Introduction
Magnetic resonance imaging (MRI) is a commonly used
advanced inspection technique in clinical and scientific research.
At present, the new technology mainly includes functional MRI,
magnetic resonance spectroscopy, three-dimensional spoiled gradient
recalled echo and diffusion tensor imaging (DTI). These
technologies have been extensively applied in the brain, spinal
cord lesions and tumors.
DTI is an MR technique developed on the basis of the
diffusion-weighted imaging (DWI). DTI collects attenuated signal
intensity induced by a diffusion of water molecules in all
directions of space through a diffusion sensitive gradient in
numerous directions, quantitatively describes the three-dimensional
trajectory of spatial diffusion, and can provide a diffusion
direction characteristic of living tissue (1). Under physiological conditions, the
diffusion rate of water molecules in each direction is different,
i.e., diffusion anisotropy. DTI can measure the direction and
degree of diffusion of the water molecules in a three-dimensional
space, describe anisotropic characteristics of tissue and precisely
investigate the distribution of fibers (2,3). Diffusion
anisotropy has been found in muscle, and has also been detected in
the spinal cord and white matter of living tissue in the late
1980s. White matter anisotropy is induced by paralleled distributed
myelinated nerve fibers (4). The
diffusion of white matter was faster in paralleled fibers compared
to vertical fibers. This feature with a color mark can reflect
spatial directivity of the white matter. Thus, the fastest
diffusion direction is the direction of fiber distribution. It is
the same in DTI of skeletal muscle and myocardium. With the
advancement of studies regarding DTI, the DTI application has
become an area of interest for human functional imaging. Since
Clark et al (5) applied DWI in
the spinal cord in 1999, the application of DTI in the spinal cord
already has certain initial experience from ex vivo; in
vivo animal to human. DTI is sensitive to the appearance of
disease, but currently, studies on spinal cord DTI are scarce
(6,7).
This is mainly due to the special structure of the spinal cord. The
cross-sectional area of the spinal cord is relatively small. The
axial spinal cord is oval and uneven in thickness, so the spinal
cord DTI requires an imaging sequence of high resolution and high
signal-to-noise ratio.
DTI is the only method to non-invasively visually
reveal fiber tractography, a unique method to evaluate
pathophysiology of spinal cord diseases, and is mainly used to
diagnose and assess injury, cancer, inflammation and myelomalacia.
DTI is not only extensively applied in the diagnosis and evaluation
of spinal cord diseases, but also in other diseases. The fibrous
ring of the intervertebral disc is composed of arranged collagen
fibers, and has directivity. Therefore, DTI can exhibit the
distribution of fibers of the fibrous ring in vivo. As the
only method to exhibit a nerve fiber bundle in vivo, the
application of DTI in the central nervous system is becoming
increasingly extensive.
Materials and methods
Subjects
A total of 45 subjects with intervertebral disc
degeneration, at the age of 23–66 years (average, 55.98±11.98
years), were enrolled in the study, including 25 males and 20
females. All the subjects suffered from varying degrees of
intervertebral disc degeneration and spinal canal stenosis. Tumor
and various metabolic diseases were excluded. All the subjects
signed informed consent. The study was approved by the Ethics
Committee of the Chinese People Liberation Army (PLA) General
Hospital (Beijing, China).
Equipment
A 3.0 T superconductive MRI machine (MR750; GE
Healthcare, Milwaukee, WI, USA) was used with an 8-channel spine
coil, gradient field 40 mT/m and gradient switching rate 150
mT/m·sec.
Image collection
An axial DTI scan was conducted with a single-shot
spin-echo echo planar imaging sequence with the following
parameters: TR/TE=10,000/72.3 msec; b value, 600
sec/mm2; slice thickness, 3.0 mm; layer spacing, 0;
number of layers, 39; matrix, 128×128; FOV, 24×24 cm; and NEX 1.0,
diffusion sensitive gradient direction 15.
Experimental design and
implementation
According to the nerve root compression conditions,
samples were divided into the compressed and uncompressed groups.
The scan was performed by a technician from the Department of
Radiology (Chinese PLA General Hospital). Prior to scanning,
subjects were informed that their body would remain stationary
during the scan.
Image analysis
Original images of DTI were loaded onto the AW4.4
(Advantage Windows; GE Healthcare) workstation, and measured using
FuncTool software. The apparent diffusion coefficient (ADC) and
fractional anisotropy (FA) were calculated. The region of interest
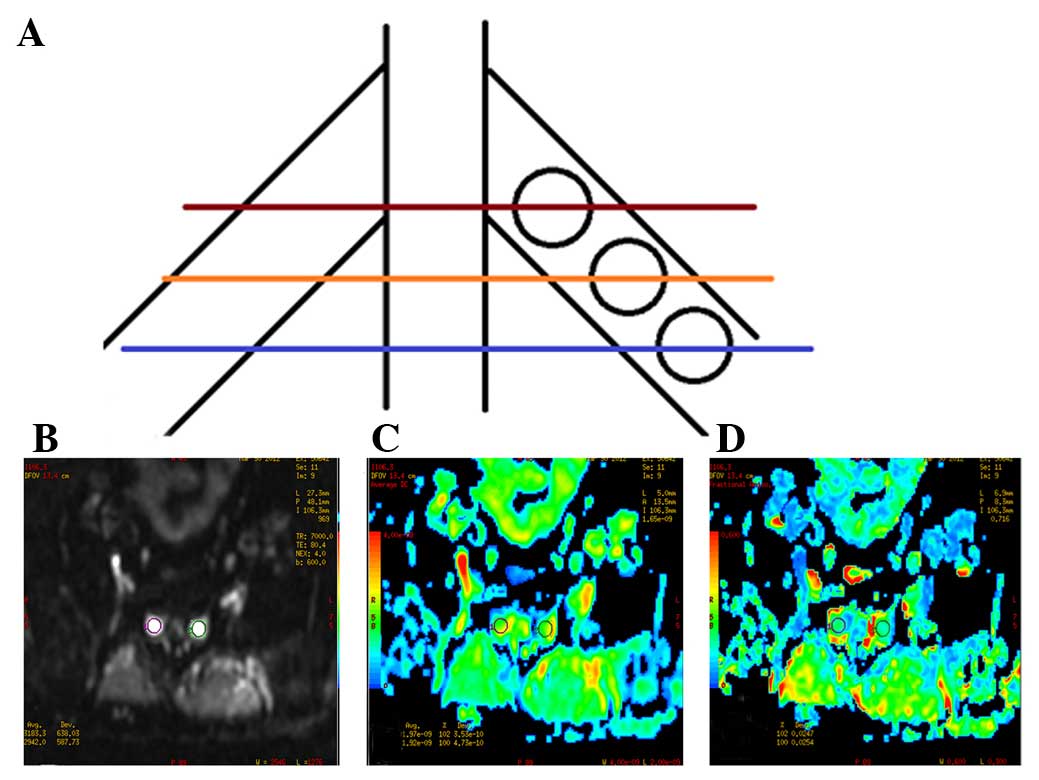
(ROI) was placed on three continuous scan planes, once every plane.
Nerve roots at the proximal, central and distal intervertebral
foramina were separately measured (Fig.
1) three times. The average value of the three measured results
was considered the final ADC and FA values. The ROI size was 20–40
mm2 (Fig. 1). ADC and FA
values of the bilateral nerve roots in L3-L4,
L4-L5 and L5-S1 were
measured in 45 subjects.
Statistical analysis
Quantitative data are expressed as mean ± standard
deviation, and analyzed using a group t-test between the two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of ADC and FA values in the
compressed and uncompressed groups
Among the 45 subjects, the number of nerve roots was
122 in the compressed group and 148 in the uncompressed group.
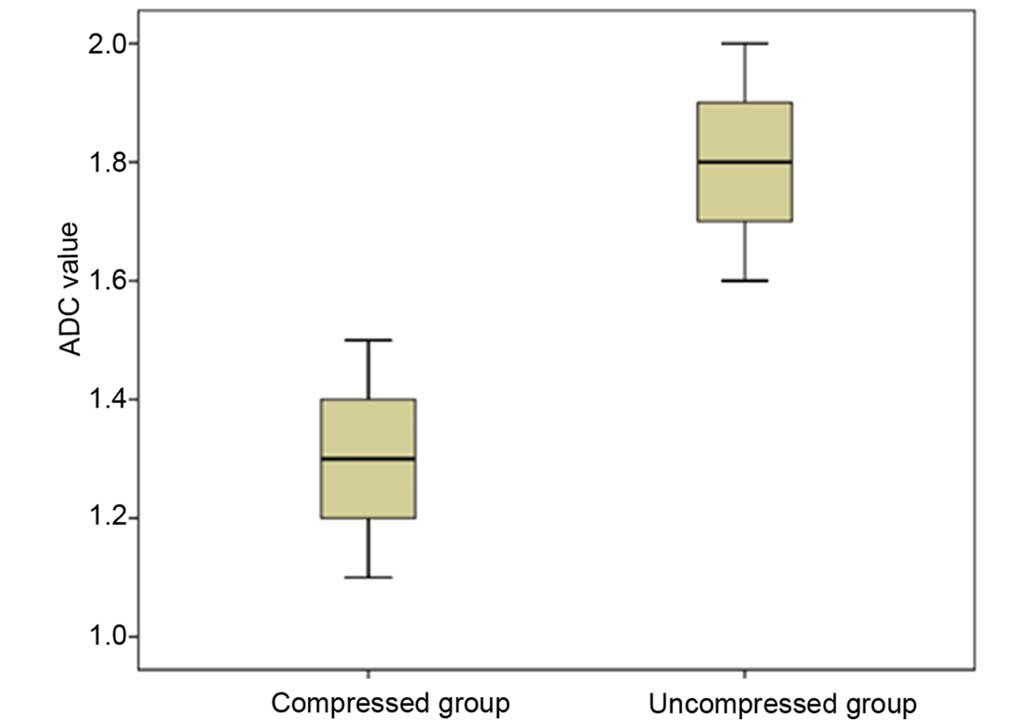
Group t-test indicated that the ADC values were significantly lower
in the compressed group (1.314±0.140×10−3
mm2/sec) compared to the uncompressed group (1.794±0.111
mm2/sec) (P=0.001) (Fig.
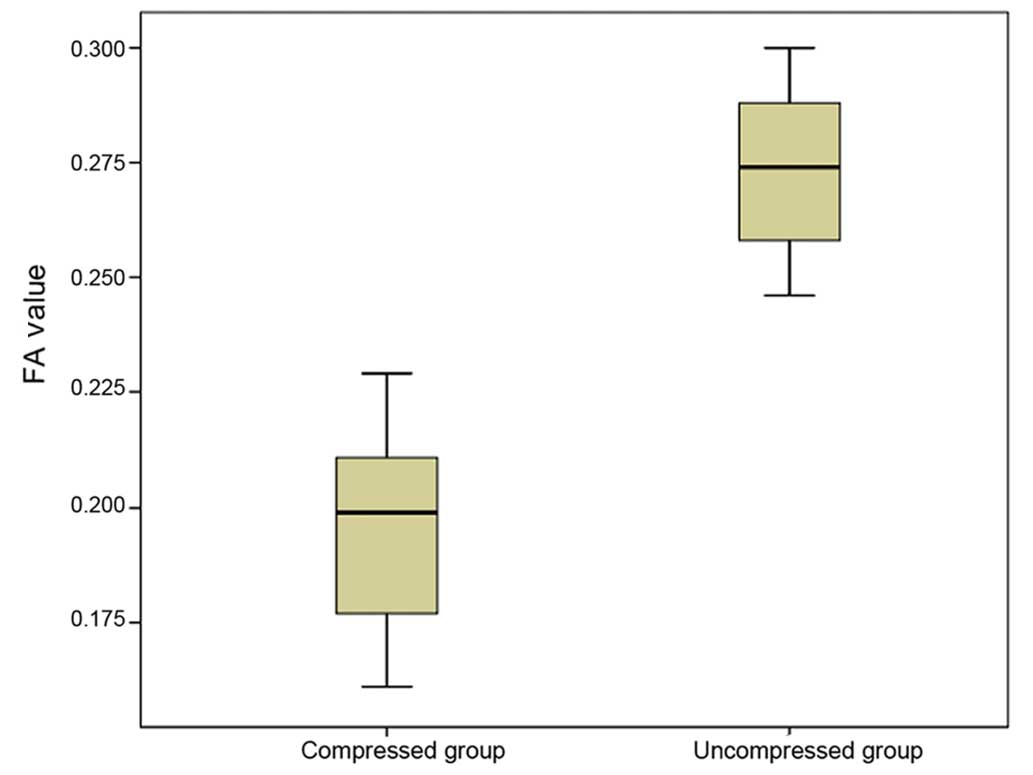
2). FA values were significantly lower in the compressed group
(0.196±0.020) compared to the uncompressed group (0.272±0.016)
(P=0.013; Fig. 3).
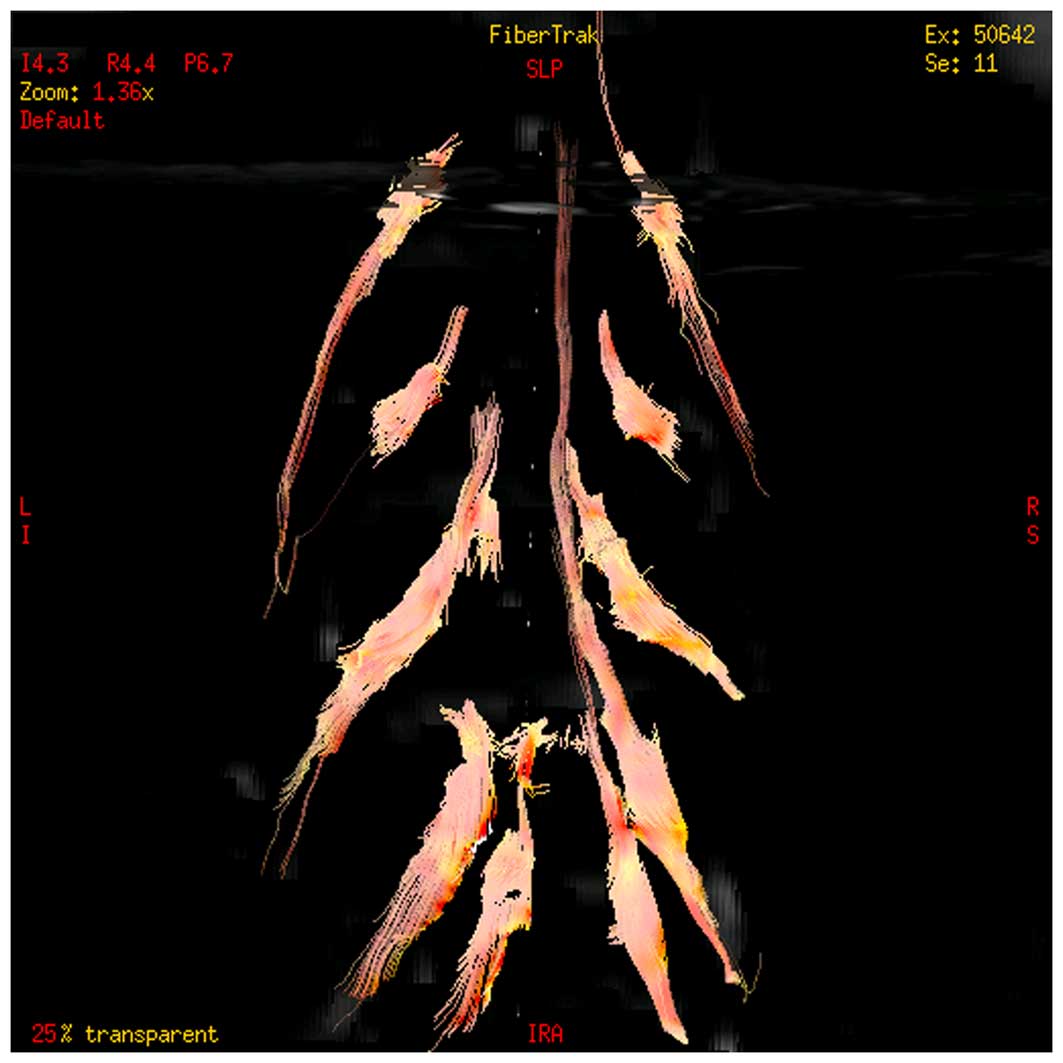
Tracer display
Among the 45 subjects, the DTI scan exhibited the
distribution and compression of the nerve root in 36 subjects
(Fig. 4).
Discussion
The spinal cord reaches the lumbar spinal canal, and
ends at the corresponding level of the L1-L2
intervertebral disc. The lumbar, sacral and caudal nerve roots of
the ventral and dorsal sections fix the spinal cord in the spinal
canal. Descending nerve roots from the spinal cord go sequentially
into their respective intervertebral foramina. Local nerve root
morphology is similar to horsetail fibers, and is therefore known
as cauda equina. Certain degenerative changes, such as
intervertebral disc protrusion and the thickening of ligamentum
flavum, can impact nerve roots and cause pain. Nucleus pulposus of
the prolapsed intervertebral disc can produce an inflammatory
mediator, impact the nerve root, and lead to pain.
With the rapid development of MRI and the increasing
maturity of image processing technologies, numerous processing
technologies of computational neuroanatomy are emerging. DTI can
objectively describe the distribution of nerve roots in morphology,
and provide a set of objective reference data on the quantitative
indicators. A previous study verified that the effects of age and
gender on ADC and FA values were not apparent in normal nerve roots
(1). DTI, as a new imaging technique,
can sensitively find the nerve root changes by measuring the ADC
and FA values. Using the conventional method on ROI, according to
the size of the nerve root cross-section at the proximal, central
and distal intervertebral foramina, ROI was generally set at 30–70
mm2, and the effect of ROI size on measurement results
was not evident (1).
DTI is a new and non-invasive MRI technique, and is
mainly used to quantitatively evaluate microscopic structural
changes of the nervous system, and to detect the blocking effect of
cell membranes and myelin sheath on the movement of water molecules
in the brain, particularly diffusion of water molecules in fiber
bundles of the white matter, such as high anisotropy (2,3). Thus, the
diffusion was faster in water molecules parallel to the direction
of axon and myelin sheath than in vertical direction (2,3). The
structure of fiber bundles observed by FA images was basically
identical to that of the real fiber bundles (4). The voxel analysis of FA value can
sensitively reflect brain lesions to a certain degree (6,7). The
structure of fiber bundles of lumbosacral nerve roots is identical
to that of intracranial nerve fibers, so their imaging principles
are identical.
The imaging method of lumbosacral nerve roots
consists of 3D FIESTA, MR PROSET technology, MRSSI (selective
excitation) technology CUBE-FLEX and DTI. Several previous methods
involve three-dimensional volumetric imaging. Occasionally, tissues
with similar signals may interfere with the image quality. For
example, vascular effects may interfere with nerve root display.
DTI of lumbosacral nerve roots has been initially used in clinical
research, but has not been extensively employed in the clinic due
to the limitation of computer processing and imaging results. This
method can provide a quantitative reference value of nerve root
(ADC and FA values). In the reconstruction of the fiber bundle, a
desired minimum threshold should be determined. If the minimum
threshold is set too low, the reconstructed nerve fibers contain
too many tissues and the edge is rough, so therefore the form of
normal nerve fibers will be lost. If the minimum threshold is set
too high, nerve fibers are rare. In the present study, the minimum
threshold was set at ~200, the obtained DTI images can objectively
reflect the real condition of nerve roots. The biggest advantage of
DTI is to quantify the research index. The present results revealed
that the ADC and FA values of the compressed nerve roots were
evidently lower compared to that of the uncompressed nerve roots,
which is consistent with a previous study (1).
In conclusion, the ADC and FA values of the nerve
roots were evidently lower in the compressed group compared to the
uncompressed group. DTI tractography can satisfactorily exhibit the
compression of nerve roots and can quantitatively assess the
compression of lumbosacral nerve roots in patients with lumbar disc
degeneration.
Acknowledgements
The present study was supported by the PLA 12th
five-year research project (grant no. CWS11J101).
References
|
1
|
Kitamura M, Eguchi Y, Inoue G, Orita S,
Takaso M, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, et
al: A case of symptomatic extra-foraminal lumbosacral stenosis
(‘far-out syndrome’) diagnosed by diffusion tensor imaging. Spine.
37:E854–E857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Basser PJ: Inferring microstructural
features and the physiological state of tissues from
diffusion-weighted images. NMR Biomed. 8:333–344. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Basser PJ, Mattiello J and LeBihan D: MR
diffusion tensor spectroscopy and imaging. Biophys J. 66:259–267.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pierpaoli C, Jezzard P, Basser PJ, Barnett
A and Di Chiro G: Diffusion tensor MR imaging of the human brain.
Radiology. 201:637–648. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clark CA, Barker GJ and Tofts PS: Magnetic
resonance diffusion imaging of the human cervical spinal cord in
vivo. Magn Reson Med. 41:1269–1273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albrecht J, Dellani PR, Müller MJ,
Schermuly I, Beck M, Stoeter P, Gerhard A and Fellgiebel A: Voxel
based analyses of diffusion tensor imaging in Fabry disease. J
Neurol Neurosurg Psychiatry. 78:964–969. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abe O, Yamasue H, Kasai K, Yamada H, Aoki
S, Iwanami A, Ohtani T, Masutani Y, Kato N and Ohtomo K:
Voxel-based diffusion tensor analysis reveals aberrant anterior
cingulum integrity in posttraumatic stress disorder due to
terrorism. Psychiatry Res. 146:231–242. 2006. View Article : Google Scholar : PubMed/NCBI
|