel pain. J Sport
Rehabil. 22:130–136. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moyne-Bressand S, Dhieux C, Dousset E and
Decherchi P: Effectiveness of foot biomechanical orthoses to
relieve patients suffering from plantar fasciitis: is the reduction
of pain related to change in neural strategy? BioMed Res Int.
2018(3594150)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wheeler PC: The addition of a tension
night splint to a structured home rehabilitation programme in
patients with chronic plantar fasciitis does not lead to
significant additional benefits in either pain, function or
flexibility: a single-blinded randomised controlled trial. BMJ Open
Sport Exerc Med. 3(e000234)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Costa IA and Dyson A: The integration of
acetic acid iontophoresis, orthotic therapy and physical
rehabilitation for chronic plantar fasciitis: A case study. J Can
Chiropr Assoc. 51:166–174. 2007.PubMed/NCBI
|
|
10
|
Osborne HR and Allison GT: Treatment of
plantar fasciitis by LowDye taping and iontophoresis: Short term
results of a double blinded, randomised, placebo controlled
clinical trial of dexamethasone and acetic acid. Br J Sports Med.
40:545–549; discussion 549. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nahin RL: Prevalence and pharmaceutical
treatment of plantar fasciitis in United States adults. J Pain.
19:885–896. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Biswas C, Pal A and Acharya A: A
comparative study of efficacy of oral nonsteroidal antiinflammatory
agents and locally injectable steroid for the treatment of plantar
fasciitis. Anesth Essays Res. 5:158–161. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Latt LD, Jaffe DE, Tang Y and Taljanovic
MS: Evaluation and Treatment of Chronic Plantar Fasciitis. Foot
Ankle Orthop. 5:1–11. 2020.
|
|
14
|
Cinar E, Saxena S, Akkurt HE and Uygur F:
Extracorporeal shockwave therapy in the management of plantar
fasciitis: A randomized controlled trial. Foot.
44(101679)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vahdatpour B, Sajadieh S, Bateni V, Karami
M and Sajjadieh H: Extracorporeal shock wave therapy in patients
with plantar fasciitis. A randomized, placebo-controlled trial with
ultrasonographic and subjective outcome assessments. J Res Med Sci.
17:834–838. 2012.PubMed/NCBI
|
|
16
|
Leão RG, Azuma MM, Ambrosio GHC, Faloppa
F, Takimoto ES and Tamaoki MJS: Effectiveness of shockwave therapy
in the treatment of plantar fasciitis. Acta Ortop Bras. 28:7–11.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun J, Gao F, Wang Y, Sun W, Jiang B and
Li Z: Extracorporeal shock wave therapy is effective in treating
chronic plantar fasciitis: A meta-analysis of RCTs. Medicine
(Baltimore). 96(e6621)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Roehrig GJ, Baumhauer J, DiGiovanni BF and
Flemister AS: The role of extracorporeal shock wave on plantar
fasciitis. Foot Ankle Clin. 10:699–712, ix. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wheeler P: The role of autologous blood
injections in the treatment for patients with chronic plantar
fasciitis - A case series and longer-term follow-up. Int
Musculoskelet Med. 37:47–53. 2013.
|
|
20
|
Roerdink RL, Dietvorst M, van der Zwaard
B, van der Worp H and Zwerver J: Complications of extracorporeal
shockwave therapy in plantar fasciitis: Systematic review. Int J
Surg. 46:133–145. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ang TW: The effectiveness of
corticosteroid injection in the treatment of plantar fasciitis.
Singapore Med J. 56:423–432. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen CM, Lee M and Lin CH, Chang CH and
Lin CH: Comparative efficacy of corticosteroid injection and
non-invasive treatments for plantar fasciitis: A systematic review
and meta-analysis. Sci Rep. 8(4033)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
McMillan AM, Landorf KB, Gilheany MF, Bird
AR, Morrow AD and Menz HB: Ultrasound guided corticosteroid
injection for plantar fasciitis: Randomised controlled trial. BMJ.
344(e3260)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tatli YZ and Kapasi S: The real risks of
steroid injection for plantar fasciitis, with a review of
conservative therapies. Curr Rev Musculoskelet Med. 2:3–9.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vahdatpour B, Kianimehr L and Ahrar MH:
Autologous platelet-rich plasma compared with whole blood for the
treatment of chronic plantar fasciitis; a comparative clinical
trial. Adv Biomed Res. 5(84)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kirmani TT, Gul IA, Manzoor QW and Kangoo
KA: Autologous whole blood injection in chronic plantar fasciitis:
A prospective clinical study. Int J Res Orthop. 4:634–637.
2018.
|
|
27
|
Martinelli N, Marinozzi A, Carnì S,
Trovato U, Bianchi A and Denaro V: Platelet-rich plasma injections
for chronic plantar fasciitis. Int Orthop. 37:839–842.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Scioli MW: Platelet-rich plasma injection
for proximal plantar fasciitis. Tech Foot Ankle Surg. 10:7–10.
2011.
|
|
29
|
Tabrizi A, Dindarian S and Mohammadi S:
The Effect of corticosteroid local injection versus platelet-rich
plasma for the treatment of plantar fasciitis in obese patients: a
single-blind, randomized clinical trial. J Foot Ankle Surg.
59:64–68. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Karimzadeh A, Raeissadat SA, Erfani Fam S,
Sedighipour L and Babaei-Ghazani A: Autologous whole blood versus
corticosteroid local injection in treatment of plantar fasciitis: A
randomized, controlled multicenter clinical trial. Clin Rheumatol.
36:661–669. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Deghady AAM, Hamid MMA, Helal AMH,
El-Sherif SM and Latief HSA: Platelet-rich plasma in treatment of
plantar fasciitis: randomized double-blinded placebo controlled
study. J Appl Clin Pathol. 2(1)2019.
|
|
32
|
Yang WY, Han YH, Cao XW, Pan JK, Zeng LF,
Lin JT and Liu J: Platelet-rich plasma as a treatment for plantar
fasciitis: A meta-analysis of randomized controlled trials.
Medicine (Baltimore). 96(e8475)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jain K, Murphy PN and Clough TM: Platelet
rich plasma versus corticosteroid injection for plantar fasciitis:
A comparative study. Foot. 25:235–237. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ersen Ö, Koca K, Akpancar S, Seven MM,
Akyıldız F, Yıldız Y and Özkan H: A randomized-controlled trial of
prolotherapy injections in the treatment of plantar fasciitis. Turk
J Phys Med Rehabil. 64:59–65. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Asheghan M, Hashemi SE, Hollisaz MT,
Roumizade P, Hosseini SM and Ghanjal A: Dextrose prolotherapy
versus radial extracorporeal shock wave therapy in the treatment of
chronic plantar fasciitis: A randomized, controlled clinical trial.
Foot Ankle Surg: Aug 25, 2020 (Epub ahead of print).
|