Introduction
Preterm birth is a major cause of neonatal mortality
and morbidity worldwide, and the leading cause of under-five
mortality is complications of preterm birth (1). Improving preterm birth outcomes is
therefore essential to reduce global under-five mortality. More
specifically, intrauterine inflammation is a major cause of preterm
labour and complications in preterm neonates (2). Ascending infection and invasion of
microorganisms into the uterine cavity may lead to intrauterine
inflammation, chorioamnionitis and foetal inflammatory response
syndrome (3). Furthermore, these
conditions are frequently associated with adverse long-term
complications, which include pulmonary and neurological impairments
(4). Thus, an approach to suppress
intrauterine inflammation would reduce the risk of severe
complications and avoid prematurity by extending the duration of
pregnancy, both of which may improve the prognosis of neonates
(5).
Previous reports by our group demonstrated that the
maternal administration of molecular hydrogen (H2),
known for its anti-oxidative and anti-inflammatory effects
(6), ameliorated damage in foetal
pulmonary and brain tissue caused by intrauterine inflammation in
lipopolysaccharide (LPS)-induced rodent models (7–9). There are
more than 300 reports outlining the utility of H2 for
the prevention or improvement of various diseases including
inflammatory disease (6).
H2 has been reported to be a novel antioxidant that
selectively scavenges hydroxyl radicals and peroxynitrates
(10), and to also have an
anti-inflammatory effect through the modulation of signalling
molecules (6). A recent report
demonstrated that H2 suppressed Ca2+
signalling (11), while
Ca2+ mobilization is important for uterine contraction
and labour onset (12). From these
findings, it may be speculated that H2 reduces
proinflammatory cytokine production, which may lead to the
suppression of uterine contractile-associated proteins (CAPs),
Ca2+ signalling and preterm uterine contractions,
ultimately resulting in the prevention of preterm delivery.
However, the potential applications of H2 for the
prevention of preterm birth remain to be elucidated.
To address this, the present study investigated the
effects of H2 administration on uterine inflammation and
aimed to determine whether H2 prevents LPS-induced
preterm delivery. The effects of H2 on the expression of
transcripts associated with inflammation, contraction and tissue
remodeling were also evaluated. Identifying an association between
the administration of H2 and preterm labour may lead to
more effective strategies for preventing preterm labour.
Materials and methods
Reagents
LPS (Escherichia coli LPS, serotype O55:B5)
was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
H2 water (HW) was gifted by Blue Mercury, Inc. (Tokyo,
Japan) and was administered to pregnant mice, as described
previously (13). Briefly, the HW was
stored in aluminium bags at a H2 concentration of
>0.4 mM. HW for drinking was made available to mice from glass
bottles with two ball bearings at the outlet to prevent
H2 degassing. The concentration of H2 was
maintained at >0.2 mM, and H2 was replaced every 24
h.
Animals and treatments
All protocols for animal experiments were approved
by the Animal Experiment Committee of Nagoya University (approval
no. 29154). A total of 38 pregnant ICR (CD-1) mice (51.01±0.86 g,
8–10 weeks old; Charles River Laboratories Japan, Kanagawa, Japan)
were maintained under a 12-h light/dark cycle (lights on 09:00
a.m., lights off 09:00 p.m.). A total of 18 of the mice were
randomly divided into the following three groups (n=6 per group):
Control, LPS and HW + LPS. In the LPS and control groups, mice were
administered an intraperitoneal injection of 500 µg LPS/kg body
weight dissolved in phosphate-buffered saline (PBS), or the same
volume of PBS, respectively, on embryonic day 15.5 (E15.5). In the
HW + LPS group, the mice were administered HW as drinking water 24
h before LPS administration (E14.5) until sacrifice (E15.5). The
mice drank approximately 200 ml/kg of regular water or HW per day,
as previously reported (8). Samples
of serum and uteri from the mice in each group were collected
(storage at −80°C for <3 months) following euthanasia 6 h after
the intraperitoneal injection of PBS or LPS (E15.5). To evaluate
the period between injection of LPS and delivery, the 20 remaining
mice allocated to LPS and HW + LPS groups (n=10 per group) were
checked for parturition every 1 h after LPS administration.
Parturition was not examined in a control group, as this is
presumed to occur at approximately E18-19, as previously reported
(14). The parturition time was
determined as the time at which mice had delivered all pups.
Measurement of progesterone
The concentration of progesterone in maternal serum
(E15.5) was evaluated with a commercially available ELISA kit
(ADI-900-011; Enzo Life Sciences, Inc., Farmingdale, NY, USA),
according to the manufacturer's instructions. The absorbance was
read at 405 nm with a microplate reader (ELx808; BioTek
Instruments, Inc., Winooski, VT, USA).
Reverse transcription-quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted from uteri samples using an
RNeasy mini kit (Qiagen K.K., Tokyo, Japan), and was reverse
transcribed using ReverTra Ace (Toyobo Co., Ltd., Osaka, Japan),
according to the manufacturer's instructions. qPCR was performed on
a Thermal Cycler Dice Real Time System TP800 (Takara Bio, Inc.,
Otsu, Japan) using SYBR Premix Ex Taq II (Takara Bio, Inc.) with
use of a crossing point method for quantification (15). The cycling profile was as follows:
Initial denaturation at 95°C for 30 sec; denaturation at 95°C for 5
sec and annealing at 60°C for 30 sec (40 cycles); and dissociation
at 95°C for 5 sec, 55°C for 10 sec and 72°C for 20 sec. The
expressions of target genes were normalized according to β-actin
expression. The primer sequences used were as follows: For mouse
β-actin forward, 5′-CGTGGGCCGCCCTAGGCACCA-3′ and reverse,
5′-ACACGCAGCTCATTGTA-3′; for mouse interleukin-6 (Il6)
forward, 5′-ACAACCACGGCCTTCCCTAC-3′ and reverse,
5′-TCCACGATTTCCCAGAGAACA-3′, as previously reported (9); for mouse tumour necrosis factor
(Tnf) forward, 5′-GTAGCCCACGTCGTAGCAAAC-3′ and reverse,
5′-CTGGCACCACTAGTTGGTTGTC-3′; for mouse Il8 forward,
5′-GCCCAGACAGAAGTCATAGAA-3′ and reverse,
5′-AGGCTCCTCCTTTCCAGGTC-3′; for mouse cyclooxygenase-2
[Cox2; also known as prostaglandin-endoperoxide synthase 2
(Ptgs2)] forward, 5′-TGCCCAGCACTTCACCCATCA-3′ and reverse,
5′-AGTCCACTCCATGGCCCAGTCC-3′; for mouse connexin-43 [Cx43;
also known as gap junction protein alpha 1 (Gja1)] forward,
5′-TAAGTGAAAGAGAGGTGCCCAGA-3′ and reverse,
5′-GTGGAGTAGGCTTGGACCTTG-3′; for mouse oxytocin receptor
(Oxtr) forward, 5′-TCATCGTGTGCTGGACGCCT-3′ and reverse,
5′-TGTTGAGGCTGGCCAAGAGCAT-3′; for mouse matrix metalloproteinase-3
(Mmp3) forward, 5′-GCTGTCTTTGAAGCATTTGGGTT-3′ and reverse,
5′-ACACAGGATGCCTTCCTTGGAT-3′; and for mouse endothelin-1
(Et1; also known as Edn1) forward,
5′-ACTTGCTGAGGACCGCGCTG-3′ and reverse, 5′-GCTCCGGTGCTGAGTTCGGC-3′.
All primers were purchased from Nippon Gene Co., Ltd. (Tokyo,
Japan).
Immunohistochemistry
The uteri samples (n=6 per group) were fixed with 4%
paraformaldehyde phosphate buffer solution (163-20145; Wako Pure
Chemical Industries, Ltd., Osaka, Japan) at 4°C for 48 h, and
maintained in 70% ethanol at 4°C for <3 months, then embedded in
paraffin. For heat-induced epitope retrieval, deparaffinised
sections at a thickness of 4 µm were placed in 1 mM citrate buffer
(pH 6.0), and heated at 90°C and 750 W using an H2500 microwave
oven for 20 min. Staining was performed using a Histofine SAB-PO
(R) kit (Nichirei Biosciences, Inc., Tokyo, Japan) with
diaminobenzidine as the chromogen, based on the manufacturer's
instructions. Rabbit polyclonal anti-Cox2 (ab52237; Abcam,
Cambridge, UK) was used at a 1:400 dilution as the primary antibody
at 4°C overnight. Finally, the slides were counterstained with
Meyer's haematoxylin at room temperature for 30 sec. For the
quantification of immunostaining, the intensity was evaluated as
intensity score (IS), as previously reported (9). Two independent examiners determined the
IS from three segments per slide at ×400 magnification with an Axio
Imager A1 (Carl Zeiss Microscopy Co., Ltd., Tokyo, Japan) using a
4-point system: 0, 1, 2 and 3 (for no, light, medium and dark
staining, respectively); the mean score from the two examiners was
used.
Statistical analysis
The data are presented as the means ± standard error
of the mean. For comparisons between two groups including
comparisons of times to parturition and progesterone levels, data
were analysed by performing a Student's t-test or Mann-Whitney U
test, according to whether distributions were normal or non-normal,
respectively. For comparison of the rate of preterm birth, a
Fisher's exact test was used. To compare three groups, one-way
analysis of variance (ANOVA) with Kruskal-Wallis post hoc analysis
was used, and P-values were also calculated between two of the
three groups, using a Student's t-test or Mann-Whitney U test, as
above. When the distribution of data was normal, P-values were
calculated by performing one-way ANOVA with a Tukey's honest
significant difference or Games-Howell test, according to whether
variance was equal or unequal, respectively. Statistical analyses
were performed using the SPSS 24 software package (IBM Corp.,
Armonk, NY, USA). Differences between values with P<0.05 were
considered statistically significant.
Results
Effect of H2 on preterm
labour
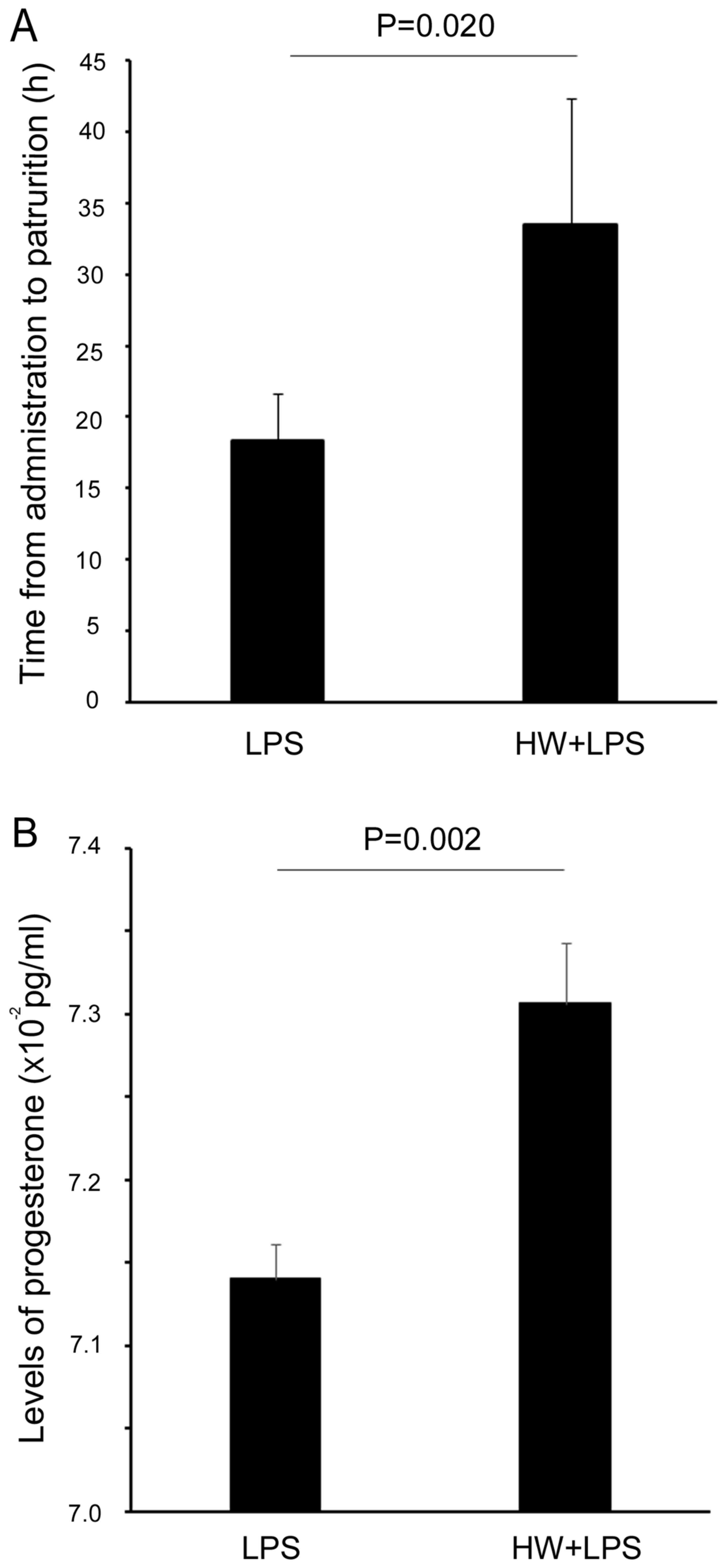
The period between injection of LPS and delivery was
significantly increased in the HW + LPS group compared with the LPS
alone group (33.5±3.4 vs. 18.3±8.8 h, respectively, P=0.020;
Fig. 1A). However, the rate of
preterm birth in the LPS group was not significantly different from
that of the HW + LPS group [number of dams that delivered pups
within 24 h after LPS administration/total number = 9/10 (90%) vs.
6/10 (60%), respectively, P=0.303; data not shown]. The
concentration of progesterone in the maternal serum of the HW + LPS
group was significantly higher than that in the LPS group
(7.30±0.04 vs. 7.14±0.02 × 10−2 pg/ml, respectively,
P=0.002; Fig. 1B).
H2 pretreatment reduces
LPS-induced proinflammatory cytokine levels in the uterus
As depicted in Fig. 2,
the expression of Tnf, Il6, and Il8 mRNA in the uteri
of the LPS group was significantly increased compared with that in
the control group (P=0.006, P=0.004 and P=0.004, respectively).
Maternal HW administration inhibited the expression of Tnf
to levels equivalent to those observed in the control group
(P=0.337; Fig. 2A). The expression of
Il6 in the uteri of the HW + LPS group was significantly
lower than that in the LPS group (P=0.037), but remained higher
than that in the control group (P=0.016; Fig. 2B). The expression of Il8 mRNA
in the uteri of the HW + LPS group was not significantly lower
compared to that in the LPS group (P=0.749; Fig. 2C).
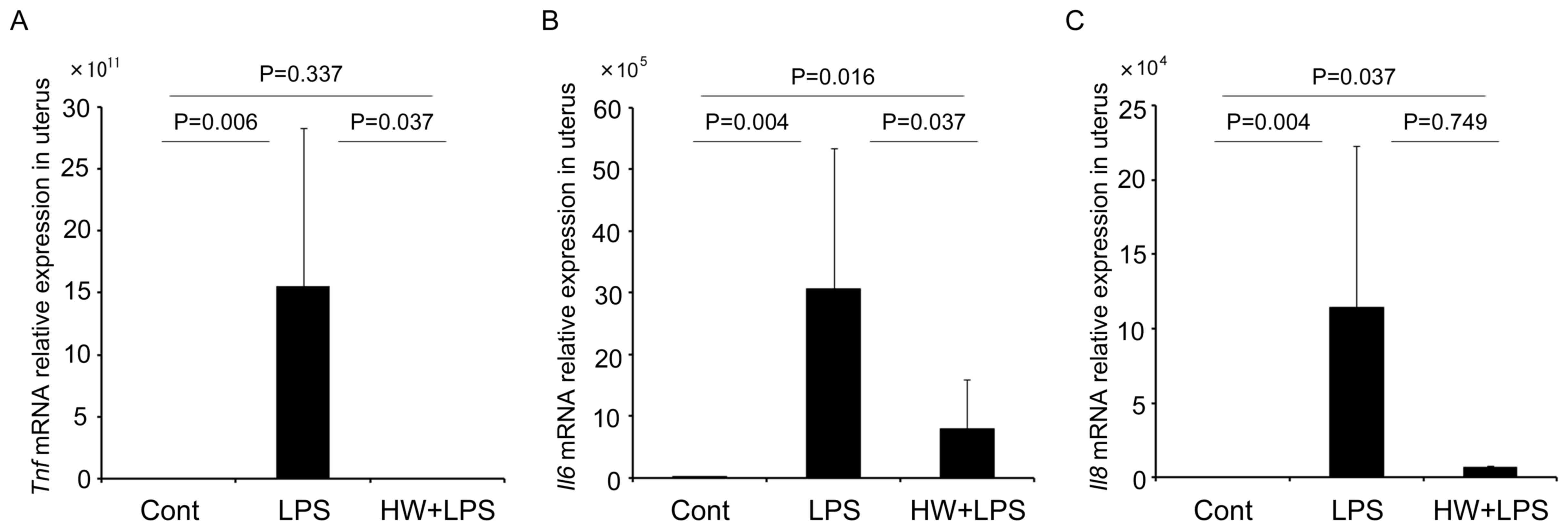 | Figure 2.Levels of Tnf, Il6 and
Il8 mRNA measured by reverse transcription-quantitative
polymerase chain reaction. The data were normalized to the
expression of β-actin and are expressed as the means ± standard
error of the mean (n=6 per group). (A) Tnf, (B) Il6
and (C) Il8 mRNA levels in the uterus of the LPS group were
significantly higher compared with those in the control group;
whereas, the levels in the HW + LPS group were lower compared with
those in the LPS group. All indicated P-values were calculated
using the Mann-Whitney U test. The data among the three groups were
also statistically analysed by the Kruskal-Wallis test, and
P-values for Tnf, Il6, and Il8 were P=0.013, P=0.002
and P=0.015, respectively. Tnf, tumour necrosis factor; Il6/8,
interleukin-6/8; LPS, lipopolysaccharide; HW, H2
water. |
H2 pretreatment
downregulates LPS-induced CAPs in the uterus
As indicated in Fig.
3, the expression of several contractile-associated proteins
(CAPs) transcripts including Cox2 (Ptgs2),
Cx43 (Gja1) and Oxtr was significantly higher
in the uteri of the LPS group compared with that in the control
group (P=0.004, P=0.004 and P=0.006, respectively). Conversely,
their levels were lower in the uteri of the HW + LPS group compared
with those in the LPS group (all P=0.004). Furthermore, the level
of Cx43 mRNA in the HW + LPS group did not differ
significantly different from that in the control group (P=0.423;
Fig. 3B). The expression of
Cox2 in the uteri of the HW + LPS group remained higher than
that in the control group (P=0.004; Fig.
3A). Meanwhile, Oxtr mRNA expression in the uteri of the
HW + LPS group was lower than that in the control group (P=0.041;
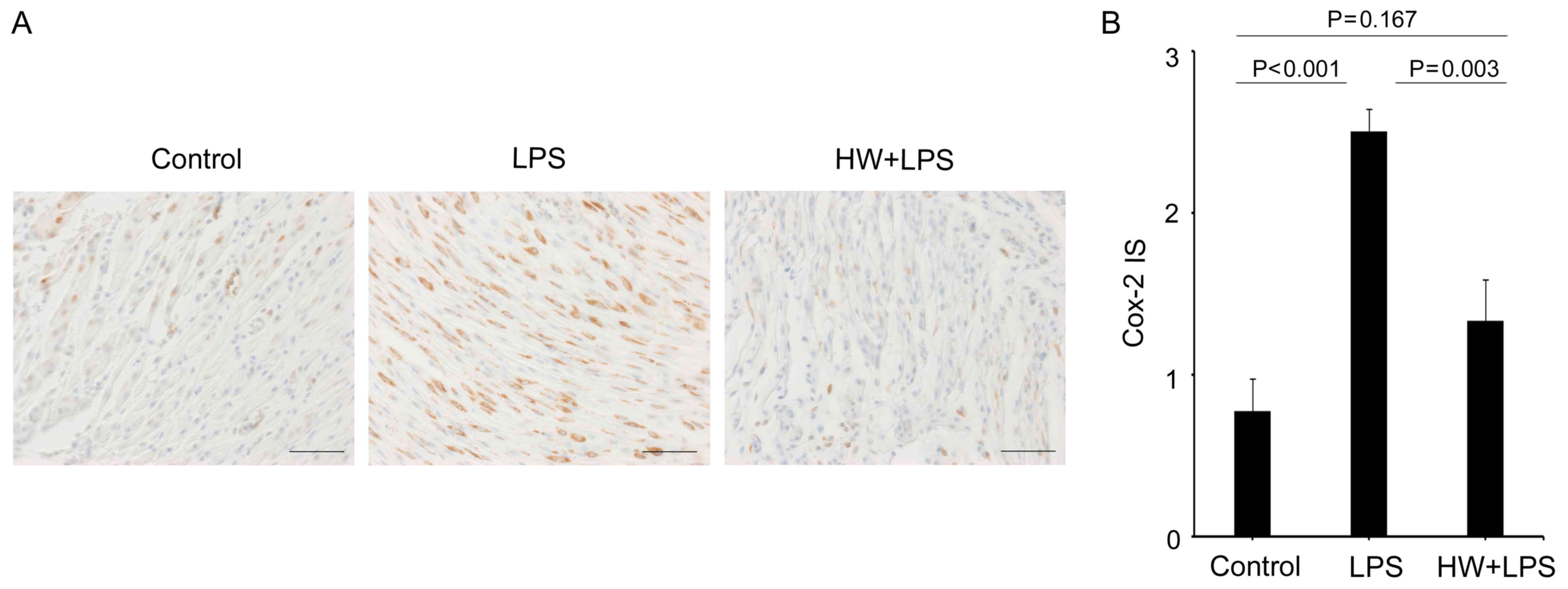
Fig. 3C). The protein expression of
Cox2 (Ptgs2) was significantly higher in the uteri of the LPS group
compared with that in the control group (P<0.001; Fig. 4A and B), while the expression level
was lower in the uteri of the HW + LPS group (P=0.003 vs. LPS
group; Fig. 4A and B). In addition,
the expression of Cox2 in the HW + LPS group did not differ
significantly different from that in the control group (P=0.167;
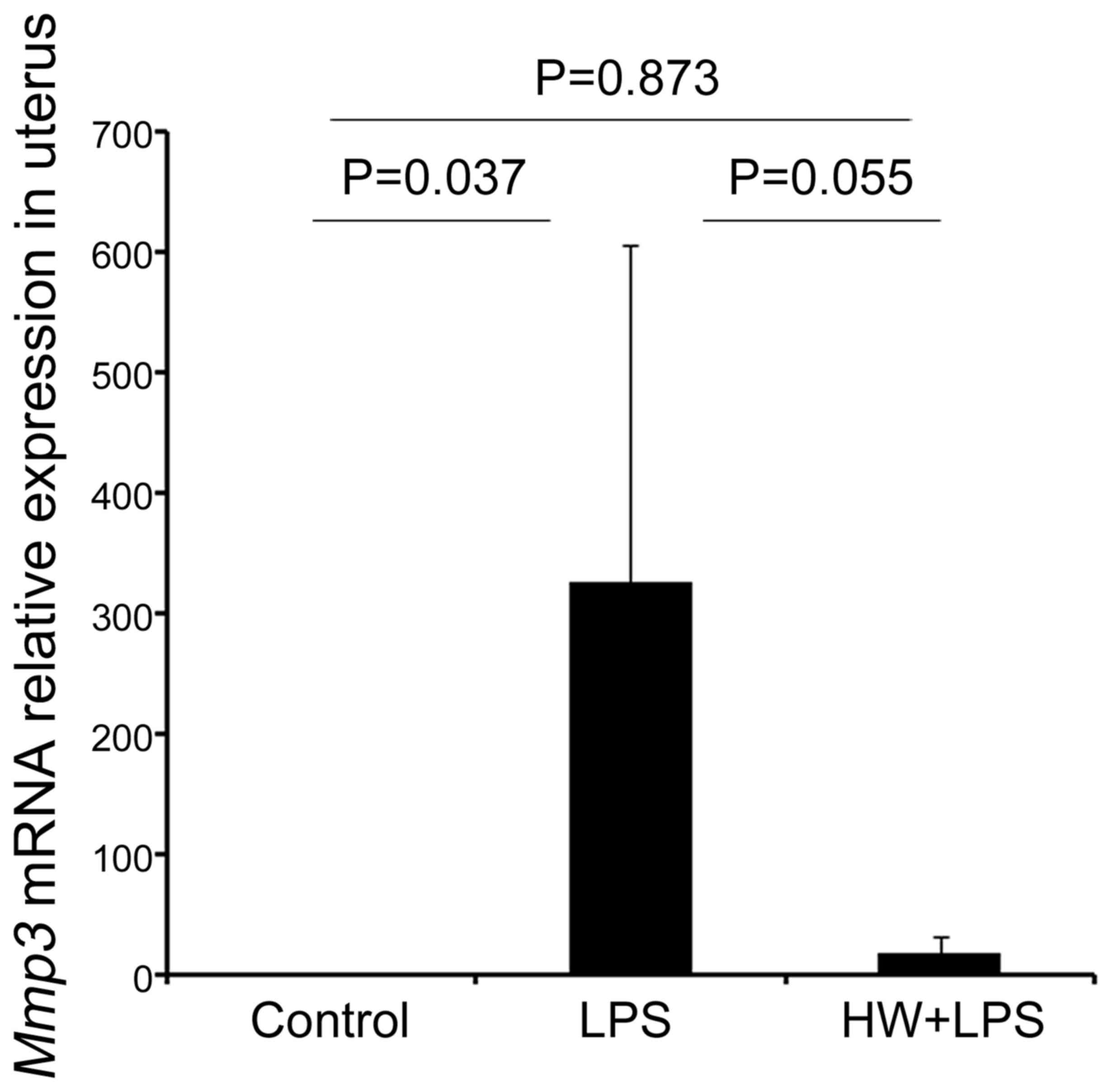
Fig. 4A and B). Mmp3
expression was also significantly higher in the LPS group compared
with that in the control group (P=0.037), and was lower in the HW +
LPS group compared with that in the LPS group, but the difference
was not significant (P=0.055; Fig.
5). Although notably, the expression of Mmp3 in the HW +
LPS group did not differ significantly different from that in the
control group (P=0.873; Fig. 5).
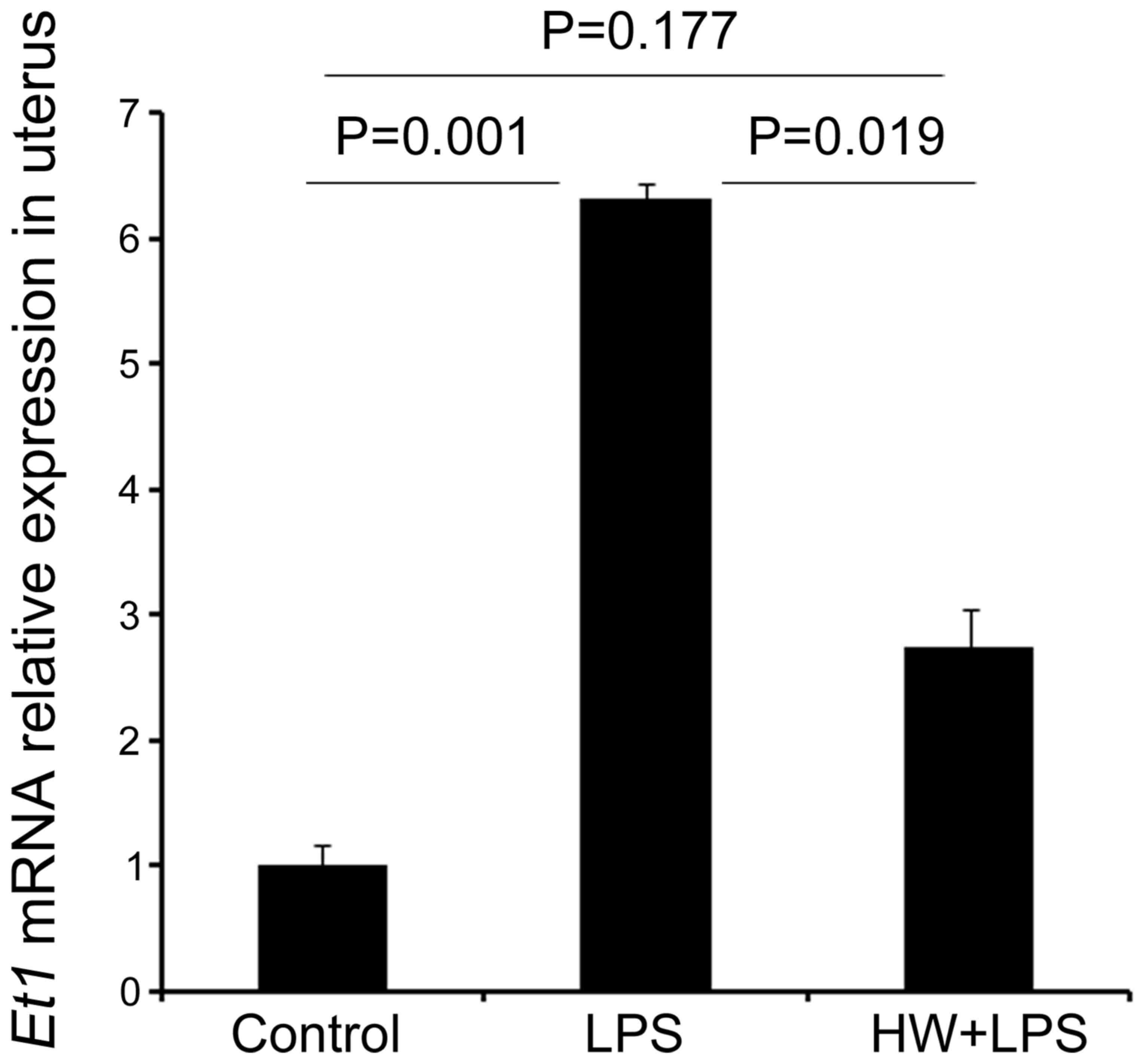
Et1 (Edn1) expression was significantly higher in the
uteri of the LPS group relative to that in the control group
(P=0.001), whereas levels in the HW + LPS group were significantly
lower compared with those in the LPS group (P=0.019; Fig. 6). Et1 mRNA levels did not
differ significantly different between the control and HW + LPS
groups (P=0.177; Fig. 6).
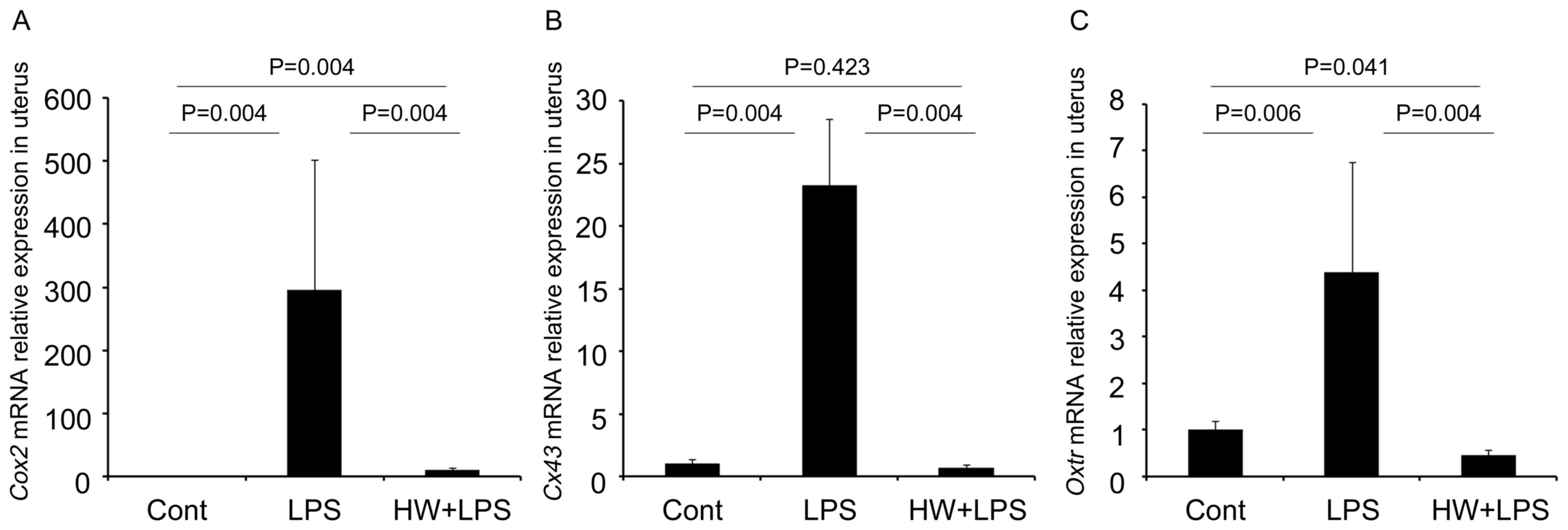 | Figure 3.mRNA levels of contractile-associated
proteins measured by reverse transcription-quantitative polymerase
chain reaction. The data were normalized to the expression of
β-actin. The results are expressed as the means ± standard error of
the mean (n=6 per group). The mRNA levels of (A) Cox2, (B)
Cx43 and (C) Oxtr were significantly increased in the
uteri of the LPS group compared with those in the control group;
whereas, levels in the HW + LPS group were significantly lower
compared with those in the LPS group. All indicated P-values were
calculated using the Mann-Whitney U test, with the exception of
Oxtr (control vs. HW + LPS), which was calculated using the
Student's t-test. The data among the three groups were also
statically analysed by the Kruskal-Wallis test, and the P-values
for Cox2, Cx43 and Oxtr were P=0.001, P=0.003, and
P=0.002, respectively. Cox2, cyclooxygenase-2; Cx43, connexin-43;
Oxtr, oxytocin receptor; LPS, lipopolysaccharide; HW, H2
water. |
Discussion
Previously, studies by our group have identified
that H2 had the potential to prevent foetal brain and
lung impairment due to maternal inflammation. Notably,
H2 was demonstrated to exert anti-oxidative and
anti-inflammatory effects in foetal organs following
intraperitoneal injection of LPS in rodent models (7–9). It is
established that preterm labour is associated with inflammation,
but the associated mechanism is not fully understood.
Pro-inflammatory cytokines in the uterus, including IL6, increase
CAPs, which initiates the onset of labour (16). To our knowledge, the present study is
the first to report that H2 may also significantly
prevent uterine inflammation, potentially extending the duration of
pregnancy in a murine model of LPS-induced preterm birth.
This effect may be partially dependent on a
significant increase in progesterone levels. However, while
H2 pretreatment did not reduce the rates of LPS-induced
preterm birth in the present study, it did extend the duration of
pregnancy. The reason for this effect is unknown. However, it is
notable that the expression of certain transcripts including
Tnf, Cx43 (Gja1), Oxtr and Et1
(Edn1) was reduced to levels equivalent to those observed in
the control group, despite no observable effects on Il8 and
Mmp3, by H2 administration. IL8 is expressed only
by the myometrium during active labour, whereas IL6 is present in
the preterm myometrium prior to and during labour (17), and no significant effect on Il8
expression by H2 pretreatment may lead to a continuation
of active labour, leading to preterm birth while extending the
period of delivery.
Progesterone, a steroid hormone that is essential
for the maintenance of pregnancy, has been demonstrated to decrease
preterm birth risk in high-risk populations, including in women
with short cervices (18). However,
while the underlying molecular mechanisms are not fully understood,
maternal plasma progesterone has been reported to be lower in
LPS-induced preterm birth models (19), and unaltered by probiotics (20). H2 may therefore be superior
in this regard, as a previous study reported that progesterone
exerted anti-inflammatory effects at the maternal-foetal interface
and increased the proportion of decidual regulatory T cells
(21). These findings suggest that
H2 may also exert an anti-inflammatory effect in
combination with increasing maternal serum progesterone.
Additionally, CAPs are an important family of
proteins that are involved in the onset of labour. For example, the
expression of certain CAPs, including Cox2 (Ptgs2), Cx43 (Gja1) and
Oxtr, has been reported to be elevated in the murine uterus at
preterm birth to an equivalent level to that observed at term
(22). More specifically, Oxtr has
been reported to be increased in the human uterus following the
onset of labour, both at term and preterm (23). Furthermore, an Oxtr antagonist,
atosiban, elicited similar outcomes to nifedipine during tocolysis
(24). Increased Cox2 expression has
also been detected in the human myometrium during preterm labour
(25). Finally, Cx43 protein
expression has been identified to increase at term and with the
onset of labour in humans (26),
although the expression of Cx43 during preterm labour remains
poorly understood in humans. Collectively, these findings indicate
that a reduction in the levels of CAPs in the uterus may inhibit
preterm labour. In the present study, H2 pretreatment
suppressed the levels of LPS-induced CAPs in the uterus.
N-acetylcysteine (NAC), which may serve as an antioxidant alike
H2, has also been reported to decrease the ratio of
preterm births in an animal model of LPS-induced preterm birth
(27), although NAC did not reduce
Cx43 or Oxtr expression (26).
MMP3 serves an important role in the tissue
remodelling that occurs in the human myometrium during labour.
Accordingly, levels of MMP3 have been reported to be increased at
labour (28). Furthermore, LPS
treatment increased MMP3 levels in human cervical smooth-muscle
cells (29) and rabbit cervical
tissues (30). In the present study,
maternal administration of H2 did not significantly
decrease LPS-induced Mmp3 mRNA expression levels. Thus, the
rates of preterm birth may not have been decreased by H2
pretreatment because the preventive effect of H2 on
cervical remodelling in the uterus was incomplete.
Et1 (Edn1) also serves a pathological role in
inflammation-induced preterm labour (31) and is increased in the amniotic fluid
of women with infection-induced preterm labour (32). Wang et al (33) demonstrated that the levels of ET1 were
increased in the gestational tissues of a murine model of
LPS-induced preterm birth, supporting the results of the present
study. These authors additionally observed that RNA silencing of
endothelin-converting enzyme-1 and a selective endothelin receptor
A antagonist (BQ-123) were effective at preventing LPS-induced
preterm labour, and decreased the percentages of preterm births to
approximately 14 and 16%, respectively; while in the
intraperitoneally injected LPS group, 70–90% of pregnant mice
delivered pups preterm (33). In the
present study, Et1 (Edn1) levels also decreased
significantly following maternal H2 administration.
However, this did not prevent preterm birth in the present study.
The inconsistency in the prevention of preterm birth may be due to
differences in the mouse strain used or the dose of LPS
administered.
The present study suggests that, while H2
pretreatment may elicit effects on uterine molecules involved in
preterm labour, it only has weak effects on the prevention of
preterm birth. Our previous reports demonstrated that may
H2 prevent foetal inflammation and oxidative damage
(7–9),
and compared to the effects of H2 on foetal damage, its
effects on preterm labour were limited. These findings suggest that
H2 pretreatment may be appropriate for preventing foetal
injury from maternal inflammation at preterm labour, but has low
potential to inhibit preterm birth. However, as H2
significantly altered the expression of a number of important
molecules expressed in the uterus, it may be effective for the
prevention of mild or chronic inflammation-related preterm birth.
The model used in the present study mimics an acute
inflammation-induced preterm birth, and resulted in preterm birth
at 18.3±8.8 h after intraperitoneal LPS administration. A recent
study reported that chronic inflammation also serves a role in
preterm labour (34) and thus further
investigation is required to fully understand the effects of
H2 on chronic inflammation-induced preterm birth.
However, the present study included a limitation in
that H2 was administered prior to LPS injection as a
preventive protocol. Most anti-oxidants and anti-inflammatory
agents are known to be effective when administered prior to injury.
In an unpublished preliminary study by our group, H2
exerted only a minimal effect on the foetus when the timing of
administration was delayed to after the onset of injury. This
finding highlights the importance of the early detection of
intrauterine inflammation. In addition, the mechanism associated
with the effect of H2, including signalling pathways in
the uterine wall remain unknown, and further investigations are
required.
In conclusion, prolonging pregnancy in response to
maternal administration of H2 for preterm labour in a
model of acute inflammation was minimal. However, the
anti-inflammatory effects and the reduction in the levels of
expression of several CAPs were observed. Maternal administration
of H2 may therefore be ineffective in preventing preterm
birth associated with acute inflammation, although the effects of
maternal H2 administration on chronic
inflammation-induced preterm birth should be investigated
further.
Acknowledgements
The authors are thankful to Blue Mercury, Inc.,
Tokyo, Japan for contributing the H2 water.
Funding
The current study was supported by the Japan Society
for the Promotion of Science Grants-in-Aid for Scientific Research
program (KAKENHI; grant nos. 15H06282 and 26462484).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TN performed experiments and data analyses, and
wrote the manuscript. TK designed the study, analysed and
interpreted data and wrote the manuscript. KI, YI, TU, HT and HL
performed experiments and data analyses. AI, ST and FK aided with
data interpretation, and edited the manuscript. The final version
of the manuscript has been read and approved by all authors.
Ethics approval and consent to
participate
All protocols for animal experiments were approved
by the Animal Experiment Committee of Nagoya University (approval
no. 29154).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ANOVA
|
analysis of variance
|
|
CAP
|
contractile-associated protein
|
|
Cox2 (Ptgs2)
|
cyclooxygenase-2
(prostaglandin-endoperoxide synthase 2)
|
|
Cx43 (Gja1)
|
connexin-43 (gap junction protein
alpha 1)
|
|
E
|
embryonic day
|
|
Et1 (Edn1)
|
endothelin-1
|
|
HW
|
H2 water
|
|
Il6/8
|
interleukin-6/8
|
|
IS
|
intensity score
|
|
LPS
|
lipopolysaccharide
|
|
Mmp3
|
matrix metalloproteinase-3
|
|
NAC
|
N-acetylcysteine
|
|
Oxtr
|
oxytocin receptor
|
|
PBS
|
phosphate-buffered saline
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
Tnf
|
tumour necrosis factor
|
References
|
1
|
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu
J, Lawn JE, Cousens S, Mathers C and Black RE: Global, regional,
and national causes of under-5 mortality in 2000–15: An updated
systematic analysis with implications for the sustainable
development goals. Lancet. 388:3027–3035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyle AK, Rinaldi SF, Norman JE and Stock
SJ: Preterm birth: Inflammation, fetal injury and treatment
strategies. J Reprod Immunol. 119:62–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim CJ, Romero R, Chaemsaithong P,
Chaiyasit N, Yoon BH and Kim YM: Acute chorioamnionitis and
funisitis: Definition, pathologic features, and clinical
significance. Am J Obstet Gynecol. 213 Suppl:S29–S52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chau V, McFadden DE, Poskitt KJ and Miller
SP: Chorioamnionitis in the pathogenesis of brain injury in preterm
infants. Clin Perinatol. 41:83–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keelan JA and Newnham JP: Editorial:
Advances in the prevention and treatment of inflammation-associated
preterm birth. Front Immunol. 7:2642016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ichihara M, Sobue S, Ito M, Ito M,
Hirayama M and Ohno K: Beneficial biological effects and the
underlying mechanisms of molecular hydrogen - comprehensive review
of 321 original articles. Med Gas Res. 5:122015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hattori Y, Kotani T, Tsuda H, Mano Y, Tu
L, Li H, Hirako S, Ushida T, Imai K, Nakano T, et al: Maternal
molecular hydrogen treatment attenuates lipopolysaccharide-induced
rat fetal lung injury. Free Radic Res. 49:1026–1037. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imai K, Kotani T, Tsuda H, Mano Y, Nakano
T, Ushida T, Li H, Miki R, Sumigama S, Iwase A, et al:
Neuroprotective potential of molecular hydrogen against perinatal
brain injury via suppression of activated microglia. Free Radic
Biol Med. 91:154–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakano T, Kotani T, Mano Y, Tsuda H, Imai
K, Ushida T, Li H, Miki R, Sumigama S, Sato Y, et al: Maternal
molecular hydrogen administration on lipopolysaccharide-induced
mouse fetal brain injury. J Clin Biochem Nutr. 57:178–182. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iuchi K, Imoto A, Kamimura N, Nishimaki K,
Ichimiya H, Yokota T and Ohta S: Molecular hydrogen regulates gene
expression by modifying the free radical chain reaction-dependent
generation of oxidized phospholipid mediators. Sci Rep.
6:189712016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herington JL, Swale DR, Brown N, Shelton
EL, Choi H, Williams CH, Hong CC, Paria BC, Denton JS and Reese J:
High-throughput screening of myometrial calcium-mobilization to
identify modulators of uterine contractility. PLoS One.
10:e01432432015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakashima-Kamimura N, Mori T, Ohsawa I,
Asoh S and Ohta S: Molecular hydrogen alleviates nephrotoxicity
induced by an anti-cancer drug cisplatin without compromising
anti-tumor activity in mice. Cancer Chemother Pharmacol.
64:753–761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rinaldi SF, Catalano RD, Wade J, Rossi AG
and Norman JE: Decidual neutrophil infiltration is not required for
preterm birth in a mouse model of infection-induced preterm labor.
J Immunol. 192:2315–2325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Larionov A, Krause A and Miller W: A
standard curve based method for relative real time PCR data
processing. BMC Bioinformatics. 6:622005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sivarajasingam SP, Imami N and Johnson MR:
Myometrial cytokines and their role in the onset of labour. J
Endocrinol. 231:R101–R119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sehringer B, Schäfer WR, Wetzka B, Deppert
WR, Brunner-Spahr R, Benedek E and Zahradnik HP: Formation of
proinflammatory cytokines in human term myometrium is stimulated by
lipopolysaccharide but not by corticotropin-releasing hormone. J
Clin Endocrinol Metab. 85:4859–4865. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Romero R, Nicolaides KH, Conde-Agudelo A,
O'Brien JM, Cetingoz E, Da Fonseca E, Creasy GW and Hassan SS:
Vaginal progesterone decreases preterm birth ≤ 34 weeks of
gestation in women with a singleton pregnancy and a short cervix:
An updated meta-analysis including data from the OPPTIMUM study.
Ultrasound Obstet Gynecol. 48:308–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aisemberg J, Vercelli CA, Bariani MV,
Billi SC, Wolfson ML and Franchi AM: Progesterone is essential for
protecting against LPS-induced pregnancy loss. LIF as a potential
mediator of the anti-inflammatory effect of progesterone. PLoS One.
8:e561612013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang S, Li W, Challis JR, Reid G, Kim SO
and Bocking AD: Probiotic Lactobacillus rhamnosus GR-1
supernatant prevents lipopolysaccharide-induced preterm birth and
reduces inflammation in pregnant CD-1 mice. Am J Obstet Gynecol.
211:44.e1–44.e12. 2014. View Article : Google Scholar
|
|
21
|
Filipovich Y, Klein J, Zhou Y and Hirsch
E: Maternal and fetal roles in bacterially induced preterm labor in
the mouse. Am J Obstet Gynecol. 214:386.e1–386.e9. 2016. View Article : Google Scholar
|
|
22
|
Cook JL, Zaragoza DB, Sung DH and Olson
DM: Expression of myometrial activation and stimulation genes in a
mouse model of preterm labor: Myometrial activation, stimulation,
and preterm labor. Endocrinology. 141:1718–1728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuchs AR, Fuchs F, Husslein P and Soloff
MS: Oxytocin receptors in the human uterus during pregnancy and
parturition. Am J Obstet Gynecol. 150:734–741. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Vliet EOG, Nijman TAJ, Schuit E, Heida
KY, Opmeer BC, Kok M, Gyselaers W, Porath MM, Woiski M, Bax CJ, et
al: Nifedipine versus atosiban for threatened preterm birth
(APOSTEL III): A multicentre, randomised controlled trial. Lancet.
387:2117–2124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitsuya K, Singh N, Sooranna SR, Johnson
MR and Myatt L: Epigenetics of human myometrium: DNA methylation of
genes encoding contraction-associated proteins in term and preterm
labor. Biol Reprod. 90:982014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chow L and Lye SJ: Expression of the gap
junction protein connexin-43 is increased in the human myometrium
toward term and with the onset of labor. Am J Obstet Gynecol.
170:788–795. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang EY, Zhang J, Sullivan S, Newman R
and Singh I: N-acetylcysteine attenuates the maternal and fetal
proinflammatory response to intrauterine LPS injection in an animal
model for preterm birth and brain injury. J Matern Fetal Neonatal
Med. 24:732–740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Brien M, O'Shaughnessy D, Ahamide E,
Morrison JJ and Smith TJ: Differential expression of the
metalloproteinase MMP3 and the alpha5 integrin subunit in human
myometrium at labour. Mol Hum Reprod. 13:655–661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watari M, Watari H, Nachamkin I and
Strauss JF III: Lipopolysaccharide induces expression of genes
encoding pro-inflammatory cytokines and the elastin-degrading
enzyme, cathepsin S, in human cervical smooth-muscle cells. J Soc
Gynecol Investig. 7:190–198. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakayama K, Otsuki K, Yakuwa K, Hasegawa
A, Sawada M, Mitsukawa K, Chiba H, Nagatsuka M and Okai T:
Recombinant human lactoferrin inhibits matrix metalloproteinase
(MMP-2, MMP-3, and MMP-9) activity in a rabbit preterm delivery
model. J Obstet Gynaecol Res. 34:931–934. 2008.PubMed/NCBI
|
|
31
|
Breuiller-Fouché M, Morinière C, Dallot E,
Oger S, Rebourcet R, Cabrol D and Leroy MJ: Regulation of the
endothelin/endothelin receptor system by interleukin-1{beta} in
human myometrial cells. Endocrinology. 146:4878–4886. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Romero R, Avila C, Edwin SS and Mitchell
MD: Endothelin-1,2 levels are increased in the amniotic fluid of
women with preterm labor and microbial invasion of the amniotic
cavity. Am J Obstet Gynecol. 166:95–99. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang W, Yen H, Chen CH, Soni R, Jasani N,
Sylvestre G and Reznik SE: The endothelin-converting
enzyme-1/endothelin-1 pathway plays a critical role in
inflammation-associated premature delivery in a mouse model. Am J
Pathol. 173:1077–1084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim CJ, Romero R, Chaemsaithong P and Kim
JS: Chronic inflammation of the placenta: Definition,
classification, pathogenesis, and clinical significance. Am J
Obstet Gynecol. 213:S53–S69. 2015. View Article : Google Scholar : PubMed/NCBI
|