Introduction
Cerebral vasospasm (CV) represents the most crucial
complication encountered following an aneurysmal subarachnoid
hemorrhage (aSAH). However, despite the use of different
therapeutic procedures, 16-65% of these patients consequently
develop delayed cerebral ischemia (DCI). The early detection of CV
in aSAH may be difficult both clinically and radiographically
(1).
Despite all existing treatment approaches, aSAH, and
the ensuing CV and DCI are the leading causes of morbidity and
mortality in affected patients (1,2).
Although 40-70% of patients exhibit substantial arterial narrowing
(on a doppler ultrasound or in angiography), only 20 to 30% of
these patients present with DCI (3,4).
Intra-arterial digital subtraction angiography (DSA) is commonly
used as a screening device for the identification of CV and focal
cerebral ischemia (CI) (5).
Furthermore, DSA allows for therapeutic endovascular interventions,
such as balloon angioplasty and stenting in each intracranial
artery if required (5). Still, the
detailed delineation of the vessels may not be obtained when
multiple stenoses are present, and DSA also constitutes an invasive
method. By contrast, computed tomography (CT) perfusion (CTP) can
identify damage which is not recognized by other methods and may be
beneficial for assessing CI related to aSAH (6). CTP allows for the early detection of CI
and provides essential information on the ischemic penumbra, which
is of utmost importance for the early identification and management
of CI (7). In addition, CTP can be
used in daily practice, or it can be used as a separate diagnostic
tool without the need for magnetic resonance imaging (MRI) data to
predict the outcomes of patients with aSAH (7,8). With
conventional MRI, performed pre- and post-contrast administration,
irreversibly damaged brain tissue cannot be discriminated from
infarcted brain tissue (penumbra), which is under the risk of
ischemia (7). Even though a
conventional MRI provides anatomical details, it does not provide
functional details on the dynamic process of ischemia and its
elongation. Thus, CTP has currently become an important technique
(7). However, there are insufficient
data on its association with other non-invasive techniques, such as
transcranial Doppler (TCD) ultrasound (3,9,10).
Although several studies have only confirmed the
efficacy of nimodipine in patients with CV and subsequent CI,
multiple centers have included papaverine, hemodynamic (triplex)
therapy and balloon angioplasty in their treatment algorithms for
severe CV, despite the fact that these have yet to be established
as life-saving therapies (2,11,12). In
addition, since 1982, the effect of locally applied nimodipine on
vasospasm has been widely used and is well-known (13). However, to the best of our knowledge,
no study to date has demonstrated the efficacy of combined
intrathecal (IT) and intravenous (IV) nimodipine therapy for
cerebral vasospasm. In this respect, the present retrospective
study aimed to evaluate the efficacy of combined IV and IT
nimodipine therapy for preventing permanent neurological
deterioration in patients with severe post-hemorrhagic CV. The
present study also aimed to assess the effectiveness of this
technique in the clinical outcomes of patients with aSAH.
Patients and methods
Patient information
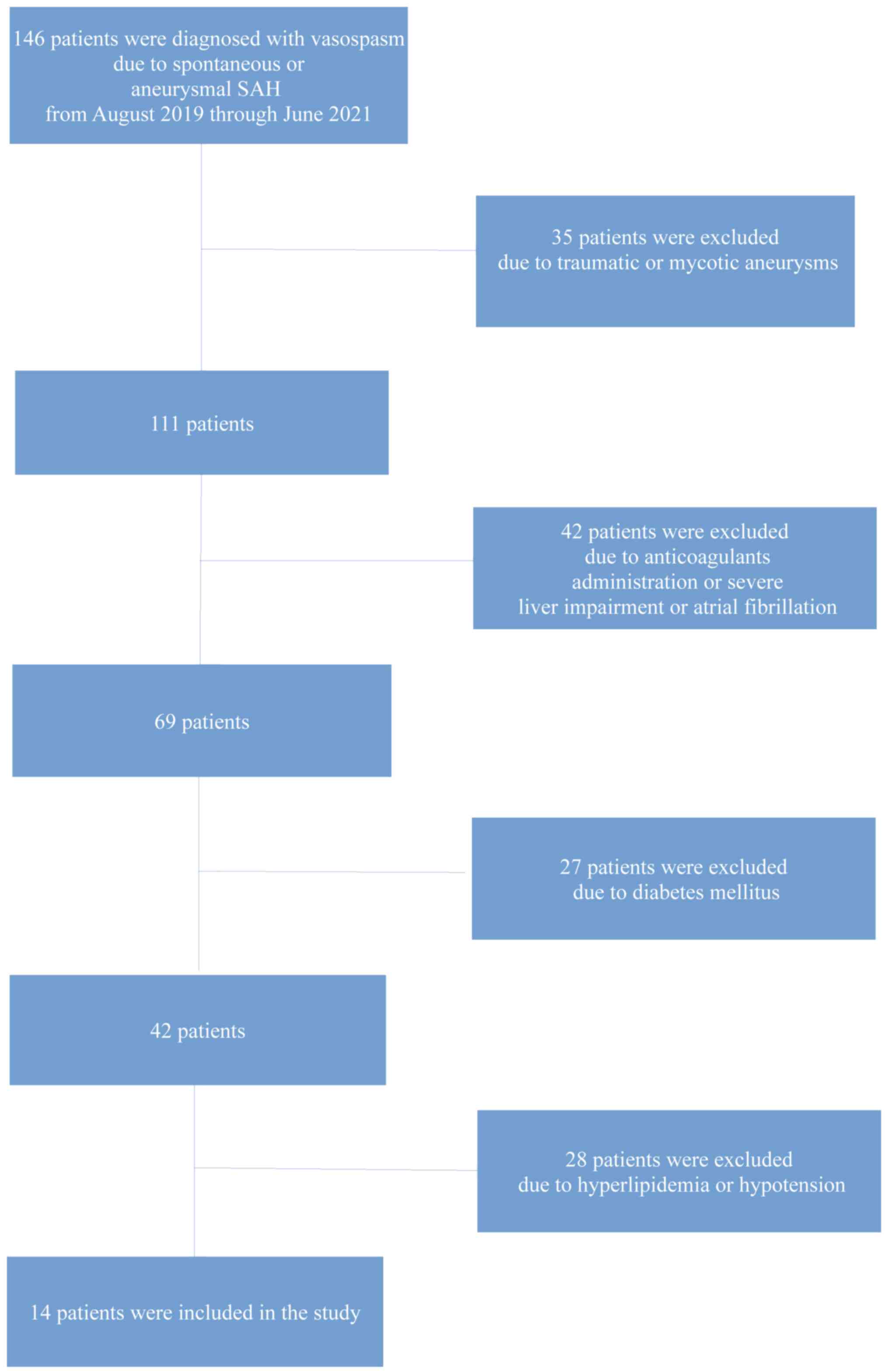
The present retrospective cohort study analyzed 14
out of 146 patients diagnosed with vasospasm due to spontaneous or
aneurysmal SAH from August, 2019 through June, 2021 at Nicosia
General Hospital, Cyprus. The inclusion criteria included the
following: An age ≥21 years, surgical or endovascular repair of the
ruptured aneurysm, severe vasospasm and CI on a CTP and TCD
evaluation. The Institutional Review Board (IRB) of Nicosia General
Hospital, Cyprus approved the study (IRB no. EEBK EΠ 2019.02.110).
The study was in line with the Declaration of Helsinki in 1995 (as
revised in Edinburgh 2000). Written informed was obtained from the
patients.
The patients were divided into two groups as
follows: i) The IV group, which included patients treated with only
IV nimodipine; and ii) the IV + IT group, which included those
patients who received IV nimodipine together with IT nimodipine [20
ml (0.4 mg) bolus via the external ventricular drain (EVD)
system].
Following clinical deterioration due to severe CV,
all patients were placed in the intensive care unit (ICU) with
intracranial pressure (ICP) monitoring. Overall, the target
parameters for the prophylactic pathway were a central venous
pressure >4 mm Hg and a cerebral perfusion pressure >60 mm
Hg. All patients were administered prophylactic IV nimodipine at 2
mg/h for at least 21 days. The exclusion criteria are presented in
Table I. The methods used for the
final inclusion of patients in the study are illustrated in
Fig. 1.
 | Table IExclusion criteria used in the present
study. |
Table I
Exclusion criteria used in the present
study.
| Factors predisposing
to cerebral ischemia apart from vasospasm |
|
Atrial
fibrillation or other arrhythmias |
|
Diabetes
mellitus |
|
Hyperlipidemia |
|
Hypotension |
| Factors predisposing
to bleeding |
|
Anticoagulant
agents |
|
Severe liver
impairment |
Follow-up
All participants had a follow-up for 30 days or
until the day of discharge from the hospital. At 30 days, outcomes
were evaluated using a CT scan and a complete neurological
examination; a Glasgow Coma Scale (GCS) assessment was also
performed. The clinical outcome was categorized according to
neurological or radiological evidence as improved or adverse
(unaltered, worse, or mortality).
Procedure for IT nimodipine
administration
An initial IT bolus of nimodipine (Nimotop, Bayer
AG) was administered in Ringer's solution (Demo S.A.) at 2 mg/100
ml (14-16).
20 ml (0.4 mg) were used for an IT bolus via the EVD after
releasing 20 ml cerebrospinal fluid (CSF). The IT bolus was
repeated every 24 h during the first 7 days of the event. The EVD
overflow level was placed 10 cm above ear level to provide CSF
outflow. The intracranial pressure values were recorded using an
online ICU data management system and ICP monitoring.
Radiological CV or CI evaluation
During the 3rd to the 6th day following aSAH, CTP
and TCD were performed on all the participants to identify a
quantifiable index of CI after CV, given that angiographically
detectable cerebral artery constriction is most commonly present
3-10 days after the onset. Cerebral blood volume (CBV) and cerebral
blood flow (CBF) values were documented and assessed after
receiving two contiguous 10-mm-thick slices placed at the
anatomical point of the basal ganglia with similar angulation as
for native CT. A bolus of 50 ml non-ionic contrast medium (Imeron
400, Bracco Imaging Deutschland GmbH) accompanied by 30 ml saline
was then infused using a power injector (Medcomp USA) at a flow
rate of 4 ml/sec. Subsequently, 40 images were captured at each
slice level at a rate of two images per second (120 kV, 110 mAs,
512x512 matrices). CTP color maps were qualitatively assessed using
a visual grading scale, and CTP parameters were established
utilizing software platforms (Perfusion CT, Siemens). A positive
visual measurement was recorded for side-to-side discrepancies or
apparent bilateral anomalies, suggesting a decline in CBF, CBV and
the mean transit time (MTT), which were related to the central
volume principle: CBF=CBV/MTT (3,17). CBV
was determined in milliliters of blood per 100 g of the brain and
was established as the volume of blood flow for a certain amount of
brain tissue (3,18). MTT was determined as the average time
needed for blood to move through a particular brain volume and was
calculated in seconds (3).
Moreover, 15-20 min prior to CTP, TCD via a
trans-temporal window was performed to illustrate and assess flow
velocities in the anterior cerebral artery (ACA), middle cerebral
artery (MCA), posterior cerebellar artery (PCA) and posterior
communicating artery. Peak systolic velocity (PSV, in cm/sec),
which is the maximum flow systolic velocity value, was calculated
at the peak of the waveform. End-diastolic velocity (EDV, in
cm/sec) was measured at the end of diastole, traditionally at the
lowest point before the start of a new waveform, and was found to
be between 20 and 50% of the PSV value (3,19). The
Lindegaard ratio, defined as the MCA mean CBF velocity divided by
the extracranial internal carotid artery mean CBF velocity, was
used to indicate systemic hemodynamic alterations. The mean flow
velocity (in cm/sec) was calculated as the average of the edge
frequency across a cardiac cycle, which was calculated as the EDV
plus one-third of the variance between PSV and EDV [MFV
(cm/sec)=(PSV + 2EDV/3)] (3,19). Sonographic CV was defined as an MFV
>140 cm/sec in the MCA, ACA, and/or a PCA or >90 cm/sec in
the basilar artery. As an index for intracranial pressure
elevation, the pulsatility index (PI)=PSV-EDV/MFV was used
(3,19).
Statistical analysis
The normality of the distribution of variables was
determined using the Shapiro-Wilk test. Categorical variables were
compared between groups using the Fisher's exact test or the
Chi-squared test, and continuous data were compared using the
Mann-Whitney U test. A P-value <0.05 was considered to indicate
a statistically significant difference. Statistical analysis was
performed using the Statistical Package for the Social Sciences
(SPSS 11; SPSS, Inc.).
Results
A total of 14 patients were enrolled in the study
(Table II). Of these, 7 patients
were males (50%), and the mean age of the patients was 50.9 years
(SD ±19 years). Of these, 8 patients (57.1%) underwent surgical and
6 patients (42.8%) underwent endovascular procedures. The outcomes
and baseline characteristics of the participants are presented in
Table II. Overall, 6 patients
[42.8%; 5 (35.7%) from the IV group and 1 (7.1%) from the IV + IT
group], who experienced clinical symptoms with severe CV, such as a
decline in the level of consciousness, were administered
hypervolemic hypertensive and intra-arterial calcium channel
therapy or IT nimodipine following the early identification of
symptomatic vasospasm with CTP or TCD findings. These patients
presented with permanent neurological deficits, as detected using a
CT scan following 1 month of follow-up. The TCD and CTP data of the
patients are presented in Tables II
and III. In the 6 patients (42.8%)
who presented with permanent neurological deficits due to
vasospasm, CTP revealed reduced a CBF and prolonged MTT. However,
TCD also revealed elevated PSV and PI values in the same patients
(Table III).
 | Table IIBaseline characteristics of the
patients in the present study. |
Table II
Baseline characteristics of the
patients in the present study.
| Parameters | All patients, n=14
(100%) | IV group, n=7
(50%) | IV + IT group, n=7
(50%) | P-value |
|---|
| Age, years | 50.9±19 | 52.7±16 | 49.1±23 | 0.848 |
| Sex (male), n
(%) | 7(50) | 2 (14.2) | 5 (35.7) | 0.109 |
| GCS of admission | 11.6±3 | 11.2±3 | 12.0±3 | 0.896 |
| Procedure | | | | |
|
Surgical, n
(%) | 8 (57.1) | 3 (21.4) | 5 (35.7) | 0.280 |
|
Endovascular,
n (%) | 6 (42.8) | 4 (28.5) | 2 (14.2) | |
| ICU stay, days | 38.7±17 | 40.0±11 | 37.5±23 | 0.522 |
| Duration of
intrathecal nimodipine treatment, days | 6.3±1 | 6.7±0 | 6.0±1 | 0.410 |
| Hunt and Hess
grade | | | | |
|
I, n
(%) | 4 (28.5) | 2 (14.2) | 2 (14.2) | NS |
|
II, n
(%) | 4 (28.5) | 1 (7.1) | 3 (21.4) | 0.237 |
|
III, n
(%) | 4 (28.5) | 3 (21.4) | 1 (7.1) | 0.237 |
|
IV, n
(%) | 1 (7.1) | 1 (7.1) | 0 (0) | 0.299 |
|
V, n
(%) | 1 (7.1) | 0 (0) | 1 (7.1) | 0.299 |
| Modified Fisher
scale | | | | |
|
I, n
(%) | 4 (28.5) | 2 (14.2) | 2 (14.2) | NS |
|
II, n
(%) | 7(50) | 2 (14.2) | 5 (35.7) | 0.109 |
|
III, n
(%) | 2 (14.2) | 2 (14.2) | 0 (0) | 0.127 |
|
IV, n
(%) | 1 (7.1) | 1 (7.1) | 0 (0) | 0.299 |
| Aneurysm
location | | | | |
|
ACoA | 2 (14.2) | 1 (7.1) | 1 (7.1) | NS |
|
MCA | 6 (42.8) | 2 (14.2) | 4 (28.5) | 0.280 |
|
PICA | 2 (14.2) | 1 (7.1) | 1 (7.1) | 1.000 |
|
Pcom | 2 (14.2) | 2 (14.2) | 0 (0) | 0.127 |
|
ICA | 2 (14.2) | 1 (7.1) | 1 (7.1) | NS |
| CT perfusion (white
matter) parameters | | | | |
| CBF mean ± SD
(mlblood/100gtissue) | 15.8±10 | 12.7±10 | 18.8±10 | 0.084 |
| CBVmean ± SD
(mlblood/100gtissue) | 1.5±1 | 1.3±0. | 1.7±1 | 0.949 |
| MMTmean ±
SD(sec) | 4.4±1 | 4.6±2 | 4.3±1 | 0.848 |
| TCD parameters | | | | |
| Total PSV mean ± SD
(cm/sec) | 94.3±30 | 108.8±36 | 79.9±12 | 0.110 |
| Total PI mean ± SD
(cm/sec) | 0.9±0 | 0.9±0 | 0.9±0 | 0.794 |
 | Table IIIUnivariate analysis (outcome:
Ischemic event at 1 month). |
Table III
Univariate analysis (outcome:
Ischemic event at 1 month).
| Parameters | Patients with
ischemic event, n=6 (42.8%) | Patients without
ischemic event, n=8 (57.2%) | P-value |
|---|
| Groups | | | |
|
IV
group | 5 (35.7) | 2 (14.2) | 0.031 |
|
IV + IT
group | 1 (7.1) | 6 (42.8) | |
| Age, years | 53.1±18 | 49.2±21 | 0.796 |
| Sex (male), n
(%) | 2 (14.2) | 5 (35.7) | 0.280 |
| GCS of
admission | 11.8±3 | 11.5±3 | 0.740 |
| Procedure | | | |
|
Surgical, n
(%) | 2 (14.2) | 6 (42.8) | 0.119 |
|
Endovascular,
n (%) | 4 (28.5) | 2 (14.2) | 0.119 |
| ICU stay, days | 45.8±15 | 33.5±18 | 0.155 |
| Duration of
intrathecal nimodipine treatment, days | 6.6±0 | 6.1±1 | 0.650 |
| Hunt and Hess
grade | | | |
|
I, n
(%) | 2 (14.2) | 2 (14.2) | 0.733 |
|
II, n
(%) | 1 (7.1) | 3 (21.4) | 0.393 |
|
III, n
(%) | 3 (21.4) | 1 (7.1) | 0.124 |
|
IV, n
(%) | 0 (0) | 1 (7.1) | 0.369 |
|
V, n
(%) | 0 (0) | 1 (7.1) | 0.369 |
| Modified Fisher
scale | | | |
|
I, n
(%) | 1 (7.1) | 3 (21.4) | 0.393 |
|
II, n
(%) | 3 (21.4) | 4 (28.5) | NS |
|
III, n
(%) | 2 (14.2) | 0 (0) | 0.078 |
|
IV, n
(%) | 0 (0) | 1 (7.1) | 0.369 |
| Aneurysm
location | | | |
|
ACoA | 1 (7.1) | 1 (7.1) | 0.825 |
|
MCA | 2 (14.2) | 4 (28.5) | 0.533 |
|
PICA | 1 (7.1) | 1 (7.1) | 0.825 |
|
Pcom | 1 (7.1) | 1 (7.1) | 0.825 |
|
ICA | 1 (7.1) | 1 (7.1) | 0.825 |
| CT perfusion (white
matter) parameters | | | |
| CBFmean ± SD
(mlblood/100gtissue) | 10.3±8 | 19.9±10 | 0.039 |
| CBVmean ± SD
(mlblood/100gtissue) | 1.3±1 | 1.6±1 | 0.846 |
| MMTmean ±
SD(sec) | 4.7±2 | 4.2±1 | 0.897 |
| TCD parameters | | | |
| Total PSV mean ± SD
(cm/sec) | 114.4±35 | 79.3±13 | 0.039 |
| Total PI mean ± SD
(cm/sec) | 1.0±0. | 0.9±0. | 0.692 |
Diagnostics
Univariate analysis revealed that there was a
statistically significant difference in the mean values of CBF and
PSV between participants who developed adverse ischemic events and
those who did not develop adverse ischemic events (P<0.05,
Table III). The rate of adverse
ischemic events was lower with IT nimodipine management during 1
month of follow-up (6 vs. 2 events; odds ratio, 15.00; 95%
confidence interval, 1.03-218.31; P=0.047) (Tables III and IV). All the variables with a statistically
significant association with the adverse ischemic events of 1 month
(the therapy group, CBF and PSV were included in the multivariate
analysis. Multivariate analysis (Table
IV) revealed that CBF, PSV and the therapy group were
independent factors in detecting an ischemic event in 1 month
(P<0.05).
 | Table IVMultivariate analysis (outcome:
Ischemic event at 1 month). |
Table IV
Multivariate analysis (outcome:
Ischemic event at 1 month).
| | 95% CI for
Exp(B) |
|---|
| Parameter | P-value | Exp(B)/OR | Lower | Upper |
|---|
| Groups | 0.047 | 15.00 | 1.030 | 218.310 |
| CBFmean ± SD
(mlblood/100gtissue) | 0.047 | -0.456 | -0.044 | -0.001 |
| Total PSV mean ± SD
(cm/sec) | 0.023 | 0.579 | 0.002 | 0.017 |
| Group | 0.042 | 0.345 | 0.004 | 0.012 |
Discussion
The of the findings present study suggested that the
combined use of IT nimodipine with IV therapy in post-aSAH patients
who develop severe CV is a safe procedure that may prevent
permanent neurological deterioration and delay unfavorable ischemic
incidents. Until 2008, the only clinical trial evaluating the
effectiveness of the local IT administration of nimodipine was
reported by Auer et al (13).
In the present study, a 2.4x10-5 M solution of
nimodipine was applied either during the surgery directly to the
cerebral vessels or through a catheter post-operatively, with
favorable results for vasospasm (13). To date, the effectiveness of IT
applied nimodipine has been tested in other clinical trials,
demonstrating a notable improvement in cerebral perfusion as
detected using CTP and follow-up DSA in the majority of patients
(12,20-22).
However, no significant adverse effects have been observed
following the IT administration of calcium blocking agents
(nimodipine/nicardipine) or magnesium sulfate (13). Although the present study included a
small sample size, the results obtained with the IT nimodipine
administration using an EVD demonstrated a favorable outcome in
patients who developed severe CV following aSAH. Moreover, avoiding
the risks of the invasive DSA procedure, the utility of CTP and TCD
are the most common and studied imaging techniques (3,23).
According to previous studies, the use of IT
vasodilating agents is time-dependent, with better effects
associated with early drug placement (24). In the present study, the IT bolus was
repeated every 24 h during the first 7 days of the event with
positive results. Pharmacodynamics diverge between the CSF and the
systemic installation, and thus, drug efficacy may vary
considerably (25). Although an IT
drug placement has numerous benefits, such as direct access to
affected vessels, a thick clot may block the stream of drugs from
the ventricle to basal blood vessels in vasospasm (26). Moreover, no existing technique can
evaluate the effectiveness of nimodipine administered IT vs.
systemically. To the best of our knowledge, no study to date, has
demonstrated the efficacy of combined IT and IV nimodipine therapy
for CV. The present study evaluated two groups of patients
depending on IT nimodipine therapy uptake. The clinical outcomes
without ischemic damage in CTP at 1 month of follow-up were related
to those of the group treated with a combination of IV and IT
nimodipine therapy administered in Ringer's solution at a
concentration of 2 mg/100 ml. By contrast, the group treated with
only IV nimodipine administration exhibited unfavorable clinical
outcomes with ischemic damage shown in the CTP and TCD.
The present study has certain limitations. First,
the present study was a small, single-center study. Another
limitation is its retrospective design. Therefore, firm conclusions
regarding the role of IT nimodipine in managing severe vasospasm
following aSAH cannot be reached. However, the findings presented
herein may serve as a basis for more extensive clinical studies in
the future.
In conclusion, although in recent years, notable
efforts have been made to gain a better understanding of the
mechanisms of CV and the further development of therapeutic agents,
IT nimodipine administration is not yet considered a mainstream
standard of care. The findings of the present study suggest that
the combination of IT and IV nimodipine therapy may be a viable
option for treating CV following aSAH. However, the findings of the
present small study should be interpreted cautiously and serve as a
basis for a future, more extensive clinical investigations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GF, AAF and VEG conceptualized the study. KF, VEG,
AAF, PP, IT, DAS, NT, VT, NM and KT made a substantial contribution
to the analysis and interpretation of the data, and wrote and
prepared the draft of the manuscript. VEG and GF analyzed the data
and provided critical revisions. VT and GF confirm the authenticity
of all the data. All authors contributed to manuscript revision,
and have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board (IRB) of Nicosia
General Hospital, Cyprus approved the study (IRB no. EEBK EΠ
2019.02.110). The study was in line with the Declaration of
Helsinki in 1995 (as revised in Edinburgh 2000). Written informed
was obtained from all the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Treggiari-Venzi MM, Suter PM and Romand
JA: Review of medical prevention of vasospasm after aneurysmal
subarachnoid hemorrhage: A problem of neurointensive care.
Neurosurgery. 48:249–261; discussion 261-2. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weyer GW, Nolan CP and Macdonald RL:
Evidence-based cerebral vasospasm management. Neurosurg Focus.
21(E8)2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fotakopoulos G, Makris D, Kotlia P,
Kapsalaki E, Papanikolaou J, Georgiadis I, Zakynthinos E and
Fountas K: The value of computed tomography perfusion &
transcranial Doppler in early diagnosis of cerebral vasospasm in
aneurysmal & traumatic subarachnoid hemorrhage. Future Sci OA.
4(FSO313)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ko NU, Rajendran P, Kim H, Rutkowski M,
Pawlikowska L, Kwok PY, Higashida RT, Lawton MT, Smith WS, Zaroff
JG and Young WL: Endothelial nitric oxide synthase polymorphism
(-786T->C) and increased risk of angiographic vasospasm after
aneurysmal subarachnoid hemorrhage. Stroke. 39:1103–1108.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Foley WD, Smith DF, Milde MW, Lawson TL,
Towne JB and Bandyk DF: Intravenous DSA examination of patients
with suspected cerebral ischemia. Radiology. 151:651–659.
1984.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Provenzale JM, Shah K, Patel U and McCrory
DC: Systematic review of CT and MR perfusion imaging for assessment
of acute cerebrovascular disease. AJNR Am J Neuroradiol.
29:1476–1482. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Menzilcioglu MS, Mete A and Ünverdi Z:
Effectiveness of CT computed tomography perfusion in diagnostics of
acute ischemic stroke. Pol J Radiol. 80:549–554. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Parsons MW, Barber PA, Chalk J, Darby DG,
Rose S, Desmond PM, Gerraty RP, Tress BM, Wright PM, Donnan GA and
Davis SM: Diffusion- and perfusionweighted MRI response to
thrombolysis in stroke. Ann Neurol. 51:28–37. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kunze E, Pham M, Raslan F, Stetter C, Lee
JY, Solymosi L, Ernestus RI, Vince GH and Westermaier T: Value of
perfusion CT, transcranial doppler sonography, and neurological
examination to detect delayed vasospasm after aneurysmal
subarachnoid hemorrhage. Radiol Res Pract.
2012(231206)2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Westermaier T, Pham M, Stetter C, Willner
N, Solymosi L, Ernestus RI, Vince GH and Kunze E: Value of
transcranial Doppler, perfusion-CT and neurological evaluation to
forecast secondary ischemia after aneurysmal SAH. Neurocrit Care.
20:406–412. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Barker FG II and Ogilvy CS: Efficacy of
prophylactic nimodipine for delayed ischemic deficit after
subarachnoid hemorrhage: A metaanalysis. J Neurosurg. 84:405–414.
1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rinkel GJ, Feigin VL, Algra A, van den
Bergh WM, Vermeulen M and van Gijn J: Calcium antagonists for
aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev.
2005(CD000277)2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Auer LM, Ito Z, Suzuki A and Ohta H:
Prevention of symptomatic vasospasm by topically applied
nimodipine. Acta Neurochir (Wien). 63:297–302. 1982.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gioia AE, White RP, Bakhtian B and
Robertson JT: Evaluation of the efficacy of intrathecal nimodipine
in canine models of chronic cerebral vasospasm. J Neurosurg.
62:721–728. 1985.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lewis PJ, Weir BK, Nosko MG, Tanabe T and
Grace MG: Intrathecal nimodipine therapy in a primate model of
chronic cerebral vasospasm. Neurosurgery. 22:492–500.
1988.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Voldby B, Petersen OF, Buhl M, Jakobsen P
and Ostergaard R: Reversal of cerebral arterial spasm by
intrathecal administration of a calcium antagonist (nimodipine).
Acta Neurochir (Wien). 70:243–254. 1984.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meier P and Zierler KL: On the theory of
the indicator-dilution method for measurement of blood flow and
volume. J Appl Physiol. 6:731–744. 1954.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Konstas AA, Goldmakher GV, Lee TY and Lev
MH: Theoretic basis and technical implementations of CT perfusion
in acute ischemic stroke, part 1: Theoretic basis. AJNR Am J
Neuroradiol. 30:662–668. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lysakowski C, Walder B, Costanza MC and
Tramèr MR: Transcranial Doppler versus angiography in patients with
vasospasm due to a ruptured cerebral aneurysm: A systematic review.
Stroke. 32:2292–2298. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang YP, Shields LB, Yao TL, Dashti SR
and Shields CB: Intrathecal treatment of cerebral vasospasm. J
Stroke Cerebrovasc Dis. 22:1201–1211. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hänggi D, Beseoglu K, Turowski B and
Steiger HJ: Feasibility and safety of intrathecal nimodipine on
posthaemorrhagic cerebral vasospasm refractory to medical and
endovascular therapy. Clin Neurol Neurosurg. 110:784–790.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Velat GJ, Kimball MM, Mocco JD and Hoh BL:
Vasospasm after aneurysmal subarachnoid hemorrhage: Review of
randomized controlled trials and meta-analyses in the literature.
World Neurosurg. 76:446–454. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cremers CH, van der Schaaf IC, Wensink E,
Greving JP, Rinkel GJ, Velthuis BK and Vergouwen MD: CT perfusion
and delayed cerebral ischemia in aneurysmal subarachnoid
hemorrhage: A systematic review and meta-analysis. J Cereb Blood
Flow Metab. 34:200–207. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Thomas JE and McGinnis G: Safety of
intraventricular sodium nitroprusside and thiosulfate for the
treatment of cerebral vasospasm in the intensive care unit setting.
Stroke. 33:486–492. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Patel MM, Goyal BR, Bhadada SV, Bhatt JS
and Amin AF: Getting into the brain: Approaches to enhance brain
drug delivery. CNS Drugs. 23:35–58. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shibuya M, Suzuki Y, Enomoto H, Okada T,
Ogura K and Sugita K: Effects of prophylactic intrathecal
administrations of nicardipine on vasospasm in patients with severe
aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien).
131:19–25. 1994.PubMed/NCBI View Article : Google Scholar
|