1. Introduction
Hearing loss of variable etiology represents one of
the most challenging public health concerns affecting the worldwide
population (1). The integrity of the
auditory system is one of the prerequisites for the acquisition and
proper development of oral language. An individual suffering from
hypoacusis is more likely to have poorer professional results than
his colleagues, will be less competitive in the labor market and
will have less chances to complete a higher education (2,3).
Etiologically, hearing loss is defined as having two main
mechanisms: Conductive hearing loss (CHL) following the pathology
of the external and middle ear with air-conduction impairment and
sensorineural hearing loss following inner ear pathology with
bone-conduction impairment. When both mechanisms are involved,
mixed hearing loss is diagnosed. The middle ear represents the
anatomic space between the external auditory canal (EAC) and the
inner ear (cochlea). It is comprised of the tympanic membrane (TM),
ossicular chain [malleus (hammer), incus (anvil) and stapes
(stirrup)] with the corresponding muscles and ligaments and the
cavity of the middle ear. The mastoid process and the Eustachian
tube are accessories to the middle ear and play critical roles in
aeration and pressure equalizing. The ossicular chain contains the
smallest bones in the human body (4,5). Their
highly specialized joints allow for soundwave transmission to the
inner ear, but also for the augmentation of sound wave amplitude
(matching the impedance the sound waves suffer, hence maintaining
the intensity). The malleus resembles a club rather than a hammer
and consists of three sections: The handle, neck and head. The
handle is firmly attached to the TM from the umbo to the upper
margin (tympanic annulus). The head of the malleus and the body of
the incus form a tight joint and are suspended by three ligaments,
which leave the chain free to vibrate and transmit sound from the
TM to the inner ear (6,7).
The Incus has the appearance of a premolar tooth
with uneven roots than an anvil. The short process of the incus
(the crus) is also fixed by a ligament to the posterior wall of the
cavity, whilst the long process of the incus is bent near its end
and bears a small bony knob that forms a loose ligament-enclosed
joint with the head of the stapes. The stapes is the smallest bone
in the body and barely weighs 3 mg. It consists of a base
(footplate), resting on the oval window, as well as a head that
articulates with the incus and is positioned at a 90˚ angle to the
long process of the incus. The footplate has a diameter of
2.96±0.15 mm. and aligns well in the oval window. It is surrounded
by an elastic annular ligament, which allows for the free vibration
and transmission of sound to the cochlea. The incudomallear joint,
is a gliding type of synovial joint, whilst the incudostapedial
joint is a ball- and socket-type of synovial joint. Contrary to
other synovial joints, the incudostapedial joint has a very limited
range of motion and is usually only of springy character. The
ossicles form a unit that moves together (8,9). CHL is
the result of afflicting the transmission of sound waves from the
external ear to the inner ear liquids which, in turn, displaces the
basilar membrane of the organ of Corti (8). The causes for this are multiple and can
reside with EAC obstruction, as well as, more frequently, with TM
or middle ear disease.
The term tympanoplasty describes the procedures that
re-establishes the continuity of the ossicular chain [usually
concomitant ossicular chain reconstruction (OCR) and TM grafting].
This procedure involves the use of various types of ossicular
prosthesis to replace damaged ossicles, in the attempt of providing
the patient with more functional results and a higher level of
social integration (3).
Since, for example, the stapes is the smallest bone
in the human body, these prostheses are, in turn, very small in
size (2-3 mm.) and can only be implanted and properly positioned
via microscope guidance. Extensive research involving doctors,
engineers, chemists and material resistance specialists has been
performed in order to obtain the ideal material for use in OCR. The
most important landmarks of this research are described in the
present review. However, this goal has not yet been reached,
although the theoretical conclusions are that such a material
should maintain its shape, rigidity and acoustic properties, and
should also be cost-effective and readily available.
Biocompatibility, safety, the ease of insertion and shaping should
also be amongst the properties of an OCR prosthesis (9). An ideal ossicular prosthesis should
concomitantly fulfill all following conditions: An optimal similar
shape to the replaced ossicle, small size, bio-integrated material,
lightweight, cost-effectiveness and ease in modifying to the
required shape. One cannot define an ideal prosthesis without
considering its acoustic properties. A prosthesis is well-designed
if it combines high stiffness and a low mass (10-13).
Another parameter to consider in evaluating the functional results
of OCR is the appearance of uncontrollable situations, since even
optimally positioned prothesis can migrate post-operatively and
dislocate from the adjacent structures (malleus handle, TM or
stapes). Thus, the human factor becomes paramount, as the surgeon
should achieve optimal functional results by placing the prosthesis
properly, which is also dependent on experience, anatomy and a
number of other uncontrollable factors (14,15).
From a practical and experimental point of view, the most useful
parameter to measure in order to obtain the quality control of a
prosthesis is the middle ear transfer function which, ideally, is
determined by directly measuring stapes velocity in response to a
constant sound pressure at the TM using a trans-mastoid or
trans-tegmen approach (14).
The two main types of design for ossicular
prosthesis are the following: Partial ossicular reconstruction
prosthesis (PORP) used to replace an interrupted ossicular chain
with intact stapes superstructure and total ossicular
reconstruction prosthesis (TORP), required when the stapes
superstructure is absent, and the footplate is mobile.
2. Data collection
The present review aimed to present, in a
chronological sequence, the evolution of knowledge regarding the
field of otology (OCR), as well as the advantages and disadvantages
of different materials and designs of ossicular prostheses. The
constant search for more effective, easily tolerated and lighter
materials has improved the acoustic rehabilitation process and has
markedly reduced the rate of functional failure. An extensive
review of the literature was accomplished by collecting data from
various scientific databases, such as PubMed, Global Health
Archive, BIOSIS Previews and MEDLINE.
3. History and evolution of ossicular chain
reconstruction
From the very first documented medical records
(Egyptian healers), ear discharge was associated with severe
complications (16) and even
Hippocrates observed that acute pain of the ear with continued high
fever was a serious condition, due to the risk of the patient
becoming delirious and even dying (17). The first attempts of ear surgery were
documented in 1640 when Banzer attempted a TM reconstruction using
a pig's bladder. Jasser was a pioneer of mastoid surgery in 1776;
however, after an unfortunate incident in which the personal
physician of the King of Denmark died of sepsis following this type
of surgery, ear operations were discredited and went into oblivion
for at least another hundred years (18,19). In
1853, Toynbee rediscovered mastoid surgery and several attempts at
a tympanic reconstruction were made by physicians, such as Blake in
1877 and Berthold in 1878 (18,19).
However, none of these pioneers considered the reconstruction of
the ossicular chain, probably due to the lack of adequate optic
equipment and anatomical knowledge of the area. The era of OCR
began at the turn of the 20th century.
In 1901, the first documented attempt at repairing
the ossicular chain was made by Matte, who named this procedure
myringostapediopexy (20).
Between 1955-1956, the modern era of middle ear
reconstruction began with Zollner and Wullstein. They emphasized
the idea of creating a differential between sound pressure at the
oval and round window by adapting each type of surgery to a
specific ossicular problem. For example, in the case of a missing
incus, the graft was connected to the stapes head (type III or
columellar tympanoplasty) (19,21).
The technique introduced in 1957 by Hall and Rytzner
(22) for the treatment of
otosclerosis was promising. It reconnected the stapes footplate to
the TM by using autogenous grafts of incus or malleus (22). During this period, autogenous and
alloplastic materials were at the height of their popularity.
Wullstein introduced an artificial material (Palavit-vinyl-acryl
plastic) in 1952, to connect the TM directly to the stapes
footplate (19).
In 1958, Shea described the connection of the TM to
the stapes head by using a polyethylene tube (19). Other researchers continued his work
by using various types of polyethylene (PE) and other inert
materials, such as Polytef (Teflon) and silicone elastomer
(SILASTIC™) (23). The initial
short-term hearing results were excellent; however, the harsh
alloplastic materials had a large potential for extrusion, middle
ear reactivity and/or penetration into the inner ear (23). As a result, numerous surgeons
preferred more compatible, autogenous prostheses.
Several otologists began using autografts
(transplantation of organs or tissues from one part of the body to
another in the same person) for ossicular reconstruction. The body
of the incus was most frequently harvested for ORC, although
cartilage and cortical bone were also frequently used. These
natural materials were well-tolerated and provided reliable
functional results; however, they were also very brittle and
required laborious and time-consuming sculpturing. There were also
scars in chronically afflicted middle ears (23).
Homografts (transplantation of cells, tissues, or
organs to a recipient from a genetically non-identical donor of the
same species) (24) were first used
for middle ear reconstruction in 1966 by House et al
(25). This line of research later
included novel solutions, such as irradiated tissues (ossicles,
cartilage) and even harvesting ‘en bloc’ ossicles and TM (24,25). The
hearing results and biocompatibility of the homografts proved to be
similar to autografts; however, other concerns appeared, such as
the risk of disease transmission (i.e., HIV or Creutzfeldt-Jakob
disease) which led to a decline in their use.
Thus, the search for a safe, reliable, and easily
available prosthesis continued. Plastipore, a high-density
polyethylene sponge was first used by Shea (26) in 1976. Due to the porous nature of
polyethylene and its lack of reactiveness, the in-growth of tissue
is possible. The material is available commercially on a large
scale and is very easy to trim with surgical instruments. Later on,
a more refined yet similar thermal-fused porous polyethylene
(Polycel) was developed. It allowed for the easy coupling of the
prosthesis to other materials, such as stainless steel, which
enabled various prosthetic designs (23). The main issue associated with the use
of this material was the high rate of extrusion when in direct
contact with the TM. A possible solution was proposed by Shea
(26), and Brackmann and Sheehy
(27) in 1979, which protected the
TM with a disc of cartilage. As a result, Plastipore and Polycel
TORPs and PORPs continue to be used with good long-term success
today (23).
Ceramics made an appearance in ossicular
reconstruction in 1979. These alloplastic materials were defined as
either bioinert or bioactive (28).
An extensive presentation of all biological and synthetic materials
used for ossiculoplasty has been published in recent studies
(28,29).
High-density aluminum oxide ceramic (bioinert
implants) did not react with surrounding tissues and were popular
in Germany and Japan. Glass ceramics (Ceravital) were used to
create bioactive implants, which were bio-compatible and reacted
with surrounding soft tissue and adjacent bone allowing for an
excellent coupling between the implant and the ossicle (30). These bioactive implants were used,
with various results obtained worldwide, even in the former
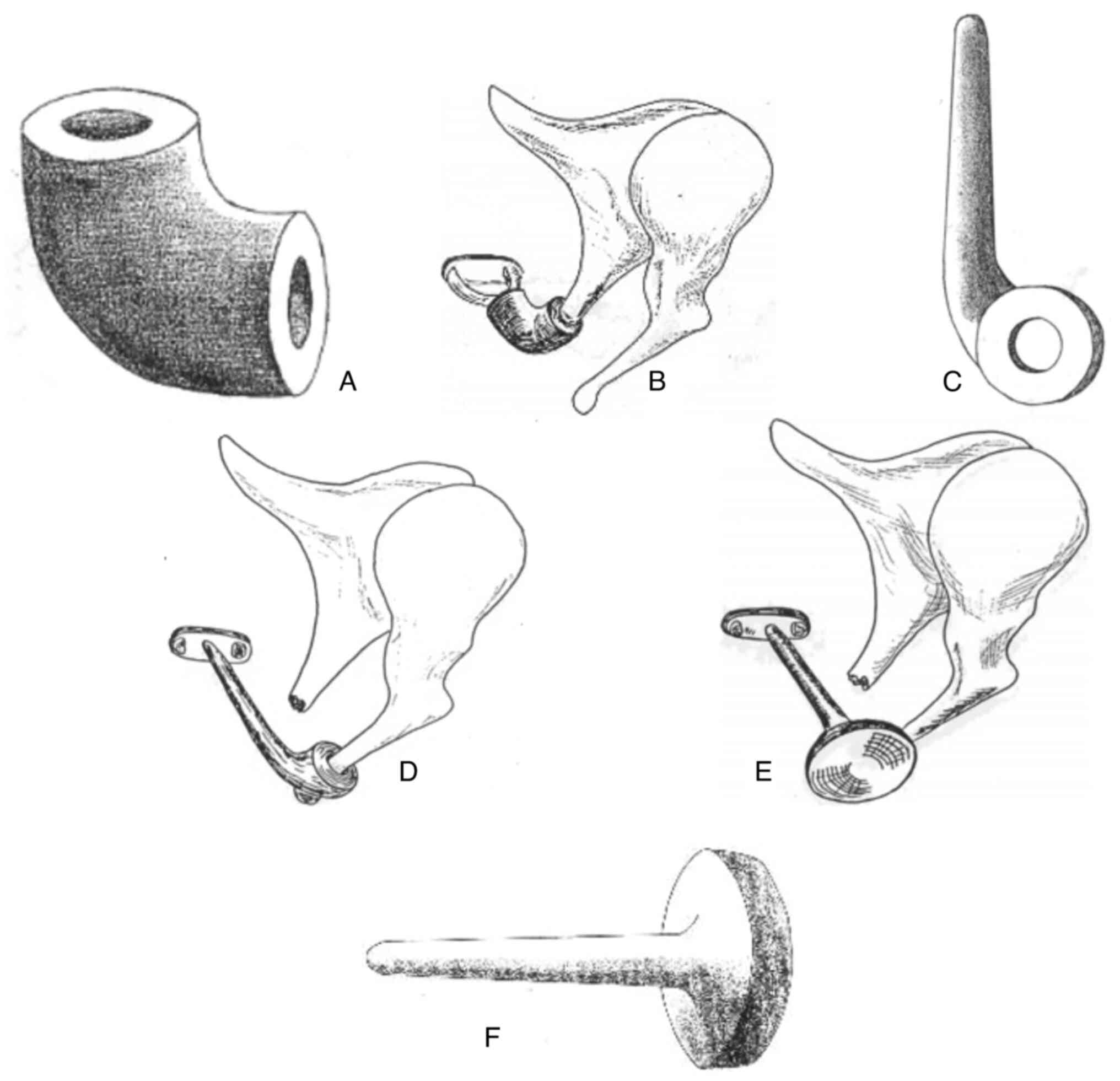
communist bloc, including Romania (31). Bio-vitro-ceramic PAW-1 (Fig. 1) is a solid, bio reactive, synthetic
biomaterial, comprised of fluorescent hydroxyapatite (HA) and
wollastonite microcrystals encompassed into a vitreous mass
(glass); the material is obtained by the controlled crystallization
of a glass from the silicium-calcium-magnesium-phosphorus system
with limited additions of boron trioxide and molecular fluoride. It
represents a locally developed product (31).
Compared to other synthetic materials, ceramic
implants allowed for direct contact with the TM without requiring a
cartilage bridge; however, they were difficult to handle and shape
because of their glass nature (23).
HA
[Ca10(PO4)6(OH)2] is a
bioactive material, easily integrated in the surrounding tissues
and was first used by Grote (32)
(calcium phosphate ceramic). A collagen-HA composite material,
characterized by a strong interaction between the collagen fibers
and the hydroxyapatite crystals, can be successfully used as a bone
substitute. The shortcomings of HA are represented by its
brittleness, which renders it technically difficult to sculpt
(33). A possible solution came from
chemically combining HA with other materials, such as Silastic or
polyethylene. HA was also used in other types of biological
prosthesis. Coralline porous HA became the most common material in
ocular implants following primary enucleation. Made from a specific
genus of reef-building coral, porous HA has a similar architecture
to human cancellous bone, with interconnecting channels. Per se, HA
represents the primary inorganic portion of human bones and the
process by which implants of HA are made from sea coral, imply
intense heat, which denatures proteins to reduce the immune
response. When it is implanted in soft tissues, porous HA allows
for the ingrowth of fibrovascular tissues in pores, and it has been
demonstrated that unwrapped HA does not become encapsulated, as
poly(methyl methacrylate) spheres or silicone (34).
In 1985, the incus and stapes prosthesis designed by
Wehrs (24) was manufactured from HA
and provided successful hearing results and a low extrusion rate 4
years after implantation. The advantage of good sound transfer
function due to the high rigidity of the material was
counterbalanced by the large mass of the prosthesis, which created
high input impedance, and potentially obstructed the surgeon's view
(24). A more recent development
brought forward the combination of HA and polyethylene (HAPEX) to
create an allograft material that approaches the mechanical
strength of bone. but is soft enough to be cut with a knife
(23).
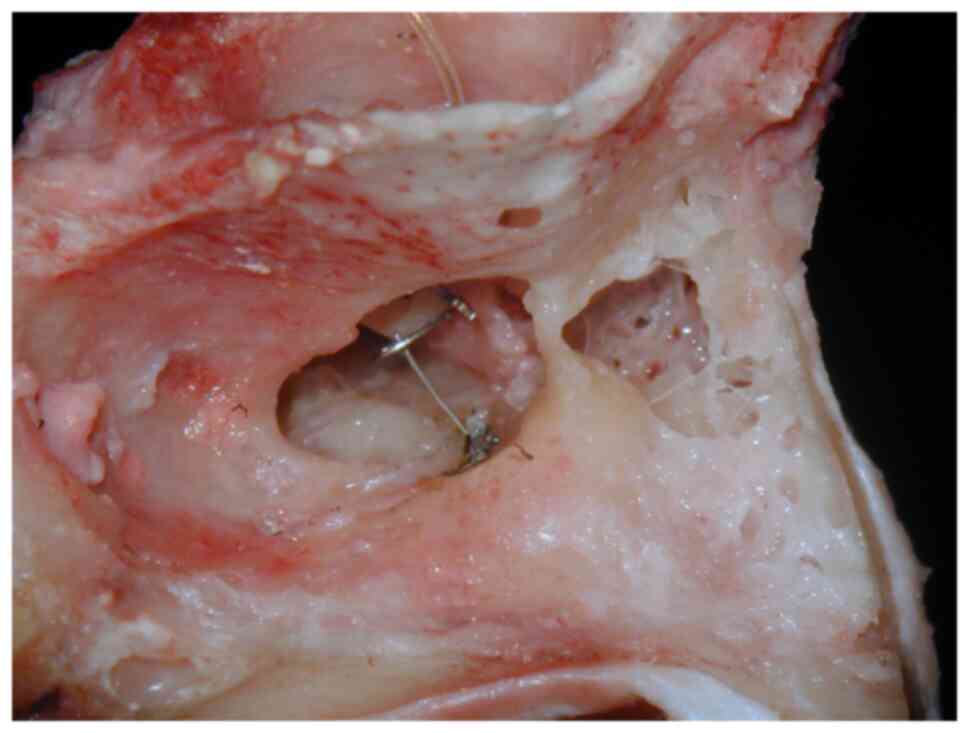
Titanium prostheses made an appearance in 1993 in
Germany (Fig. 2) and have remained
the most popular choice for otologists ever since. They combine the
rigidity and biocompatible of HA with lightweight (the specific
density of titanium is <57% that of stainless steel). It is also
non-magnetic, has excellent biocompatibility, and can be
manufactured in any required shape and size. The characteristics of
this material allow for various designs and the majority of the
titanium prostheses possess an open head which provides better
visualization during placement (23). Cartilage protection for the TM is
still necessary, and this brings about an interesting conclusion
that we should consider all materials for ossicular reconstruction
experiments. Several authors to date have published favorable
hearing results with titanium prostheses and compared them with HA.
Titanium may provide improved hearing responses at higher
frequencies because of its low mass (35-38).
After a long and interesting history of material
research, experimentation and evaluation, titanium prevailed among
the alloplastic materials and is by far the most used for
ossiculoplasty over the past years. This fact is due to its
favorable properties of the material: Excellent acoustic
transmission characteristics, very good biocompatibility and the
possibility to develop particularly filigree design. This last
property also allows integrating functional elements in the middle
ear prostheses (29).
Although extensively used in the past for ossicular
reconstruction, biomaterials (autografts), particularly the body of
the incus and cartilage (tragal and/or conchal) have decreased in
popularity as of late, due to the fact that they are not always
available for harvest and they do not have the required mass,
stiffness and thickness (28).
Autologous materials have a high degree of tolerance within the
middle ear and provide reliable hearing results, but are also
difficult to sculpt and fixate to the soft tissues of the middle
ear and prolong intraoperative time. Modern tympanoplasty requires
less biomaterials, usually conchal cartilage and temporal fascia,
but their importance still remains high in repairing TM
perforations and as interposed between soft tissues and synthetic
(titanium) prosthesis in both overlay and underlay techniques.
4. Conclusions and future perspectives
Considering the present state of otologic surgery,
it can be safely stated that the ideal middle ear implant does not
exist. For all patients, follow-ups need to be performed regularly
to detect the first signs of potential complications, regardless of
the used implant. Future research and material development may
provide an ideal prosthesis for OCR.
Present-day surgery uses autogenous and alloplastic
prostheses with equally good outcomes. It is considered that the
choice of prosthesis and material remains an issue for personal
preference and experience, and it is recommended that each surgeon
should try several variants and select the one that provides
consistent, favorable results. It is also clear that other factors,
such as material quality, surgical technique, the experience of a
surgeon or the environment in which the prosthesis is placed,
influences the functional results.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HM, ITD and MR contributed equally to this work and
should, therefore, be considered co-first authors. HM and MR
conceptualization. MR, HM and ITD were involved in the data search
and selection for inclusion in the review. HM, MAS and AIM were
involved in the organization and integration of the data collected
from various studies for the purposes of the present review, as
well as in the graphical representations. MR and HM were involved
in the writing and preparation of the original draft. MR and ITD
were involved in the writing, reviewing and editing of the
manuscript. MR, HM and ITD were involved in visualization. HM and
MR supervised the study. HM, MR and ITD were involved in project
administration. AIM and MAS made substantial contributions to the
acquisition, analysis and interpretation of the data for inclusion
in the review and provided a final review of the article. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
The patient whose implant is depicted in Fig. 2 provided informed consent for
inclusion in the present study.
Patient consent for publication
The patient whose implant is depicted in Fig. 2 provided consent for the publication
of data and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neagu A, Mocanu AI, Bonciu A, Coadă G and
Mocanu H: Prevalence of GJB2 gene mutations correlated to presence
of clinical and environmental risk factors in the etiology of
congenital sensorineural hearing loss of the Romanian population.
Exp Ther Med. 21(612)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Neagu AC, Budișteanu M, Gheorghe DC,
Mocanu AI and Mocanu H: Rare gene mutations in romanian hypoacusis
patients: Case series and a review of the literature. Medicina
(Kaunas). 58(1252)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mocanu H and Oncioiu I: The influence of
clinical and environmental risk factors in the etiology of
congenital sensorineural hearing loss in the Romanian population.
Iran J Public Health. 48:2301–2303. 2019.PubMed/NCBI
|
|
4
|
Gan RZ, Yang F, Zhang X and Nakmali D:
Mechanical properties of stapedial annular ligament. Med Eng Phys.
33:330–339. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gan RZ, Reeves BP and Wang X: Modeling of
sound transmission from ear canal to cochlea. Ann Biomed Eng.
35:2180–2195. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Merchant SN, Incesulu A, Glynn RJ and
Nadol JB Jr: Histologic studies of the posterior stapediovestibular
joint in otosclerosis. Otol Neurotol. 22:305–310. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hüttenbrink KB: The mechanics of the
middle-ear at static air pressures: The role of the ossicular
joints, the function of the middle-ear muscles and the behavior of
stapedial prostheses. Acta Otolaryngol Suppl. 451:1–35.
1988.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hirsch BE: Ossicular Chain Reconstruction.
In: Head and Neck Surgery. Myers E (ed). 2nd Edition. Saunders
Elsevier, Philadelphia, PA, pp1-3, 2008.
|
|
9
|
Bojrab DI and Babu SC: Ossiculoplasty I.
In: Middle Ear, Mastoid Surgery. Habermann RS (ed). Thieme Verlag,
New York, NY, pp151-158, 2004.
|
|
10
|
Eiber A, Freitag HG, Burkhardt G, Hemmert
W, Maassen M, Rodriguez-Jorge J and Zenner HP: Dynamics of middle
ear prostheses-simulations and measurements. Audiol Neurotol.
4:178–184. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Goode RL and Nishihara S: Experimental
models of ossiculoplasty. Otolaryngol Clin North Am. 27:663–675.
1994.PubMed/NCBI
|
|
12
|
Hudde H and Weistendörfer C: A
three-dimensional circuit model of the middle ear. Acustica.
83:535–549. 1997.
|
|
13
|
Hüttenbrink KB and Hudde H: Studies of
sound condition in the reconstructed middle ear with a hydrophone.
Initial results. HNO. 42:49–57. 1994.PubMed/NCBI(In German).
|
|
14
|
Neudert M, Bornitz M, Mocanu H,
Lasurashvili N, Beleites T, Offergeld C and Zahnert T: Feasibility
study of a mechanical real-time feedback system for optimizing the
sound transfer in the reconstructed middle ear. Otol Neurotol.
39:e907–e920. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mocanu H, Mocanu AI, Coada G, Bonciu A,
Schipor MA and Radulescu M: Analysis of long-term anatomic results
of radical mastoidectomy. Exp Ther Med. 23(156)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Caley ER: The Leyden Papyrus X: An English
translation with brief notes. J Chem Educ. 3(1149)1926.
|
|
17
|
Benmoussa N, Fabre C, Deo S, Pretre C and
Charlier P: Further arguments confirming the first description of
cholesteatoma by Hippocrates. Eur Arch Otorhinolaryngol.
277:2387–2388. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Malhotra M, Malhotra R, Varshney S, Priya
M, Bhardwaj A, Tyagi A, Kumar A and Gupta S: A historical review of
indian perspectives on techniques of tympanoplasty. Int J
Otolaryngol. 2020(1408270)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sarkar S: A review on the history of
tympanoplasty. Indian J Otolaryngol Head Neck Surg. 65 (Suppl
3):S455–S460. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chavan SS, Jain PV, Vedi JN, Rai DK and
Kadri H: Ossiculoplasty: A prospective study of 80 cases. Iran J
Otorhinolaryngol. 26:143–150. 2014.PubMed/NCBI
|
|
21
|
Mudry A: Tympanoplasty before
tympanoplasty: Alea jacta erat! Otol Neurotol. 43:276–280.
2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hall A and Rytzner C: Stapedectomy and
autotransplantation of ossicles. Acta Otolaryngol. 47:318–324.
1957.PubMed/NCBI View Article : Google Scholar
|
|
23
|
McElveen JT Jr, Cunningham CD III and
Sheehy JL: Ossicular Reconstruction. In: Otologic Surgery.
Brackmann D (ed). 3rd Edition. Elsevier Canada, Toronto, ON,
2001.
|
|
24
|
Wehrs RE: Homograft ossicles in middle ear
surgery. Am J Otol. 6:33–34. 1985.PubMed/NCBI
|
|
25
|
House WJ, Patterson ME and Linthicum FH
Jr: Incus homografts in chronic ear surgery. Arch Otolaryngol.
84:148–153. 1966.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shea JJ: Plastipore total ossicular
replacement prosthesis. Laryngoscope. 86:239–240. 1976.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Brackmann DE and Sheehy JL: Tympanoplasty:
TORPs and PORPs. Laryngoscope. 89:108–114. 1979.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Radulescu M and Mocanu AI: A review of
biovitroceramic PAW1-Romanian bioactive implant material for
ossiculoplasty. Rom J Mil Med. 125:190–195. 2022.
|
|
29
|
Neudert M and Zahnert T:
Tympanoplasty-news and new perspectives. GMS Current Topics in
Otorhinolaryngology-Head and Neck Surgery 2017, Vol. 16.
|
|
30
|
Niparko JK, Kemink JL, Graham MD and
Kartush JM: Bioactive glass ceramic in ossicular reconstruction: A
preliminary report. Laryngoscope. 98 (8 Pt 1):822–825.
1988.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mocanu H, Mocanu AI, Bonciu A, Coada G,
Schipor MA and Radulescu M: Analysis of long-term functional
results of radical mastoidectomy. Exp Ther Med.
22(1216)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Grote JJ: Tympanoplasty with calcium
phosphate. Arch Otolaryngol. 110:197–199. 1984.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ficai A, Andronescu E, Ghitulicã C, Voicu
G, Trandafir V, Mânzu D, Ficai M and Pall S: Colagen/Hydroxyapatite
Interactions in Composite Biomaterials. Mat Plast. 46:11–15.
2009.
|
|
34
|
Costea CF, Bogdanici CM, Carauleanu A,
Dimitriu G, Sava A, Dumitrescu N, Turliuc MD, Cucu A, Ciocoiu M,
Dragomir R and Buzduga CM: Updates of Ocular Prostheses-A review of
biomaterials and design in anophthalmic socket. Rev Chim.
70:239–244. 2019.
|
|
35
|
Krueger WW, Feghali JG, Shelton C, Green
JD, Beatty CW, Wilson DF, Thedinger BS, Barrs DM and McElveen JT:
Preliminary ossiculoplasty results using the Kurz titanium
prostheses. Otol Neurotol. 24:836–839. 2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gardner EK, Jackson CG and Kaylie DM:
Results with titanium ossicular reconstruction prostheses.
Laryngoscope. 114:65–70. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Martin AD and Harner SG: Ossicular
reconstruction with titanium prosthesis. Laryngoscope. 114:61–64.
2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zenner HP, Stegmaier A, Lehner R, Baumann
I and Zimmermann R: Open Tubingen titanium prostheses for
ossiculoplasty: A prospective clinical trial. Otol Neurotol.
22:582–589. 2001.PubMed/NCBI View Article : Google Scholar
|