Introduction
Acute myeloid leukemia (AML) is a heterogeneous
clonal hematopoietic progenitor cell disorder characterized by
immature myeloid cell proliferation and bone marrow failure,
exhibiting a spectrum of morphological, immunophenotypic,
cytogenetic and molecular characteristics (1).
Moreover, AML is an aggressive disease with a poor
prognosis (2,3). In addition, >50% of patients with
AML are not candidates for intensive chemotherapy therapy due to
their age, performance status and/or associated comorbidities
(4). Although the long-term overall
survival (OS) rates of patients <65 years of age have
significantly improved over the past years owing to improved
supportive care and an increased use of allogeneic hematopoietic
stem cell transplantation (alloHSCT), the prognosis for the elderly
AML population is still poor, with a 5-year OS rate of <10%
(5).
Furthermore, two-thirds of patients with AML who
achieve a complete remission (CR) will relapse within the following
18 months (6), and regrettably,
there are no safe and effective curative treatments, apart from
alloHSCT, which is a rather aggressive therapeutic modality with
high treatment-related morbidity and mortality (5). Therefore, given the significant
incidence of relapsed AML and the frequent toxicities associated
with standard intensive chemotherapy, an optimal treatment strategy
for this population remains unsatisfactory and has yet to be
established (4,7). In addition, although several new drugs
for the treatment of AML, particularly for elderly patients, have
been approved in recent years, such as the FMS-like tyrosine kinase
3 inhibitors, venetoclax, glasdegib or Vyxeos, the medical needs of
patients with relapsed or refractory (RR) AML continue to be unmet
(1,2,8,9).
Signal transducer and activator of transcription
(STAT) is a seven-member family group of latent cytoplasmic
transcription factors that are involved in hematopoietic cytokine
receptor signaling pathways that mediate several biological
processes, such as cell proliferation, differentiation, survival
and immune response, by transferring signals from cell-surface
cytokines and growth factor receptors to the cell nucleus and
subsequently by regulating the transcription of target genes
(10). The persistent and aberrant
activation of specific STAT factors, particularly STAT3, often
results in the growth and survival of tumor cells and,
consequently, in the development of a wide range of cancers
(11). STAT3 is the STAT family
member most strongly associated with tumorigenesis. There are two
main mechanisms through which STAT3 promotes tumorigenesis: By
acting as a nuclear transcription factor (12) and as a regulator of oxidative
phosphorylation (OXPHOS) via interaction with components of the
electron transport chain (13).
STAT3 is constitutively activated in leukemic cells
from patients with AML. It is now clear that the activation of
STAT3 contributes to the development and resistance of AML
(10). Furthermore, the assessment
of bone marrow samples from patients with AML has demonstrated that
the activation of STAT3 is significantly associated with a reduced
OS and progression-free survival (PFS) (14).
It has been demonstrated that the blockade of
aberrant STAT3 signaling induces tumor cell apoptosis and inhibits
tumor growth, confirming its critical role in the molecular
pathogenesis of several tumors. It has also been identified as a
potential target for the discovery and development of novel
anticancer drugs (10,15).
OPB-111077 is a novel orally bioavailable
low-molecular-weight compound discovered and developed by Otsuka
Pharmaceutical Co., Ltd. as an orally active antitumor agent for
the treatment of various types of cancer. In preclinical analyses,
it has been shown to be a potent and highly specific STAT3
inhibitor with a good efficacy and safety profile, supporting the
initiation of early clinical investigation in humans (16). In fact, a first in-human study of
OPB-111077 demonstrated that it could be administered safely, and
its pharmacokinetic profile was acceptable for further clinical
development (16). Mechanistic
analyses have demonstrated that OPB-111077 significantly inhibits
the STAT3 activation pathway, with antitumor effects against a wide
range of human solid and blood tumor cell lines. Furthermore,
OPB-111077 has been shown to exhibit efficacy against several solid
and blood cancers both in vitro and in vivo (16,17).
Although in a phase I study, the activity of
OPB-111077 in a range of solid tumors was limited, this drug
exhibited clinical activity in one subject with diffuse large
B-cell lymphoma (16), and hence, it
could be more efficient in tumor cells with a high proliferative
index, such as AML.
Biomarker-based treatment selection is a popular
topic in oncology. However, few successful biomarkers have been
discovered thus far, with the majority of these being molecular,
such as tyrosine kinase inhibitors in chronic myeloid leukemia
(18).
Previous studies have analyzed the association
between ex vivo drug testing and clinical outcomes in adult
patients with AML. Functional ex vivo assays that predict a
patient's clinical response to anticancer drugs for guiding cancer
treatment have long been a goal, but few have yet proven to be
reliable (19,20).
The present study conducted a phase Ib
dose-escalation and biomarker-driven study to assess the safety and
efficacy profiles of OPB-111077 in patients with RR AML. In order
to identify and select the subpopulation most sensitive to the
study drug and optimize disease management, a precision medicine,
personalized, ex vivo test was first performed that
evaluated the pharmacological activity of OPB-111077 directly in
individual patient bone marrow samples.
Patients and methods
Ethics approval
The present study was approved by the Research
Ethics Committee of Hospital Universitario 12 de Octubre, Madrid,
Spain, and was conducted according to all the local regulatory
requirements, as well as in accordance with the Declaration of
Helsinki. Informed consent was provided by all the study
participants. This trial was registered at www.clinicaltrials.gov as # NCT03197714.
Study population
Patients were eligible for the study if they met the
following inclusion criteria: A diagnosis of RR non-M3-AML, newly
diagnosed non-M3-AML not eligible for or willing to undergo
intensive induction chemotherapy, and the highest sensitivity
(>70% of the samples analyzed) in the bone marrow analysis of
the OPB-111077 ex vivo sensitivity test. The other selection
criteria are presented in Table SI.
The following patient characteristics were collected: Age, weight,
height, sex, Eastern Cooperative Oncology Group (ECOG) performance
status, blast infiltration, FMS-like tyrosine kinase (FLT),
nucleophosmin 1 (NPM1), French-American-British (FAB
classification), the presence of concomitant disease, refractory
AML and the number of relapses.
Study design and treatment
This phase 1b, open-label, non-randomized,
dose-escalation clinical trial comprised two stages. The first
dose-escalation stage aimed to characterize the safety,
tolerability and maximum tolerated dose (MTD) of OPB-111077 in
patients with high-risk AML. Subsequently, following the
determination of the MTD, an expansion stage further evaluated the
safety and preliminary antitumor activity of OPB-111077 in the
study population.
OPB-111077 was administered orally on a once daily
dose schedule in 28-day cycles until intolerable toxicity or
disease progression, with two dosing schemes as follows: A starting
dose or dose level (DL)1 of 200 mg/day and a DL2 of 250 mg/day. A
3+3 dose-escalation schedule based on the dose-limiting toxicity
(DLT) assessment following the first dose of OPB-111077 was
implemented.
Patients were enrolled in the study between
September 7, 2017 and March 31, 2020 at three Spanish sites:
Hospital La Fe (Valencia), Hospital 12 de Octubre (Madrid), and
Hospital San Pedro de Alcántara (Cáceres). Patients fulfilling the
study selection criteria were included in the trial after
evaluating their anti-proliferative activity to OPB-111077 with a
Vivia Biotech ex vivo PharmaFlow precision medicine (PM)
test (Vivia Biotech, S.L.) (21).
This tool is a cell-based multicolor screening flow cytometry
platform that evaluates the pharmacological activity of drug
treatments on individual patient bone marrow samples, assessing the
patient's cell sensitivity or resistance to a specific drug. Its
methodology has been previously described in detail (22). The Vybrant® CFDA SE Cell
Tracer lit (Thermo Fisher Scientific, Inc.) was used to distinguish
between proliferating and non-proliferating cells, and StemSpan™
Serum-Free Expansion Medium II (SFEM II; StemCell Technologies,
Inc.) supplemented with StemSpan™ CC100 (StemCell Technologies,
Inc.) and autologous plasma was used as the culture medium for
proliferation ex vivo assays in both the preliminary
preclinical phase, where the approved drug, decitabine, was also
used as an anti-proliferative control, and later in the clinical
trial. The leukemic cells were identified using a gating strategy
based on forward scatter and/or side scatter and the expression of
different surface markers. The response effect was measured by
counting the number of live leukemic cells remaining following
exposure to increasing concentrations of OPB-111077 in both the
proliferating and non-proliferating fractions based on
carboxyfluorescein diacetate (CFDA) expression. Dose response
curves for the drug were measured for each proliferative subset
based on the CFDA peak signal. A criterion to consider the results
valid was set based on the culture behavior of tumor cells. Thus,
tumor cells must be viable in culture (net difference with
preincubation basal measure) and >40% confluent in control wells
without the drug. In addition, the ratio of non-induced apoptosis
could not be increased by >60%.
Data analysis for the estimation of the drug effect
on pathological cells from bone marrow samples was carried out
using a population modeling approach and a non-linear mixed effect
regression analysis using NONMEM software version 7.2 (version VII,
ICON Development Solutions). By this methodology, dose-response
curves from all samples were calculated and processed
simultaneously. Residual errors and interindividual variability
were calculated to determine the population standard profile for
the drug. The normalized value of the area under the dose-response
curve (PERCENT_AUC) was used as the optimal activity marker that
was derived from the estimated individual model parameters.
Patients whose ex vivo results to OPB-111077 fell within the
highest 30% (range, >70th percentile of the OPB-111077 profile)
were classified as sensitive and enrolled in the study. In total,
26 out of 47 patients were initially discarded due to acceptance
criteria of the 70th percentile (Fig.
S1).
Optimal culture conditions were typically observed
at 72 h; thus, the results were preferably evaluated at this
incubation time. If an insufficient number of proliferative cells
was counted or a high uncertainty was associated with the result
estimations, the results were then evaluated at longer time periods
of 96 or 120 h. Only single values with an acceptable range 95%
confidence interval (CI) <40% were considered in any case.
Safety assessments
The MTD level was defined as the maximum dose level
below the maximum administered dose at which less than one-third of
the patients experienced DLT. The study patients (between a minimum
of 3 and a maximum of 12 patients) began on level 1, and they were
assessed weekly during the first 28 days following the first dose
of OPB-111077.
DLT was defined as one of the following toxicities
occurring during the DLT assessment window and was considered by
the investigator to be related to study treatment: Any grade ≥3 or
4 non-hematological toxicity or any unexpected non-tolerable grade
II adverse event possibly related to the treatment regimen that
requires a delay beyond 1 week until recovery.
The tolerability and safety of OPB-111077 assessment
was assessed by recording the incidence of treatment-emergent
adverse events (TEAEs) and by grading them according to the Common
Terminology Criteria for Adverse Events (CTCAE) Version
4.03(23).
Efficacy assessments
Bone marrow aspiration was performed on the 1st day
of each cycle until the end of treatment (EOT). Following bone
marrow aspiration, the clinical response was assessed with the
overall response rate (ORR), which was defined as the percentage of
patients who reached CR, morphological complete remission with
incomplete blood count recovery (CrCRi) or partial remission (PR)
(24). In the case of CR or CrCRi
after cycle 3, bone marrow aspiration was performed every 3
months.
The EOT visit took place within 14 days after the
final administration of the study drug or at the time of
discontinuation from the trial. Patients discontinued the study if
they experienced intolerable toxicity, suffered disease
progression, withdrew their consent, or did not benefit from the
trial therapy in the opinion of the investigator.
PFS was defined as the time from the date of the
informed consent form to the date of progression or death (from any
cause), whichever occurred first. OS was defined as the time from
the date of the informed consent form to the date of death due to
any cause.
Statistical analysis
Exploratory and descriptive methods were used to
describe all the study variables. Continuous variables are
summarized as the mean, median, standard deviation and
interquartile range, and categorical variables are presented as
absolute and relative distributions of frequencies. Baseline
categorical characteristics for enrolled and excluded patients due
to screening failure were compared using the Chi-squared test
(Table SII). The associations
between ORR and the half maximal effective concentration (EC50) and
the area under the curve (AUC), as determined using the Vivia
Biotech ex vivo sensitivity test, were evaluated with an
unpaired t-test. PFS and OS time-to-event analyses were performed
using the Kaplan-Meier method; no comparisons were made for
time-to-event outcomes and, therefore, no P-values are
provided.
All analyses were performed using the SPSS
Statistics software package, version 22.0 (IBM Corporation).
Results
To analyze the mechanisms of action of the compound,
in a preliminary preclinical phase, a total of 19 patients with AML
were analyzed at the Vivia Biotech laboratories in a proliferation
assay. This was the starting point to further expand the number of
samples reaching statistical significance and converging in
population models in order to achieve a better characterization of
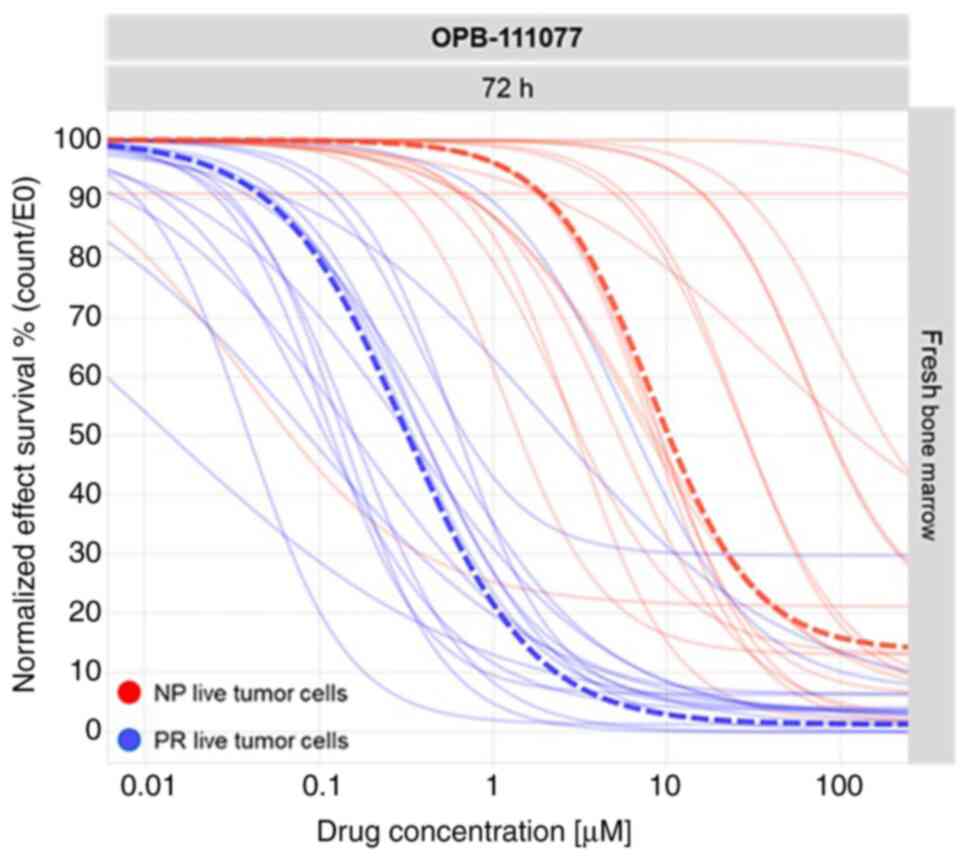
OPB-111077. As shown in Fig. 1,
OPB-111077 exerted anti-proliferative rather than cytotoxic
activity, as it exerted a more prominent effect on proliferating
cells than on the population of non-proliferative cells. A
comparison between a reference anti-proliferative approved drug in
AML, decitabine, was performed. Population dose response curves of
the proliferating cells were generated using both the novel
OPB-111077 compound and decitabine. The pharmacological profiles
revealed a high interpatient variability in the patient samples
incubated with OPB-111077 and in those incubated with decitabine
(Figs. S2 and S3), suggesting the need for a precision
medicine (PM) test to select the best patient candidates. The
overlapping population curves of the proliferating cells showed
similar activity of OPB-111077 vs. decitabine.
Once the pharmacodynamic model of OPB-111077 in the
AML patient samples was established, a phase Ib
investigator-sponsored trial using this assay as a selection
criterion was launched. A total of 47 patients with RR AML were
screened, and 12 were ultimately enrolled in the study between
September 7, 2017 and March 31, 2020 at three Spanish sites
(Fig. S1): Hospital La Fe
(Valencia), Hospital 12 de Octubre (Madrid), and Hospital San Pedro
de Alcántara (Cáceres). In total, 26 patients were excluded using
the personalized medicine sensitivity test, as their results were
below the primary acceptance criteria of the 70th percentile.
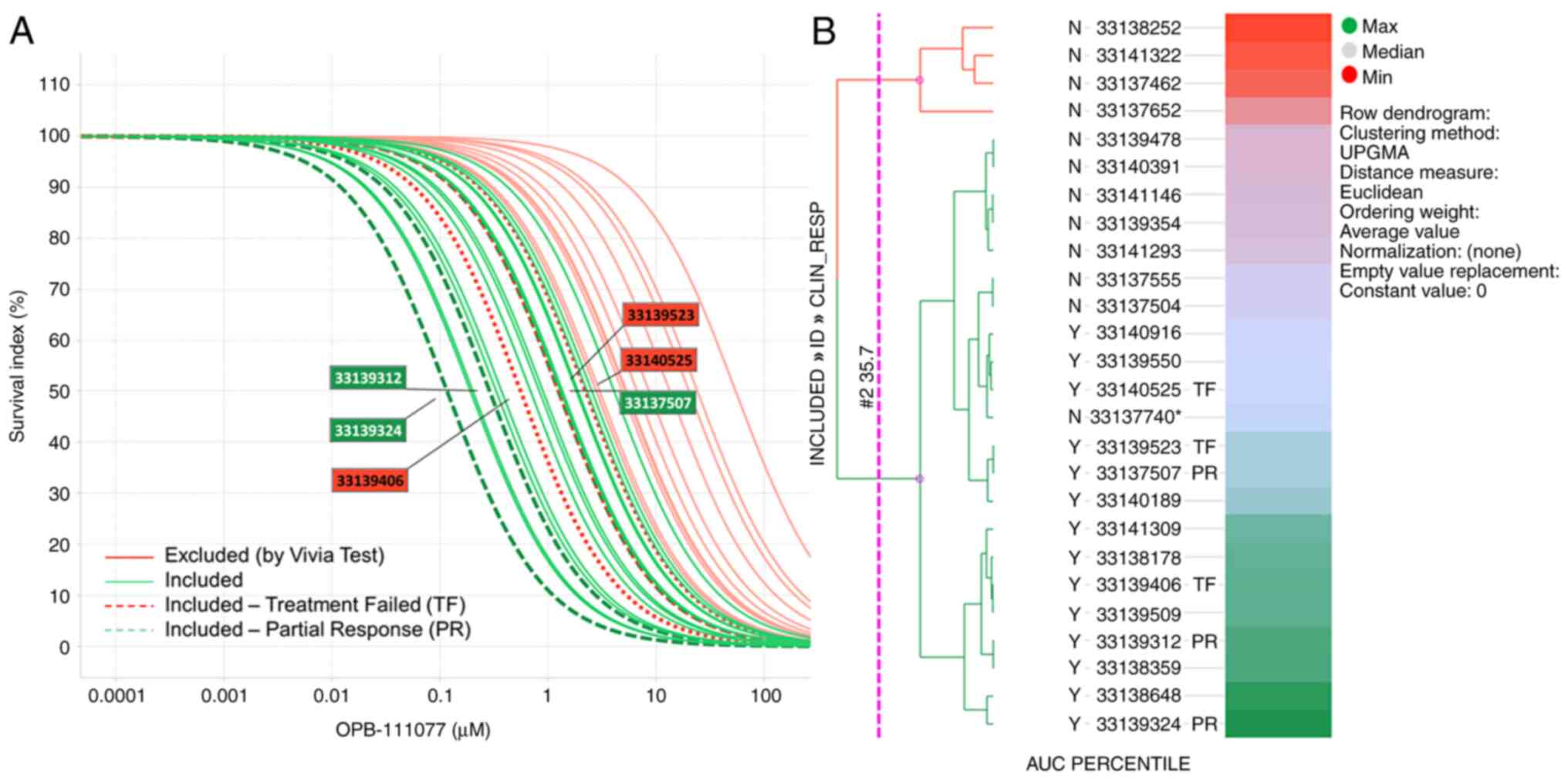
Dose response curves of OPB-111077 in bone marrow
samples from the screened subjects, highlighting those sensitive
and resistant treated patients, are displayed in Fig. 2A. A stratification based on the
percentile AUC and represented in a heatmap was performed to aid in
the selection of patients to be included in this phase Ib clinical
trial (Fig. 2B). Few samples crossed
the sensitive (green) vs. resistant (red) threshold. These samples
near the threshold may have slightly shifted their activity from 72
h shown to 96 h or 120 h (data not shown), which were also measured
and could serve to decide on patient inclusion.
The patient demographics and baseline clinical
characteristics are summarized in Table
I. The median age was 76 years, and 91.7% were male. No
differences were observed in the patient screening failure, except
in the frequency of the NPM1 mutation (Table SII). A total of 5 (42%) patients
with AML were refractory; the median (range) of relapse was 2
(1-6)
(Table I). In addition, 5 (42%)
patients were treated with the first level dose (DL1) of OPB-111077
(200 mg), while 7 patients (58%) were escalated the second dose
level (DL2) of 250 mg. The median total doses administered were
17,000 mg and 8,250 mg for DL1 and DL2, respectively. The study
treatment dose was only reduced in 1 patient treated with DL2.
 | Table IClinical and demographic
characteristics. |
Table I
Clinical and demographic
characteristics.
| Characteristic | Value |
|---|
| Age, years, median
(range) | 76.0
(72.0-79.0) |
| Weight, kg, median
(range) | 69.0
(64.8-79.3) |
| Height, cm, median
(range) | 165.5
(160.0-169.5) |
| Sex, n (%) | |
|
Female | 1 (8.3) |
|
Male | 11 (91.7) |
| ECOG, n (%) | |
|
0 | 5 (41.7) |
|
1 | 6 (50.0) |
|
Unknown | 1 (8.3) |
| Blast Infiltration,
%, median (range) | 62.0
(47.0-71.0) |
| FLT-3 ITD, n
(%) | |
|
Not
mutated | 8 (66.7) |
|
Mutated | 2 (16.7) |
|
Unknown | 2 (16.7) |
| NPM1, n (%) | |
|
Not
mutated | 4 (33.3) |
|
Mutated | 2 (16.7) |
|
Unknown | 6 (50.0) |
| FAB, n (%) | |
|
M0 | 1 (12.5) |
|
M1 | 3 (37.5) |
|
M2 | 1 (12.5) |
|
M4 | 1 (12.5) |
|
M4 eos | 1 (12.5) |
|
M5 | 1 (12.5) |
| Concomitant
disease, n (%) | |
|
Yes | 12 (100.0) |
| Refractory AML, n
(%) | 5 (41.7) |
| Relapses, median
(range) | 2 (1-6) |
Safety MTD
Dose-limiting toxicity was not observed in any of
the patients treated with either DL1 (200 mg) or DL2 (250 mg);
hence, the MTD was not reached.
Safety assessments
The most frequently reported serious adverse events
(SAEs) in the study population, ranging from grade 3 (G3) to grade
5 (G5), were febrile neutropenia, pneumonia and respiratory tract
infection (Table II).
 | Table IISerious adverse events per
subject. |
Table II
Serious adverse events per
subject.
| |
Grade |
|---|
| | 2 | 3 | 4 | 5 | Total |
|---|
| Dose level | SOC | PT | n | % | n | % | n | % | n | % | n | % |
|---|
| Level 1: 200 mg
daily (n=5) | Blood and lymphatic
system disorders | Febrile
neutropenia | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 |
| | Gastrointestinal
disorders | Colitis | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 |
| | Infections and
infestations | Pneumonia | 0 | 0.0 | 2 | 40.0 | 0 | 0.0 | 0 | 0.0 | 2 | 40.0 |
| | | Respiratory
syncytial virus | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 | 1 | 20.0 |
| | | infection | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 |
| | | Respiratory tract
infection | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 |
| | | Skin infection | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 |
| | | Soft tissue
infection | | | | | | | | | | |
| | Respiratory,
thoracic and mediastinal disorders | Dyspnea | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 |
| | | Pulmonary
hemorrhage | 0 | 0.0 | 0 | 0 | 1 | 20.0 | 0 | 0.0 | 1 | 20.0 |
| | | Respiratory
failure | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 1 | 20.0 | 1 | 20.0 |
| Level 2: 250 mg
daily (n=7) | Blood and lymphatic
system disorder | Febrile
neutropenia | 0 | 0.0 | 2 | 28.6 | 0 | 0.0 | 0 | 0.0 | 2 | 28.6 |
| | Cardiac
disorders | Extrasystoles | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| | Infections and
infestations | Pneumonia | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 | 1 | 14.3 |
| | | Respiratory tract
infection | 0 | 0.0 | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| | | Sepsis | 0 | 0.0 | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| | | Septic shock | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 | 1 | 14.3 |
| | | Tonsillitis | 0 | 0.0 | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| | Injury, poisoning
and procedural complications | Medication
error | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| | Renal and urinary
disorders | Acute kidney
injury | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| Total (n=12) | Blood and lymphatic
system disorders | Febrile
neutropenia | 0 | 0.0 | 3 | 25.0 | 0 | 0.0 | 0 | 0.0 | 3 | 25.0 |
| | Cardiac
disorders | Extrasystoles | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 |
| | Gastrointestinal
disorders | Colitis | 0 | 0.0 | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 |
| | Infections and
infestations | Pneumonia | 0 | 0.0 | 2 | 16.7 | 0 | 0.0 | 1 | 8.3 | 3 | 25.0 |
| | | Respiratory
syncytial virus infection | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 | 1 | 8.3 |
| | | Respiratory tract
infection | 0 | 0.0 | 2 | 16.7 | 0 | 0.0 | 0 | 0.0 | 2 | 16.7 |
| | | Sepsis | 0 | 0.0 | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 |
| | | Septic shock | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 | 1 | 8.3 |
| | | Skin infection | 0 | 0.0 | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 |
| | | Soft tissue
infection | 0 | 0.0 | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 |
| | | Tonsillitis | 0 | 0.0 | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 |
| | Injury, posioning
and procedural complications | Medical error | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 |
| | Renal and urinary
disorders | Acute kidney
injury | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 |
| | Respiratory,
thoracic and mediastinal disorders | Dyspnoea | 0 | 0.0 | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 |
| | | Pulmonary
haemorrhage | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 | 0 | 0.0 | 1 | 8.3 |
| | | Respiratory
failure | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 | 1 | 8.3 |
Moreover, seven TEAEs were reported in 3 patients,
all with grades 1 or 2: One patient treated with DL1 experienced
vomiting (G2); a second patient treated with DL2 had extrasystoles
(G2); and a third patient treated with DL2 reported anorexia,
diarrhea, epigastric discomfort, nausea and vomiting, all with G1.
Only extrasystoles (G2) were regarded as a severe TEAEs.
All enrolled patients (n=12) discontinued the study
treatment (Fig. S1). In total, 6
patients (50%) did so due to disease progression, and 3 (25%) did
so as a result of adverse events [respiratory failure (G5),
respiratory infection (G5), and extrasystoles (G2)]. Furthermore, 2
patients died during the treatment period due to disease
progression and respiratory infection.
Efficacy
Only 6 patients (50%) were evaluable for clinical
efficacy, assessed as the ORR. A total of 6 patients (50%) were
excluded from the clinical efficacy assessment as they either did
not have a bone marrow aspiration or they had no information about
cycle 2. Among the evaluable patients, 3 (25%) patients achieved
PR, whereas the other 3 (25%) patients presented with treatment
failure (TF) as the optimal response. ORR was therefore observed in
3 (25%) patients, with a 95% CI of 0.5-49.5%.
The biomarker AUC and EC50 values differed according
to the clinical response. Patients with PR as the optimal response
presented higher mean AUC values (80.94%) than those observed in
patients with TF (59.91%), with a mean difference (95% CI) of
21.033 (-8.361-50.428). Likewise, and as shown in Fig. 2A, the median EC50 was lower in
patients with PR as the best response (0.45 µM) than in patients
with TF (1.28 µM), with a mean difference (95% CI) of 0.831
(-0.563-2.226). However, none of the observed differences reached
statistical significance (P>0.05) (Table III).
 | Table IIIClinical response according to
biomarker AUC and EC50 in the sensitivity test. |
Table III
Clinical response according to
biomarker AUC and EC50 in the sensitivity test.
| Association between
the optimal response and the mean AUC |
|---|
| Optimal
response | No. of
patients | Mean AUC | SD AUC | Mean difference
(95% CI) |
P-valuea |
|---|
| PR | 3 | 80.943 | 12.584 | 21.033
(-8.361-50.428) | 0.118 |
| TF | 3 | 59.910 | 13.338 | | |
| Association between
the optimal response and the EC50 in the sensitivity test |
| Best response | No. of
patients | Mean EC50 | SD EC50 | Mean difference
(95% CI) |
P-valuea |
| PR | 3 | 0.453 | 0.445 | 0.831
(-0.563-2.226) | 0.173 |
| TF | 3 | 1.285 | 0.748 | | |
Finally, the estimated median PFS and OS were 57
days (95% CI, 37-77) and 95 days (95% CI, 27-163), respectively, as
shown in Figs. S4 and S5.
Discussion
The present phase I dose-escalation trial was
performed to assess the safety, tolerability and efficacy of
OPB-111077 in patients with RR AML treated with doses ranging from
200-250 mg/day for 4 weeks.
In the current trial, no DLTs were observed, and
therefore, the MTD (primary study endpoint) was not reached,
confirming the good safety profile of OPB-111077. This good safety
and tolerability profile has also been reported in previously
published studies with OPB-111077 (16,17).
Likewise, the most frequently reported TEAEs were nausea, vomiting
and fatigue.
Although the clinical activity (i.e., an ORR of 25%)
may be considered modest (25,26), it
was much higher than the response observed in the aforementioned
published phase I studies with OPB-111077 (i.e., an ORR of 1/145)
(16,17). It is also an even higher response
rate compared to other new drugs with different mechanisms of
action, such as MDM2 antagonist RO6839921. Uy et al
(27) reported a response rate of
7.7% in their phase 1 study. The same occurred in the phase 1 study
on CWP232291, in which Lee et al (28) described a low number of responses.
However, it should be noted that the patients included in the
present clinical trial had a very poor prognosis; they were elderly
(many of them >70 years of age), a difficult-to-treat population
(29), and the majority were
refractory to standard therapy (30). Tolcher et al (16) reported clinical activity (durable PR)
in only one subject, with diffuse large B-cell lymphoma, from a
population of 18 patients with unselected and mostly solid tumors,
while in the study conducted by Yoo et al (17), no patients with hepatocellular
carcinoma achieved complete or partial responses with OPB-111077. A
plausible explanation for this finding is that, unlike other phase
I trials, in the present study, the population was selected based
on a biomarker that enabled the upfront identification and
enrollment of those AML patients with the highest sensitivity to
the study drug, discarding those hypothetically resistant ones and
thus minimizing the likelihood of treatment failures. This is
supported by the differences in both the AUC and EC50 values that
were found between patients achieving PR or TF as the optimal
responses. However, those differences did not meet the statistical
significance criteria, probably due to the small sample size. Other
research groups have also implemented this ex vivo
personalized medicine sensitivity test in the AML population to
improve prognostic risk stratification, tailor treatments, and
minimize drug resistance. As in the current analysis based on the
expression of a biomarker, other researchers have found strong
correlations between the ex vivo sensitivity test and the
clinical response to chemotherapy in AML patients in their
respective studies (31,32).
One of the mechanisms through which STAT3 promotes
oncogenesis is through the activation of OXPHOS (16). Of note, OXPHOS has been reported to
be involved as a mechanism of resistance to chemotherapy in AML
(33). Therefore, the use of drugs
targeting OXPHOS may be an appropriate therapeutic approach for the
treatment of refractory and relapsed AML (34,35).
Other drugs have been proposed to function through the OXPHOS of
leukemic cells, such as IACS-010759(36) and ME-344(37) (4).
However, in contrast to OPB-111077, phase 1 studies of the use of
these drugs in relapsed/refractory acute myeloid leukemia have not
yet been conducted.
As demonstrated in the present study, drugs such as
decitabine, similar to OPB-111077, exert an anti-proliferative
effect on tumor cells. Therefore, the combination of both can
increase anti-tumor activity. In this regard, the authors of an
ongoing trial evaluating the combination of OPB-111077 with
decitabine and venetoclax for the treatment of AML have suggested
that the combination of OPB-111077 and venetoclax reduces tumor
cell proliferation and increases apoptosis rates to a greater
extent than exposure to any single study drug (38). Notably, the effects obtained with the
combination were even more pronounced in AML cells that were
genetically engineered to increase OXPHOS (38). Pollyea et al (39) also demonstrate that the combination
of venetoclax and a hypomethylating agent such as azacitidine can
eradicate leukemic cells by disrupting energy metabolism through
suppression of OXPHOS. This is in line with the similar activity
and weak toxicity found in the preliminary preclinical study we
performed, which may suggest a similar clinical profile; thus,
their use in combination could increase the chance of achieving an
overall response.
Certain limitations of the present study are the
small number of patients included, although this is due to of the
nature of a phase I clinical trial and the strategy used for
patient selection. The employment of an ex vivo test for
selection could hinder patient treatment in this aggressive
disease.
In conclusion, OPB-111077 as a monotherapy has
exhibited a good safety and tolerability profile in patients with
RR AML. Additionally, some clinical response was found compared to
previous studies performed with the same study drug (16,17). The
innovative biomarker-driven design used in the present study to
select the patient population upfront based on their sensitivity to
the study drug may partly explain these improved results over
previous studies. This innovative phase IB biomarker selection
design may help to lower the high attrition rate of new drugs.
Supplementary Material
Patient enrollment flow chart. ST,
sensitivity test (<70% of analyzed samples); DF, diagnosis
failure (without histological evidence of relapsed or refractory
acute myeloid leukemia); LE, life expectancy ≤3 months; SI,
systemic antineoplastic therapy within 14 days of study treatment;
IC, insufficient cellularity; OR, other reason (death caused by
COVID-19 infection).
Population curves for OPB-111077 and
decitabine. O.F.V*, objective function; parameters
typical and random (variability and residual error) are shown
together with the corresponding relative standard error calculated
as the ratio between the standard error provided by NONMEM and the
estimate. Estimates of inter-patient variability (IPV) are
expressed as the coefficient of variation (%).
Overlapped populational curves of
OPB-111077 and decitabine.
Cumulative progression-free survival.
Kaplan-Meier survival curve. Median PFS (95% CI), 57.000
(36.631-77.369).
Overall survival. Kaplan-Meier
survival curve. Median OS (95% CI), 95.000 (26.545-163.455).
Study selection criteria.
Patient characteristics.
Acknowledgements
The authors would like to thank Vivia Biotech for
performing the ex vivo assays. The authors would also like
to thank Mr. Juan Luis Sanz and Mrs. Susana Vara (APICES, Madrid,
Spain) for their support with the study design, setup, coordination
and project management, monitoring, statistical analysis and
medical writing assistance.
Funding
Funding: The present study was partially funded by Otsuka and
the CRIS Cancer Foundation (Grant nos. CRIS 18001 and CRIS
28001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JML participated in the conception and design of the
study, as well as in data acquisition, data analysis, and in the
interpretation of the data and the drafting of the manuscript. PM,
RA, PMS, JMBB, MC, EAC, JAPS, ADLF, JPDO, RRV, JSP, BB, IC and MLPC
participated in data acquisition. NLM participated in data
acquisition and data analysis. JG, JLRR and DP participated in the
conception of the study. JB participated in the conception and
design of the study, and in data interpretation. All authors have
revised the manuscript, and all authors have read and approved the
final manuscript and ensure its accuracy or integrity. JML and JB
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Hospital Universitario 12 de Octubre, Madrid,
Spain. Informed consent was provided by all the study
participants.
Patient consent for publication
Not applicable.
Competing interests
JML has had stocks and been a member of the board of
directors for Vivia Biotech. JPO has received research funding from
Vivia Biotech. JG, JLRR, DP and JB are employees of Vivia Biotech.
JAPS, AF, RR-V, PM, NLM, PMS, JMBB, MC, EAC, BB, IC and MLPC
declare that they have no competing interests.
References
|
1
|
Dohner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Löwenberg B: Prognostic factors in acute
myeloid leukaemia. Best Pract Res Clin Haematol. 14:65–75.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stone RM: The difficult problem of acute
myeloid leukemia in the older adult. CA Cancer J Clin. 52:363–371.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Griffiths EA, Carraway HE, Chandhok NS and
Prebet T: Advances in non-intensive chemotherapy treatment options
for adults diagnosed with acute myeloid leukemia. Leuk Res.
91(106339)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heuser M, Ofran Y, Boissel N, Brunet Mauri
S, Craddock C, Janssen J, Wierzbowska A and Buske C: Esmo
Guidelines Committee. Electronic address: simpleclinicalguidelines@esmo.org.
Acute myeloid leukaemia in adult patients: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
31:697–712. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yilmaz M, Wang F, Loghavi S, Bueso-Ramos
C, Gumbs C, Little L, Song X, Zhang J, Kadia T, Borthakur G, et al:
Late relapse in acute myeloid leukemia (AML): Clonal evolution or
therapy-related leukemia? Blood Cancer J. 9(7)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Park S, Cho BS and Kim HJ: New agents in
acute myeloid leukemia (AML). Blood Res. 55:S14–S18.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guerra VA, DiNardo C and Konopleva M:
Venetoclax-based therapies for acute myeloid leukemia. Best Pract
Res Clin Haematol. 32:145–153. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tiong IS and Wei AH: New drugs creating
new challenges in acute myeloid leukemia. Genes Chromosomes Cancer.
58:903–914. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brachet-Botineau M, Polomski M, Neubauer
HA, Juen L, Hedou D, Viaud-Massuard MC, Prie G and Gouilleux F:
Pharmacological inhibition of oncogenic STAT3 and STAT5 signaling
in hematopoietic cancers. Cancers (Basel). 12(240)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gu Y, Mohammad IS and Liu Z: Overview of
the STAT-3 signaling pathway in cancer and the development of
specific inhibitors. Oncol Lett. 19:2585–2594. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Furqan M, Akinleye A, Mukhi N, Mittal V,
Chen Y and Liu D: STAT inhibitors for cancer therapy. J Hematol
Oncol. 6(90)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Meier JA and Larner AC: Toward a new
STATe: The role of STATs in mitochondrial function. Semin Immunol.
26:20–28. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Benekli M, Baer MR, Baumann H and Wetzler
M: Signal transducer and activator of transcription proteins in
leukemias. Blood. 101:2940–2954. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ogura M, Uchida T, Terui Y, Hayakawa F,
Kobayashi Y, Taniwaki M, Takamatsu Y, Naoe T, Tobinai K, Munakata
W, et al: Phase I study of OPB-51602, an oral inhibitor of signal
transducer and activator of transcription 3, in patients with
relapsed/refractory hematological malignancies. Cancer Sci.
106:896–901. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tolcher A, Flaherty K, Shapiro GI, Berlin
J, Witzig T, Habermann T, Bullock A, Rock E, Elekes A, Lin C, et
al: A first-in-human phase I study of OPB-111077, a small-molecule
STAT3 and oxidative phosphorylation inhibitor, in patients with
advanced cancers. Oncologist. 23:658–e672. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoo C, Kang J, Lim HY, Kim JH, Lee MA, Lee
KH, Kim TY and Ryoo BY: Phase I dose-finding study of OPB-111077, a
novel STAT3 inhibitor, in patients with advanced hepatocellular
carcinoma. Cancer Res Treat. 51:510–518. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kalia M: Biomarkers for personalized
oncology: Recent advances and future challenges. Metabolism. 64
(Suppl 1):S16–S21. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kuusanmäki H, Leppä AM, Pölönen P, Kontro
M, Dufva O, Deb D, Yadav B, Brück O, Kumar A, Everaus H, et al:
Phenotype-based drug screening reveals association between
venetoclax response and differentiation stage in acute myeloid
leukemia. Haematologica. 105:708–720. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Martínez-Cuadrón D, Gil C, Serrano J,
Rodríguez G, Pérez-Oteyza J, García-Boyero R, Jiménez-Bravo S,
Vives S, Vidriales MB, Lavilla E, et al: A precision medicine test
predicts clinical response after idarubicin and cytarabine
induction therapy in AML patients. Leuk Res. 76:1–10.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vivia Biotech: PharmaFlow PM Test. 2020.
https://www.viviabiotech.com/pharmaflow-precision-medicine-test/.
Accessed October, 2021.
|
|
22
|
Bennett TA, Montesinos P, Moscardo F,
Martinez-Cuadron D, Martinez J, Sierra J, García R, de Oteyza JP,
Fernandez P, Serrano J, et al: Pharmacological profiles of acute
myeloid leukemia treatments in patient samples by automated flow
cytometry: A bridge to individualized medicine. Clin Lymphoma
Myeloma Leuk. 14:305–318. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 4.0. 2010. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Accessed September, 2020.
|
|
24
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, et al: Revised recommendations of the international working
group for diagnosis, standardization of response criteria,
treatment outcomes, and reporting standards for therapeutic trials
in acute myeloid leukemia. J Clin Oncol. 21:4642–4649.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bejanyan N, Weisdorf DJ, Logan BR, Wang
HL, Devine SM, de Lima M, Bunjes DW and Zhang MJ: Survival of
patients with acute myeloid leukemia relapsing after allogeneic
hematopoietic cell transplantation: A center for international
blood and marrow transplant research study. Biol Blood Marrow
Transplant. 21:454–459. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Breems DA, Van Putten WL, Huijgens PC,
Ossenkoppele GJ, Verhoef GE, Verdonck LF, Vellenga E, De Greef GE,
Jacky E, Van der Lelie J, et al: Prognostic index for adult
patients with acute myeloid leukemia in first relapse. J Clin
Oncol. 23:1969–1978. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Uy GL, Assouline S, Young AM, Blotner S,
Higgins B, Chen LC and Yee K: Phase 1 study of the MDM2 antagonist
RO6839921 in patients with acute myeloid leukemia. Invest New
Drugs. 38:1430–1441. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee JH, Faderl S, Pagel JM, Jung CW, Yoon
SS, Pardanani AD, Becker PS, Lee H, Choi J, Lee K, et al: Phase 1
study of CWP232291 in patients with relapsed or refractory acute
myeloid leukemia and myelodysplastic syndrome. Blood Adv.
4:2032–2043. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Webster JA and Pratz KW: Acute myeloid
leukemia in the elderly: Therapeutic options and choice. Leuk
Lymphoma. 59:274–287. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Martinez-Lopez J, Montesinos P, Martinez
Sanchez P, Gorrochategui J, Rojas JL, Primo D, Bergua JM, Ayala RM,
Calbacho M, López Muñoz N, et al: Biomarker-driven phase Ib
clinical trial of OPB-111077 in acute myeloid leukemia increases
overall response rates. Blood. 136 (Suppl 1):18–19. 2020.
|
|
31
|
Lin L, Tong Y, Straube J, Zhao J, Gao Y,
Bai P, Li J, Wang J, Wang H, Wang X, et al: Ex-vivo drug testing
predicts chemosensitivity in acute myeloid leukemia. J Leukoc Biol.
107:859–870. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Onecha E, Ruiz-Heredia Y, Martinez-Cuadron
D, Barragan E, Martinez-Sanchez P, Linares M, Rapado I,
Perez-Oteyza J, Magro E, Herrera P, et al: Improving the prediction
of acute myeloid leukaemia outcomes by complementing mutational
profiling with ex vivo chemosensitivity. Br J Haematol.
189:672–683. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wilde L, Martinez-Outschoorn U, Palmisiano
N and Kasner M: OPB-111077 in combination with decitabine and
venetoclax for the treatment of acute myeloid leukemia. Blood.
134:2597. 2019.
|
|
34
|
Ashton TM, McKenna WG, Kunz-Schughart LA
and Higgins GS: Oxidative phosphorylation as an emerging target in
cancer therapy. Clin Cancer Res. 24:2482–2490. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee H, Jeong AJ and Ye SK: Highlighted
STAT3 as a potential drug target for cancer therapy. BMB Rep.
52:415–423. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tsuji A, Akao T, Masuya T, Murai M and
Miyoshi H: IACS-010759, a potent inhibitor of glycolysis-deficient
hypoxic tumor cells, inhibits mitochondrial respiratory complex I
through a unique mechanism. J Biol Chem. 295:7481–7491.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jeyaraju DV, Hurren R, Wang X, MacLean N,
Gronda M, Shamas-Din A, Minden MD, Giaever G and Schimmer AD: A
novel isoflavone, ME-344, targets the cytoskeleton in acute myeloid
leukemia. Oncotarget. 7:49777–49785. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wilde L, Martinez-Outschoorn U, Palmisiano
N, Keiffer G and Kasner M: Results of the phase 1b dose escalation
study of OPB-111077, decitabine, and venetoclax for the treatment
of newly diagnosed or relapsed/refractory AML. Blood.
136(10)2020.
|
|
39
|
Pollyea DA, Stevens BM, Jones CL, Winters
A, Pei S, Minhajuddin M, D'Alessandro A, Culp-Hill R, Riemondy KA,
Gillen AE, et al: Venetoclax with azacitidine disrupts energy
metabolism and targets leukemia stem cells in patients with acute
myeloid leukemia. Nat Med. 24:1859–1866. 2018.PubMed/NCBI View Article : Google Scholar
|