Introduction
Papillary thyroid carcinoma (PTC) is the most common
pathological type of thyroid malignant tumor, accounting for
>80% of all thyroid cancers and typically occurs in young women
or children (1). PTC usually
presents as palpable thyroid mass or nodule and may be associated
with hoarseness, dysphagia, stridor or pain (2). Although the majority of the PTCs have
a good prognosis by surgical resection combined with radioiodine
and levothyroxine treatment, metastasis and recurrence occur for
certain PTCs (3). Certain clinical
and pathological characteristics have been associated with a poor
prognosis, such as advanced age at diagnosis, larger primary tumor
(≥3 cm), extrathyroidal invasion, lymph node metastasis and
advanced tumor-node-metastasis (TNM) stage (4,5).
Therefore, a clear understanding of the pathogenesis of PTC is
intrinsically valuable in the identification of novel diagnostic,
prognostic and therapeutic targets.§
MicroRNAs (miRNAs) are a class of non-protein-coding
RNAs that regulate gene expression at the post-transcriptional and
translational levels (6).
Accumulating evidence suggests that miRNAs play essential roles in
the regulation of tumor cell proliferation, differentiation,
apoptosis and metastasis (7). It is
well known that microRNA-101 (miR-101) is a tumor-suppressive miRNA
that is downregulated in several cancer types. For example, miR-101
was significantly downregulated in gastric cancer in comparison
with normal gastric mucosas (8).
Enhanced miR-101 expression suppressed colony formation ability as
well as tumor cell motility of colorectal cancer (9). miR-101 expression is also involved in
other cancer types including breast, liver and prostate cancer
(10). However, the expression and
biological functional roles of miR-101 in PTC are largely
unknown.
The family of Ras homologue (Rho) plays a vital role
in multiple cell functions as a molecular switch (11). Ras-related C3 botulinum toxin
substrate 1 (Rac1), a member of the Rho family, plays a vital role
in multiple cell functions including tumor cell proliferation,
apoptosis, angiogenesis and metastasis (12). These findings underline the
involvement of Rac1 in the development and progression of cancer
types.
The role of miR-101 in PTC via targeting of Rac1 was
therefore investigated. The results demonstrated that miR-101 was
clearly downregulated in PTC tissue compared with that in adjacent
normal thyroid tissue and restoration of miR-101 expression was
able to reduce the proliferation of K1 PTC cells. Furthermore, we
found that miR-101 is involved in PTC progression by directly
targeting Rac1. To the best of our knowledge, this is the first
study to support the hypothesis that miR-101 downregulation is
involved in the development of PTC.
Materials and methods
Cell culture
The K1 PTC cell line was purchased from the European
Collection of Animal Cell Cultures (ECACC, Salisbury, UK). The cell
line was maintained in RPMI-1640 medium supplemented with 10% fetal
bovine serum.
Patients and tissue specimens
A total of 10 cases of PTC, which had been
clinically and histologically diagnosed at the First Affiliated
Hospital of Sun Yat-sen University (China), were obtained following
patient consent and approval from the Institutional Research Ethics
Committee of Sun Yat-sen University.
Plasmids and constructs
pCMV-miR-101 was purchased from OriGene (Rockville,
MD, USA). pCMV-hygro-Rac1 was purchased from Sino Biological
(Beijing, China). 3′-UTRs of Rac1 were amplified and then cloned
into a modified pGL3 control vector where SacII and
PstI sites were introduced into the original XbaI
site downstream of the luciferase gene. To mutate the binding sites
within the 3′-UTR, a QuikChange site-directed mutagenesis kit from
Stratagene (La Jolla, CA, USA) was used according to the
manufacturer’s instructions.
Western blot analysis
Cells were harvested, washed with 1X
phosphate-buffered saline and lysed in mammalian protein extraction
reagent RIPA (Beyotime, Jiangsu, China). A bicinchoninic acid
protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA)
was used for protein quantification. Proteins were separated in
SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad,
Richmond, CA, USA) and immunoblotted with antibodies. The primary
antibodies used were anti-Rac1 (Epitomics, Burlingame, CA, USA) and
anti-α-tubulin (Sigma-Aldrich, St. Louis, MO, USA).
RNA extraction and quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. The primers used for
detection of mature miR-101 and U6 were designed by and purchased
from Riobio Biotech Corporation (Guangzhou, China). The primers
used for mRNAs were: Rac1, forward: 5′-CTGATGCAGGCCATCA AGT-3′ and
reverse: 5′-TCTCCAGGAAATGCATTGGT-3′; GAPDH, forward:
5′-TGGTGGACCTCATGGCCTAC-3′ and reverse:
5′-CAGCAACTGAGGGCCTCTCT-3′.
Transient transfection
For siRNA transfection, Lipofectamine 2000
(Invitrogen Life Technologies) was used following the
manufacturer’s instructions. The siRNA sequence for Rac1 was
5′-GAGGAAGAGAAAAUGCCUG-3′ (13).
MTT assay
Cell proliferation was measured by [3-(4,5-
dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT)
assay.
Colony formation
The indicated cells were plated in 6-well plates
(500 cells per plate), cultured for 10 days and the colonies were
stained with crystal violet.
Luciferase assay
Dual-luciferase reporter assays were performed as
per the manufacturer’s instructions (Promega Corporation, Madison,
WI, USA), as previously described (14).
Statistical analysis
Data were expressed as mean ± SD. Statistical
significance was determined with t-test or analysis of variance by
the SPSS 13.0 software. P<0.05 was considered statistically
significant difference.
Results
miR-101 is downregulated in PTC
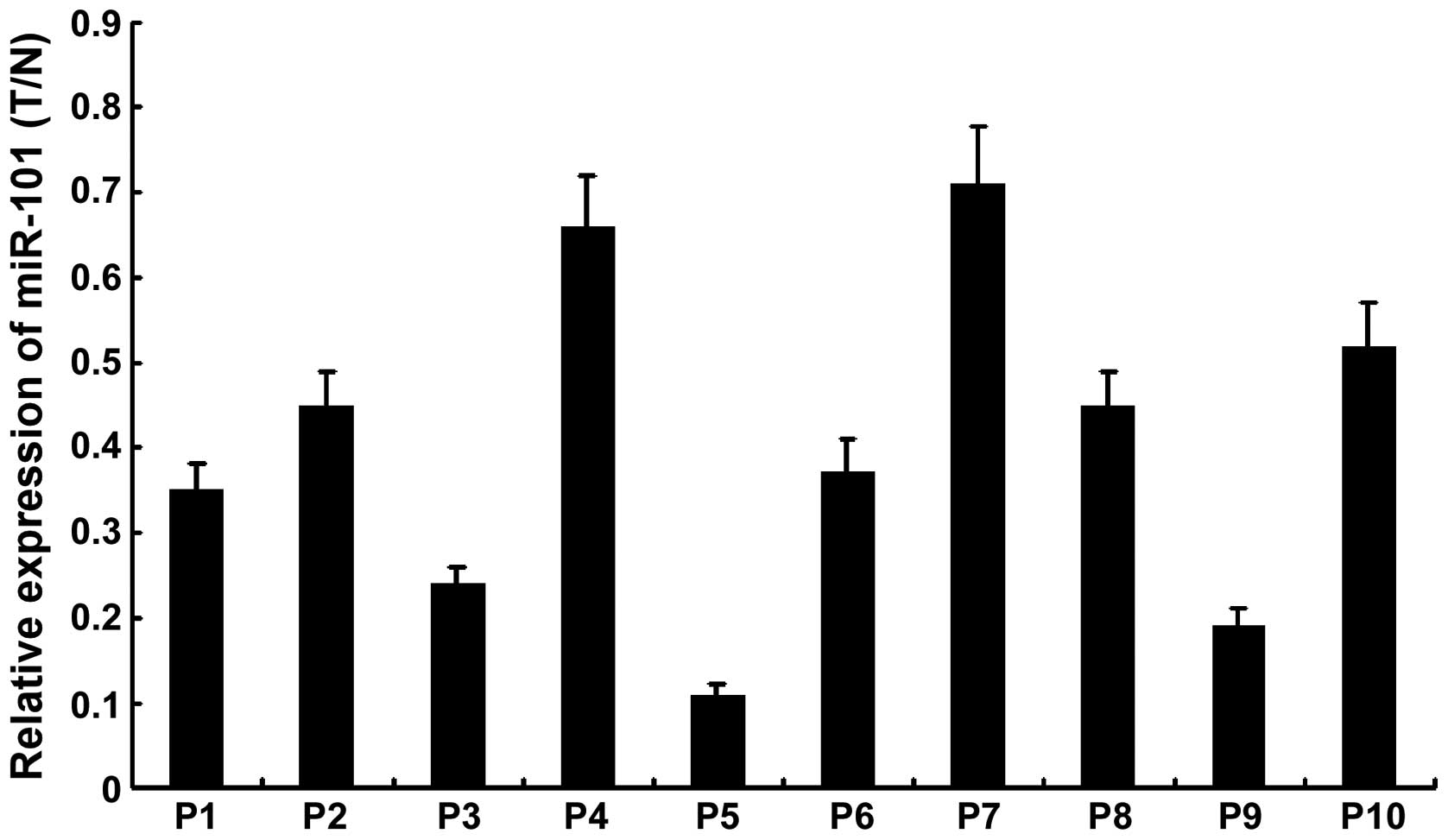
To detect a possible involvement of miR-101 in PTC,
a quantitative RT-PCR assay was performed to examine the expression
level of miR-101 in 5 pairs of PTC tissues (T) and their adjacent
non-cancerous thyroid tissues (N). As shown in Fig. 1, miR-101 was significantly
downregulated in PTC tissues as compared with that in the adjacent
non-cancerous thyroid tissues.
miR-101 inhibits K1 cells
proliferation
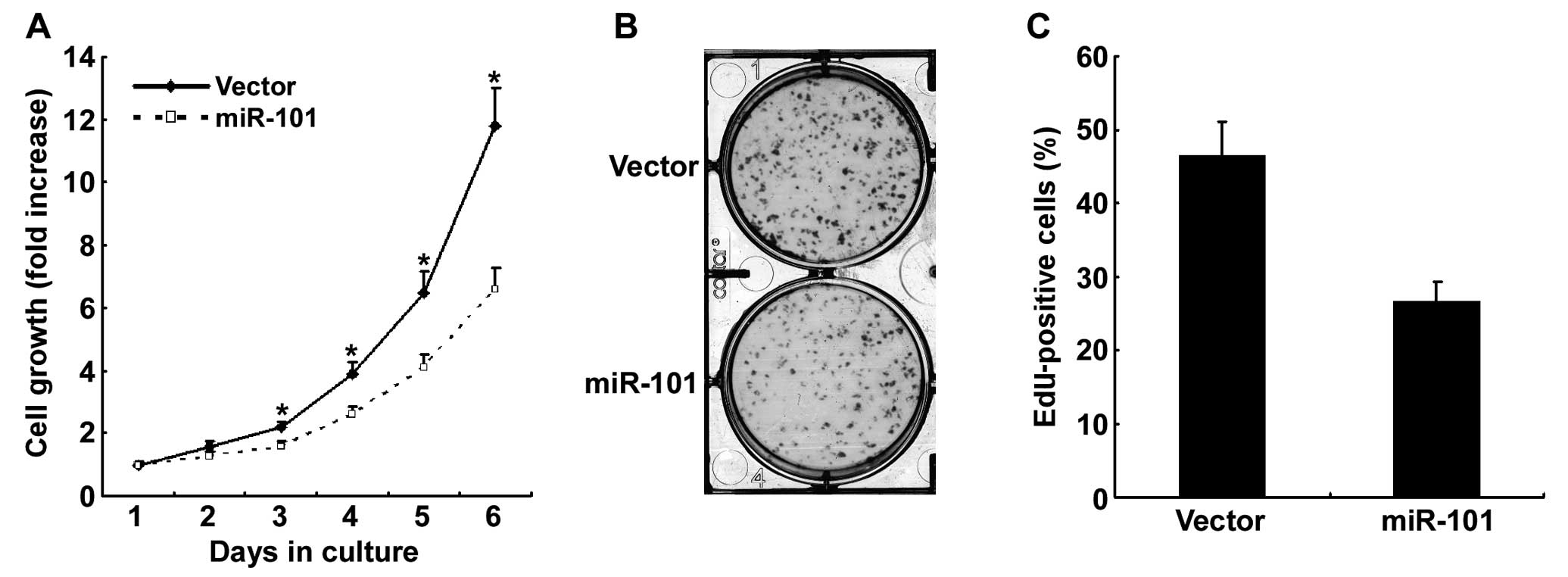
To investigate the possible function of miR-101 in
PTC, we transfected miR-101 into the K1 PTC cell line and examined
its effect on cell growth. Results of MTT assay showed that the
restoration of miR-101 expression resulted in the inhibition of the
growth of K1 cells as compared with the vector control (Fig. 2A). In addition, the colony formation
assay exhibited that miR-101-overexpressing K1 cells generated a
significantly lower number of colonies in comparison with vector
control cells (Fig. 2B). The EdU
incorporation assay revealed that the restored expression of
miR-101 resulted in reduced proliferation of thyroid cancer cells
(Fig. 2C). These data demonstrate
miR-101 is involved in thyroid cancer cell proliferation.
miR-101 directly targets Rac1 expression
in PTC
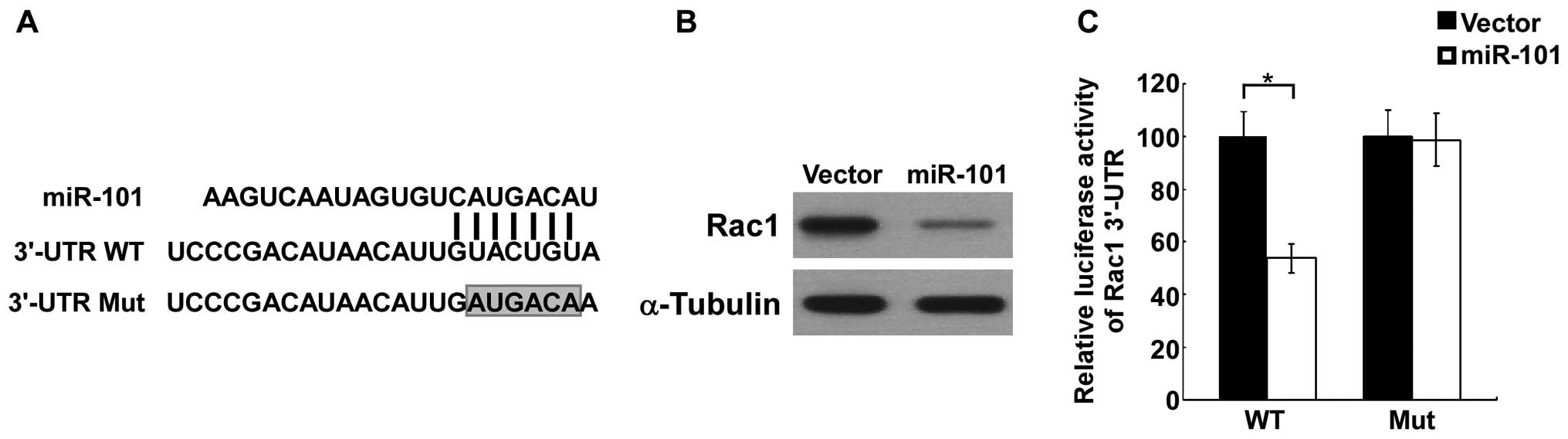
To explore the mechanism of miR-101-induced cell
growth inhibition, we searched putative targets of miR-101 using
TargetScan (http://www.targetscan.org/) and miRanda (www.microrna.org/miranda_new.html). The conserved
target gene Rac1 was identified and used for subsequent
investigation (Fig. 3A). The
protein level of Rac1 was significantly downregulated in
miR-101-overexpressing K1 cells (Fig.
3B), whereas no significant change in the mRNA levels of Rac1
was observed (data not shown). Luciferase reporter assays were
performed to investigate the direct interaction between miR-101 and
Rac1. As shown in Fig. 3C, ectopic
expression of miR-101 significantly inhibited the luciferase
acitivity of a reporter construct containing the 3′-UTR Rac1.
Moreover, when a mutation was introduced into the predicted miR-101
site in 3′-UTR Rac1, the reporter failed to response to miR-101,
suggesting the silencing was associated with the predicted target
site. Taken together, these data suggest that Rac1 is a direct
target of miR-101.
Rac1 is functionally related with the
effect of miR-101
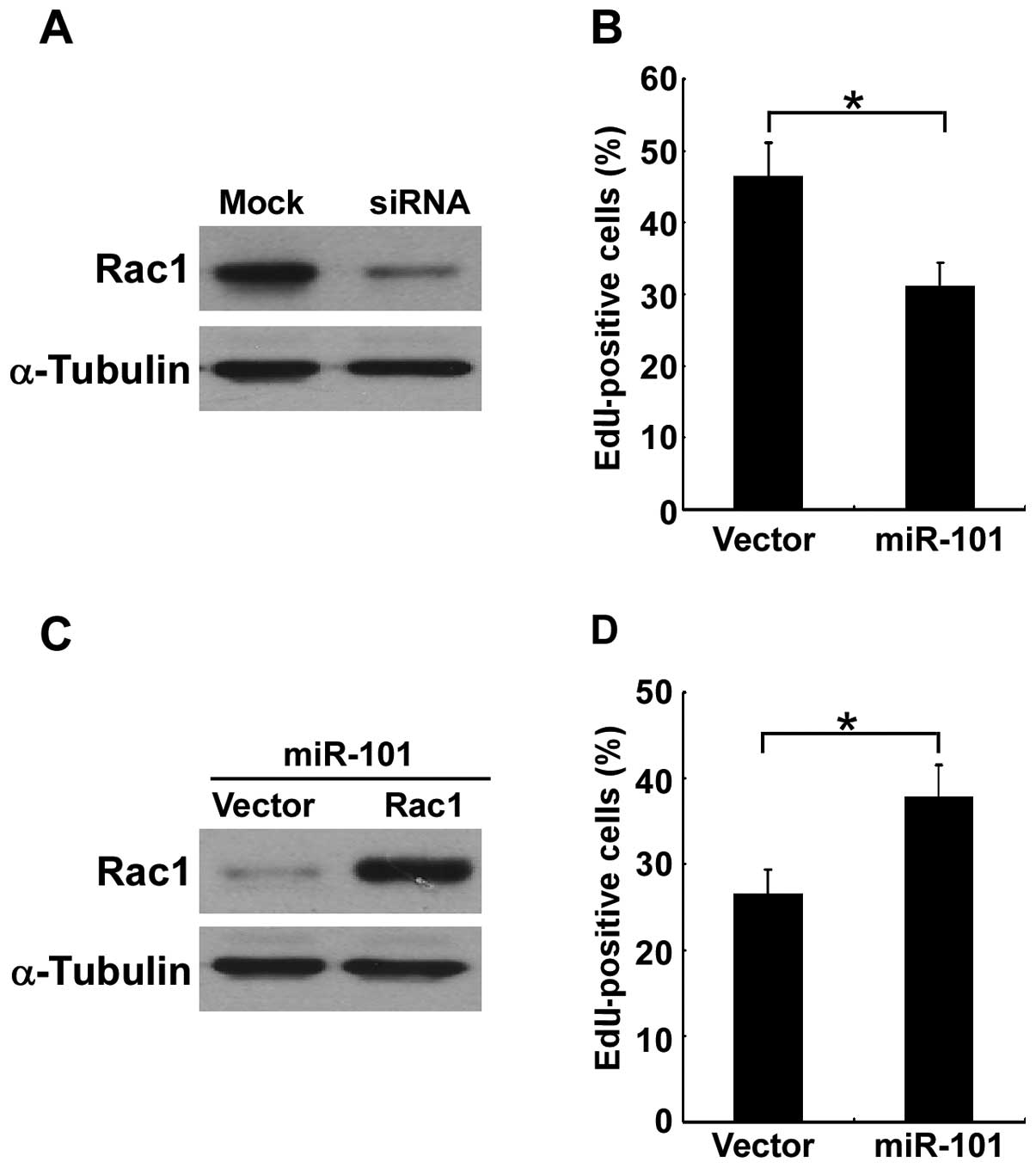
In order to investigate the role of Rac1 in PTC cell
proliferation, we knocked down the expression of Rac1 in K1 cells
(Fig. 4A). It was shown that the
suppression of miR-101 led to the inhibition of cell proliferation
(Fig. 4B). Moreover, re-expression
of Rac1 in miR-101-expressing K1 cells (Fig. 4C) partially antagonized the
miR-101-induced inhibition of cell proliferation (Fig. 4D. These data suggest that Rac1 is a
functionally relevant target of the effect of miR-101 on PTC cell
proliferation.
Discussion
A vital biological feature of tumors is their
potential for unrestrained growth. The activation of oncogenes and
the aberrant expression of tumor suppressor genes are the main
factors leading to tumors (15).
miRNAs are small, single-stranded RNAs that regulate gene
expression (16). In recent
studies, miRNAs have been known to be involved in the pathogenesis
of cancer and miRNA expression profiles are associated with
prognosis in various types of cancer (17). miRNAs with increased expression
levels in tumors may function as oncogenes and promote cancer
development by negatively regulating tumor suppressor genes. By
contrast, the miRNAs frequently downregulated in cancer may
function as tumor suppressor and inhibit cancer development by
downregulating oncogenes (18).
miR-101 is a well-known tumor suppressive miRNA that is
downregulated in several cancer types. Nevertheless, the expression
and biological function of miR-101 in PTC remains to be
clarified.
Liu et al(19) have identified that 248 miRNAs were
significantly deregulated (P<0.01) in PTC tissues when compared
with their matching normal thyroid tissues. Subsequently,
hsa-miR-101 was identified. Consistent with results of that study,
we found that miR-101 was downregulated in PTC, suggesting that
miR-101 is a tumor suppressor. Furthermore, we investigated the
role of miR-101 in the growth of PTC cells by MTT assay, colony
formation and EdU incorporation assay. As expected, the ectopic
expression of miR-101 inhibited the growth of PTC cells. Our
results therefore indicate that miR-101 is involved in the negative
regulation of PTC cell proliferation. However, the mechanism
underlying the effect of miR-101 on tumor growth remains to be
elucidated.
Target genes of miR-101 were identified to examine
the molecular mechanism underlying the miR-101-induced suppression
of PTC cell growth. According to the in silico analyses, we
predicted Rac1 as a direct target, which encodes a small G-protein
that is an important member of the Rho family (20). Rac1 is overexpressed in various
types of cancer including breast, colon and lung carcinomas
(21–23) and participates in all stages of
tumor formation (24). However, the
effect of Rac1 on PTC remains preliminary and whether miR-101
affects the growth of PTC cells through Rac1 remains to be
determined. We have clearly demonstrated that miR-101 induced the
inhibition of PTC cell proliferation by directly targeting Rac1.
Depletion of Rac1 mimics the anti-proliferative effect of miR-101,
indicating that Rac1 has a functional contribution to PTC cell
proliferation. The results also showed that suppression of Rac1
cannot fully recapitulate the miR-101 effect on PTC cell
proliferation and together with the fact that many other target
genes modulated by miR-101 are found in other types of cancer,
additional studies should be conducted to investigate other target
genes regulated by miR-101 that contribute to PTC
tumorigenesis.
Accumulated evidence have identified miRNAs that aid
in the diagnosis of disease and serve as potential targets for
therapy in the future (25). Recent
successful preclinical therapeutic trials in cancers include
miR-380-5p replacement in neuroblastoma (26) and miR replacement/anti-miR
combination therapy in hepatoblastoma (27). Notably, certain miR-based therapies
have been applied in the clinic, including blockade of miR-122 for
chronic viral hepatitis (28–29).
Our findings provide insight into the therapeutic implications of
miR-101 in PTC.
Acknowledgements
This study was supported by grants from the 5010
project of Sun Yat-sen University (no. 2010002), the Science and
Technology Fund of Guangzhou (no. 1346000270), the Industrial
Technology Research and Development Funds of Guangdong Province,
the Natural Science Foundation of China (no. 81370076) and the
Guangdong Provincial Key Laboratory of Medicine.
References
|
1
|
Lloyd RV, Buehler D and Khanafshar E:
Papillary thyroid carcinoma variants. Head Neck Pathol. 5:51–56.
2011. View Article : Google Scholar
|
|
2
|
Singh A, Butuc R and Lopez R: Metastatic
papillary thyroid carcinoma with absence of tumor focus in thyroid
gland. Am J Case Rep. 14:73–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silver CE, Owen RP, Rodrigo JP, et al:
Aggressive variants of papillary thyroid carcinoma. Head Neck.
33:1052–1059. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Voutilainen PE, Multanen MM, Leppaniemi
AK, et al: Prognosis after lymph node recurrence in papillary
thyroid carcinoma depends on age. Thyroid. 11:953–957. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou CK, Chen RF, Chou FF, et al: miR-146b
is highly expressed in adult papillary thyroid carcinomas with high
risk features including extrathyroidal invasion and the BRAF(V600E)
mutation. Thyroid. 20:489–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen PS, Su JL and Hung MC: Dysregulation
of microRNAs in cancer. J Biomed Sci. 19:902012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O’Day E and Lal A: MicroRNAs and their
target gene networks in breast cancer. Breast Cancer Res.
12:2012010.
|
|
8
|
Carvalho J, van Grieken NC, Pereira PM, et
al: Lack of microRNA-101 causes E-cadherin functional deregulation
through EZH2 up-regulation in intestinal gastric cancer. J Pathol.
228:31–44. 2012.PubMed/NCBI
|
|
9
|
Chandramouli A, Onyeagucha BC,
Mercado-Pimentel ME, et al: MicroRNA-101 (miR-101)
post-transcriptionally regulates the expression of EP4 receptor in
colon cancers. Cancer Biol Ther. 13:175–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang R, Wang HB, Hao CJ, et al: MiR-101 is
involved in human breast carcinogenesis by targeting Stathmin1.
PLoS One. 7:e461732012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fritz G, Just I and Kaina B: Rho GTPases
are over-expressed in human tumors. Int J Cancer. 81:682–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Silva AL, Carmo F and Bugalho MJ: RAC1b
overexpression in papillary thyroid carcinoma: a role to unravel.
Eur J Endocrinol. 168:795–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lewis-Saravalli S, Campbell S and Claing
A: ARF1 controls Rac1 signaling to regulate migration of MDA-MB-231
invasive breast cancer cells. Cell Signal. 25:1813–1819. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan H, Wei G, Wu J, et al:
Down-regulation of miR-218-2 and its host gene SLIT3 cooperate to
promote invasion and progression of thyroid cancer. J Clin
Endocrinol Metab. 98:E1334–El1344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu D, Xia P, Diao D, et al: MiRNA-429
suppresses the growth of gastric cancer cells in vitro. J Biomed
Res. 26:389–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar
|
|
18
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, He M, Hou Y, et al: Expression
profiles of microRNAs and their target genes in papillary thyroid
carcinoma. Oncol Rep. 29:1415–1420. 2013.PubMed/NCBI
|
|
20
|
Wu YJ, Tang Y, Li ZF, Li Z, Zhao Y, Wu ZJ
and Su Q: Expression and significance of Rac1, Pak1 and Rock1 in
gastric carcinoma. Asia Pac J Clin Oncol. Jan 8–2013.(Epub ahead of
print). View Article : Google Scholar
|
|
21
|
Schnelzer A, Prechtel D, Knaus U, et al:
Rac1 in human breast cancer: overexpression, mutation analysis, and
characterization of a new isoform, Rac1b. Oncogene. 19:3013–3020.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jordan P, Brazao R, Boavida MG, Gespach C
and Chastre E: Cloning of a novel human Rac1b splice variant with
increased expression in colorectal tumors. Oncogene. 18:6835–6839.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Wang Y, Zhang Y, et al: Abnormal
expression of p120-catenin, E-cadherin, and small GTPases is
significantly associated with malignant phenotype of human lung
cancer. Lung Cancer. 63:375–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bosco EE, Mulloy JC and Zheng Y: Rac1
GTPase: a ‘Rac’ of all trades. Cell Mol Life Sci. 66:370–374.
2009.
|
|
25
|
Sempere LF: Integrating contextual miRNA
and protein signatures for diagnostic and treatment decisions in
cancer. Expert Rev Mol Diagn. 11:813–827. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Swarbrick A, Woods SL, Shaw A, et al:
miR-380-5p represses p53 to control cellular survival and is
associated with poor outcome in MYCN-amplified neuroblastoma. Nat
Med. 16:1134–1140. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cairo S, Wang Y, de Reynies A, et al: Stem
cell-like micro-RNA signature driven by Myc in aggressive liver
cancer. Proc Natl Acad Sci USA. 107:20471–20476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho WC: MicroRNAs in cancer - from
research to therapy. Biochim Biophys Acta. 1805:209–217.
2010.PubMed/NCBI
|
|
29
|
Wahid F, Shehzad A, Khan T and Kim YY:
MicroRNAs: synthesis, mechanism, function, and recent clinical
trials. Biochim Biophys Acta. 1803:1231–1243. 2010. View Article : Google Scholar : PubMed/NCBI
|