Introduction
De Quervain's tenosynovitis (DQT) is a painful
stenosing tenosynovitis of the first dorsal compartment of the
wrist that contains tendons of the abductor pollicis longus (APL)
and extensor pollicis brevis (EPB). The disease limits wrist
movement and is also known as de Quervain's disease, de Quervain's
syndrome and de Quervain's tendinopathy (1,2). It is
considered one of the most frequent types of wrist tendonitis in
athletes, and it is also more prevalent among women between the
ages of 30 and 50 years (3).
Although the exact cause of DQT remains unclear, overuse or
repetitive activity involving the wrist is one of the common causes
(4,5).
Non-surgical conservative therapy is considered a
first-line treatment for DQT. It includes decreased activity and
physiotherapy to reduce pain and inflammation, splinting to reduce
tendon friction, the use of non-steroidal anti-inflammatory drugs
(NSAIDs), and the injection of corticosteroids (6). The majority of cases (83%) recover
following a single corticosteroid injection (7). In the case that conservative therapy
fails, which is often due to an inaccurate injection and anatomical
variations in the first dorsal compartment, a surgical approach
through decompression is considered (8).
Platelet-rich plasma (PRP) therapy is the injection
of a patient's own platelet-concentrated plasma that contains
growth factors and possesses regenerative characteristics that
stimulate tissue healing (9).
Ultrasound (US) guidance allows for the accurate injection of PRP
(10). Previous studies have
demonstrated the efficacy of PRP in the management of other
tendinopathies (11). Currently, PRP
injection therapy is used as alternative management in patients
with DQT who have failed to respond to other conservative treatment
strategies (12). However, there are
insufficient studies regarding its efficacy, with or without US
guidance. The present study aimed to evaluate the efficacy of the
use of US-guided PRP injection in the management of DQT.
Patients and methods
Registration
The current study was registered as per the
Declaration of Helsinki - ‘Every research study involving human
subjects must be registered in a publicly accessible database
before recruitment of the first subject’ (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/).
The study was recorded at Research Registry, with a registration
number of: researchregistry8593.
Setting and study design
The present study was a prospective interventional
study that included 12 patients with DQT. It was conducted over a
period of 13 months, from January, 2020 until February, 2021 at the
Sulaimani Teaching Hospital and Shar Teaching Hospital (Sulaimani,
Iraq). Ethics committee approval was obtained from the Ethics
Committee of the University of Sulaimani. Verbal and signed written
consents were acquired from all the patients for US-guided PRP
injection and for the use of their data.
Inclusion and exclusion criteria
The inclusion criteria included patients with DQT
who failed to respond to conservative treatments. Patients who were
had a history of rheumatoid arthritis, trauma or fractures in the
hands or the wrist joints, shoulders or elbow problems, or those
who had received previous corticosteroid injection therapy for DQT
within the last 6 weeks were excluded from the study.
Pre-treatment assessment
A short history and demographic information were
collected from the patients, and they were given a 10-point visual
analog scale (VAS) score to assess pain intensity and ability to
perform daily tasks. All the patients were diagnosed clinically
using the Finkelstein test. A US examination was used to confirm
the diagnosis of DQT. In addition, the examination of the opposite
hand was also performed for comparison. A B-mode US examination
with a sufficient amount of gel was performed in both the
transverse and sagittal planes to allow for the proper evaluation
and visualization of anatomical structures, followed by a color
Doppler US mode to detect peri-tendinous hyperemia. The B-mode gain
was decreased and the color gain was increased at a threshold just
below aliasing to optimize the visualization of low-velocity flow.
Complete data on the baseline sonographic findings were collected,
including the thickness of the extensor retinaculum, tendon sheath
effusion, paratendinous hyperemia and anatomical variation.
Procedure
For the preparation of the PRP, 10 ml of blood were
drawn from each patient and placed in a Hightop PRP tube (Lora).
The blood was centrifuged at 1,792 x g for 10 min at a temperature
of 24˚C. Finally, 2 ml PRP were obtained from each blood sample,
which was ready for injection. PRP injections were performed under
local anesthesia using an aseptic technique with the patient in a
sitting position, with the hand resting on a pillow and slight
ulnar deviation of the wrist. Under the US guide, 1 ml of the
anesthetic agent (lidocaine) was diffused subcutaneously. After
5-10 min, 2 ml PRP were injected into the affected area under US
guidance. The injection was made by inserting a 22-gauge needle at
a 45˚ angle to the transducer into the tendon sheath, followed by
needle tenotomy of the tendons to induce intra-tendinous micro
tear, promoting faster healing.
In the case of sub-compartmentalization, half of the
PRP (1 ml) was injected into each compartment. To ensure this, once
the first compartment was injected, either the septum between the
sheaths was pierced with the needle, or the needle was drawn back
and the remaining half was injected around the other tendon. The
injection area was then cleaned and a plaster was applied.
Each patient was monitored for 10 min after the
injection, then discharged from the department. Patients were
recommended to avoid straining and repetitive movements of the
treated wrist for at least 7 days and to wear a wrist splint for
2-3 days. They were also advised to use an ice pack or paracetamol
as a painkiller when necessary and to avoid the use of other
NSAIDs.
Patient follow-up
All patients were followed-up at 1 week after the
injection and were examined for any complications at the injection
site, including the presence of infection, loss of function and
tendon stiffness or rupture. In addition, the patients were
scheduled to visit after 1 and 3 months to determine the pain
severity level based on the VAS score, and to evaluate the efficacy
and durability of the treatment using a US examination. None of the
patients received any other treatment for DQT during the follow-up
period.
Data collection and analysis
Microsoft excel 2019 was used to register the data.
The Statistical Package for the Social Sciences (SPSS)
program-version (25) (IBM Corp.)
was used to code and conduct data analysis. The outcomes of the
procedure were analyzed using one-way ANOVA test with Tukey's post
hoc test being performed when significant results were observed (as
the periodic groups had the same sample size). The results are
presented as the mean ± standard deviation. Qualitative data are
presented as frequencies and percentages, and McNemar's test was
used to make comparisons (as data for the same variable were
obtained from the same individual in different time periods). A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Demographic and baseline
characteristics
A total of 12 hands of 12 female patients with DQT
were examined in the present study. All the patients were
housewives with an average age of 43 years, ranging from 28 to 68
years. Amongst the affected hands, 8 (66.6%) were dominant, and 4
(33.3%) were non-dominant, as presented in Table I.
 | Table IDemographics and history of the
patients with DQT in the present study. |
Table I
Demographics and history of the
patients with DQT in the present study.
| Patient no. | Age, years | Duration of
symptoms | Affected hand | Previous
treatment |
|---|
| 1 | 28 | 12 months | Dominant | Rest, NSAID, and
corticosteroid injection |
| 2 | 65 | 2 months | Dominant | Rest, NSAID |
| 3 | 35 | 2 months | Non-dominant | Rest, NSAID |
| 4 | 45 | 3 months | Dominant | Rest, NSAID |
| 5 | 30 | 4 months | Dominant | Rest, NSAID |
| 6 | 68 | 4 months | Non-dominant | Rest, NSAID, and
corticosteroid injection |
| 7 | 30 | 2 months | Dominant | Rest, NSAID |
| 8 | 53 | 3 months | Dominant | Rest, NSAID |
| 9 | 43 | 4 months | Non-dominant | Rest, NSAID |
| 10 | 26 | 2 months | Dominant | Rest, NSAID |
| 11 | 60 | 6 months | Dominant | Rest, NSAID, and
corticosteroid injection |
| 12 | 33 | 2 months | Non-dominant | Rest, NSAID |
Clinical assessment
Upon a clinical examination, all the patients
presented with tenderness over the radial styloid process, 4
patients had swelling, and the results of the Finkelstein's test
were positive for all the cases. The patients had an average VAS
score of 8.66 prior to treatment, and post-treatment, the score
decreased to 4.5 and 1.91 (P<0.001) at the 1- and 3-month
follow-up periods, respectively. The VAS scores of the patients
before and after treatment are presented in Table II.
 | Table IIVAS scores of patients for pain
intensity. |
Table II
VAS scores of patients for pain
intensity.
| Patient no. | Pre-treatment VAS
score | VAS score at
1-month follow-up | VAS score at
3-month follow-up |
P-valuea |
|---|
| 1 | 9 | 1 | 0 | <0.001 |
| 2 | 9 | 8 | 8 | |
| 3 | 9 | 5 | 0 | |
| 4 | 9 | 4 | 1 | |
| 5 | 9 | 5 | 2 | |
| 6 | 8 | 3 | 0 | |
| 7 | 9 | 7 | 7 | |
| 8 | 7 | 2 | 0 | |
| 9 | 9 | 6 | 1 | |
| 10 | 8 | 5 | 2 | |
| 11 | 9 | 4 | 1 | |
| 12 | 9 | 4 | 1 | |
| Mean | 8.66±0.65 | 4.5±1.97 | 1.91±2.71 | |
No procedure-related complications occurred during
the injection; however, 2 patients had mild vasovagal signs after
the procedure, which may be due to side-effects of lidocaine or
pain at the time of the injection. Amongst the patients, complete
recovery was observed in 4 patients (33.3%), 6 patients (50%) had
recovered to a degree where they returned to their daily activities
with minimal pain, and no significant improvement was observed in 2
patients (16.6%).
Sonographic evaluation
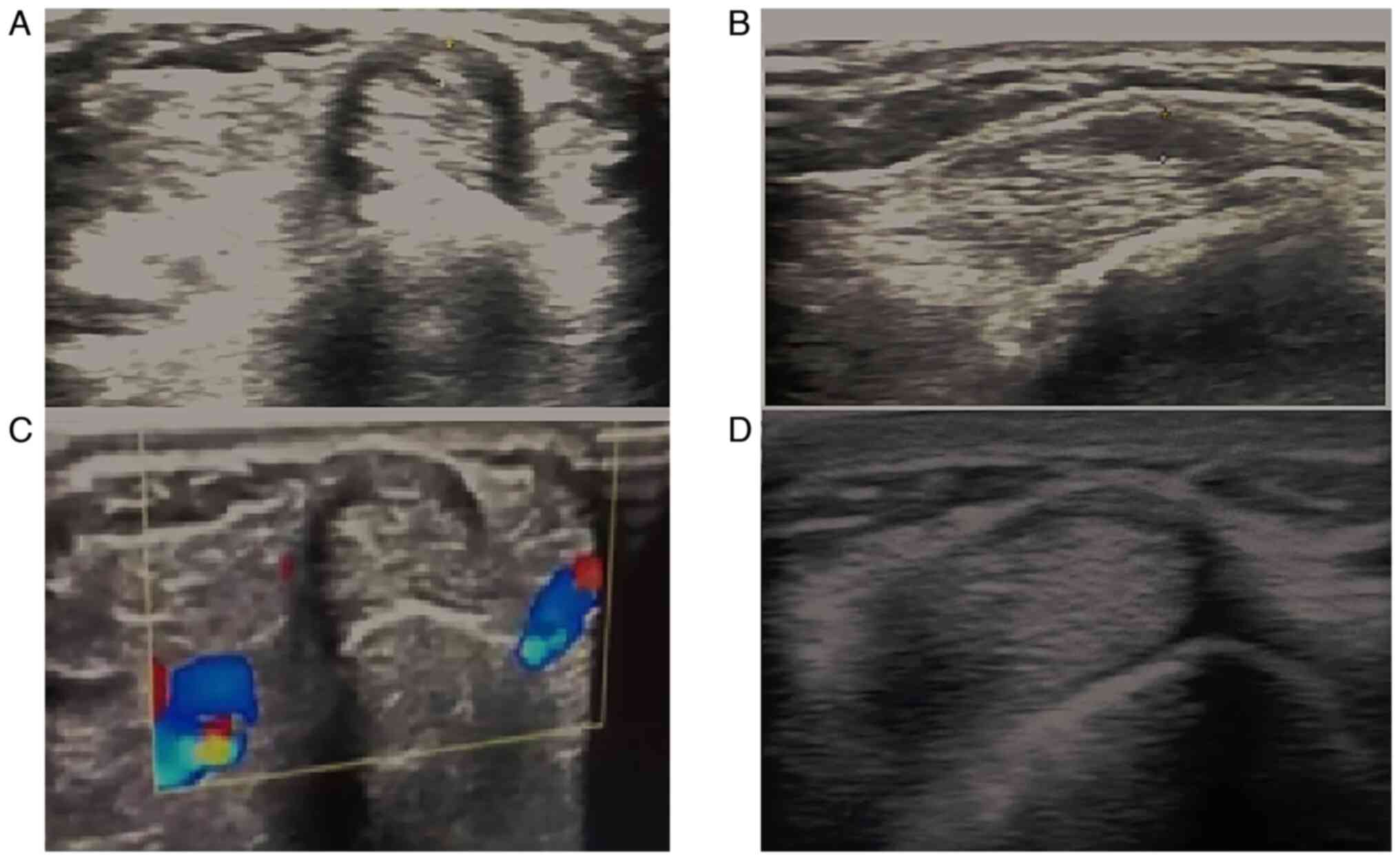
Baseline sonographic findings (as presented in
Table III) revealed a thickened
retinaculum (1.89±0.5; ranging from 1.3-3 mm) and tendon sheath
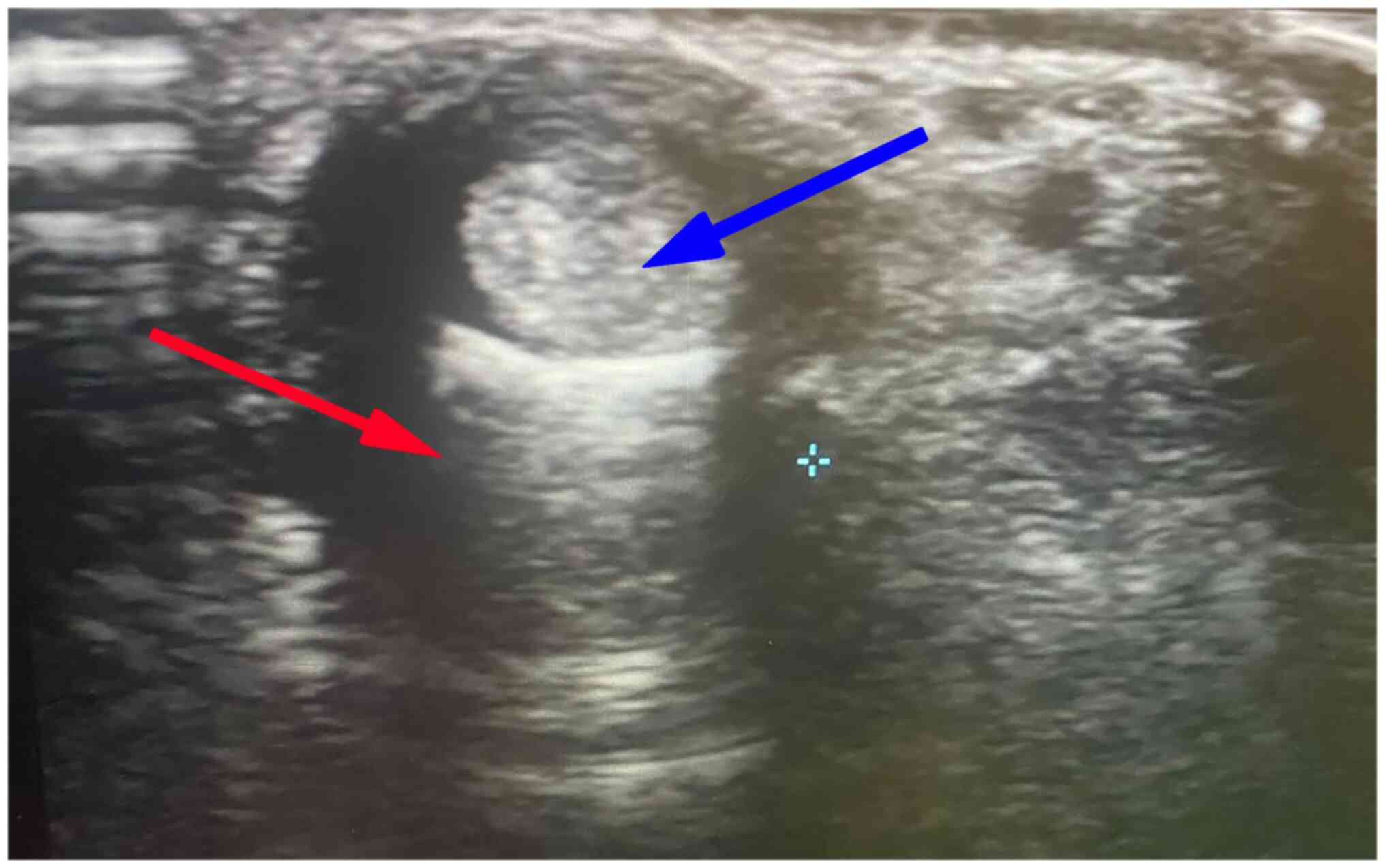
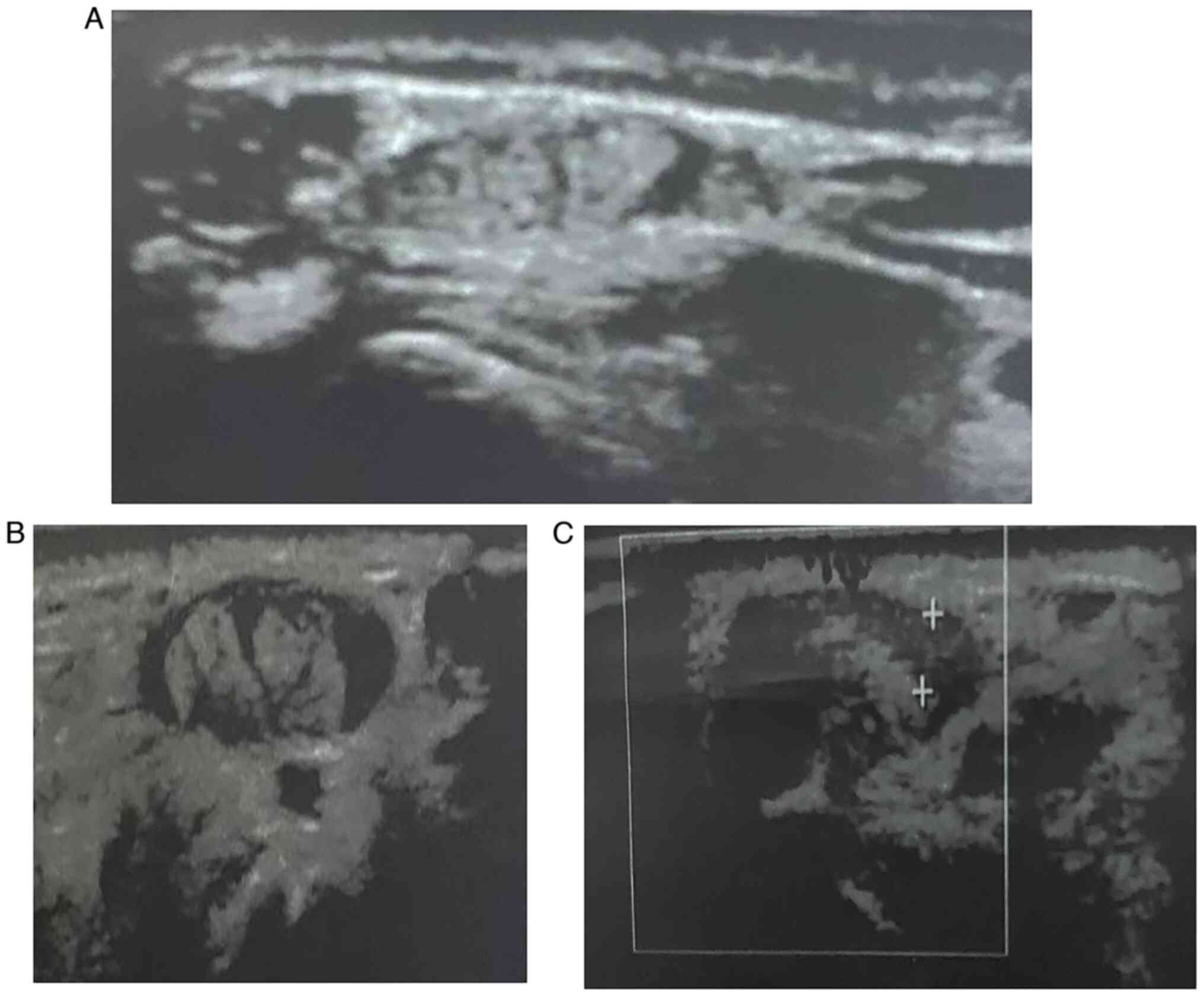
effusion (2.07±0.52) in all patients (illustrated in Figs. 1 and 2). As regards anatomical variations, 5
patients (41.7%) had septum between APL and EPB, and 4 patients
(33.3%) had accessory tendon slips (example illustrated in Fig. 1). However, post-PRP injection, a US
examination at the 1- and 3-month follow-up periods revealed a
significant improvement in the patients. The thickness of the
extensor retinaculum had progressively decreased, from a mean of
1.89 mm pre-injection to a mean of 1.3 mm and 0.96 mm at the 1- and
3-month follow-up, respectively (P<0.001). The tendon sheath
effusion observed in all the patients had a mean thickness of 2.07
mm pre-injection. At the 1-month follow-up, effusion was observed
in 11 cases (91%) with a mean thickness of 1.6 mm, and at the
3-month follow-up, only 7 of the cases had effusion (58%) with a
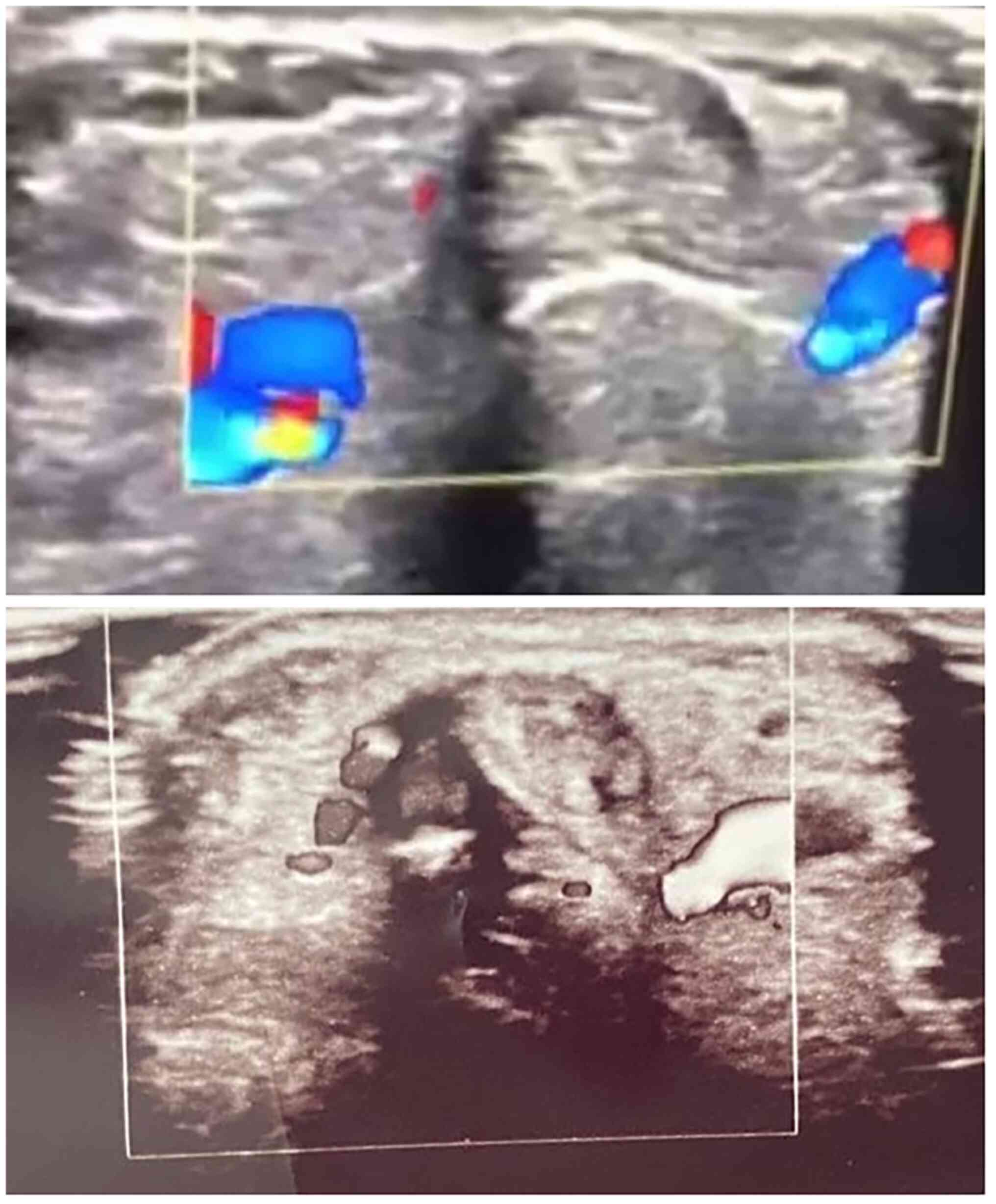
mean thickness of 0.73 mm (P<0.001). Peri-tendinous hyperemia
was initially observed in 7 patients (58.33%), and after the PRP
injection this was only observed in 2 patients (16.7%) at the
1-month follow-up (P<0.063) and in no patients (0%) (P<0.001)
at the 3-month follow-up (Table
III; examples illustrated in Fig.
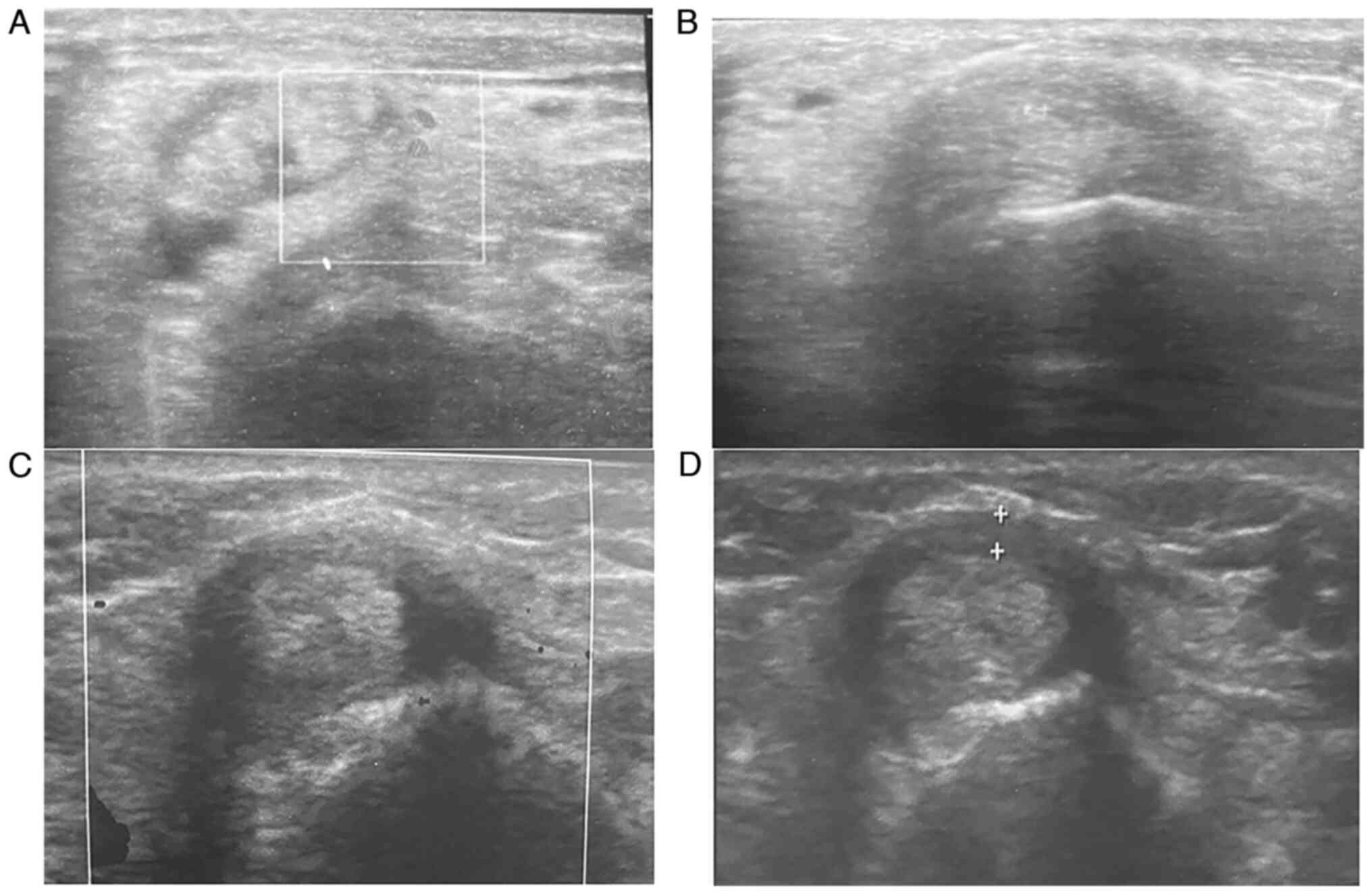
3). Sonographic improvements observed in two different patients
are illustrated in Figs. 4 and
5.
 | Table IIIUltrasound findings at baseline and
at the 1- and 3-month follow-up periods. |
Table III
Ultrasound findings at baseline and
at the 1- and 3-month follow-up periods.
| | Tendon sheath
effusion (mm) | Retinaculum
thickness (mm) | Peri-tendinous
hyperemia |
|---|
| Patient no. | Septum between EPB
and APL | Multiple tendon
slips | Baseline | 1-Month
follow-up | 3-Month
follow-up | P-value | Baseline | 1-Month
follow-up | 3-Month
follow-up | P-value | Baseline | 1-Month
follow-up | P-value | 3-Month
follow-up | P-value |
|---|
| 1 | Yes | - | 1.8 | 1 | 0.5 | <0.001 | 1.6 | 1.4 | 0.9 | <0.001 | - | - | 0.063 | - | <0.001 |
| 2 | - | - | 2 | 2 | 1.8 | | 2.4 | 2.4 | 2.4 | | Yes | Yes | | - | |
| 3 | - | Yes | 1.4 | 1.2 | 0 | | 1.8 | 0.9 | 0.5 | | - | - | | - | |
| 4 | Yes | - | 2.3 | 2 | 1 | | 2.5 | 1.8 | 1.2 | | - | - | | - | |
| 5 | - | - | 2 | 1.6 | 0 | | 1.9 | 1.6 | 1 | | Yes | - | | - | |
| 6 | Yes | - | 3 | 3 | 2 | | 1.6 | 1.4 | 1 | | Yes | - | | - | |
| 7 | - | - | 2.3 | 2 | 1 | | 1.8 | 1.6 | 1 | | Yes | - | | - | |
| 8 | Yes | Yes | 1.5 | 1.5 | 1.5 | | 1.9 | 1 | 1 | | Yes | Yes | | - | |
| 9 | - | Yes | 2 | 1.8 | 0 | | 1.5 | 0.9 | 0.9 | | - | - | | - | |
| 10 | - | - | 1.5 | 1 | 0 | | 1.3 | 1 | 0.6 | | Yes | - | | - | |
| 11 | Yes | Yes | 3 | 2.1 | 1 | | 3 | 1.6 | 1 | | Yes | - | | - | |
| 12 | - | - | 2 | 0 | 0 | | 1.4 | 0 | 0 | | - | - | | - | |
| Overall | 5/12 (41.7%) | 4/12 (33.3%) | 2.07±0.52 | 1.6±0.75 | 0.73±0.76 | | 1.89±0.5 | 1.3±0.6 | 0.96±0.56 | | 7/12 (58.3%) | 2/12 (16.7%) | | 0/12 (0%) | |
Discussion
DQT is a common disorder that was first mentioned in
Gray's Anatomy in 1893 as washerwoman's sprain. The condition was
named after the Swiss surgeon, Fritz de Quervain, after he reported
5 cases of first compartment tenosynovitis in 1895(13). It occurs in 1.3 and 0.5% of working
women and men, respectively (14).
DQT affects the APL and EPB tendons in the first dorsal compartment
of the wrist, which become inflamed and injured as a result of
repetitive wrist movements, resulting in pain and reduction in the
wrist's range of motion. Its symptoms can be elicited by
Finkelstein's test (15,16). DQT may also occur as a consequence of
certain wrist fractures, dislocations of the wrist, or in the
setting of systemic diseases such as rheumatoid arthritis (17,18).
Although DQT mainly affects the dominant hand, the
involvement of the non-dominant hand has been stated in previous
research (19). In their study,
Lutsky et al (20) reported
an equal involvement of dominant and non-dominant hands in DQT
cases. In the present study, the dominant hand was involved more
frequently (66.6%).
Usually, the APL and EPB tendons are in a single
compartment; however, certain anatomic variations may be risk
factors for the disease, such as the presence of a fibrous septum
and multiple tendon slips (21).
These anatomic variations may play a role in the development of DQT
by resulting in overcrowding and increased tendon friction
(22). In addition, early
motherhood, pregnancy and the post-menopausal status are considered
predisposing factors in women (14).
Chiavaras et al (23)
reported the presence of an inter-compartment septum in the first
extensor compartment in 47% of cadaveric wrists; moreover, this
prevalence is greater (59%) in the wrists of patients with DQT. In
the present study, out of the 12 patients examined, 5 patients
(41.7%) had septum between APL and EPB, and 4 patients (33.3%) had
accessory tendon slips; this is slightly lower than what has been
previously mentioned by Chiavaras et al (23).
Generally, the inflammation and pain caused by DQT
can be reduced using a wrist splint to limit wrist movement, and
oral analgesics, such as NSAIDs. The injection of steroids into the
first dorsal compartment of the wrist is considered as the next
line of treatment prior to surgery (24). Furthermore, US-guided PRP injection
has emerged as a new non-operative treatment alternative for DQT
(10).
The visualization of compartmental anatomy and
needle placement with US-guided injection enhances the injection
accuracy and clinical outcomes (12). US-guided PRP injection prevents
intra-tendinous injection, diminishes the risk of subsequent tear,
precisely introduces the injectate into the affected region in
cases of sub-compartmentalization, and prevents injection-related
complications, such as superficial radial nerve injury (25,26).
Peck and Ely (12) used US-guided
percutaneous tenotomy and PRP injection in their study to
successfully treat a case of DQT, with no reported complications.
Moreover, Güleç et al (27)
used anatomical landmarks for the percutaneous release of the first
dorsal compartment and reported several complications, including a
39.6% laceration rate. In the present study, the majority of the
cases (83.3%) experienced symptomatic improvement, and no
procedure-related complications occurred; however, 2 patients had
mild vasovagal signs after the procedure.
Previously, a cohort study by Deb et al
(28) revealed a good clinical
outcome of PRP injection in the treatment of DQT without US
guidance; however, they were only able to decrease the VAS scores
of patients from 8.98±0.57 to 4.91±1.01 and 3.96±1.94 at the 1- and
6-month follow-up periods, respectively. In addition, Deb et
al (28) used a blind approach
with a 4-ml PRP injection. In the present study, an improved
clinical outcome was achieved with the use of US guidance and half
the amount of PRP (2 ml); the mean VAS scores of the patients
decreased from 8.66 to 4.5 and 1.91 at 1 and 3 months
post-treatment. Another study by Sobhia et al (29) attempted to determine the efficacy of
PRP injection in comparison to steroid injection and revealed a
significant improvement in the pathological manifestations of DQT,
such as peri-tendinous hyperemia, thickening of the retinaculum,
and tendon sheath effusion. These improvements were also achieved
in the present study.
Despite the advantages of the present study, it
still has multiple limitations, including a small sample size and
the lack of long-term follow-up. In addition, the levels of
inflammatory markers were not determined, and the lack of a control
group without US guidance is also a limitation. Thus, further
studies are required in the future to validate the current
findings.
In conclusion, US plays a critical role in the
treatment of DQT, as improved clinical outcomes can be obtained
with US-guided injections, particularly in cases with
sub-compartmentalization. Hence, a US-guided PRP injection with
needle tenotomy can be used as an alternative non-surgical therapy
for patients who do not respond to conventional conservative
treatments. In order to better understand the efficacy of this
technique in refractory DQT, further more dedicated and controlled
research trials are required.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AMS was a major contributor to the conception of the
study. KKM, KMS and SKA were involved in the literature review, the
design of the study, in the revision of the manuscript and in the
processing of the figures. KAM and SOA are the radiologists who
performed the assessments of the patients. FHK and BAA were
involved in the literature review, in the writing of the
manuscript, and in data analysis and interpretation. SHM and RQS
were involved in designing the study. SHM and RQS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the University of Sulaimani (Sulaimani, Iraq; no. 2019:33). Written
informed consent was obtained from all the patients and/or the
families of the patients.
Patient consent for publication
Patient consent was obtained regarding the
publication of their data and any related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dehghan M and Salehitali SH: Comparing the
efficacy of local injection of methylprednisolone and lidocaine
with and without splint, and with splinting alone in treating
patients with De Quervain's tenosynovitis. JQUMS. 16:4–9. 2012.
|
|
2
|
Hadianfard M, Ashraf A, Fakheri M and
Nasiri A: Efficacy of acupuncture versus local methylprednisolone
acetate injection in De Quervain's tenosynovitis: A randomized
controlled trial. J Acupunct Meridian Stud. 7:115–121.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Avci S, Yilmaz C and Sayli U: Comparison
of nonsurgical treatment measures for de Quervain's disease of
pregnancy and lactation. J Hand Surg Am. 27:322–324.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Memon R and Patel N: Outcomes of
Intrasheath steroid injection for treatment of De Quervains
Tenosynovitis. National J Integrated Res Med. 10:58–60. 2019.
|
|
5
|
Novikov AV, Shchedrina MA and Petrov SV:
De Quervain's disease (etiology, pathogenesis, diagnosis and
treatment). Part II. NN Priorov J Traumatology Orthopedics.
26:55–68. 2019.
|
|
6
|
Allam AE, Al-Ashkar DS, Negm AA, Eltawab
BA, Wu WT and Chang KV: Ultrasound-guided methotrexate injection
for De Quervain disease of the wrist: What lies beyond the horizon?
J Pain Res. 10:2299–2302. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Richie CA III and Briner WW Jr:
Corticosteroid injection for treatment of de Quervain's
tenosynovitis: A pooled quantitative literature evaluation. J Am
Board Fam Pract. 16:102–106. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mirzanli C, Ozturk K, Esenyel CZ, Ayanoglu
S, Imren Y and Aliustaoglu S: Accuracy of intrasheath injection
techniques for de Quervain's disease: A cadaveric study. J Hand
Surg Eur Vol. 37:155–160. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fortier LA, Mohammed HO, Lust G and Nixon
AJ: Insulin-like growth factor-I enhances cell-based repair of
articular cartilage. J Bone Joint Surg Br. 84:276–288.
2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McDermott JD, Ilyas AM, Nazarian LN and
Leinberry CF: Ultrasound-guided injections for De Quervain's
tenosynovitis. Clin Orthop Relat Res. 470:1925–1931.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou Y and Wang JH: PRP treatment efficacy
for tendinopathy: A review of basic science studies. BioMed Res
Int. 2016(9103792)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Peck E and Ely E: Successful treatment of
de Quervain tenosynovitis with ultrasound-guided percutaneous
needle tenotomy and platelet-rich plasma injection: A case
presentation. PM R. 5:438–441. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Quervain F: On a form of chronic
tendovaginitis by Dr. Fritz de Quervain in la Chaux-de-Fonds. 1895.
Am J Orthop (Belle Mead NJ). 26:641–644. 1997.PubMed/NCBI
|
|
14
|
De Maeseneer M, Marcelis S, Jager T,
Girard C, Gest T and Jamadar D: Spectrum of normal and pathologic
findings in the region of the first extensor compartment of the
wrist: Sonographic findings and correlations with dissections. J
Ultrasound Med. 28:779–786. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Danda RS, Kamath J, Jayasheelan N and
Kumar P: Role of guided ultrasound in the treatment of De Quervain
tenosynovitis by local steroid infiltration. J Hand Microsurg.
8:34–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rowland P, Phelan N, Gardiner S, Linton KN
and Galvin R: The effectiveness of corticosteroid injection for de
Quervain's stenosing tenosynovitis (DQST): a systematic review and
meta-analysis. Open Orthop J. 9:437–444. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zingas C, Failla JM and Van Holsbeeck M:
Injection accuracy and clinical relief of de Quervain's tendinitis.
J Hand Surg. 23:89–96. 1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Harvey FJ, Harvey PM and Horsley MW: De
Quervain's disease: Surgical or nonsurgical treatment. J Hand Surg.
15:83–87. 1990.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kuo YL, Hsu CC, Kuo LC, Wu PT, Shao CJ, Wu
KC, Wu TT and Jou IM: Inflammation is present in de Quervain
disease-correlation study between biochemical and histopathological
evaluation. Ann Plast Surg. 74:S146–S151. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lutsky K, Kim N, Medina J, Maltenfort M
and Beredjiklian PK: Hand dominance and common hand conditions.
Orthopedics. 39:e444–e448. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rousset P, Vuillemin-Bodaghi V, Laredo JD
and Parlier-Cuau C: Anatomic variations in the first extensor
compartment of the wrist: Accuracy of US. Radiology. 257:427–433.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Choi SJ, Ahn JH, Lee YJ, Ryu DS, Lee JH,
Jung SM, Park MS and Lee KW: de Quervain disease: US identification
of anatomic variations in the first extensor compartment with an
emphasis on subcompartmentalization. Radiology. 260:480–486.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chiavaras MM, Jacobson JA, Yablon CM,
Brigido MK and Girish G: Pitfalls in wrist and hand ultrasound. AJR
Am J Roentgenol. 203:531–540. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
McKenzie JM: Conservative treatment of de
Quervain's disease. Br Med J. 4:659–660. 1972.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Adams JE and Habbu R: Tendinopathies of
the hand and wrist. J Am Acad Orthop Surg. 23:741–750.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cheong IY, Rhyu IJ, Kim KH, Chung PW, Kim
D, Park BK and Kim DH: Anatomical basis for injection around first
dorsal compartment of the wrist: A fresh cadaveric study. Pain
Physician. 19:E893–E900. 2016.PubMed/NCBI
|
|
27
|
Güleç A, Türkmen F, Toker S and Acar MA:
Percutaneous release of the first dorsal extensor compartment: A
cadaver study. Plast Reconstr Surg Glob Open. 4:1–6.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Deb D, Singh YN, Singh NB and Das R: A
study to compare the efficacy, feasibility and durability of
conservative and physical therapy, corticosteroid therapy and
platelet rich plasma therapy in patients suffering from de
Quervain's tenosynovitis: A prospective cohort study. Int J Med Sci
Diagnosis Res. 4:6–10. 2020.
|
|
29
|
Sobhia AM, Eman A and Abd El-Rahim M: The
role of platelet rich plasma in comparison with corticosteroids in
the treatment of De Quervain Tenosynovitis. Med J Cairo Univ.
88:141–148. 2020.
|