Introduction
Neuroblastoma is a type of childhood cancer arising
from the sympathetic ganglia of the trunk and adrenal medulla
(1,2). It is the second most common solid tumor
observed in childhood following leukemia and brain tumors, with an
exceptionally high incidence in children <5 years of age
(3-5).
Half of the patients with neuroblastoma have metastases at the time
of diagnosis (6). However, infants
<18 months of age often have a better prognosis, and sometimes
differentiate and regress spontaneously (7). These features distinguish neuroblastoma
from other solid tumors.
A culture system for human neuroblastoma has been
established and has been used for experiments on neuroblastoma
(8,9). Lactoferrin, found in abundance in
breast milk and as a protein in cow's milk, is virtually non-toxic
when ingested orally and crosses the blood-brain barrier 50-fold
more rapidly than transferrin (10).
Lactoferrin has been found to induce neuroblastoma differentiation
with the expression of β-tubulin III and neurofilaments, and to
decrease survivin expression (11).
Furthermore, lactoferrin recruits PI3K signaling, while both PI3K
and ERK signaling are involved in inducing differentiation
(11). The radioprotective effects
of lactoferrin have also been studied in mice exposed to X-rays,
exhibiting higher survival rates, reduced DNA damage and increased
levels of superoxide dismutase following treatment with lactoferrin
(12-14).
These findings may be related to the mechanism of spontaneous
neuroblastoma regression in infants more likely to ingest
lactoferrin through the milk and indicate a potential novel
application of lactoferrin; however, the underlying mechanisms
remain unclear.
The author has previously examined the effects of
bioactive substances on cells constituting the human brain.
Recently, it was suggested that nicotine induces cellular
dysfunction in human glioblastoma under lithium carbonate
administration (15) and that
platinum nano-colloids affect human glioblastoma cell growth in a
coexisting neurotransmitter-dependent manner (16). The present study investigated the
potential of lactoferrin to inhibit the proliferation of IMR-32
human neuroblastoma cells compared to doxorubicin and dibutyryl
cyclic AMP (db-cAMP), including under X-ray irradiation
conditions.
Materials and methods
Cells and cell culture
The IMR-32 human neuroblastoma cell line was
obtained from the JCRB cell bank (cat. no. JCRB9050). The IMR-32 is
a fibroblast-like cell line established by W.W. Nichols in 1970,
which was obtained during exploratory surgery from an abdominal
mass of a 13-month-old boy (8). The
proto-oncogene N-myc (MYCN), a genetic signature of
neuroblastoma, is amplified in IMR-32 cells (17,18). The
IMR-32 cells were cultured in Eagle's minimum essential medium
(E-MEM) supplemented with non-essential amino acids (056-08385,
FUJIFILM Wako Pure Chemical Corp.), L-glutamine (073-05391,
FUJIFILM Wako Pure Chemical Corp.), 10% fetal bovine serum
(S-FBS-NL-015, Serana Europe GmbH) and
penicillin-streptomycin-amphotericin B suspension (161-23181,
FUJIFILM Wako Pure Chemical Corp.) at 37˚C with 5%
CO2.
Lactoferrin
Lactoferrin from bovine milk (123-04124, FUJIFILM
Wako Pure Chemical Corp.) has a molecular weight of ~83,000 kDa and
an iron saturation of 3.6-25.0%. Lactoferrin was dissolved in PBS
(-) of pH 7.4, phosphate-buffered saline without Ca and Mg
(164-23551, FUJIFILM Wako Pure Chemical Corp.), at a concentration
of 1 mg/ml, and subjected to light scattering measurement using a
zeta-potential and particle size analyzer (ELSZneo, Otsuka
Electronics Co. Ltd.), and resulted in electrophoretic mobility of
-2.71±0.51x10-5 cm2/Vsec, a particle size of
14.3±0.1 nm and a molecular weight of 1.669x105,
suggesting that lactoferrin exists as an aggregate in the PBS (-)
solution.
Cell proliferation assay
The
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
(WST-8) assay with a water-soluble tetrazolium salt was employed to
assess cell proliferation (19). The
IMR-32 cells were seeded at 3,000 cells/well in a 96-well culture
plate (Sumitomo Bakelite Co., Ltd.) as n=5 and pre-incubated for 24
h at 37˚C with 5% CO2. Lactoferrin was added to each
well at 0-120 µM. Following a 1-day incubation, the cells were
exposed to X-rays at 1 Gy (CAX-150-20; Chubu Medical Co., Ltd.; 150
kV-20 mA, 1 mm Al + 0.1 mm Cu filters, 0.60 Gy/min) and incubated
for 6 days at 37˚C with 5% CO2. The medium was then
replaced with 5% of WST-8 solution (cat. no. 347-07621, Dojindo
Laboratories, Inc.) diluted with E-MEM and incubated for 1.5 h at
37˚C with 5% CO2. Subsequently, the absorbance was
measured at λ=450 nm with a multi-spectrophotometer (Viento,
Dainippon Sumitomo Pharma, Co. Ltd.). The amount of formed formazan
is proportional to the number of viable cells, as intracellular
mitochondrial dehydrogenase reduces WST-8 to yellowish-orange
formazan (19). In addition, was
conducted in a single administration with db-cAMP at 0-4.8 nM (cat.
no. sc-201567, Santa Cruz Biotechnology, Inc.), an inducer of
neuroblastoma cell differentiation, and doxorubicin hydrochloride
at 0-3.84 mM (040-21521, FUJIFILM Wako Pure Chemical Corp.), an
anticancer drug. The doubling time of the IMR-32 cells was ~36 h,
and the cells were in a logarithmic growth phase during 7 days of
culture. Cell morphology was observed under a phase contrast
microscope (CKX-53, Olympus Corp.) at a magnification of x200. Cell
proliferation was evaluated by repeating the experiment five times,
and the IC50 value for each reagent was determined. The
IC50 values were estimated by plotting a series of
dose-response data with the logarithm of the dose and using a
fitted straight line.
Membrane lipid peroxidation and
leakage of lactate dehydrogenase
Cell membrane disruption was evaluated by membrane
lipid peroxidation and lactate dehydrogenase leakage. The IMR-32
cells were seeded at 5,000 cells/well in a 96-well culture plate as
n=5 and pre-incubated for 24 h at 37˚C with 5% CO2.
Lactoferrin was added to each well at 0-30 µM. Following a 1-day
incubation, the cells were exposed to X-rays at 1 Gy and incubated
for 24 h at 37˚C with 5% CO2. The cell culture medium
was then replaced with 1 µmol/l
N-(4-diphenylphosphinophenyl)-N'-(3,6,9,12-tetraoxatridecyl)perylene-3,4,9,10-tetracarboxydiimide
(Liperfluo, Dojindo Laboratories, Inc.) for the detection of lipid
hydroperoxides (20). Following 1.5
h of incubation at room temperature in the dark, the fluorescence
intensity, proportional to lipid peroxide in membrane lipids, was
measured at Ex/Em=485 nm/535 nm using a microplate reader (TriStar
LB941, Berthold Technologies GmbH & Co. KG). On the other hand,
the cell culture medium was replaced with E-MEM containing
water-soluble formazan of the cytotoxicity LDH-assay kit (Dojindo
Laboratories, Inc.). Following 0.5 h of incubation at room
temperature in the dark, the absorbance at 490 nm, proportional to
lactate dehydrogenase leakage (21),
was measured using the multi-spectrophotometer (Viento, Dainippon
Sumitomo Pharma, Co. Ltd.).
Levels of apoptosis-mediating
caspase-3/7
The IMR-32 cells were seeded at 5,000 cells/well in
a 96-well culture plate (SPL Life Sciences Co., Ltd.) as n=5 and
pre-incubated for 24 h at 37˚C with 5% CO2. Lactoferrin
was added to each well at 0-120 µM. Following a 1-day incubation,
the cells were exposed to X-rays at 1 Gy and incubated for 24 h at
37˚C with 5% CO2. The cell culture medium was then
replaced with E-MEM containing the Caspase-Glo 3/7 assay system
(Promega Corp.). Following 0.5-h of incubation at room temperature
in the dark, the luminescence intensity, proportional to
caspase-3/7 activity, was measured using the microplate reader
(TriStar LB941, Berthold Technologies GmbH & Co. KG).
Measurement of intracellular reactive
oxygen species
The nitroblue tetrazolium (NBT) reduction method was
employed to assess the production of superoxide anion radicals
(O2·-) in cells (22,23). The
IMR-32 cells were seeded at 12,000 cells/well in a 96-well culture
plate as n=5 and pre-incubated for 24 h at 37˚C with 5%
CO2. Lactoferrin was added to each well at 0-30 µM.
Following a 1-day incubation, the medium was replaced with 0.2% NBT
(Tokyo Chemical Industry, Co., Ltd.)-containing medium filtered
<0.22 µm. The cells were exposed to X-rays at 1 Gy and incubated
at 37˚C with 5% CO2. Following a 3-h incubation, the
absorbance of NBT-formazan was measured at l=620 nm using the
multi-spectrophotometer (Viento) and cell morphology was observed
using a phase contrast microscope (CKX-53, Olympus Corp.) at a
magnification of x200.
Intracellular uptake of
lactoferrin
The intracellular uptake of lactoferrin was assessed
by immunostaining with a goat anti-bovine lactoferrin antibody. The
IMR-32 cells were seeded at 36,000 cells/well in a chamber slide
(Nalge Nunc International Corp.) and pre-incubated for 3 days at
37˚C with 5% CO2. Lactoferrin was added to each well at
a dose of 1.2 µM with no cytotoxicity. The cells were rinsed with
E-MEM following incubation for 0.2, 6 and 24 h at 37˚C.
Subsequently, 4% paraformaldehyde phosphate buffer solution at pH
7.4 (FUJIFILM Wako Pure Chemical Corp.) was added, and the cells
were allowed to stand for 15 min at room temperature. The cells
were then washed with PBS(-) (FUJIFILM Wako Pure Chemical Corp.)
and permeabilized with 0.1% Triton X-100 (FUJIFILM Wako Pure
Chemical Corp.) for 5 min on ice. Blocking was carried out with 2%
rabbit serum (Cedarlane Laboratories, Inc.) in PBS(-) for 30 min at
room temperature, and the cells were then allowed to react with a
goat anti-bovine lactoferrin antibody (1:200 dilution; cat. no.
A10-126, Bethyl Laboratories, Inc.) as a primary antibody overnight
at 4˚C. After washing with PBS(-), an FITC-conjugated rabbit
anti-goat IgG antibody (1:200 dilution; cat. no. SA00003-4,
Proteintech Group, Inc.) was added as a secondary antibody to react
for 1 h at room temperature. The cell nuclei were then stained with
DAPI (D523, 1:500 dilution, Dojindo Laboratories, Inc.) for 15 min
at room temperature, and the cells were observed under a phase
contrast fluorescence microscope (CKX-53) at Ex/Em: 330-385 nm/420
nm and 460-495 nm/510 nm, and a magnification of x400.
Measurement of hydroxyl radical (OH·)
formation using electron spin resonance spectroscopy (ESR)
The formation of hydroxyl radicals (OH·) in the
Fenton reaction system with
Fe2Cl2/H2O2 was
measured using ESR with a spin trap method with
5,5-dimethyl-1-pyrroline N-oxide (DMPO, MilliporeSigma). A hydrogen
peroxide solution (35%, Nacalai Tesque, Inc.) of 0.5 ml was added
to a glass vial, followed by a drop of 2.5% iron chloride
(FeCl2)·4H2O solution (Nacalai Tesque, Inc.),
0.5 ml of lactoferrin solution of 120 µM. Subsequently, 0.01 g DMPO
was added, which required ~30 sec. The mixed solution was then
filled into a flat quartz cell and measured using a ESR
spectrometer (JES-FA200, JEOL Ltd.). The ESR spectra were obtained
at a microwave power level of 0.4 and 100 kHz filed modulation at
room temperature. The magnetic field was calibrated with the
well-known splitting constants of Mn2+ in MgO.
Statistical analysis
Cell proliferation, membrane lipid peroxidation, the
leakage of lactate dehydrogenase and the levels of caspase-3/7
activity are expressed as the mean ± SD, n=5. The data were
analyzed using one-way ANOVA followed by Dunnett's test with
KaleidaGraph 4.5J software (HULINKS Inc.). A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Proliferation of IMR-32 neuroblastoma
cells
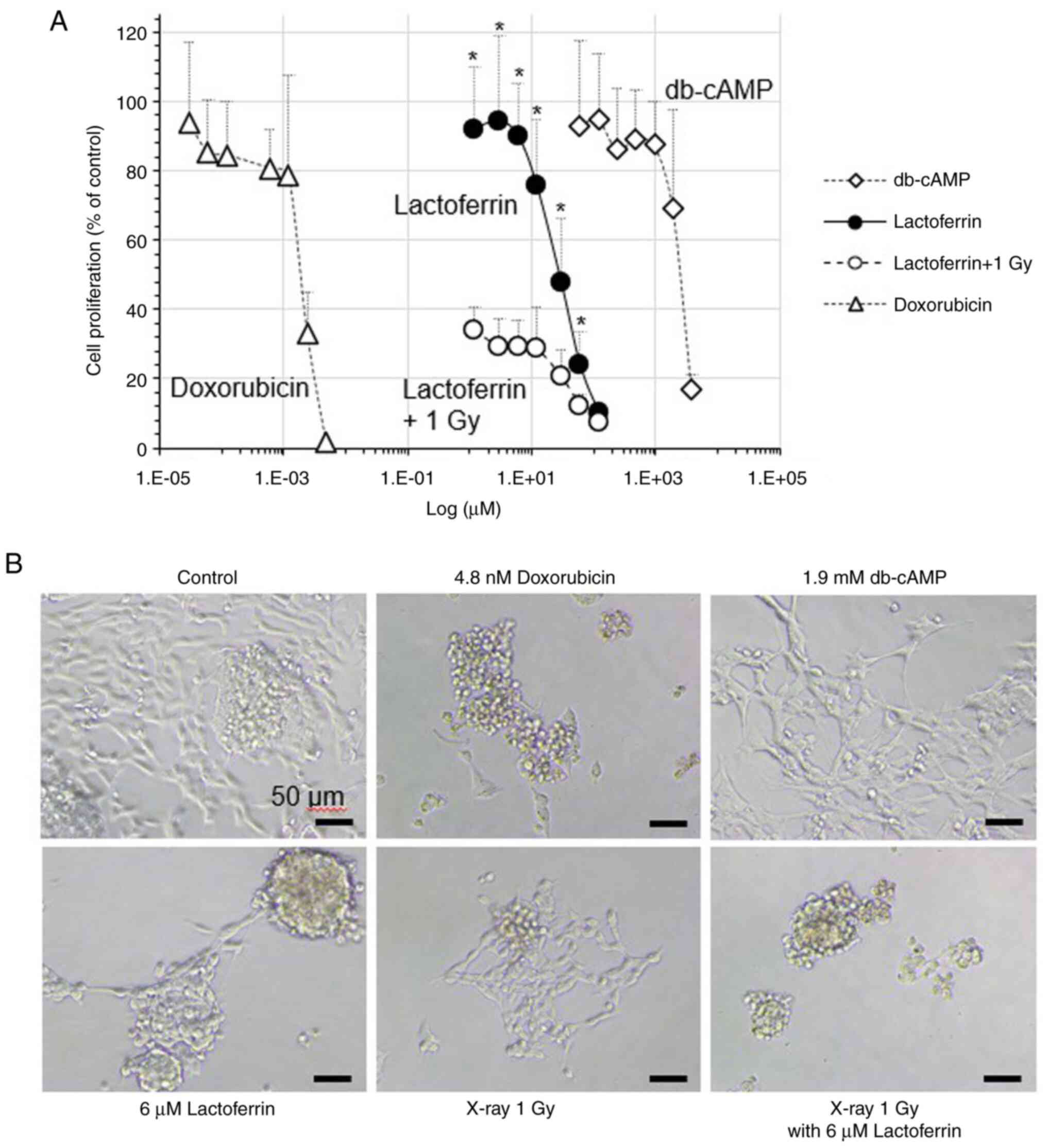
In the IMR-32 cells treated with 1.2-120 µM
lactoferrin, the cell proliferation rate (% of control) decreased
from 92.8 to 10.3% in a concentration-dependent manner (Fig. 1). The cell proliferation rate
decreased to 33.9% following X-ray irradiation at 1 Gy, and
treatment with 120 µM lactoferrin prior to X-ray exposure led to a
cell proliferation rate of 7.2%, indicating that lactoferrin has no
radioprotective effect. When doxorubicin, an anticancer drug, was
administered at concentrations of 0.03-4.8 nM, cell proliferation
decreased from 93.7 to 1.5%. In addition, db-cAMP, a
differentiation inducer, at concentrations of 60-3.84 mM, reduced
cell proliferation from 92.8 to 17.1%. The IC50 values
for cell proliferation were ~2.0 nM for doxorubicin, 2.7 mM for
db-cAMP and 45.9 µM for lactoferrin. IC50 values were
calculated from experimental data repeated five times. The
differentiation inducer db-cAMP induced neurite outgrowth, whereas
lactoferrin treatment did not increase neurite outgrowth (Fig. 1).
Intracellular reactive oxygen
species
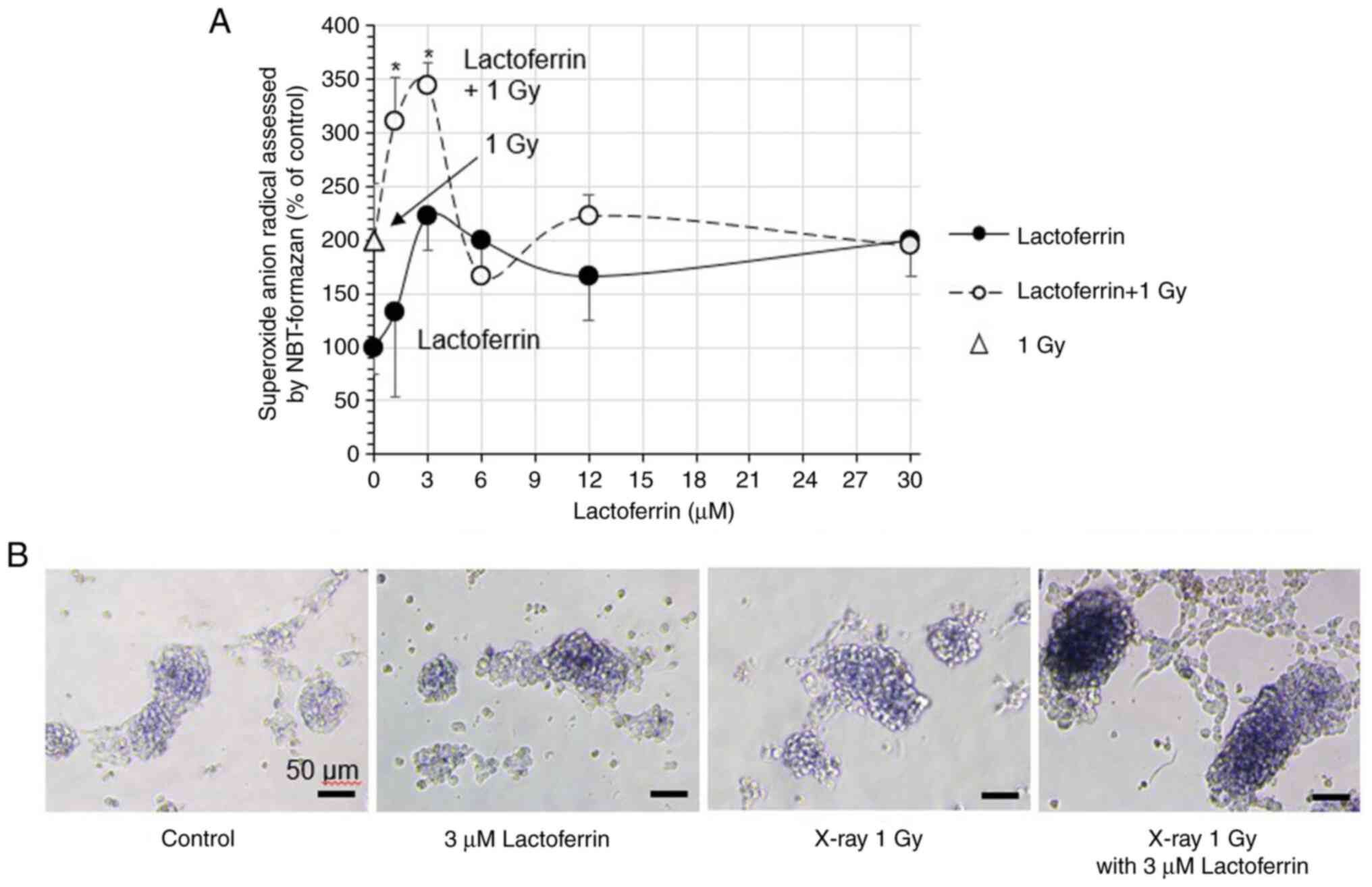
In the IMR-32 cells treated with 1.2-30 µM
lactoferrin, superoxide anion radicals (O2·-;
% of control) increased in a concentration-dependent manner to
194.4%, with a peak of 222.2% at 3 µM (Fig. 2A). X-ray irradiation at 1 Gy
increased superoxide anion radicals to 200.0%, and treatment with 3
µM lactoferrin increased them to a peak of 344.4%. Micrographs of
the cells exhibited a blue color of NBT formazan corresponding to
the formation of superoxide anion radicals (Fig. 2B).
Intracellular uptake of
lactoferrin
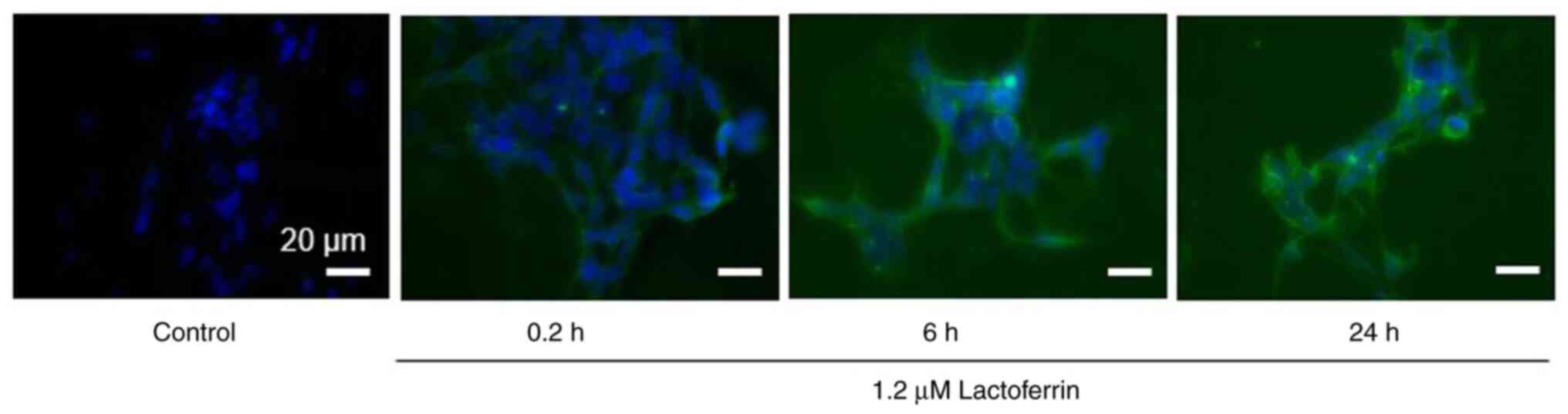
Fluorescence microscopy images of the IMR-32 cells
treated with 1.2 µM lactoferrin for 0.2, 6 and 24 h revealed that
lactoferrin was gradually incorporated into the cells over time
(Fig. 3).
Membrane lipid peroxidation and
leakage of lactate dehydrogenase
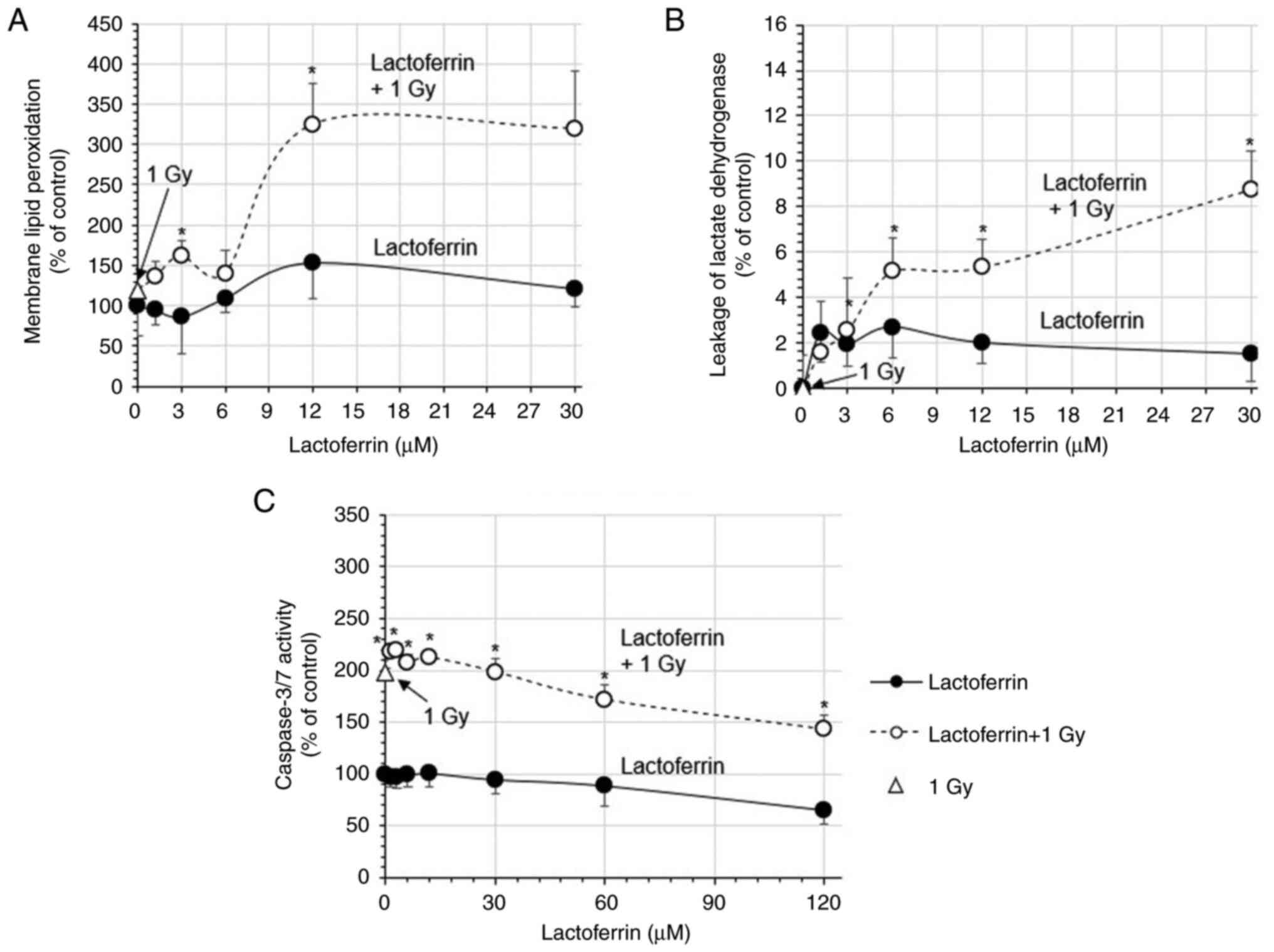
In the IMR-32 cells treated with 1.2-30 µM
lactoferrin, membrane lipid peroxidation (% of control) gradually
increased to 121.2% (Fig. 4A). X-ray
irradiation at 1 Gy increased membrane lipid peroxidation to
118.8%, which increased to 324.8% with 12 µM lactoferrin (Fig. 4A). The leakage of lactate
dehydrogenase also gradually increased by 1.5-2.7%. Although X-rays
at 1 Gy did not increase it, following X-ray irradiation at 1 Gy
with lactoferrin, the leakage of lactate dehydrogenase was
increased by 5.1% at 6 µM and 8.8% at 30 µM, indicating a
significant synergic effect of X-rays and lactoferrin (Fig. 4B).
The apoptosis-mediating caspase-3/7 activity
decreased gradually from 97.4 to 65.5% in cells treated with
1.2-120 µM lactoferrin, but increased rapidly to 198.0% following
X-ray irradiation at 1 Gy. In the cells treated with lactoferrin
and X-rays at 1 Gy, caspase-3/7 activity decreased gradually from
218.7 to 143.2% (Fig. 4C).
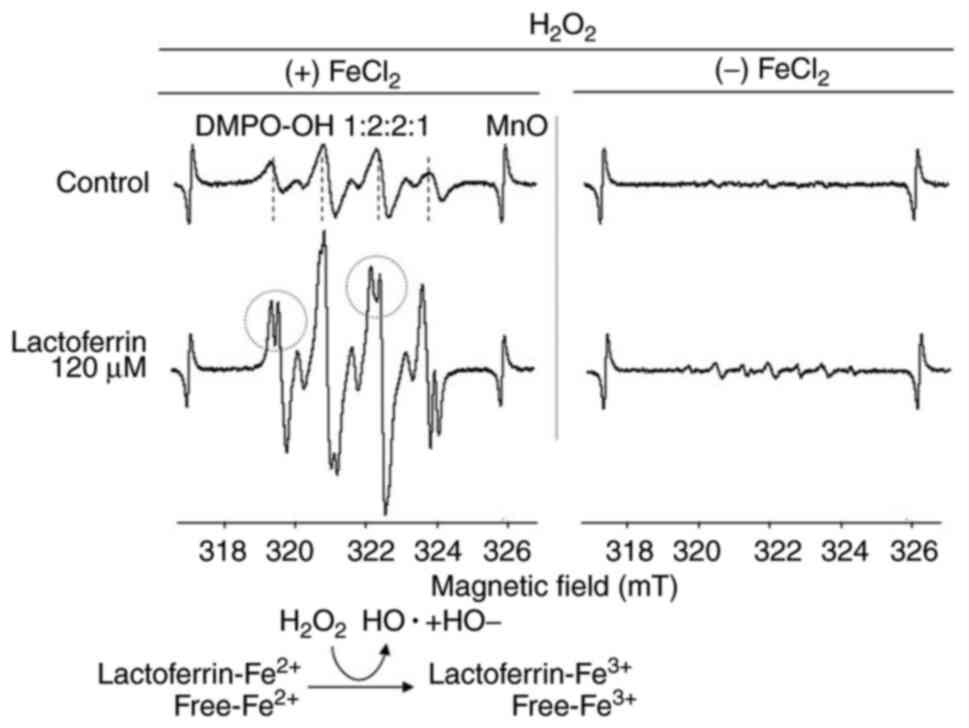
Hydroxyl radical (OH·) formation
measured using ESR
When lactoferrin was added in the reaction system
without iron chloride, the levels of hydroxyl radicals (OH·) did
not markedly increase. However, in the Fenton reaction system with
iron chloride, the coexistence of lactoferrin resulted in a
considerable increase in the formation of hydroxyl radicals (OH·)
compared with the control, which split into two peaks (Fig. 5).
Discussion
The present study investigated the inhibition of the
proliferation of IMR-32 human neuroblastoma cells by lactoferrin,
including under X-ray irradiation. In IMR-32 human neuroblastoma
cells, a concentration-dependent decrease in cell proliferation was
observed. X-ray irradiation at 1 Gy reduced cell proliferation to
~30% and cell proliferation was not restored by lactoferrin
treatment prior to X-ray irradiation. The IC50 values
were ~2.0 nM for doxorubicin, 2.7 mM for db-cAMP and 45.9 µM for
lactoferrin. Neurite outgrowth was observed with db-cAMP, although
no increase in neurite outgrowth was observed with lactoferrin
treatment. This may be due to the fact that the medium containing
10% FBS was unfavorable for inducing differentiation. Thus,
lactoferrin inhibited the growth of neuroblastoma cells, although
not as markedly as the anticancer drug, doxorubicin.
Lactoferrin increased intracellular superoxide anion
radicals (O2·-), further augmented by X-ray
irradiation at 1 Gy with lactoferrin, reaching a peak at 1.2-3 µM.
Membrane lipid peroxidation was also increased by X-rays with
lactoferrin, peaking at a relatively low concentration of 12 µM. In
addition, cellular immunostaining revealed that lactoferrin was
gradually taken into the cells over a period of 24 h following
administration. Based on previous studies, the lactoferrin receptor
is highly expressed on the apical surface of respiratory epithelial
cells, as well as brain endothelial cells and neurons (24,25).
Although lactoferrin has a large molecular weight, the cellular
uptake of lactoferrin nanoparticles has been observed in SH-SY5Y
neuroblastoma cells (26). Recently,
the radioprotective effects of lactoferrin have been reported. Wei
et al (14) reported that
lactoferrin prolonged the survival rate of mice exposed to 8 Gy of
X-rays, which was attributed to the suppression of intestinal
injury through the reduction of inflammatory cytokines and the
downregulation of NF-κB. Feng et al (12) reported that in the hepatic tissue of
mice exposed to 7 Gy X-rays, treatment with lactoferrin increased
the levels of superoxide dismutase and decreased those of
malondialdehyde, suggesting that lactoferrin may prevent radiation
damage in patients undergoing radiotherapy. In contrast to these
reports, the results of the present study indicated that
intracellularly incorporated lactoferrin did not exert an
antioxidant effect, but promoted intracellular oxidation. Moreover,
lactoferrin slightly increased the leakage of lactate dehydrogenase
from cells, which was significantly increased by X-rays in
combination with lactoferrin. The bovine lactoferrin used in the
experiments in the present study had an electrophoretic mobility of
-2.71x10-5 cm2/Vsec, which was slightly
negatively charged and may have caused damage by binding to the
cell membrane or being taken up into the cell. On the other hand,
the levels of apoptosis-mediating caspase-3/7 activity were
significantly increased by X-ray irradiation at 1 Gy, but not by
lactoferrin, suggesting that lactoferrin does not actively induce
the apoptosis of IMR-32 cells. These results indicate that the
mechanism of cell growth inhibition by lactoferrin involves
membrane damage rather than apoptosis in the cells.
ESR measurements revealed that the hydroxyl radical
signal increased and split in two upon the addition of lactoferrin.
The two split peaks indicate multiple environments for forming
hydroxyl radicals (OH·), suggesting the involvement of ferrous iron
trapped in lactoferrin and free ferrous ions in the reaction
system. In the ESR measurements of lactoferrin-mediated radicals,
Nishimura et al (13)
demonstrated that lactoferrin scavenged superoxide anion radicals
(O2·-) generated in the hypoxanthine-xanthine
oxidase system and hydroxyl radicals (OH·) generated in the
Cu(en)2 or H2O2/ultraviolet-ray
system. On the other hand, hydroxyl radical production, measured
using ESR, has been demonstrated to be produced by a Fenton-type
Haber-Weiss reaction catalyzed by lactoferrin (27). It has also been reported that
oxidation of Fe2+ is accelerated in the presence of
lactoferrin and that Fe2+ and lactoferrin produces ·OH
via an H2O2 intermediate with toxicity to
microorganisms (28). Of note, the
present study focused on a reaction system where lactoferrin and
ferrous ion coexist. The results in the Fenton reaction system with
Fe2Cl2/H2O2 indicated
that lactoferrin increased the hydroxyl radical (OH·) formation via
H2O2. Bovine lactoferrin, a glycoprotein with
two symmetric lobes, can bind one ferric ion per lobe and prevent
the echovirus-induced cytopathic effect (29). Chung and Raymond (30) reported that apoproteins prefer an
‘open’ conformation in which the iron-binding site is close to the
protein surface and exposed to the surrounding solution, whereas
lactoferrin becomes a closed, stable form when the iron is bound
and is less likely to release iron than transferrin. It is known
that bovine lactoferrin and natural human lactoferrin have similar
three-dimensional structures, with human lactoferrin consisting of
691 and bovine lactoferrin composed of 689 amino acids (31). The iron saturation of bovine
lactoferrin is 15-20%, and that of natural human lactoferrin is
~10% (32). The bovine lactoferrin
used in the present study has an iron saturation of 3.6-25.0%,
indicating that it has extra capacity to capture iron ions.
Generally, it has been considered that when lactoferrin is
administered to cells, lactoferrin removes iron, thereby decreasing
oxidative stress in the cells (33,34);
however, this was not the case in the present study. It was
hypothesized that when lactoferrin takes up ferrous irons, the
ferrous ions are immediately oxidized and stabilized as ferric
irons, and along with that oxidation process, reactive oxygen
species are generated in cells.
In the present study, the author first tried DCFH-DA
to detect a wide range of reactive oxygen species in cells;
however, as there was a problem with cells peeling off, the author
switched to the NBT method. Since superoxide anion radicals are
also critical intracellular reactive oxygen species, this has been
discussed as much as possible with the data in the present study.
In the future, the author would also like to examine how
lactoferrin treatment affects drug-resistant neuroblastoma cells,
such as SK-N-Be2c and KCNR.
In conclusion, the present study demonstrated that
lactoferrin inhibited the proliferation of neuroblastoma cells even
under X-rays, accompanied by cell membrane disruption. In the
Fenton reaction system with
Fe2Cl2/H2O2,
lactoferrin increased hydroxyl radical (OH·) formation via
H2O2, as confirmed by ESR spectra.
Lactoferrin, which is found abundantly in milk and a food component
in dairy products, may help to prevent or treat neuroblastoma in
infants with modest efficacy, and it did not exert a protective
effect against X-rays.
Acknowledgements
The author would like to thank Otsuka Electronics
Co., Ltd. (Osaka, Japan) for their technical assistance in
measuring electrophoretic mobility and other properties of
lactoferrin. The measurement of electron spin resonance spectra was
supported by the Equipment Sharing Division, Organization for
Co-Creation Research and Social Contributions, Nagoya Institute of
Technology.
Funding
Funding: The present study was supported by an intramural
research grant from Mie University (Mie, Japan).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author upon reasonable
request.
Author's contributions
SK was involved in the conceptualization,
methodology, investigation and writing of the study. SK confirms
the authenticity of all the raw data. The author has read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that he has no competing
interests.
References
|
1
|
Ertugrul MS, Nadaroglu H, Nalci OB,
Hacimuftuoglu A and Alayli A: Preparation of CoS
nanoparticles-cisplatin bio-conjugates and investigation of their
effects on SH-SY5Y neuroblastoma cell line. Cytotechnology.
72:885–896. 2020.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
2
|
Ito R, Asami S, Kagawa S, Motohashi S,
Shichino H, Chin M, Yoshida Y, Nemoto N, Mugishima H and Suzuki T:
Usefulness of tyrosine hydroxylase mRNA for diagnosis and detection
of minimal residual disease in neuroblastoma. Biol Pharm Bull.
27:315–318. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Qiao Y, Sunada NK, Hatada AE, Lange I,
Khutsishvili M, Alizade V, Atha D, Ko'omoa-Lange DL and Borris RP:
Potential anti-neuroblastoma agents from Juniperus oblonga. Biochem
Biophys Res Commun. 516:733–738. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pohl A, Erichsen M, Stehr M, Hubertus J,
Bergmann F, Kammer B and von Schweinitz D: Image-defined risk
factors correlate with surgical radicality and local recurrence in
patients with neuroblastoma. Klin Padiatr. 228:118–123.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boyd JE, Parmley RT, Langevin AM and
Saldívar VA: Neuroblastoma presenting as acute monoblastic
leukemia. J Pediatr Hematol Oncol. 18:206–212. 1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zheng T, Ménard M and Weiss WA:
Neuroblastoma metastases: Leveraging the avian neural crest. Cancer
Cell. 32:395–397. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
London WB, Castleberry RP, Matthay KK,
Look AT, Seeger RC, Shimada H, Thorner P, Brodeur G, Maris JM,
Reynolds CP and Cohn SL: Evidence for an age cutoff greater than
365 days for neuroblastoma risk group stratification in the
children's oncology group. J Clin Oncol. 23:6459–6465.
2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tumilowicz JJ, Nichols WW, Cholon JJ and
Greene AE: Definition of a continuous human cell line derived from
neuroblastoma. Cancer Res. 30:2110–2118. 1970.PubMed/NCBI
|
|
9
|
Imashuku S, Inui A, Nakamura T, Tanaka J
and Miyake S: Catecholamine metabolism in tissue culture cells of a
neuroblastoma. J Clin Endocrinol Metab. 36:931–936. 1973.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ji B, Maeda J, Higuchi M, Inoue K, Akita
H, Harashima H and Suhara T: Pharmacokinetics and brain uptake of
lactoferrin in rats. Life Sci. 78:851–855. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sriramoju B, Kanwar RK and Kanwar JR:
Lactoferrin induced neuronal differentiation: A boon for brain
tumours. Int J Dev Neurosci. 41:28–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng L, Li J, Qin L, Guo D, Ding H and
Deng D: Radioprotective effect of lactoferrin in mice exposed to
sublethal X-ray irradiation. Exp Ther Med. 16:3143–3148.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nishimura Y, Homma-Takeda S, Kim HS and
Kakuta I: Radioprotection of mice by lactoferrin against
irradiation with sublethal X-rays. J Radiat Res. 55:277–282.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wei YL, Xu JY, Zhang R, Zhang Z, Zhao L
and Qin LQ: Effects of lactoferrin on X-ray-induced intestinal
injury in Balb/C mice. Appl Radiat Isot. 146:72–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kato S: Under lithium carbonate
administration, nicotine triggers cell dysfunction in human
glioblastoma U-251MG cells, which is distinct from cotinine. Med
Int (Lond). 2(19)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kato S: Effects of platinum-coexisting
dopamine with X-ray irradiation upon human glioblastoma cell
proliferation. Hum Cell. 34:1653–1661. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zaatiti H, Abdallah J, Nasr Z, Khazen G,
Sandler A and Abou-Antoun TJ: Tumorigenic proteins upregulated in
the MYCN-amplified IMR-32 human neuroblastoma cells promote
proliferation and migration. Int J Oncol. 52:787–803.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tanimoto T, Tazawa H, Ieda T, Nouso H,
Tani M, Oyama T, Urata Y, Kagawa S, Noda T and Fujiwara T:
Elimination of MYCN-amplified neuroblastoma cells by
telomerase-targeted oncolytic virus via MYCN suppression. Mol Ther
Oncolytics. 18:14–23. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ishiyama M, Tominaga H, Shiga M, Sasamoto
K, Ohkura Y and Ueno K: A combined assay of cell viability and in
vitro cytotoxicity with a highly water-soluble tetrazolium salt,
neutral red and crystal violet. Biol Pharm Bull. 19:1518–1520.
1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Soh N, Ariyoshi T, Fukaminato T, Nakajima
H, Nakano K and Imato T: Swallow-tailed perylene derivative: A new
tool for fluorescent imaging of lipid hydroperoxides. Org Biomol
Chem. 5:3762–3768. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Wu L, Oshima T, Shan J, Sei H, Tomita T,
Ohda Y, Fukui H, Watari J and Miwa H: PAR-2 activation enhances
weak acid-induced ATP release through TRPV1 and ASIC sensitization
in human esophageal epithelial cells. Am J Physiol Gastrointest
Liver Physiol. 309:G695–G702. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Baehner RL and Nathan DG: Leukocyte
oxidase: Defective activity in chronic granulomatous disease.
Science. 155:835–836. 1967.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi HS, Kim JW, Cha YN and Kim C: A
quantitative nitroblue tetrazolium assay for determining
intracellular superoxide anion production in phagocytic cells. J
Immunoassay Immunochem. 27:31–44. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Elfinger M, Maucksch C and Rudolph C:
Characterization of lactoferrin as a targeting ligand for nonviral
gene delivery to airway epithelial cells. Biomaterials.
28:3448–3455. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Suzuki YA, Lopez V and Lönnerdal B:
Mammalian lactoferrin receptors: Structure and function. Cell Mol
Life Sci. 62:2560–2575. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bi C, Wang A, Chu Y, Liu S, Mu H, Liu W,
Wu Z, Sun K and Li Y: Intranasal delivery of rotigotine to the
brain with lactoferrin-modified PEG-PLGA nanoparticles for
Parkinson's disease treatment. Int J Nanomedicine. 11:6547–6559.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bannister JV, Bannister WH, Hill HA and
Thornalley PJ: Enhanced production of hydroxyl radicals by the
xanthine-xanthine oxidase reaction in the presence of lactoferrin.
Biochim Biophys Acta. 715:116–120. 1982.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Klebanoff SJ and Waltersdorph AM:
Prooxidant activity of transferrin and lactoferrin. J Exp Med.
172:1293–1303. 1990.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pietrantoni A, Ammendolia MG, Tinari A,
Siciliano R, Valenti P and Superti F: Bovine lactoferrin peptidic
fragments involved in inhibition of echovirus 6 in vitro infection.
Antiviral Res. 69:98–106. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chung TDY and Raymond KN: Lactoferrin: The
role of conformational changes in its iron binding and release. J
Am Chem Soc. 115:6765–6768. 1993.
|
|
31
|
Lorget F, Clough J, Oliveira M, Daury MC,
Sabokbar A and Offord E: Lactoferrin reduces in vitro osteoclast
differentiation and resorbing activity. Biochem Biophys Res Commun.
296:261–266. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gibbons JA, Kanwar JR and Kanwar RK:
Iron-free and iron-saturated bovine lactoferrin inhibit survivin
expression and differentially modulate apoptosis in breast cancer.
BMC Cancer. 15(425)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shoji H, Oguchi S, Shinohara K, Shimizu T
and Yamashiro Y: Effects of iron-unsaturated human lactoferrin on
hydrogen peroxide-induced oxidative damage in intestinal epithelial
cells. Pediatr Res. 61:89–92. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wakabayashi H, Matsumoto H, Hashimoto K,
Teraguchi S, Takase M and Hayasawa H: Inhibition of
iron/ascorbate-induced lipid peroxidation by an N-terminal peptide
of bovine lactoferrin and its acylated derivatives. Biosci
Biotechnol Biochem. 63:955–957. 1999.PubMed/NCBI View Article : Google Scholar
|