Introduction
Coronary atherosclerosis is a chronic inflammatory
vascular disease and is initiated as a result of endothelial damage
and dysfunction, which lead to the accumulation and oxidation of
low density lipoprotein (LDL)-cholesterol in the arterial wall
(1,2)
Monocytes migrate from the blood into the subendothelial intima and
transform into macrophages, which then accumulate lipid particles
(foam cells) to form the lipid core of atherosclerotic plaques
(2,3).
Inflammatory and thrombotic processes serve central roles in the
formation of atherosclerotic lesions and subsequent plaque rupture,
which lead toward acute coronary syndrome (2,3).
Coronary artery disease (CAD) and myocardial
infarction (MI) are serious clinical conditions that remain the
leading cause of mortality in the United States (4). Disease prevention is an important
strategy for reducing the overall burden of CAD and MI, with the
identification of biomarkers for disease risk being key for risk
prediction and for potential intervention, in order to reduce the
chance of future adverse coronary events. In addition to
conventional risk factors for CAD, including hypertension, diabetes
mellitus and dyslipidemia, the importance of genetic factors has
been highlighted (5–7). Genes responsible for familial
hypercholesterolemia and Tangier disease are prototypical examples
of monogenic forms of CAD and MI with Mendelian inheritance
(5,8).
Familial hypercholesterolemia is an autosomal dominant disorder
characterized by marked increases in the circulating concentrations
of total cholesterol and LDL-cholesterol caused by mutations of the
genes for LDL receptor (LDLR), apolipoprotein B
(APOB), proprotein convertase subtilisin/kexin type 9
(PCSK9), cytochrome P450 family 7 subfamily A member 1
(CYP7A1) or LDL receptor adaptor protein 1 (LDLRAP1)
(9,10). Tangier disease is an autosomal
recessive disorder characterized by a decrease in the circulating
concentration of high density lipoprotein (HDL)-cholesterol as a
result of loss-of-function mutations in the ATP-binding cassette
subfamily A member 1 gene (ABCA1) (11–13). The
etiology of common forms of CAD is multifactorial and includes
genetic components, as well as environmental and lifestyle factors
(5–8).
The heritability of common forms of CAD has been estimated to be
40–60% on the basis of family and twin studies (6,7,14).
Genome-wide association studies (GWASs) in
European-ancestry (15–21), African American (22) or Han Chinese populations (23,24) have
identified various genes and loci that confer susceptibility to CAD
or MI. A meta-analysis of GWASs for CAD among European-ancestry
populations, including low-frequency variants, identified 202
independent genetic variants at 129 loci with a false discovery
rate (FDR) of <5% (25). These
genetic variants together accounted for ~28% of the heritability of
CAD, demonstrating that genetic susceptibility to this condition is
largely determined by common variants with small effect sizes
(6,25). A more recent meta-analysis for CAD in
European-ancestry populations identified 304 independent genetic
variants with an FDR of <5%, and these variants accounted for
21.2% of the heritability of CAD (26). In total, GWASs identified 163 loci
associated with CAD at a genome-wide significance level and >300
possible loci for this condition with an FDR of <5% (7). Although several single nucleotide
polymorphisms (SNPs) have been revealed to be significantly
associated with MI in Japanese patients (27,28),
genetic variants that contribute toward susceptibility to CAD and
MI in Japanese patients remain to be definitively identified.
A study of monozygotic and dizygotic twins revealed
that mortality from CAD at younger ages was significantly
influenced by genetic factors in males and females, whereas the
genetic effect was smaller at older ages (29,30). A
family history of MI is also more apparent in individuals with
early-onset MI than in those with late-onset MI, suggestive of a
greater heritability in the former (31,32).
The present study included exome-wide association
studies (EWASs) for CAD with the use of human exome array-based
genotyping methods in order to identify genetic variants that
confer susceptibility to this condition in Japanese patients. In
order to increase the statistical power of the EWAS, patients with
early-onset CAD were examined.
Materials and methods
Study subjects
In our previous EWAS, the median age of subjects
with CAD was 69 years (33).
Therefore, patients with an age of ≤65 years were defined as
individuals with early-onset CAD in the present study. A total of
7,256 Japanese subjects aged ≤65 years [mean age, 51.7 years; age
range, 18–65 years; males/females (%), 58.3/41.7; 1,482 with CAD,
including 1,152 with MI, and 5,774 controls] were enrolled in the
present study. The subjects were individuals who either visited
outpatient clinics or were admitted to participating hospitals in
Japan (Gifu Prefectural Tajimi Hospital, Tajimi; Gifu Prefectural
General Medical Center, Gifu; Japanese Red Cross Nagoya First
Hospital, Nagoya; Northern Mie Medical Center Inabe General
Hospital, Inabe; and Hirosaki University Hospital and Hirosaki
Stroke and Rehabilitation Center, Hirosaki, Japan) due to various
symptoms or for an annual health check-up between October 2002 and
March 2014, or who were community-dwelling individuals recruited to
a population-based cohort study in Inabe between March 2010 and
September 2014 (34).
The diagnosis of CAD was based on the detection of
stenosis of >50% in any major coronary artery or in the left
main trunk by coronary angiography. The diagnosis of MI was based
on typical electrocardiographic changes and on increases in the
serum activity of creatine kinase (MB isozyme) and in the serum
concentration of troponin T. The diagnosis was confirmed by
identification of the responsible stenosis in any of the major
coronary arteries or in the left main trunk by coronary
angiography. The control individuals had no history of MI, CAD,
aortic aneurysm or peripheral artery disease; of ischemic or
hemorrhagic stroke; or of other atherosclerotic, thrombotic,
embolic or hemorrhagic disorders. Although certain control
individuals had conventional risk factors for CAD, including
hypertension, diabetes mellitus, dyslipidemia and CKD, they did not
have any cardiovascular complications.
EWAS
Venous blood (5 or 7 ml) was collected into tubes
containing 50 mmol/l ethylenediaminetetraacetic acid (disodium
salt), peripheral blood leukocytes were isolated, and genomic DNA
was extracted from these cells with the use of a DNA extraction kit
(Genomix; Talent SRL, Trieste, Italy; or SMITEST EX-R&D;
Medical & Biological Laboratories, Co., Ltd., Nagoya, Japan).
The EWASs for CAD (1,482 cases and 5,774 controls) was performed
with the use of a Human Exome-12 v1.2 DNA Analysis BeadChip or
Infinium Exome-24 v1.0 BeadChip (Illumina, Inc., San Diego, CA,
USA). These exome arrays include putative functional exonic
variants selected from ~12,000 individual exome and whole-genome
sequences. The exonic content consists of ~244,000 SNPs from
European, African, Chinese and Hispanic individuals (35). SNPs contained in only one of the exome
arrays (~2.6% of all SNPs) were excluded from analysis. Quality
control was performed as follows (36): i) Genotyping data with a call rate of
<97% were discarded, with the mean call rate for the remaining
data being 99.9%; ii) gender specification was checked for each
sample, and those for which gender phenotype in the clinical
records was inconsistent with genetic sex were discarded; iii)
duplicate samples and cryptic relatedness were checked by
calculation of identity by descent, and all pairs of DNA samples
exhibiting an identity by descent of >0.1875 were inspected and
one sample from each pair was excluded; iv) the frequency of
heterozygosity for SNPs was calculated for all samples, and those
with extremely low or high heterozygosity (>3 standard
deviations from the mean) were discarded; v) SNPs in sex
chromosomes or mitochondrial DNA were excluded from the analysis,
as were nonpolymorphic SNPs or SNPs with a minor allele frequency
of <1.0%; vi) SNPs whose genotype distributions deviated
significantly (P<0.01) from Hardy-Weinberg equilibrium in
control individuals were discarded; and vii) genotype data were
examined for population stratification by principal components
analysis (37), and population
outliers were excluded from the analysis. A total of 31,465 SNPs
passed quality control for the EWASs of CAD and these SNPs were
subjected to analyses.
Statistical analysis
For analysis of the characteristics of the study
subjects, quantitative data were compared between subjects with CAD
and controls using the unpaired Student's t-test. Categorical data
were compared between the two groups using the Pearson's
χ2 test. Allele frequencies were estimated by the gene
counting method, and Fisher's exact test was applied to identify
departure from the Hardy-Weinberg equilibrium. In the EWAS, the
association between allele frequencies of each SNP and CAD was
examined using the Fisher's exact test. The genomic inflation
factor (λ) was 0.93. To compensate for multiple comparisons of
genotypes with CAD, an FDR was applied for statistical significance
of association (38). The
significance level was set at an FDR of <0.05 for the EWAS.
Multivariable logistic regression analysis was performed with CAD
as a dependent variable and independent variables, including age,
sex (0, female and 1, male), the prevalence of hypertension,
diabetes mellitus, and dyslipidemia (0, no history of these
conditions; 1, positive history), as well as the genotype of each
SNP. Genotypes of the SNPs were assessed according to dominant [0,
AA; 1, AB + BB (A, major allele; B, minor allele)] and recessive
(0, AA + AB; 1, BB) genetic models, and the P-value, odds ratio and
95% confidence interval were calculated. A stepwise forward
selection procedure was also performed to examine the effects of
genotypes on CAD. The P-levels for inclusion in and exclusion from
the model were 0.25 and 0.1, respectively. In the stepwise forward
selection procedure, each genotype was examined according to a
dominant or recessive model on the basis of statistical
significance in the multivariable logistic regression analysis. The
association between genotypes of SNPs and intermediate phenotypes
of CAD was examined using the Pearson's χ2 test. With
the exception of the initial EWAS by the Fisher's exact test (FDR
<0.05), P<0.05 was considered to indicate a statistically
significant difference. Statistical tests were performed using JMP
Genomics version 9.0 software (SAS Institute, Inc., Cary, NC,
USA).
Association between genes, chromosomal
loci and SNPs identified in the present study and phenotypes
previously reported by GWASs
The genes, chromosomal loci, and SNPs identified in
the present study were compared with the cardiovascular
disease-related phenotypes previously reported by GWASs available
in the Genome-Wide Repository of Associations Between SNPs and
Phenotypes (GRASP) Search database v. 2.0.0.0 (https://grasp.nhlbi.nih.gov/Search.aspx), developed by
the Information Technology and Applications Center at the National
Center for Biotechnology Information (National Heart, Lung, and
Blood Institute, National Institutes of Health, Bethesda, MD, USA)
(39,40).
Gene Ontology analysis
Biological functions of the genes were examined by
the use of the Gene Ontology and GO Annotations databases (QuickGO
version 2018; https://www.ebi.ac.uk/QuickGO/; European
Bioinformatics Institute, European Molecular Biology Laboratory,
Hinxton, Cambridgeshire, UK) (41,42).
Network analysis of gene-gene
interactions
Network analyses were performed to predict
functional gene-gene interactions by the use of GeneMANIA Cytoscape
plugin (http://apps.cytoscape.org/apps/genemania; Donnelly
Centre for Cellular and Biomolecular Research, University of
Toronto, Toronto, Canada) (43–45) using
Cytoscape v3.4.0 software (http://www.cytoscape.org/; The Cytoscape Consortium,
San Diego, CA, USA) (46). To begin
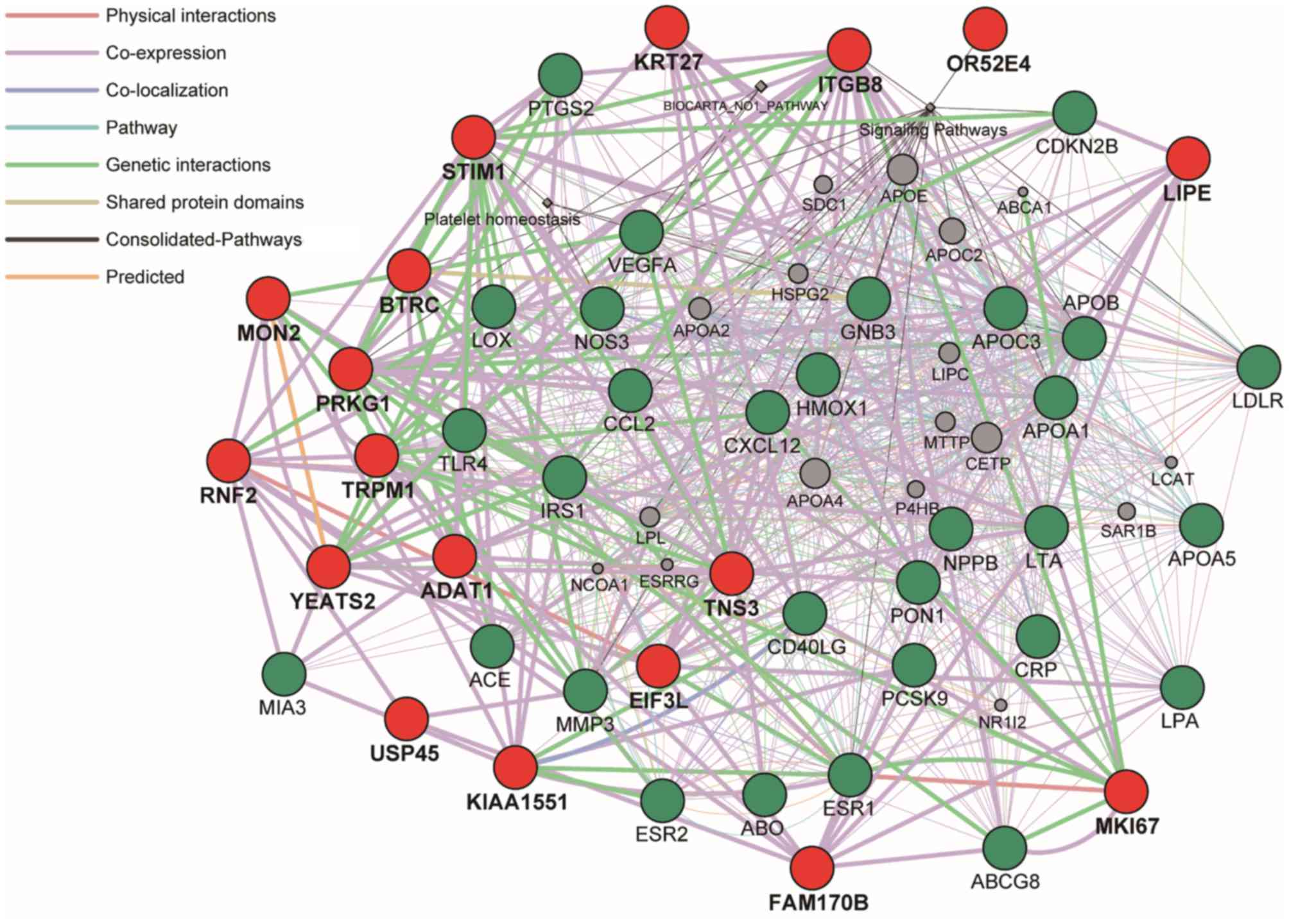
with, the 30 genes (ACE, NOS3, CCL2, PON1, CD40LG, LOX, APOB,
CRP, APOA1, LPA, ESR1, LDLR, APOC3, VEGFA, LTA, HMOX1, MMP3, APOA5,
PCSK9, CDKN2B, TLR4, GNB3, PTGS2, NPPB, ABCG8, ESR2, CXCL12, MIA3,
IRS1 and ABO) were selected from the DisGeNET database
(http://www.disgenet.org/web/DisGeNET;
Integrative Biomedical Informatics Group, Research Programme on
Biomedical Informatics, Barcelona Biomedical Research Park,
Barcelona, Spain) (47,48), according to the rank order of high
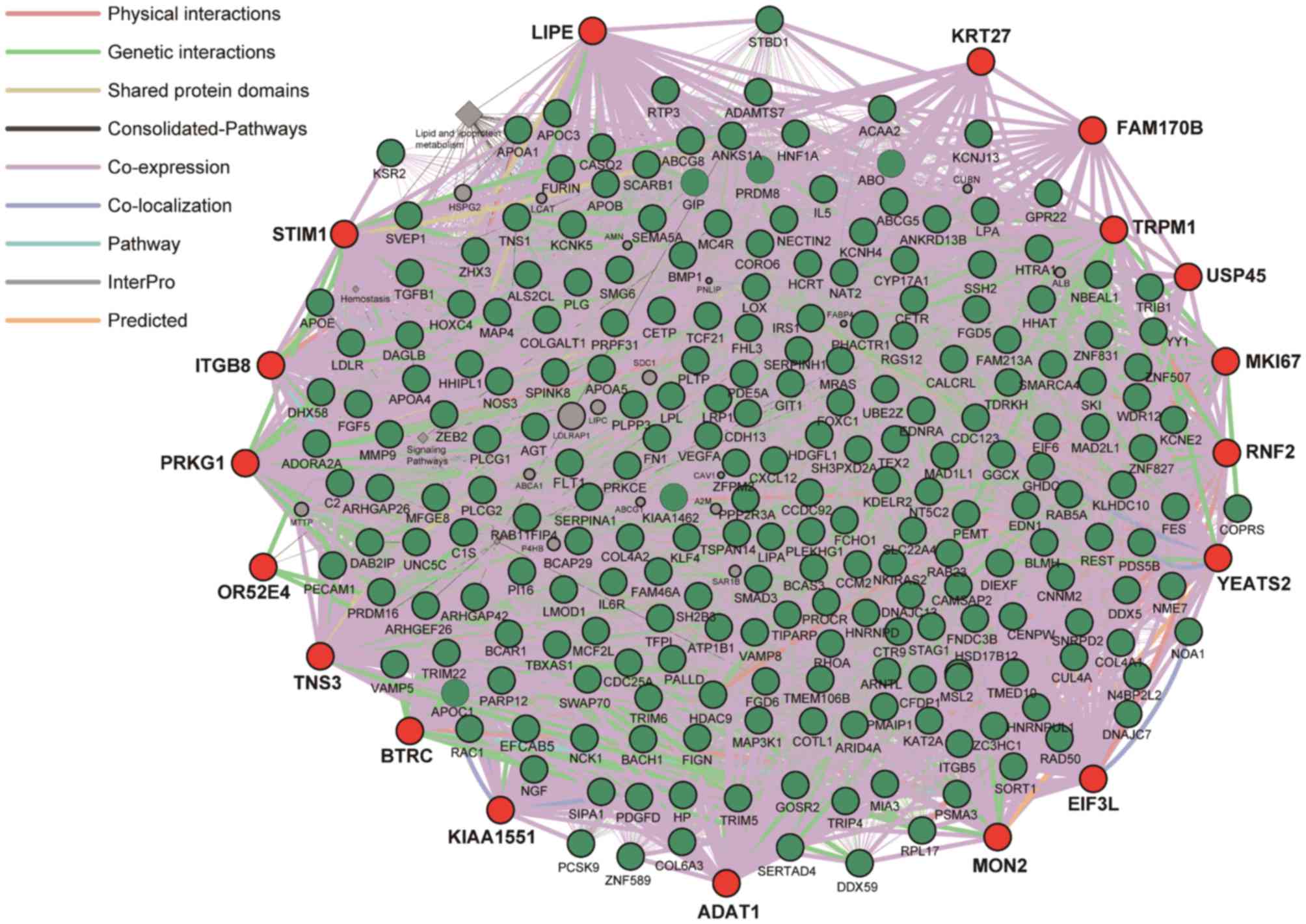
scores in association with CAD. Next, the 234 genes previously
identified by GWASs (7) were
selected, among which six genes were not included in GeneMANIA
database and had no interaction with other genes. Therefore, the
228 genes (SKI, PRDM16, FHL3, PCSK9, PPAP2B, SORT1, NGF, CASQ2,
TDRKH, IL6R, ATP1B1, NME7, DDX59, CAMSAP2, LMOD1, HHAT, SERTAD4,
DIEXF, MIA3, AGT, APOB, ABCG5, ABCG8, PRKCE, VAMP5, VAMP8, GGCX,
ZEB2, FIGN, CALCRL, TFPI, WDR12, NBEAL1, FN1, TNS1, IRS1, KCNJ13,
COL6A3, FGD5, ALS2CL, RTP3, CDC25A, SPINK8, MAP4, ZNF589, RHOA,
ITGB5, DNAJC13, STAG1, MSL2, NCK1, PPP2R3A, MRAS, ARHGEF26, TIPARP,
FNDC3B, RGS12, REST, NOA1, STBD1, PRDM8, FGF5, HNRNPD, UNC5C,
MAD2L1, PDE5A, ZNF827, EDNRA, PALLD, SEMA5A, MAP3K1, LOX, SLC22A4,
IL5, RAD50, ARHGAP26, FOXC1, PHACTR1, EDN1, HDGFL1, C2, ANKS1A,
PI16, KCNK5, VEGFA, RAB23, FAM46A, CENPW, TCF21, PLEKHG1, LPA, PLG,
MAD1L1, DAGLB, RAC1, KDELR2, TMEM106B, HDAC9, CCM2, BCAP29, GPR22,
CFTR, ZC3HC1, KLHDC10, PARP12, TBXAS1, NOS3, NAT2, LPL, BMP1,
ZFPM2, TRIB1, KLF4, SVEP1, DAB2IP, ABO, CDC123, KIAA1462, CXCL12,
TSPAN14, FAM213A, LIPA, CYP17A1, CNNM2, NT5C2, SH3PXD2A, HTRA1,
TRIM5, TRIM22, TRIM6, SWAP70, CTR9, ARNTL, HSD17B12, SIPA1,
SERPINH1, ARHGAP42, PDGFD, APOA1, APOC3, APOA4, APOA5, C1S, PRPF31,
HOXC4, LRP1, FGD6, SH2B3, KSR2, HNF1A, CCDC92, SCARB1, FLT1,
N4BP2L2, PDS5B, COL4A1, COL4A2, MCF2L, CUL4A, ARID4A, PSMA3,
TMED10, SERPINA1, HHIPL1, YY1, TRIP4, SMAD3, ADAMTS7, MFGE8, FURIN,
FES, CETP, HP, CFDP1, BCAR1, PLCG2, CDH13, SMG6, PEMT, CORO6, BLMH,
ANKRD13B, GIT1, SSH2, EFCAB5, COPRS, RAB11FIP4, DHX58, KAT2A, RAB5,
NKIRAS2, DNAJC7, KCNH4, HCRT, GHDC, GOSR2, UBE2Z, GIP, BCAS3,
PECAM1, DDX5, TEX2, ACAA2, RPL17, PMAIP1, MC4R, LDLR, SMARCA4,
FCHO1, COLGALT1, ZNF507, HNRNPUL1, TGFB1, APOE, APOC1, PVRL2,
COTL1, SNRPD2, PROCR, EIF6, ZHX3, PLCG1, PLTP, MMP9, ZNF831, BACH1,
KCNE2 and ADORA2A) were applied to analysis.
Results
Characteristics of subjects
The characteristics of the 7,256 subjects enrolled
in the present study are presented in Table I. The age, the frequency of males, and
the prevalence of obesity, hypertension, diabetes mellitus (DM),
dyslipidemia, chronic kidney disease (CKD) and hyperuricemia, as
well as body mass index, systolic and diastolic blood pressure,
fasting plasma glucose level, blood glycosylated hemoglobin
(hemoglobin A1c) content, and the serum concentrations of
triglycerides, creatinine, and uric acid were greater, whereas the
serum concentration of HDL-cholesterol and estimated glomerular
filtration rate were lower, in patients with CAD than in
controls.
 | Table I.Characteristics of control subjects
and patients with coronary artery disease. |
Table I.
Characteristics of control subjects
and patients with coronary artery disease.
| Characteristic | Control | Coronary artery
disease | P-value |
|---|
| No. subjects | 5,774 | 1,482 |
|
| Age, years |
50.6±10.2 | 55.9±7.4 | <0.0001 |
| Sex, males/females,
% | 52.1/47.9 | 82.5/17.5 | <0.0001 |
| Smoking, % | 42.5 | 43.0 |
0.7719 |
| Obesity, % | 31.0 | 43.0 | <0.0001 |
| Body mass index,
kg/m2 | 23.2±3.5 | 24.5±3.5 | <0.0001 |
| Hypertension,
% | 31.7 | 70.0 | <0.0001 |
| Systolic BP,
mmHg | 121±18 | 139±27 | <0.0001 |
| Diastolic BP,
mmHg |
75±13 |
78±15 | <0.0001 |
| Diabetes mellitus,
% | 12.7 | 58.7 | <0.0001 |
| Fasting plasma
glucose, mmol/l |
5.66±1.78 |
7.55±3.39 | <0.0001 |
| Blood hemoglobin
A1c, % |
5.72±0.96 |
6.89±1.75 | <0.0001 |
| Dyslipidemia,
% | 56.9 | 84.1 | <0.0001 |
| Serum
triglycerides, mmol/l |
1.32±0.98 |
1.84±1.34 | <0.0001 |
| Serum
HDL-cholesterol, mmol/l |
1.65±0.45 |
1.20±0.36 | <0.0001 |
| Serum
LDL-cholesterol, mmol/l |
3.18±0.83 |
3.18±0.98 |
0.9770 |
| Chronic kidney
disease, % | 10.3 | 29.4 | <0.0001 |
| Serum creatinine,
µmol/l |
69.8±61.0 |
95.5±119.3 | <0.0001 |
| eGFR, ml
min−1 1.73 m−2 |
78.7±17.1 |
70.7±26.9 | <0.0001 |
| Hyperuricemia,
% | 15.2 | 25.5 | <0.0001 |
| Serum uric acid,
µmol/l | 321±89 |
353±102 | <0.0001 |
EWAS for CAD
The association between allele frequencies for
31,465 SNPs that passed quality control and CAD was examined using
the Fisher's exact test, and the 170 SNPs were significantly (FDR
<0.05) associated with CAD (Table
II).
 | Table II.170 SNPs significantly (FDR <0.5)
associated with coronary artery disease in the exome-wide
association study. |
Table II.
170 SNPs significantly (FDR <0.5)
associated with coronary artery disease in the exome-wide
association study.
| Gene | SNP | Nucleotide
substitutiona | Amino acid
substitution | Chromosome | Position | MAF, % | Allele OR | P-value, allele
frequency | FDR, allele
frequency |
|---|
| PLCB2 | rs200787930 | C/T | E1106K | 15 | 40289298 | 1.2 | 0.03 |
1.24×10−29 |
1.56×10−26 |
| MARCH1 | rs61734696 | G/T | Q137K | 4 | 164197303 | 1.2 | 0.03 |
2.09×10−29 |
2.54×10−26 |
| VPS33B | rs199921354 | C/T | R80Q | 15 | 91013841 | 1.2 | 0.03 |
2.76×10−29 |
3.30×10−26 |
| CXCL8 | rs188378669 | G/T | E31* | 4 | 73741568 | 1.2 | 0.03 |
3.15×10−29 |
3.70×10−26 |
| TMOD4 | rs115287176 | G/A | R277W | 1 | 151170961 | 1.2 | 0.03 |
1.21×10−28 |
1.39×10−25 |
| COL6A3 | rs146092501 | C/T | E1386K | 2 | 237371861 | 1.2 | 0.04 |
2.93×10−28 |
3.27×10−25 |
| ZNF77 | rs146879198 | G/A | R340* | 19 | 2934109 | 1.2 | 0.04 |
2.92×10−28 |
3.27×10−25 |
| ADGRL3 | rs192210727 | G/T | R580I | 4 | 61909615 | 1.3 | 0.10 |
2.92×10−23 |
3.06×10−20 |
| OR52E4 | rs11823828 | T/G | F227L | 11 | 5884973 | 36.6 | 1.54 |
3.40×10−21 |
3.35×10−18 |
| ALDH2 | rs671 | G/A | E504K | 12 | 111803962 | 27.6 | 1.41 |
4.12×10−15 |
3.78×10−12 |
| ACAD10 | rs11066015 | G/A |
| 12 | 111730205 | 27.5 | 1.41 |
4.92×10−15 |
4.45×10−12 |
| BRAP | rs3782886 | A/G |
| 12 | 111672685 | 29.3 | 1.37 |
4.38×10−13 |
3.71×10−10 |
| HECTD4 | rs11066280 | T/A |
| 12 | 112379979 | 29.0 | 1.37 |
6.94×10−13 |
5.73×10−10 |
| HECTD4 | rs2074356 | C/T |
| 12 | 112207597 | 25.4 | 1.36 |
1.21×10−11 |
9.78×10−9 |
| NAA25 | rs12231744 | C/T | R876K | 12 | 112039251 | 35.1 | 0.77 |
1.68×10−9 |
1.24×10−6 |
| GOSR2 | rs1052586 | T/C |
| 17 | 46941097 | 48.7 | 0.79 |
3.94×10−8 |
2.61×10−5 |
| ATXN2 | rs7969300 | T/C | N248S | 12 | 111555908 | 38.8 | 0.79 |
4.41×10−8 |
2.87×10−5 |
| LILRB2 | rs73055442 | C/T | R103H | 19 | 54279838 | 1.6 | 44.10 |
2.00×10−7 |
1.20×10−4 |
|
| rs12229654 | T/G |
| 12 | 110976657 | 22.5 | 1.28 |
2.09×10−7 |
1.24×10−4 |
|
LOC107987429 | rs2844533 | T/C |
| 6 | 31383025 | 15.3 | 1.32 |
3.49×10−7 |
1.95×10−4 |
| MTFR2 | rs143974258 | G/A | R360* | 6 | 136231355 | 3.3 | 0.05 |
6.66×10−7 |
3.60×10−4 |
|
PSORS1C1 | rs3130559 | C/T |
| 6 | 31129524 | 44.2 | 0.82 |
1.51×10−6 |
7.74×10−4 |
|
| rs2596548 | G/T |
| 6 | 31362769 | 5.4 | 1.51 |
1.83×10−6 |
9.21×10−4 |
| EIF3L | rs9466 | T/C |
| 22 | 37877742 | 21.6 | 1.28 |
1.96×10−6 |
9.77×10−4 |
| LPGAT1 | rs150552771 | T/C | K200E | 1 | 211783358 | 5.0 | 7.14 |
2.26×10−6 | 0.0011 |
| LAIR2 | rs34429135 | T/A | F115Y | 19 | 54508164 | 2.5 | ND |
2.70×10−6 | 0.0013 |
|
| rs2523644 | A/G |
| 6 | 31374707 | 8.1 | 1.40 |
2.75×10−6 | 0.0013 |
|
| rs10757278 | A/G |
| 9 | 22124478 | 49.5 | 0.83 |
2.92×10−6 | 0.0014 |
| CCHCR1 | rs130067 | T/G | E328D | 6 | 31150734 | 33.2 | 0.81 |
3.10×10−6 | 0.0015 |
| TCHP | rs74416240 | G/A |
| 12 | 109904793 | 13.3 | 1.30 |
3.25×10−6 | 0.0015 |
|
| rs1333049 | G/C |
| 9 | 22125504 | 49.4 | 1.20 |
3.95×10−6 | 0.0018 |
|
CDKN2B-AS1 | rs4977574 | A/G |
| 9 | 22098575 | 47.1 | 1.21 |
4.18×10−6 | 0.0019 |
|
CDKN2B-AS1 | rs2383207 | G/A |
| 9 | 22115960 | 33.7 | 0.81 |
4.86×10−6 | 0.0022 |
| SLC16A1 | rs1049434 | T/A | D490E | 1 | 112913924 | 34.7 | 0.82 |
5.76×10−6 | 0.0025 |
| GIT2 | rs925368 | T/C | N389S | 12 | 109953174 | 12.5 | 1.30 |
6.02×10−6 | 0.0026 |
|
| rs1333048 | A/C |
| 9 | 22125348 | 49.6 | 1.20 |
6.46×10−6 | 0.0028 |
|
| rs2523578 | T/C |
| 6 | 31360765 | 8.1 | 1.39 |
6.54×10−6 | 0.0028 |
|
| rs404890 | G/T |
| 6 | 32231090 | 30.5 | 1.22 |
8.90×10−6 | 0.0037 |
| APOE | rs7412 | C/T | R176C | 19 | 44908822 | 4.3 | 0.60 |
1.06×10−5 | 0.0043 |
| CCHCR1 | rs130071 | G/A |
| 6 | 31148433 | 5.1 | 1.52 |
1.06×10−5 | 0.0043 |
|
| rs602633 | C/A |
| 1 | 109278889 | 7.6 | 0.69 |
1.15×10−5 | 0.0046 |
| CELSR2 | rs12740374 | G/T |
| 1 | 109274968 | 7.7 | 0.69 |
1.15×10−5 | 0.0046 |
| MKI67 | rs145121731 | G/A | S2722L | 10 | 128102595 | 1.5 | 2.04 |
1.20×10−5 | 0.0047 |
| CUBN | rs78201384 | C/T | E304K | 10 | 17111024 | 2.7 | 0.52 |
1.38×10−5 | 0.0054 |
|
PSORS1C3 | rs887466 | T/C |
| 6 | 31175734 | 41.1 | 1.20 |
1.38×10−5 | 0.0054 |
|
PSORS1C1 | rs3094663 | G/A |
| 6 | 31139310 | 30.9 | 1.20 |
1.40×10−5 | 0.0054 |
|
| rs10853110 | A/G |
| 17 | 49241052 | 39.2 | 1.20 |
1.49×10−5 | 0.0057 |
| WDR37 | rs10794720 | C/T |
| 10 | 1110225 | 8.5 | 0.71 |
1.52×10−5 | 0.0057 |
| CELSR2 | rs629301 | A/C |
| 1 | 109275684 | 7.8 | 0.70 |
1.52×10−5 | 0.0057 |
| SKIV2L | rs592229 | G/T |
| 6 | 31962664 | 42.4 | 1.20 |
1.57×10−5 | 0.0058 |
|
| rs12182351 | T/C |
| 6 | 32233930 | 29.8 | 1.22 |
1.59×10−5 | 0.0059 |
| POU5F1 | rs3130503 | G/A |
| 6 | 31169388 | 29.5 | 1.20 |
1.64×10−5 | 0.0060 |
|
PSORS1C3 | rs1265155 | T/C |
| 6 | 31175917 | 41.1 | 1.19 |
1.68×10−5 | 0.0061 |
| CELSR2 | rs646776 | A/G |
| 1 | 109275908 |
7.7 | 0.70 |
1.70×10−5 | 0.0062 |
|
| rs2596503 | C/T |
| 6 | 31353033 | 19.3 | 1.24 |
1.75×10−5 | 0.0063 |
| TRPM1 | rs2241493 | T/C | N54S | 15 | 31070149 | 12.6 | 0.76 |
1.81×10−5 | 0.0065 |
| CCDC141 | rs13419085 | T/C | N1170S | 2 | 178837710 |
1.8 | 0.46 |
1.92×10−5 | 0.0068 |
| VARS2 | rs9394021 | A/G | Q777R | 6 | 30925350 | 44.9 | 0.84 |
1.98×10−5 | 0.0069 |
| SFTA2 | rs2286655 | T/C |
| 6 | 30931969 | 44.9 | 1.19 |
1.99×10−5 | 0.0069 |
|
| rs3873334 | T/C |
| 6 | 30928370 | 44.9 | 1.19 |
1.98×10−5 | 0.0069 |
|
| rs9261800 | C/G |
| 6 | 30408822 |
2.8 | 7.21 |
2.02×10−5 | 0.0069 |
| TCF19 | rs3130453 | C/T |
| 6 | 31157072 | 34.4 | 0.83 |
2.10×10−5 | 0.0072 |
|
C21orf59 | rs76974938 | C/T | D67N | 21 | 32609946 |
2.4 | 0.00 |
2.14×10−5 | 0.0073 |
| DDR1 | rs2239518 | T/C |
| 6 | 30897948 | 44.9 | 1.19 |
2.19×10−5 | 0.0074 |
| CDSN | rs3130984 | C/T | S143N | 6 | 31117187 | 13.4 | 1.29 |
2.20×10−5 | 0.0074 |
|
| rs197932 | T/C |
| 17 | 46896981 | 26.9 | 0.82 |
2.23×10−5 | 0.0075 |
| CDSN | rs3130981 | C/T | D527N | 6 | 31116036 | 13.6 | 1.29 |
2.30×10−5 | 0.0075 |
| MICB-DT | rs3132469 | C/T |
| 6 | 31488790 |
5.3 | 1.46 |
2.41×10−5 | 0.0078 |
|
HLA-DQB1 | rs1049056 | C/A | A6S | 6 | 32666592 | 11.9 | 1.30 |
2.51×10−5 | 0.0081 |
| DDR1 | rs2239517 | A/G |
| 6 | 30897338 | 44.6 | 1.19 |
2.59×10−5 | 0.0083 |
| CCHCR1 | rs1265110 | G/A |
| 6 | 31151645 | 30.2 | 0.83 |
2.69×10−5 | 0.0085 |
| CCDC63 | rs10774610 | T/C |
| 12 | 110902439 | 23.7 | 1.22 |
2.76×10−5 | 0.0087 |
| GTF2H4 | rs2284176 | C/T |
| 6 | 30907845 | 44.6 | 1.19 |
2.80×10−5 | 0.0088 |
| GTF2H4 | rs3909130 | G/A |
| 6 | 30906388 | 44.6 | 1.19 |
2.84×10−5 | 0.0089 |
| GTF2H4 | rs916920 | G/A |
| 6 | 30909425 | 44.7 | 1.19 |
2.85×10−5 | 0.0089 |
|
| rs1264569 | A/G |
| 6 | 30397543 |
4.6 | 1.49 |
2.98×10−5 | 0.0092 |
| CACNA1D | rs35874056 | G/A | G460S | 3 | 53702798 |
2.0 | 25.00 |
3.09×10−5 | 0.0094 |
|
| rs9468845 | A/G |
| 6 | 30901816 | 44.7 | 1.19 |
3.12×10−5 | 0.0094 |
| DDR1 | rs8408 | C/T |
| 6 | 30899889 | 44.7 | 1.19 |
3.11×10−5 | 0.0094 |
| DDR1 | rs7756521 | C/T |
| 6 | 30880476 | 44.7 | 1.19 |
3.10×10−5 | 0.0094 |
|
CDKN2B-AS1 | rs1011970 | G/T |
| 9 | 22062135 |
5.6 | 1.41 |
3.15×10−5 | 0.0095 |
| ADAT1 | rs145161932 | T/C | R57G | 16 | 75612670 |
1.4 | 0.39 |
3.29×10−5 | 0.0098 |
| POU5F1 | rs885950 | T/G |
| 6 | 31172375 | 34.0 | 0.83 |
3.28×10−5 | 0.0098 |
| DDR1 | rs4618569 | A/G |
| 6 | 30887474 | 44.7 | 1.19 |
3.41×10−5 | 0.0101 |
| KRT13 | rs146918776 | A/G | Y281H | 17 | 41502993 |
1.5 | 1.94 |
3.51×10−5 | 0.0103 |
|
| rs2523638 | G/A |
| 6 | 31376496 | 43.1 | 1.19 |
3.53×10−5 | 0.0103 |
| PSRC1 | rs599839 | A/G |
| 1 | 109279544 |
7.9 | 0.71 |
3.52×10−5 | 0.0103 |
|
| rs9275141 | G/T |
| 6 | 32683340 | 26.4 | 1.21 |
3.62×10−5 | 0.0105 |
| CCDC63 | rs10849915 | T/C |
| 12 | 110895818 | 23.6 | 1.22 |
3.63×10−5 | 0.0105 |
| HLA-DRA | rs3177928 | G/A |
| 6 | 32444658 |
5.9 | 1.41 |
3.81×10−5 | 0.0108 |
| OAS3 | rs2072134 | C/T |
| 12 | 112971371 | 17.6 | 1.24 |
4.06×10−5 | 0.0114 |
| USP45 | rs41288947 | C/G | T521R | 6 | 99446210 | 14.9 | 1.26 |
4.11×10−5 | 0.0115 |
| CCHCR1 | rs1265109 | A/C |
| 6 | 31151812 | 48.2 | 1.18 |
4.16×10−5 | 0.0116 |
|
LOC101929163 | rs6930777 | C/T |
| 6 | 32383789 |
5.5 | 1.43 |
4.45×10−5 | 0.0122 |
|
| rs7333181 | G/A |
| 13 | 111568950 |
2.5 | 0.54 |
4.45×10−5 | 0.0122 |
| DDR1 | rs1264323 | T/C |
| 6 | 30888130 | 38.8 | 1.19 |
4.48×10−5 | 0.0122 |
|
LINC00243 | rs3094111 | G/A |
| 6 | 30820414 | 14.7 | 1.25 |
4.52×10−5 | 0.0123 |
|
| rs10484561 | T/G |
| 6 | 32697643 |
5.9 | 1.41 |
4.55×10−5 | 0.0123 |
|
PSORS1C1 | rs3130558 | G/C |
| 6 | 31129406 | 13.7 | 1.27 |
4.59×10−5 | 0.0124 |
|
HLA-DQB1 | rs1049060 | T/A | S27T | 6 | 32666529 | 28.8 | 1.20 |
4.92×10−5 | 0.0131 |
|
| rs2844650 | G/A |
| 6 | 30934756 |
4.7 | 1.47 |
4.99×10−5 | 0.0131 |
| DDR1 | rs3132572 | T/C |
| 6 | 30893952 |
4.7 | 1.47 |
4.99×10−5 | 0.0131 |
| CCHCR1 | rs1265115 | T/G |
| 6 | 31149298 | 47.7 | 1.18 |
4.96×10−5 | 0.0131 |
| CCHCR1 | rs3094225 | T/C |
| 6 | 31145275 | 48.4 | 1.18 |
4.93×10−5 | 0.0131 |
|
LOC107987453 | rs3129987 | C/T |
| 6 | 30798427 | 14.5 | 1.25 |
5.05×10−5 | 0.0132 |
| DPCR1 | rs2517451 | A/G |
| 6 | 30946974 |
4.7 | 1.47 |
5.11×10−5 | 0.0133 |
|
KIAA1551 | rs10771894 | A/G | S352G | 12 | 31982009 | 32.4 | 1.19 |
5.18×10−5 | 0.0134 |
|
| rs13427905 | C/T |
| 2 | 71846585 | 18.5 | 0.80 |
5.22×10−5 | 0.0134 |
| ABCA1 | rs1883025 | G/A |
| 9 | 104902020 | 28.8 | 0.83 |
5.46×10−5 | 0.0139 |
| SFTA2 | rs2253705 | G/A |
| 6 | 30932317 | 18.0 | 1.23 |
5.60×10−5 | 0.0141 |
| PLUT | rs954750 | G/A |
| 13 | 27889801 | 48.3 | 1.18 |
5.86×10−5 | 0.0146 |
| TCF19 | rs1419881 | T/C |
| 6 | 31162816 | 48.1 | 1.18 |
6.37×10−5 | 0.0156 |
|
| rs13209234 | G/A |
| 6 | 32448198 |
5.9 | 1.41 |
6.47×10−5 | 0.0158 |
|
PSORS1C1 | rs1265100 | T/C |
| 6 | 31137533 | 32.2 | 0.83 |
6.55×10−5 | 0.0159 |
| YEATS2 | rs76174573 | G/T | C1232F | 3 | 183804099 |
3.7 | 0.61 |
6.74×10−5 | 0.0162 |
| ABO | rs1053878 | C/T | P156L | 9 | 133256264 | 22.8 | 1.20 |
6.78×10−5 | 0.0162 |
|
| rs4014195 | C/G |
| 11 | 65739351 | 16.6 | 1.24 |
6.78×10−5 | 0.0162 |
| SFTA2 | s2253588 | C/G |
| 6 | 30931600 | 23.6 | 1.21 |
6.93×10−5 | 0.0165 |
| CYP4F8 | rs201166643 | C/A | R488S | 19 | 15629257 |
1.1 | ND |
7.00×10−5 | 0.0165 |
| NAXE | rs7516274 | C/G | L19V | 1 | 156591859 |
1.8 | 0.48 |
7.18×10−5 | 0.0169 |
|
| rs10757283 | T/C |
| 9 | 22134173 | 33.8 | 0.84 |
7.25×10−5 | 0.0170 |
| BTNL2 | rs28362680 | G/A | A202V | 6 | 32403039 | 39.7 | 0.85 |
7.40×10−5 | 0.0171 |
| BTNL2 | rs10947262 | C/T |
| 6 | 32405535 | 39.7 | 0.85 |
7.40×10−5 | 0.0171 |
| KRT27 | rs17558532 | C/T | A284T | 17 | 40779624 |
3.6 | 0.62 |
7.71×10−5 | 0.0176 |
| GTF2H4 | rs3130780 | G/T |
| 6 | 30906531 | 18.0 | 1.23 |
7.71×10−5 | 0.0176 |
|
| rs2532934 | T/C |
| 6 | 30926982 | 24.1 | 1.20 |
7.74×10−5 | 0.0176 |
| VARS2 | rs753725 | G/A |
| 6 | 30923094 | 24.1 | 1.20 |
7.68×10−5 | 0.0176 |
| PLUT | rs11619319 | A/G |
| 13 | 27913462 | 48.1 | 1.18 |
7.64×10−5 | 0.0176 |
|
| rs3095273 | C/T |
| 6 | 29598592 |
5.5 | 1.41 |
8.16×10−5 | 0.0184 |
| TNS1 | rs918949 | C/T | V1590I | 2 | 217809974 | 42.8 | 0.85 |
8.39×10−5 | 0.0188 |
|
LINC00243 | rs3130785 | C/T |
| 6 | 30828961 | 14.6 | 1.24 |
8.37×10−5 | 0.0188 |
| VARS2 | rs2249464 | C/T | R309W | 6 | 30920384 | 24.1 | 1.20 |
9.39×10−5 | 0.0207 |
|
| rs3095345 | A/G |
| 6 | 30854636 | 17.9 | 1.22 |
9.37×10−5 | 0.0207 |
| ITGB8 | rs80015015 | G/A | C481Y | 7 | 20401881 |
7.1 | 1.35 |
1.01×10−4 | 0.0220 |
| VARS2 | rs885905 | C/T |
| 6 | 30922654 | 23.4 | 1.20 |
1.07×10−4 | 0.0232 |
| LIPE | rs34052647 | G/A | R611C | 19 | 42407617 |
5.5 | 1.39 |
1.16×10−4 | 0.0249 |
| PHACTR1 | rs9369640 | A/C |
| 6 | 12901209 |
9.1 | 0.74 |
1.30×10−4 | 0.0275 |
| BTNL2 | rs41417449 | T/C | M295V | 6 | 32396234 | 23.0 | 0.83 |
1.35×10−4 | 0.0280 |
| BTNL2 | rs41441651 | C/T | D336N | 6 | 32396111 | 23.0 | 0.83 |
1.35×10−4 | 0.0280 |
| BTNL2 | rs28362675 | C/A | E454* | 6 | 32394744 | 23.0 | 0.83 |
1.35×10−4 | 0.0280 |
| BTNL2 | rs78587369 | G/A | T165I | 6 | 32403150 | 23.0 | 0.83 |
1.35×10−4 | 0.0280 |
| BTNL2 | rs3763315 | G/T |
| 6 | 32408877 | 23.0 | 0.83 |
1.35×10−4 | 0.0280 |
| BTNL2 | rs2076528 | T/G |
| 6 | 32396417 | 23.0 | 0.83 |
1.35×10−4 | 0.0280 |
| PRKG1 | rs9414827 | G/A |
| 10 | 51137314 | 10.1 | 0.76 |
1.37×10−4 | 0.0282 |
|
| rs6537384 | T/G |
| 4 | 145949613 | 28.8 | 1.19 |
1.43×10−4 | 0.0294 |
|
| rs6067640 | G/A |
| 20 | 51092837 | 38.5 | 0.85 |
1.48×10−4 | 0.0302 |
|
| rs10514995 | A/G |
| 5 | 66443611 | 48.7 | 1.16 |
1.51×10−4 | 0.0306 |
| BTNL2 | rs34423804 | T/A | V283D | 6 | 32396269 | 23.0 | 0.83 |
1.63×10−4 | 0.0329 |
| PHACTR1 | rs9349379 | G/A |
| 6 | 12903725 | 34.2 | 0.85 |
1.69×10−4 | 0.0341 |
| STIM1 | rs116855870 | A/G |
| 11 | 4055527 |
1.1 | 1.93 |
1.71×10−4 | 0.0343 |
| ZNF142 | rs3821033 | C/T | A1313T | 2 | 218642579 | 11.2 | 1.26 |
1.78×10−4 | 0.0355 |
|
LINC00354 | rs4907518 | G/A |
| 13 | 111898209 | 45.6 | 0.85 |
1.82×10−4 | 0.0362 |
| TNS3 | rs11763932 | G/A |
| 7 | 47567880 | 42.0 | 0.85 |
1.91×10−4 | 0.0378 |
| BTRC | rs2270439 | C/A | P566H | 10 | 101550817 |
3.5 | 0.63 |
1.94×10−4 | 0.0381 |
| MIA3 | rs2936051 | A/G | E881G | 1 | 222629862 | 40.1 | 0.85 |
1.96×10−4 | 0.0384 |
|
| rs6825911 | C/T |
| 4 | 110460482 | 45.9 | 0.86 |
2.01×10−4 | 0.0391 |
| VNN1 | rs2294757 | G/A | T26I | 6 | 132713959 | 37.4 | 0.85 |
2.02×10−4 | 0.0393 |
| ZNF860 | rs140232911 | C/T | S161L | 3 | 31989561 | 10.4 | 0.44 |
2.09×10−4 | 0.0406 |
|
| rs838880 | C/T |
| 12 | 124777047 | 47.5 | 1.16 |
2.23×10−4 | 0.0430 |
| MIA3 | rs2936052 | A/G | K605R | 1 | 222629034 | 34.4 | 0.85 |
2.26×10−4 | 0.0430 |
| DTNBP1 | rs2743868 | G/A |
| 6 | 15625577 | 31.6 | 1.18 |
2.26×10−4 | 0.0430 |
| MON2 | rs11174549 | A/G | I1385V | 12 | 62565357 |
5.0 | 1.40 |
2.26×10−4 | 0.0430 |
|
| rs507666 | G/A |
| 9 | 136149399 | 27.8 | 1.18 |
2.26×10−4 | 0.0430 |
| FAM170B | rs73302786 | G/T | D252E | 10 | 49131709 |
3.5 | 1.47 |
2.36×10−4 | 0.0445 |
|
PSORS1C3 | rs3131018 | G/T |
| 6 | 31175805 | 15.7 | 1.23 |
2.36×10−4 | 0.0445 |
| PIEZO2 | rs35033671 | C/A | C1148F | 18 | 10759842 | 11.0 | 1.27 |
2.39×10−4 | 0.0448 |
| SLC22A3 | rs1810126 | C/T |
| 6 | 160451119 | 49.1 | 0.86 |
2.46×10−4 | 0.0460 |
| PANK1 | rs11185790 | G/A |
| 10 | 89612776 | 46.9 | 1.16 |
2.57×10−4 | 0.0481 |
| GFY | rs73053944 | C/G | T203S | 19 | 49427038 |
2.9 | 1.51 |
2.58×10−4 | 0.0481 |
| RNF2 | rs1046592 | A/G |
| 1 | 185100429 | 33.9 | 0.85 |
2.63×10−4 | 0.0488 |
Multivariable logistic regression
analysis of the association between SNPs and CAD
The association between the 170 SNPs identified in
the EWAS for CAD and this condition was examined by multivariable
logistic regression analysis with adjustment for age, sex and the
prevalence of hypertension, diabetes mellitus and dyslipidemia
(Table III). The 162 SNPs were
significantly (P<0.05 in a dominant or recessive model)
associated with CAD.
 | Table III.162 SNPs associated with coronary
artery disease as determined by multivariable logistic regression
analysis. |
Table III.
162 SNPs associated with coronary
artery disease as determined by multivariable logistic regression
analysis.
|
|
|
| Dominant model | Recessive
model |
|---|
|
|
|
|
|
|
|---|
| Gene | SNP |
| P-value | OR | 95% CI | P-value | OR | 95% CI |
|---|
| PLCB2 | rs200787930 | C/T | <0.0001 | 0.02 | 0.01–0.09 |
|
|
|
| MARCH1 | rs61734696 | G/T | <0.0001 | 0.02 | 0.01–0.10 |
|
|
|
| VPS33B | rs199921354 | C/T | <0.0001 | 0.02 | 0.01–0.09 |
|
|
|
| CXCL8 | rs188378669 | G/T | <0.0001 | 0.02 | 0.01–0.09 |
|
|
|
| TMOD4 | rs115287176 | G/A | <0.0001 | 0.02 | 0.01–0.10 |
|
|
|
| COL6A3 | rs146092501 | C/T | <0.0001 | 0.02 | 0.01–0.10 |
|
|
|
| ZNF77 | rs146879198 | G/A | <0.0001 | 0.02 | 0.01–0.10 |
|
|
|
| ADGRL3 | rs192210727 | G/T | <0.0001 | 0.07 | 0.03–0.16 |
0.9959 |
|
|
| OR52E4 | rs11823828 | T/G | <0.0001 | 1.66 | 1.41–1.97 | <0.0001 | 2.44 | 2.01–2.97 |
| ALDH2 | rs671 | G/A | <0.0001 | 1.73 | 1.50–2.01 | <0.0001 | 1.80 | 1.44–2.26 |
| ACAD10 | rs11066015 | G/A | <0.0001 | 1.73 | 1.49–2.01 | <0.0001 | 1.79 | 1.42–2.25 |
| BRAP | rs3782886 | A/G | <0.0001 | 1.71 | 1.48–1.99 | <0.0001 | 1.70 | 1.36–2.12 |
| HECTD4 | rs11066280 | T/A | <0.0001 | 1.73 | 1.49–2.01 | <0.0001 | 1.73 | 1.38–2.17 |
| HECTD4 | rs2074356 | C/T | <0.0001 | 1.61 | 1.39–1.87 | <0.0001 | 1.76 | 1.38–2.26 |
| NAA25 | rs12231744 | C/T | <0.0001 | 0.63 | 0.54–0.73 | <0.0001 | 0.55 | 0.43–0.70 |
| GOSR2 | rs1052586 | T/C |
0.0003 | 0.73 | 0.62–0.87 | <0.0001 | 0.64 | 0.53–0.77 |
| ATXN2 | rs7969300 | T/C | <0.0001 | 0.63 | 0.55–0.74 | <0.0001 | 0.57 | 0.45–0.71 |
|
| rs12229654 | T/G | <0.0001 | 1.46 | 1.26–1.69 | <0.0001 | 1.72 | 1.31–2.25 |
|
LOC107987429 | rs2844533 | T/C | <0.0001 | 1.36 | 1.17–1.59 |
0.8616 |
|
|
| MTFR2 | rs143974258 | G/A |
0.0014 | 0.04 | 0.01–0.28 |
|
|
|
|
PSORS1C1 | rs3130559 | C/T |
0.0127 | 0.82 | 0.70–0.96 |
0.0629 |
|
|
|
| rs2596548 | G/T | <0.0001 | 1.76 | 1.41–2.20 |
0.2047 |
|
|
| EIF3L | rs9466 | T/C |
0.0053 | 1.24 | 1.07–1.44 |
0.0199 | 1.47 | 1.06–2.04 |
| LPGAT1 | rs150552771 | T/C |
0.9970 |
|
| <0.0001 | 2.20 | 1.83–2.64 |
|
| rs2523644 | A/G | <0.0001 | 1.59 | 1.31–1.92 |
0.9088 |
|
|
|
| rs10757278 | A/G | <0.0001 | 0.71 | 0.60–0.83 |
0.0023 | 0.77 | 0.65–0.91 |
| CCHCR1 | rs130067 | T/G |
0.0010 | 0.78 | 0.68–0.91 |
0.0183 | 0.73 | 0.57–0.95 |
| TCHP | rs74416240 | G/A |
0.0002 | 1.35 | 1.15–1.58 |
0.1725 |
|
|
|
| rs1333049 | G/C |
0.0031 | 1.29 | 1.09–1.53 | <0.0001 | 1.41 | 1.20–1.66 |
|
CDKN2B-AS1 | rs4977574 | A/G |
0.0003 | 1.36 | 1.15–1.60 | <0.0001 | 1.43 | 1.21–1.69 |
|
CDKN2B-AS1 | rs2383207 | G/A | <0.0001 | 0.75 | 0.65–0.87 |
0.0171 | 0.75 | 0.59–0.95 |
| SLC16A1 | rs1049434 | T/A |
0.0106 | 0.83 | 0.71–0.96 | <0.0001 | 0.57 | 0.45–0.73 |
| GIT2 | rs925368 | T/C |
0.0001 | 1.37 | 1.16–1.61 |
0.3189 |
|
|
|
| rs1333048 | A/C |
0.0036 | 1.29 | 1.09–1.53 | <0.0001 | 1.40 | 1.19–1.64 |
|
| rs2523578 | T/C | <0.0001 | 1.55 | 1.27–1.88 |
0.8694 |
|
|
|
| rs404890 | G/T |
0.0005 | 1.29 | 1.12–1.50 |
0.0160 | 1.35 | 1.06–1.72 |
| APOE | rs7412 | C/T |
0.0001 | 0.56 | 0.42–0.76 |
0.2259 |
|
|
| CCHCR1 | rs130071 | G/A |
0.0149 | 1.36 | 1.06–1.73 |
0.2668 |
|
|
|
| rs602633 | C/A |
0.0001 | 0.64 | 0.51–0.80 |
0.1782 |
|
|
| CELSR2 | rs12740374 | G/T | <0.0001 | 0.63 | 0.51–0.79 |
0.1708 |
|
|
| MKI67 | rs145121731 | G/A |
0.0014 | 1.94 | 1.29–2.91 |
0.9957 |
|
|
| CUBN | rs78201384 | C/T |
0.0003 | 0.50 | 0.34–0.73 |
0.9959 |
|
|
|
PSORS1C3 | rs887466 | T/C |
0.0013 | 1.29 | 1.11–1.52 |
0.1666 |
|
|
|
PSORS1C1 | rs3094663 | G/A | <0.0001 | 1.41 | 1.21–1.63 |
0.6404 |
|
|
|
| rs10853110 | A/G |
0.0026 | 1.26 | 1.09–1.47 |
0.0102 | 1.29 | 1.06–1.56 |
| WDR37 | rs10794720 | C/T |
0.0003 | 0.68 | 0.55–0.84 |
0.0734 |
|
|
| CELSR2 | rs629301 | A/C |
0.0002 | 0.65 | 0.52–0.82 |
0.1708 |
|
|
| SKIV2L | rs592229 | G/T |
0.0154 | 1.22 | 1.04–1.42 |
0.0138 | 1.26 | 1.05–1.51 |
|
| rs12182351 | T/C |
0.0008 | 1.28 | 1.11–1.48 |
0.0138 | 1.37 | 1.07–1.75 |
| POU5F1 | rs3130503 | G/A | <0.0001 | 1.41 | 1.22–1.63 |
0.6308 |
|
|
|
PSORS1C3 | rs1265155 | T/C |
0.0017 | 1.29 | 1.10–1.50 |
0.1666 |
|
|
| CELSR2 | rs646776 | A/G |
0.0002 | 0.65 | 0.52–0.82 | 0.2660 |
|
|
|
| rs2596503 | C/T |
0.0204 | 1.19 | 1.03–1.38 | 0.1828 |
|
|
| TRPM1 | rs2241493 | T/C |
0.0002 | 0.71 | 0.60–0.85 | 0.0410 | 0.49 | 0.25–0.97 |
| CCDC141 | rs13419085 | T/C |
0.0005 | 0.43 | 0.27–0.69 |
|
|
|
| VARS2 | rs9394021 | A/G |
0.0261 | 0.82 | 0.69–0.98 | 0.0117 | 0.81 | 0.69–0.95 |
| SFTA2 | rs2286655 | T/C |
0.0097 | 1.24 | 1.05–1.45 | 0.0277 | 1.22 | 1.02–1.45 |
|
| rs3873334 | T/C |
0.0117 | 1.23 | 1.05–1.44 | 0.0261 | 1.22 | 1.02–1.45 |
| TCF19 | rs3130453 | C/T |
0.0077 | 0.82 | 0.71–0.95 | 0.0024 | 0.68 | 0.53–0.87 |
| DDR1 | rs2239518 | T/C |
0.0103 | 1.23 | 1.05–1.45 | 0.0282 | 1.22 | 1.02–1.45 |
| CDSN | rs3130984 | C/T | <0.0001 | 1.39 | 1.18–1.64 | 0.1220 |
|
|
|
| rs197932 | T/C |
0.0042 | 0.81 | 0.70–0.93 | 0.0348 | 0.73 | 0.54–0.98 |
| CDSN | rs3130981 | C/T | <0.0001 | 1.39 | 1.18–1.64 | 0.1226 |
|
|
| MICB-DT | rs3132469 | C/T | <0.0001 | 1.63 | 1.30–2.05 | 0.3960 |
|
|
|
HLA-DQB1 | rs1049056 | C/A |
0.0050 | 1.28 | 1.08–1.52 | 0.1586 |
|
|
| DDR1 | rs2239517 | A/G |
0.0115 | 1.23 | 1.05–1.44 | 0.0326 | 1.21 | 1.02–1.44 |
| CCHCR1 | rs1265110 | G/A |
0.0096 | 0.83 | 0.71–0.95 | 0.0948 |
|
|
| CCDC63 | rs10774610 | T/C |
0.0006 | 1.29 | 1.12–1.49 | 0.0214 | 1.38 | 1.05–1.82 |
| GTF2H4 | rs2284176 | C/T |
0.0153 | 1.22 | 1.04–1.43 | 0.0306 | 1.21 | 1.02–1.45 |
| GTF2H4 | rs3909130 | G/A |
0.0138 | 1.22 | 1.04–1.43 | 0.0326 | 1.21 | 1.02–1.44 |
| GTF2H4 | rs916920 | G/A |
0.0139 | 1.22 | 1.04–1.43 | 0.0326 | 1.21 | 1.02–1.44 |
|
| rs1264569 | A/G |
0.0004 | 1.54 | 1.21–1.97 | 0.5339 |
|
|
|
| rs9468845 | A/G |
0.0141 | 1.22 | 1.04–1.43 | 0.0332 | 1.21 | 1.02–1.44 |
| DDR1 | rs8408 | C/T |
0.0141 | 1.22 | 1.04–1.43 | 0.0326 | 1.21 | 1.02–1.44 |
| DDR1 | rs7756521 | C/T |
0.0155 | 1.22 | 1.04–1.43 | 0.0303 | 1.21 | 1.02–1.45 |
|
CDKN2B-AS1 | rs1011970 | G/T |
0.0047 | 1.36 | 1.10–1.69 | 0.2199 |
|
|
| ADAT1 | rs145161932 | T/C |
0.0104 | 0.48 | 0.27–0.84 | 0.9960 |
|
|
| POU5F1 | rs885950 | T/G |
0.0049 | 0.81 | 0.70–0.94 | 0.0129 | 0.73 | 0.57–0.94 |
| DDR1 | rs4618569 | A/G |
0.0141 | 1.22 | 1.04–1.43 | 0.0333 | 1.21 | 1.02–1.44 |
| KRT13 | rs146918776 | A/G | <0.0001 | 2.21 | 1.50–3.26 |
|
|
|
|
| rs2523638 | G/A |
0.0197 | 1.20 | 1.03–1.41 | 0.1032 |
|
|
| PSRC1 | rs599839 | A/G |
0.0004 | 0.67 | 0.54–0.83 | 0.1023 |
|
|
|
| rs9275141 | G/T |
0.0134 | 1.20 | 1.04–1.39 | 0.0065 | 1.43 | 1.11–1.85 |
| CCDC63 | rs10849915 | T/C |
0.0005 | 1.30 | 1.12–1.50 | 0.0458 | 1.33 | 1.01–1.76 |
| HLA-DRA | rs3177928 | G/A | <0.0001 | 1.58 | 1.27–1.96 | 0.7635 |
|
|
| OAS3 | rs2072134 | C/T |
0.0004 | 1.31 | 1.13–1.53 | 0.0268 | 1.51 | 1.05–2.16 |
| USP45 | rs41288947 | C/G |
0.0002 | 1.35 | 1.15–1.58 | 0.1360 |
|
|
| CCHCR1 | rs1265109 | A/C |
0.0007 | 1.35 | 1.14–1.61 | 0.0334 | 1.20 | 1.01–1.41 |
|
LOC101929163 | rs6930777 | C/T | <0.0001 | 1.60 | 1.28–2.00 | 0.9815 |
|
|
|
| rs7333181 | G/A |
0.0219 | 0.64 | 0.44–0.94 | 0.9969 |
|
|
| DDR1 | rs1264323 | T/C |
0.0240 | 1.19 | 1.02–1.39 | 0.0423 | 1.22 | 1.01–1.48 |
|
LINC00243 | rs3094111 | G/A |
0.0061 | 1.24 | 1.06–1.45 | 0.5293 |
|
|
|
| rs10484561 | T/G | <0.0001 | 1.59 | 1.28–1.98 | 0.7637 |
|
|
|
PSORS1C1 | rs3130558 | G/C | <0.0001 | 1.39 | 1.18–1.64 | 0.2712 |
|
|
|
HLA-DQB1 | rs1049060 | T/A |
0.0676 |
|
| 0.0077 | 1.38 | 1.09–1.75 |
|
| rs2844650 | G/A | <0.0001 | 1.66 | 1.30–2.11 | 0.4729 |
|
|
| DDR1 | rs3132572 | T/C | <0.0001 | 1.66 | 1.30–2.11 | 0.4729 |
|
|
| CCHCR1 | rs1265115 | T/G |
0.0004 | 1.36 | 1.15–1.62 | 0.0443 | 1.19 | 1.00–1.40 |
| CCHCR1 | rs3094225 | T/C | <0.0001 | 1.46 | 1.23–1.74 | 0.5822 |
|
|
|
LOC107987453 | rs3129987 | C/T |
0.0037 | 1.26 | 1.08–1.47 | 0.5667 |
|
|
| DPCR1 | rs2517451 | A/G | <0.0001 | 1.65 | 1.30–2.11 | 0.4729 |
|
|
| CCHCR1 | rs1265115 | T/G |
0.0004 | 1.36 | 1.15–1.62 | 0.0443 | 1.19 | 1.00–1.40 |
| CCHCR1 | rs3094225 | T/C | <0.0001 | 1.46 | 1.23–1.74 | 0.5822 |
|
|
|
LOC107987453 | rs3129987 | C/T |
0.0037 | 1.26 | 1.08–1.47 | 0.5667 |
|
|
| DPCR1 | rs2517451 | A/G | <0.0001 | 1.65 | 1.30–2.11 | 0.4729 |
|
|
|
KIAA1551 | rs10771894 | A/G |
0.1122 |
|
| 0.0085 | 1.35 | 1.08–1.69 |
|
| rs13427905 | C/T |
0.0024 | 0.78 | 0.67–0.92 | 0.1397 |
|
|
| ABCA1 | rs1883025 | G/A |
0.0051 | 0.81 | 0.70–0.94 | 0.0080 | 0.68 | 0.51–0.90 |
| SFTA2 | rs2253705 | G/A |
0.0015 | 1.28 | 1.10–1.48 | 0.6682 |
|
|
| PLUT | rs954750 | G/A |
0.0409 | 1.19 | 1.01–1.41 | 0.0008 | 1.33 | 1.12–1.57 |
| TCF19 | rs1419881 | T/C |
0.0009 | 1.34 | 1.13–1.60 | 0.0487 | 1.18 | 1.00–1.39 |
|
| rs13209234 | G/A | <0.0001 | 1.55 | 1.25–1.93 | 0.7617 |
|
|
|
PSORS1C1 | rs1265100 | T/C |
0.0055 | 0.81 | 0.70–0.94 | 0.4135 |
|
|
| YEATS2 | rs76174573 | G/T |
0.0031 | 0.61 | 0.44–0.85 | 0.1756 |
|
|
| ABO | rs1053878 | C/T |
0.0033 | 1.25 | 1.08–1.44 | 0.1723 |
|
|
|
| rs4014195 | C/G |
0.0083 | 1.23 | 1.05–1.43 | 0.2586 |
|
|
| SFTA2 | rs2253588 | C/G |
0.0021 | 1.26 | 1.09–1.45 | 0.7016 |
|
|
|
| rs10757283 | T/C |
0.0079 | 0.82 | 0.71–0.95 | 0.0181 | 0.74 | 0.58–0.95 |
| BTNL2 | rs28362680 | G/A |
0.1143 |
|
| 0.0082 | 0.76 | 0.62–0.93 |
| BTNL2 | rs10947262 | C/T |
0.1143 |
|
| 0.0082 | 0.76 | 0.62–0.93 |
| KRT27 | rs17558532 | C/T |
0.0004 | 0.57 | 0.41–0.78 | 0.5002 |
|
|
| GTF2H4 | rs3130780 | G/T |
0.0022 | 1.26 | 1.09–1.47 | 0.6706 |
|
|
|
| rs2532934 | T/C |
0.0023 | 1.25 | 1.08–1.45 | 0.5320 |
|
|
| VARS2 | rs753725 | G/A |
0.0021 | 1.26 | 1.09–1.45 | 0.5274 |
|
|
| PLUT | rs11619319 | A/G |
0.0482 | 1.18 | 1.00–1.40 | 0.0007 | 1.33 | 1.13–1.58 |
|
| rs3095273 | C/T |
0.0045 | 1.38 | 1.11–1.73 | 0.1778 |
|
|
| TNS1 | rs918949 | C/T |
0.0028 | 0.79 | 0.68–0.92 | 0.1301 |
|
|
|
LINC00243 | rs3130785 | C/T |
0.0060 | 1.24 | 1.06–1.45 | 0.5417 |
|
|
| VARS2 | rs2249464 | C/T |
0.0028 | 1.25 | 1.08–1.44 | 0.5320 |
|
|
|
| rs3095345 | A/G |
0.0021 | 1.27 | 1.09–1.47 | 0.7469 |
|
|
| ITGB8 | rs80015015 | G/A | <0.0001 | 1.56 | 1.28–1.91 | 0.2511 |
|
|
| VARS2 | rs885905 | C/T |
0.0028 | 1.25 | 1.08–1.44 | 0.7687 |
|
|
| LIPE | rs34052647 | G/A |
0.0001 | 1.53 | 1.23–1.90 | 0.0074 | 3.70 | 1.42–9.65 |
| PHACTR1 | rs9369640 | A/C |
0.0025 | 0.73 | 0.60–0.90 | 0.1527 |
|
|
| BTNL2 | rs41417449 | T/C |
0.1641 |
|
| 0.0017 | 0.55 | 0.38–0.80 |
| BTNL2 | rs41441651 | C/T |
0.1586 |
|
| 0.0017 | 0.55 | 0.38–0.80 |
| BTNL2 | rs28362675 | C/A |
0.1584 |
|
| 0.0017 | 0.55 | 0.38–0.80 |
| BTNL2 | rs78587369 | G/A |
0.1590 |
|
| 0.0018 | 0.55 | 0.38–0.80 |
| BTNL2 | rs3763315 | G/T |
0.1637 |
|
| 0.0017 | 0.55 | 0.38–0.80 |
| BTNL2 | rs2076528 | T/G |
0.1524 |
|
| 0.0017 | 0.55 | 0.38–0.80 |
| PRKG1 | rs9414827 | G/A |
0.0003 | 0.70 | 0.58–0.85 | 0.0395 | 0.46 | 0.22–0.96 |
|
| rs6537384 | T/G |
0.0191 | 1.19 | 1.03–1.38 | 0.1787 |
|
|
|
| rs6067640 | G/A |
0.0323 | 0.85 | 0.73–0.99 | 0.0054 | 0.74 | 0.60–0.91 |
|
| rs10514995 | A/G |
0.0467 | 1.19 | 1.00–1.42 | 0.0825 |
|
|
| BTNL2 | rs34423804 | T/A |
0.1675 |
|
| 0.0018 | 0.55 | 0.38–0.80 |
| PHACTR1 | rs9349379 | G/A |
0.0029 | 0.80 | 0.69–0.93 | 0.0820 |
|
|
| STIM1 | rs116855870 | A/G |
0.0133 | 1.76 | 1.13–2.76 | 0.9967 |
|
|
| ZNF142 | rs3821033 | C/T |
0.0015 | 1.32 | 1.11–1.57 | 0.3469 |
|
|
|
LINC00354 | rs4907518 | G/A |
0.0138 | 0.82 | 0.70–0.96 | 0.0050 | 0.77 | 0.64–0.92 |
| TNS3 | rs11763932 | G/A |
0.0054 | 0.81 | 0.69–0.94 | 0.0018 | 0.73 | 0.60–0.89 |
| BTRC | rs2270439 | C/A |
0.0063 | 0.64 | 0.47–0.88 | 0.6622 |
|
|
| MIA3 | rs2936051 | A/G |
0.1239 |
|
| 0.0002 | 0.67 | 0.55–0.83 |
|
| rs6825911 | C/T |
0.0020 | 0.78 | 0.67–0.91 | 0.0446 | 0.83 | 0.70–1.00 |
| VNN1 | rs2294757 | G/A | 0.2269 |
|
| 0.0334 | 0.79 | 0.63–0.98 |
| ZNF860 | rs140232911 | C/T | 0.0013 | 0.14 | 0.04–0.46 |
|
|
|
|
| rs838880 | C/T | 0.0338 | 1.20 | 1.01–1.41 | 0.0308 | 1.20 | 1.02–1.43 |
| MIA3 | rs2936052 | A/G | 0.0927 |
|
| 0.0044 | 0.71 | 0.56–0.90 |
| DTNBP1 | rs2743868 | G/A | 0.0208 | 1.19 | 1.03–1.37 | 0.0941 |
|
|
| MON2 | rs11174549 | A/G | 0.0427 | 1.28 | 1.01–1.61 | 0.1917 |
|
|
|
| rs507666 | G/A | 0.0098 | 1.21 | 1.05–1.40 | 0.1384 |
|
|
| FAM170B | rs73302786 | G/T | 0.0003 | 1.62 | 1.25–2.11 | 0.6351 |
|
|
|
PSORS1C3 | rs3131018 | G/T | 0.0002 | 1.35 | 1.15–1.58 | 0.9209 |
|
|
| PIEZO2 | rs35033671 | C/A | 0.0035 | 1.30 | 1.09–1.54 | 0.1909 |
|
|
| PANK1 | rs11185790 | G/A | 0.0065 | 1.26 | 1.07–1.48 | 0.0457 | 1.19 | 1.00–1.41 |
| GFY | rs73053944 | C/G | 0.0011 | 1.60 | 1.21–2.13 | 0.0698 |
|
|
| RNF2 | rs1046592 | A/G | 0.0301 | 0.85 | 0.74–0.98 | 0.7186 |
|
|
Stepwise forward selection procedure
of the effects of SNPs on CAD
A stepwise forward selection procedure was performed
to examine effects of genotypes for the 162 SNPs associated with
CAD by multivariable logistic regression analysis on this condition
(Table IV). The 54 SNPs were
significant (P<0.05) and independent [coefficient of
determination (R2), 0.0008 to 0.0297] determinants of
CAD. These SNPs together accounted for 15.5% of the cause of
CAD.
 | Table IV.54 SNPs associated with coronary
artery disease as determined by a stepwise forward selection
procedure. |
Table IV.
54 SNPs associated with coronary
artery disease as determined by a stepwise forward selection
procedure.
| Gene | SNP | P-value | R2
(individual) | R2
(accumulated) |
|---|
| PLCB2 | rs200787930 | <0.0001 | 0.0297 | 0.0297 |
| ALDH2 | rs671 | <0.0001 | 0.0061 | 0.0358 |
| GOSR2 | rs1052586 | <0.0001 | 0.0053 | 0.0411 |
|
PSORS1C1 | rs3094663 | <0.0001 | 0.0052 | 0.0463 |
| CCHCR1 | rs130071 | <0.0001 | 0.0059 | 0.0522 |
|
| rs13427905 | <0.0001 | 0.0047 | 0.0569 |
| OR52E4 | rs11823828 | <0.0001 | 0.0043 | 0.0612 |
| EIF3L | rs9466 | <0.0001 | 0.0042 | 0.0654 |
|
KIAA1551 | rs10771894 | <0.0001 | 0.0039 | 0.0693 |
| CCDC141 | rs13419085 | <0.0001 | 0.0035 | 0.0728 |
| MIA3 | rs2936051 |
0.0001 | 0.0033 | 0.0761 |
|
| rs602633 |
0.0001 | 0.0033 | 0.0794 |
| KRT27 | rs17558532 |
0.0001 | 0.0032 | 0.0826 |
| TRPM1 | rs2241493 |
0.0002 | 0.0030 | 0.0856 |
|
| rs7333181 |
0.0002 | 0.0030 | 0.0886 |
| ADAT1 | rs145161932 |
0.0002 | 0.0029 | 0.0915 |
| APOE | rs7412 |
0.0003 | 0.0028 | 0.0943 |
| YEATS2 | rs76174573 |
0.0004 | 0.0026 | 0.0969 |
| SLC16A1 | rs1049434 |
0.0005 | 0.0025 | 0.0994 |
| RNF2 | rs1046592 |
0.0007 | 0.0025 | 0.1019 |
|
| rs6825911 |
0.0006 | 0.0024 | 0.1043 |
| ITGB8 | rs80015015 |
0.0007 | 0.0024 | 0.1067 |
| USP45 | rs41288947 |
0.0007 | 0.0024 | 0.1091 |
| PHACTR1 | rs9369640 |
0.0007 | 0.0024 | 0.1115 |
|
| rs1333048 |
0.0008 | 0.0024 | 0.1139 |
|
| rs838880 |
0.0011 | 0.0022 | 0.1161 |
| STIM1 | rs116855870 |
0.0017 | 0.0021 | 0.1182 |
|
| rs2523644 |
0.0016 | 0.0021 | 0.1203 |
| MKI67 | rs145121731 | 0.0020 | 0.0020 | 0.1223 |
|
FAM170B-AS1 | rs73302786 | 0.0019 | 0.0020 | 0.1243 |
|
| rs6067640 | 0.0022 | 0.0020 | 0.1263 |
| GFY | rs73053944 | 0.0024 | 0.0019 | 0.1282 |
| WDR37 | rs10794720 | 0.0033 | 0.0018 | 0.1300 |
| SKIV2L | rs592229 | 0.0037 | 0.0018 | 0.1318 |
|
| rs6537384 | 0.0041 | 0.0017 | 0.1335 |
|
| rs10757283 | 0.0058 | 0.0016 | 0.1351 |
|
CDKN2B-AS1 | rs1011970 | 0.0110 | 0.0014 | 0.1365 |
| PRKG1 | rs9414827 | 0.0087 | 0.0014 | 0.1379 |
|
| rs197932 | 0.0127 | 0.0013 | 0.1392 |
|
LINC00354 | rs4907518 | 0.0125 | 0.0013 | 0.1405 |
| LIPE | rs34052647 | 0.0151 | 0.0013 | 0.1418 |
| BTRC | rs2270439 | 0.0143 | 0.0013 | 0.1431 |
| TNS3 | rs11763932 | 0.0163 | 0.0013 | 0.1444 |
| TNS1 | rs918949 | 0.0158 | 0.0012 | 0.1456 |
|
| rs12229654 | 0.0184 | 0.0011 | 0.1467 |
|
| rs4014195 | 0.0213 | 0.0011 | 0.1478 |
| PANK1 | rs11185790 | 0.0227 | 0.0011 | 0.1489 |
|
| rs507666 | 0.0279 | 0.0010 | 0.1499 |
| MON2 | rs11174549 | 0.0359 | 0.0009 | 0.1508 |
| HECTD4 | rs2074356 | 0.0396 | 0.0009 | 0.1517 |
| CUBN | rs78201384 | 0.0484 | 0.0009 | 0.1526 |
| PLUT | rs954750 | 0.0497 | 0.0008 | 0.1534 |
| ABCA1 | rs1883025 | 0.0479 | 0.0008 | 0.1542 |
|
| rs10514995 | 0.0493 | 0.0008 | 0.1550 |
Association between SNPs associated
with CAD and intermediate phenotypes
The association between the 54 SNPs associated with
CAD and intermediate phenotypes of this condition, including
hypertension, DM, hypertriglyceridemia, hypo-HDL-cholesterolemia,
hyper-low density lipoprotein (LDL)-cholesterolemia, CKD, obesity,
and hyperuricemia, was examined using Pearson's χ2 test
(Table V).
 | Table V.Association between SNPs associated
with coronary artery disease and intermediate phenotypes. |
Table V.
Association between SNPs associated
with coronary artery disease and intermediate phenotypes.
| Gene | SNP | Hypertension | DM | Hyper-TG | Hypo-HDL | Hyper-LDL | CKD | Obesity | Hyperuricemia |
|---|
| PLCB2 | rs200787930 |
<0.0001a |
0.0004a | 0.3432 |
<0.0001a |
<0.0001a |
<0.0001a |
0.0405a |
0.9639 |
| ALDH2 | rs671 |
0.0039a |
0.0074a |
0.0298a |
<0.0001a |
<0.0001a |
0.0273a |
0.0350a |
<0.0001a |
| GOSR2 | rs1052586 |
0.3498 |
0.0167a | 0.4457 |
0.2898 |
0.2638 |
0.6185 | 0.3670 |
0.4679 |
|
PSORS1C1 | rs3094663 |
0.0069a |
0.0670 | 0.0947 |
0.0020a |
0.3869 |
0.1080 | 0.7345 |
0.5091 |
| CCHCR1 | rs130071 |
0.0865 |
0.0008a | 0.2247 |
0.0143a |
0.0141a |
0.5894 | 0.8651 |
0.0423a |
|
| rs13427905 |
0.0149a |
0.0149a | 0.0524 |
0.0545 |
0.6318 |
0.9487 | 0.1197 |
0.0920 |
| OR52E4 | rs11823828 |
0.0024a |
<0.0001a |
0.0265a |
0.1186 |
0.4445 |
0.0027a | 0.1141 |
0.2815 |
| EIF3L | rs9466 |
0.0008a |
0.0204a |
0.0054a |
0.2905 |
0.0114a |
0.2368 | 0.2435 |
0.0312a |
|
KIAA1551 | rs10771894 |
0.2439 |
0.0091a | 0.9562 |
0.0343a |
0.6934 |
0.3869 | 0.0974 |
0.5419 |
| CCDC141 | rs13419085 |
0.3387 |
0.1255 | 0.6537 |
0.1647 |
0.7447 |
0.2483 | 0.8101 |
0.7938 |
| MIA3 | rs2936051 |
0.4092 |
0.5246 | 0.9990 |
0.0475a |
0.1222 |
0.6614 | 0.8787 |
0.1949 |
|
| rs602633 |
0.4468 |
0.2375 | 0.7350 |
0.0005a |
0.0021a |
0.0842 | 0.6617 |
0.9338 |
| KRT27 | rs17558532 |
0.1706 |
0.2358 | 0.3643 |
0.3607 |
0.7663 |
0.0133a | 0.2306 |
0.5325 |
| TRPM1 | rs2241493 |
0.3861 |
0.2332 | 0.7465 |
0.7106 |
0.0815 |
0.1387 | 0.5698 |
0.9502 |
|
| rs7333181 |
0.2308 |
0.0487a |
0.0379a |
0.1185 |
0.2010 |
0.0795 | 0.2182 |
0.6544 |
| ADAT1 | rs145161932 |
0.4468 |
0.0160a | 0.3611 |
0.3357 |
0.5412 |
0.7534 | 0.5836 |
0.1202 |
| APOE | rs7412 |
0.3680 |
0.9184 | 0.6322 |
0.1157 |
<0.0001a |
0.6367 | 0.5319 |
0.2528 |
|
YEATS2 | rs76174573 |
0.1305 |
0.0687 |
0.0380a |
0.0606 |
0.8313 |
0.6458 | 0.6338 |
0.1706 |
|
SLC16A1 | rs1049434 | 0.8319 |
0.0016a | 0.1897 |
0.0686 | 0.9212 | 0.0646 | 0.8850 |
0.2672 |
| RNF2 | rs1046592 |
0.0007a |
0.0140a | 0.5319 |
0.0544 | 0.4098 | 0.7276 | 0.4643 |
0.1040 |
|
| rs6825911 |
0.0317a | 0.4070 | 0.5755 |
0.1068 | 0.1423 | 0.4050 | 0.2325 |
0.6717 |
| ITGB8 | rs80015015 | 0.4001 | 0.5178 | 0.4838 |
0.0075a | 0.5169 | 0.3341 | 0.1339 |
0.2408 |
| USP45 | rs41288947 | 0.4383 | 0.1373 | 0.2641 |
0.0162a | 0.6636 | 0.1341 |
0.0063a |
0.0682 |
|
PHACTR1 | rs9369640 | 0.1667 | 0.4673 | 0.8831 |
0.5247 | 0.8191 | 0.7417 | 0.6674 |
0.4133 |
|
| rs1333048 | 0.2947 |
0.0251a | 0.5799 |
0.0156a | 0.3204 | 0.6650 | 0.5825 |
0.2450 |
|
| rs838880 | 0.9565 |
0.0108a | 0.3044 |
0.0045a | 0.7818 | 0.7699 | 0.8126 |
0.2552 |
| STIM1 | rs116855870 | 0.1425 | 0.2455 | 0.6418 |
0.8631 | 0.7116 | 0.5285 | 0.7357 |
0.3365 |
|
| rs2523644 | 0.4105 | 0.2101 | 0.6604 |
0.0773 | 0.0627 |
0.0449a | 0.2968 |
0.2580 |
| MKI67 | rs145121731 | 0.0903 | 0.2528 | 0.2203 |
0.0138a | 0.4030 |
0.0048a | 0.2459 |
0.5059 |
|
FAM170B-AS1 | rs73302786 | 0.2662 | 0.6366 | 0.2511 |
0.2687 | 0.8989 | 0.8203 | 0.2323 |
0.5404 |
|
| rs6067640 |
0.0380a | 0.2144 | 0.7990 |
0.0077a | 0.6995 | 0.1809 | 0.6901 |
0.9347 |
| GFY | rs73053944 |
0.0145a | 0.5731 | 0.5880 |
0.2471 | 0.8788 | 0.6316 | 0.4534 |
0.9116 |
| WDR37 | rs10794720 | 0.6272 |
0.0103a | 0.9125 |
0.6954 | 0.6528 | 0.1352 | 0.7804 |
0.0458a |
|
SKIV2L | rs592229 |
0.0014a | 0.0754 | 0.0752 |
0.0157a | 0.7557 | 0.1230 | 0.3617 |
0.1727 |
|
| rs6537384 | 0.3890 | 0.1107 | 0.2980 |
0.3818 |
0.0344a | 0.0808 | 0.0620 |
0.7745 |
|
| rs10757283 | 0.8792 |
0.0082a | 0.9667 |
0.0420a | 0.4745 |
0.0342a | 0.8876 |
0.4636 |
|
CDKN2B-AS1 | rs1011970 |
0.0443a | 0.0628 | 0.6524 |
0.0834 | 0.5456 | 0.4293 | 0.6420 |
0.3237 |
| PRKG1 | rs9414827 | 0.6287 | 0.8365 | 0.6694 |
0.0424a | 0.1488 | 0.6060 |
0.0291a |
0.0549 |
|
| rs197932 | 0.1918 |
0.0272a | 0.5146 |
0.5673 | 0.2395 | 0.3102 | 0.7843 |
0.4625 |
|
LINC00354 | rs4907518 | 0.2933 | 0.8434 | 0.3915 |
0.1285 |
0.0028a | 0.6454 | 0.6846 |
0.7513 |
| LIPE | rs34052647 | 0.1525 | 0.7148 | 0.0040a |
0.4199 | 0.0801 | 0.0879 | 0.0940 |
0.0081a |
| BTRC | rs2270439 | 0.3191 | 0.9636 | 0.1684 |
0.1393 | 0.9515 | 0.2852 | 0.4689 |
0.5780 |
| TNS3 | rs11763932 | 0.4812 | 0.6129 | 0.9920 |
0.8857 | 0.1424 | 0.9598 | 0.9307 |
0.9591 |
| TNS1 | rs918949 | 0.1509 | 0.0510 | 0.3218 |
0.5993 | 0.7044 | 0.5484 | 0.6955 |
0.9461 |
|
| rs12229654 |
0.0203a | 0.3290 | 0.1080 |
<0.0001a |
0.0171a | 0.1167 | 0.2297 |
<0.0001a |
|
| rs4014195 | 0.1622 |
0.0445a |
0.0270a |
0.1811 | 0.7090 | 0.3732 | 0.5607 |
0.2233 |
| PANK1 | rs11185790 | 0.3638 | 0.4169 | 0.1750 |
0.2583 | 0.3889 |
0.0149a | 0.6355 |
0.3282 |
|
| rs507666 | 0.9872 |
0.0084a | 0.7080 |
0.0370a |
0.0129a | 0.4210 | 0.9126 |
0.6992 |
| MON2 | rs11174549 |
0.0283a |
0.0133a | 0.8790 |
0.3587 |
0.0325a | 0.2617 | 0.6406 |
0.9153 |
|
HECTD4 | rs2074356 |
0.0285a | 0.1092 |
0.0109a |
<0.0001a |
0.0002a | 0.0174 | 0.1786 |
<0.0001a |
| CUBN | rs78201384 | 0.1429 | 0.9473 | 0.7525 |
0.0027a |
0.0269a |
0.0327a | 0.9812 |
0.3888 |
| PLUT | rs954750 | 0.9214 |
0.0212a | 0.6905 |
0.8004 | 0.8585 |
0.0264a | 0.2382 |
0.8865 |
| ABCA1 | rs1883025 | 0.8085 | 0.2006 |
0.0134a |
0.3092 | 0.0667 | 0.8891 | 0.4019 |
0.3377 |
|
| rs10514995 |
0.0014a |
0.0353a | 0.2529 |
0.3708 | 0.7542 | 0.0855 | 0.5232 |
0.0365a |
The SNP rs671 of ALDH2 was significantly
(P<0.05) associated with all the intermediate phenotypes;
rs200787930 of PLCB2 and rs2074356 of HECTD4 to six
of the eight phenotypes; rs9466 of EIF3L to five of the
eight phenotypes; rs130071 of CCHCR1, rs11823828 of
OR52E4 and rs12229654 to four of the eight phenotypes;
rs11174549 of MON2, rs10514995, rs507666, rs10757283 and
rs78201384 of CUBN to three of the eight phenotypes;
rs1046592 of RNF2, rs13427905, rs3094663 of PSORS1C1,
rs6067640, rs592229 of SKIV2L, rs4014195, rs7333181,
rs838880, rs1333048, rs10771894 of KIAA1551, rs954750 of
PLUT, rs10794720 of WDR37, rs34052647 of LIPE,
rs602633, rs145121731 of MKI67, rs41288947 of USP45,
and rs9414827 of PRKG1 to two of the eight phenotypes; and
rs73053944 of GFY, rs6825911, rs1011970 of
CDKN2B-AS1, rs1049434 of SLC16A1, rs145161932 of
ADAT1, rs1052586 of GOSR2, rs197932, rs1883025 of
ABCA1, rs76174573 of YEATS2, rs80015015 of
ITGB8, rs2936051 of MIA3, rs7412 of APOE,
rs4907518 of LINC00354, rs6537384, rs17558532 of
KRT27, rs11185790 of PANK1, and rs2523644 to one of
the eight phenotypes.
Linkage disequilibrium analyses
Linkage disequilibrium (LD) was examined among SNPs
associated with CAD. There was significant LD among rs12229654 at
12q24.1, rs671 of ALDH2, and rs2074356 of HECTD4
[square of the correlation coefficient (r2), 0.564 to
0.882)].
Association between genes, chromosomal
loci and SNPs identified in the present study and phenotypes
previously reported by GWASs
The association between genes, chromosomal loci and
SNPs identified in the present study and cardiovascular
disease-related phenotypes previously reported by GWASs available
in the GRASP Search database (Table
VI). Chromosomal region 1p13.3, MIA3, PHACTR1, SKIV2L,
CDKN2B-AS1, 9p21, ALDH2 and HECTD4 were previously
revealed to be associated with CAD or MI. SLC16A1, PSORS1C1,
CCHCR1, 6p21.3, ABCA1, 9q34.2, CUBN, PANK1, 12q24.1, 12q24.31,
PLCB2 and APOE were previously associated with
circulating concentrations of LDL-cholesterol, HDL-cholesterol,
triglycerides or insulin, or type 1 diabetes mellitus. Chromosome
4q24, 17q21.3 and GOSR2 were previously associated with
systolic or diastolic blood pressure or pulse pressure. CCDC141,
TNS1, WDR37 and 11q13.1 were previously associated with
cardiac, pulmonary or renal function. The remaining 21 genes
(RNF2, YEATS2, USP45, ITGB8, TNS3, FAM170B-AS1, PRKG1, BTRC,
MKI67, STIM1, OR52E4, KIAA1551, MON2, PLUT, LINC00354, TRPM1,
ADAT1, KRT27, LIPE, GFY and EIF3L) and five chromosomal
regions (2p13, 4q31.2, 5q12, 13q34 and 20q13.2) identified in the
present study have not been revealed to be associated with CAD or
cardiovascular disease-related phenotypes in previous GWASs.
 | Table VI.Association between genes,
chromosomal loci and SNPs associated with coronary artery disease
in the present study and previously examined cardiovascular
disease-related phenotypes. |
Table VI.
Association between genes,
chromosomal loci and SNPs associated with coronary artery disease
in the present study and previously examined cardiovascular
disease-related phenotypes.
| Gene/chr.
locus | SNP | Chr. | Position | Previously
examined phenotypes |
|---|
| 1p13.3 | rs602633 | 1 | 109278889 | CAD (23202125,
20032323), LDL-cholesterol (20686565, 23063622, 19060906, 21943158,
18193043, 18262040, 19913121, 21977987, 20339536), HDL-cholesterol
(23063622, 20686565), total cholesterol (20686565, 23063622) |
|
SLC16A1 | rs1049434 | 1 | 112913924 | HDL-cholesterol
(23063622) |
| RNF2 | rs1046592 | 1 | 185100429 | None |
| MIA3 | rs2936051 | 1 | 222629862 | CAD (19198612,
21347282, 23364394, 21378990, 17554300, 22319020, 21966275), MI
(19198609) |
| 2p13 | rs13427905 | 2 | 71846585 | None |
|
CCDC141 | rs13419085 | 2 | 178837710 | Heart rate
(23583979, 20639392), left ventricular mass (19584346) |
| TNS1 | rs918949 | 2 | 217809974 | Lung function,
forced expiratory volume in |
|
|
|
|
| 1 second
(20010834, 21946350, 23284291) |
|
YEATS2 | rs76174573 | 3 | 183804099 | None |
| 4q24 | rs6825911 | 4 | 110460482 | Systolic BP
(21572416), diastolic BP (21572416) |
| 4q31.2 | rs6537384 | 4 | 145949613 | None |
| 5q12 | rs10514995 | 5 | 66443611 | None |
|
PHACTR1 | rs9369640 | 6 | 12901209 | CAD (21378988,
23202125, 22745674, 21347282, 23364394, 21378990, 22751097,
22745674), MI (19198609, 21378990), ischemic stroke (22306652) |
|
PSORS1C1 | rs3094663 | 6 | 31139310 | Type 1 diabetes
(17554300, 17632545), triglycerides (20686565), total cholesterol
(20686565) |
|
CCHCR1 | rs130071 | 6 | 31148433 | Triglycerides
(20686565) |
| 6p21.3 | rs2523644 | 6 | 31374707 | Type 1 diabetes
(17554300, 17632545), LDL-cholesterol (23063622, 20686565),
triglycerides (23063622, 20686565), total cholesterol (23063622,
20686565) |
|
SKIV2L | rs592229 | 6 | 31962664 | CAD (21971053),
type 1 diabetes (17554300, 17632545), LDL-cholesterol (20686565),
triglycerides (20686565), total cholesterol (20686565) |
| USP45 | rs41288947 | 6 | 99446210 | None |
| ITGB8 | rs80015015 | 7 | 20401881 | None |
| TNS3 | rs11763932 | 7 | 47567880 | None |
|
CDKN2B-AS1 | rs1011970 | 9 | 22062135 | CAD (21347282),
LDL-cholesterol (23063622), abdominal aortic aneurysm (20622881),
type 2 diabetes (17463249) |
| 9p21 | rs1333048 | 9 | 22125348 | CAD (23202125,
21606135, 19198612, 17634449, 20032323, 23364394), MI (17478679),
intracranial aneurysm (22961961) |
| 9p21 | rs10757283 | 9 | 22134173 | Type 2 diabetes
(20581827) |
| ABCA1 | rs1883025 | 9 | 104902020 | HDL-cholesterol
(20686565, 23505323, 23063622, 21909109, 19060911, 21347282,
19060906, 18193043, 18193044, 18193046, 22629316, 20864672,
21347282, 23726366), LDL-cholesterol (20686565), total cholesterol
(20686565, 23063622, 20339536) |
| 9q34.2 | rs507666 | 9 | 136149399 | Venous thrombosis
(22675575), VLDL-cholesterol |
|
|
|
|
| small lipoprotein
fraction concentration (19936222), LDL-cholesterol lipoprotein
fraction concentration (19936222) |
| WDR37 | rs10794720 | 10 | 1110225 | Estimated
glomerular filtration rate (20383146, 22479191), serum creatinine
(20383146) |
| CUBN | rs78201384 | 10 | 17111024 | LDL-cholesterol
(23063622), HDL-cholesterol (23063622), total cholesterol
(23063622) |
| FAM170B-AS1 | rs73302786 | 10 | 49131709 | None |
| PRKG1 | rs9414827 | 10 | 51137314 | None |
| PANK1 | rs11185790 | 10 | 89612776 | Insulin
concentration (19060910) |
| BTRC | rs2270439 | 10 | 101550817 | None |
| MKI67 | rs145121731 | 10 | 128102595 | None |
| STIM1 | rs116855870 | 11 | 4055527 | None |
|
OR52E4 | rs11823828 | 11 | 5884973 | None |
| 11q13.1 | rs4014195 | 11 | 65739351 | Serum urate
(23263486), serum creatinine (20383146), estimated glomerular
filtration rate (20383146) |
|
KIAA1551 | rs10771894 | 12 | 31982009 | None |
| MON2 | rs11174549 | 12 | 62565357 | None |
| 12q24.1 | rs12229654 | 12 | 110976657 | HDL-cholesterol
(21909109) |
| ALDH2 | rs671 | 12 | 111803962 | CAD (21971053,
21572416, 23202125), MI (21971053), LDL-cholesterol (21572416,
20686565), HDL-cholesterol |
|
|
|
|
| (21572416,
21372407), total cholesterol (20686565), systolic BP |
|
|
|
|
| (21572416),
diastolic BP (21572416, 21909115), serum creatinine |
|
|
|
|
| (22797727),
estimated glomerular filtration rate (22797727), type 1 diabetes
(17554300) |
|
HECTD4 | rs2074356 | 12 | 112207597 | CAD (21971053,
21572416, 22751097, 19820697, 23364394, 23202125), MI (19820697),
LDL-cholesterol (21572416, 20686565), HDL-cholesterol (21572416,
21909109, 22751097), total cholesterol |
|
|
|
|
| (20686565),
systolic BP (21572416, 21909115), diastolic BP |
|
|
|
|
| (21572416,
21909115, 19862010, 19430479, 22751097), hypertension (21572416),
serum creatinine (22797727), estimated |
|
|
|
|
| glomerular
filtration rate (22797727), type 1 diabetes (18978792) |
| 12q24.31 | rs838880 | 12 | 124777047 | HDL-cholesterol
(20686565) |
| PLUT | rs954750 | 13 | 27889801 | None |
| 13q34 | rs7333181 | 13 | 111568950 | None |
|
LINC00354 | rs4907518 | 13 | 111898209 | None |
| TRPM1 | rs2241493 | 15 | 31070149 | None |
| PLCB2 | rs200787930 | 15 | 40289298 | Triglycerides
(23063622) |
| ADAT1 | rs145161932 | 16 | 75612670 | None |
| KRT27 | rs17558532 | 17 | 40779624 | None |
| 17q21.3 | rs197932 | 17 | 46896981 | Pulse pressure
(21909110), systolic BP (21909110, 21909115) |
| GOSR2 | rs1052586 | 17 | 46941097 | Pulse pressure
(21909110), systolic BP (21909110, 21909115) |
| LIPE | rs34052647 | 19 | 42407617 | None |
| APOE | rs7412 | 19 | 44908822 | LDL-cholesterol
(23100282, 23063622, 20686565, 22629316, 19060911, 23067351,
23696881, 20838585), HDL-cholesterol |
|
|
|
|
| (21386085),
triglycerides (23063622, 20686565, 22629316, 19060911, 21386085),
total cholesterol (23063622, 20686565) |
| GFY | rs73053944 | 19 | 49427038 | None |
| 20q13.2 | rs6067640 | 20 | 51092837 | None |
| EIF3L | rs9466 | 22 | 37877742 | None |
Gene Ontology analysis of genes
identified in the present study
Biological functions of the 21 genes identified in
the present study were estimated using the database of Gene
Ontology and GO Annotations (QuickGO; Table VII). Given that FAM170B-AS1
is the gene for non-coding RNA, FAM170B was examined.
Various biological functions were predicted in the 18 genes
(RNF2, YEATS2, USP45, ITGB8, TNS3, FAM170B, PRKG1, BTRC, MKI67,
STIM1, OR52E4, MON2, TRPM1, ADAT1, KRT27, LIPE, GFY and EIF3L),
although those of KIAA1551, PLUT and LINC00354 were
not. Gene ontology analysis revealed that ITGB8, PRKG1,
STIM1 and LIPE may be involved in the development of
CAD.
 | Table VII.Gene ontology analysis of the 21
genes identified in the present study. |
Table VII.
Gene ontology analysis of the 21
genes identified in the present study.
| Gene | Function | Biological
process |
|---|
| RNF2 | Ubiquitin-protein
transferase activity, chromatin binding, zinc ion binding,
transferase activity, metal ion binding, ubiquitin protein ligase
activity, RING-like zinc finger domain binding | Histone H2A-K119
monoubiquitination, negative regulation of transcription by RNA
polymerase II, regulation of DNA-templated transcription, germ cell
development, negative regulation of DNA binding transcription
factor activity, negative regulation of G0 to G1 transition |
|
YEATS2 |
Modification-dependent protein binding,
RNA polymerase II transcription factor activity, sequence-specific
DNA binding | Negative
regulation of transcription by RNA polymerase II, histone H3
acetylation, negative regulation of DNA-templated
transcription |
| USP45 | Thiol-dependent
ubiquitin-specific protease activity, cysteine-type peptidase
activity, zinc ion binding, thiol-dependent ubiquitinyl hydrolase
activity | Protein
deubiquitination, ubiquitin-dependent protein catabolic process,
DNA repair, global genome nucleotide-excision repair |
| ITGB8 | Extracellular
matrix protein binding, signaling receptor binding | Ganglioside
metabolic process, cell adhesion, integrin-mediated signaling
pathway, regulation of gene expression, positive regulation of
angiogenesis, cartilage development, extracellular matrix
organization, cell-matrix adhesion |
| TNS3 | Protein binding,
focal adhesion | Positive
regulation of cell proliferation, cell migration, lung alveolus
development |
|
FAM170B | Protein binding,
outer acrosomal membrane | Positive
regulation of acrosome reaction, regulation of fertilization |
| PRKG1 | cGMP-dependent
protein kinase activity, calcium channel regulator activity,
nucleotide binding, ATP binding, transferase activity, cGMP
binding, protein serine/threonine kinase activity, cGMP-dependent
protein kinase activity | Negative
regulation of vascular smooth muscle cell proliferation and
migration, neuron migration, cGMP-mediated signaling, dendrite
development, forebrain development, relaxation of vascular smooth
muscle, regulation of GTPase activity, negative regulation of
platelet aggregation, actin cytoskeleton organization |
| BTRC | Ubiquitin-protein
transferase activity, ubiquitin protein ligase activity, β-catenin
binding, protein phosphorylated amino acid binding, protein
dimerization activity | Protein
polyubiquitination, ubiquitin-dependent protein catabolic process,
regulation of circadian rhythm, regulation of canonical Wnt
signaling pathway, protein dephosphorylation, mammary gland
epithelial cell proliferation, regulation of I-κB kinase/NF-κB
signaling, positive regulation of DNA-templated transcription, G2/M
transition of mitotic cell cycle, negative regulation of DNA
binding transcription factor activity, stress-activated MAPK
cascade, interleukin-1-mediated signaling pathway |
| MKI67 | RNA binding, DNA
binding, ATP binding, protein binding | Regulation of
mitotic nuclear division, regulation of chromosome segregation and
organization, cell proliferation |
| STIM1 | Calcium channel
regulator activity, calcium ion binding, microtubule plus-end
binding, metal ion binding, protein binding | Cellular calcium
ion homeostasis, activation of store-operated calcium channel
activity, regulation of calcium ion transport, positive regulation
of angiogenesis, regulation of cardiac conduction |
|
OR52E4 | Olfactory
receptor activity, G-protein coupled receptor activity | Signal
transduction, G-protein coupled receptor signaling pathway,
detection of chemical stimulus involved in sensory perception of
smell |
|
KIAA1551 |
Uncharacterized |
|
| MON2 | Protein binding,
protein transport | Golgi to endosome
transport |
| PLUT |
Uncharacterized |
|
|
LINC00354 |
Uncharacterized |
|
| TRPM1 | Ion channel
activity | G-protein coupled
glutamate receptor signaling pathway, ion transmembrane transport,
protein tetramerization, cellular response to light stimulus |
| ADAT1 | RNA binding,
tRNA-specific adenosine deaminase activity, hydrolase activity,
metal ion binding | tRNA
processing |
| KRT27 | Structural
molecule activity, intermediate filament | Hair follicle
morphogenesis, keratinization, cornification |
| LIPE | Triglyceride
lipase activity, serine hydrolase activity, protein kinase binding,
hormone-sensitive lipase activity | Protein
phosphorylation, lipid metabolic process, steroid metabolic
process, cholesterol metabolic process, triglyceride catabolic
process, long-chain fatty acid catabolic process, diacylglycerol
catabolic process |
| GFY | Protein
localization to non-motile cilium, non-motile cilium assembly | Sensory
perception of smell, response to stimulus |
| EIF3L | Translation
initiation factor activity, RNA binding, protein binding | Translational
initiation, viral translational termination-reinitiation |
Network analysis of newly identified
genes
Network analysis of the 21 genes identified in the
present study was performed using the GeneMANIA Cytoscape plugin
with Cytoscape v3.4.0 software (Figs.
1 and 2). FAM170B was applied to
the analysis instead of FAM170B-AS1. PLUT and
LINC00354 were not included in the GeneMANIA database.
GFY had no interaction with other genes. The network
analysis revealed that the 18 genes identified in the present study
had potential direct or indirect interactions with the 30 genes
previously revealed to be associated with CAD (Fig. 1). Similar analysis revealed that
complex networks were observed between the 18 genes identified in
the present study and the 228 genes identified in previous GWASs
(Fig. 2).
Discussion
Despite recent advances in therapy for acute
coronary syndrome, including coronary stent implantation (49), CAD remains the leading cause of
mortality and is therefore a key public health problem (4). The identification of genetic variants
that confer susceptibility to CAD is therefore clinically important
for the prevention and management of this condition.
The EWAS was performed for patients with early-onset
CAD, with genetic factors serving a greater role in such patients
compared with those with late-onset CAD. The present study
identified the 54 SNPs as significant and independent determinants
of CAD. These SNPs together accounted for 15.5% of the cause of
CAD. Among these loci, 21 genes (RNF2, YEATS2, USP45, ITGB8,
TNS3, FAM170B-AS1, PRKG1, BTRC, MKI67, STIM1, OR52E4, KIAA1551,
MON2, PLUT, LINC00354, TRPM1, ADAT1, KRT27, LIPE, GFY and
EIF3L) and 5 chromosomal regions (2p13, 4q31.2, 5q12, 13q34
and 20q13.2) that confer susceptibility to CAD have been newly
identified.
Among 26 SNPs identified, 14 SNPs were significantly
associated with two to five of the eight intermediate phenotypes.
The SNP rs9466 of EIF3L was associated with hypertension,
DM, hypertriglyceridemia, hyper-LDL-cholesterolemia and
hyperuricemia; rs11823828 of OR52E4 with hypertension, DM,
hypertriglyceridemia, and CKD; rs11174549 of MON2 with
hypertension, DM, hyper-LDL-cholesterolemia; rs10514995 at 5q12
with hypertension, DM and hyperuricemia; rs1046592 of RNF2
and rs13427905 at 2p13 with hypertension and DM; rs6067640 at
20q13.2 with hypertension and hypo-HDL-cholesterolemia; rs7333181
at 13q34 with DM and hypertriglyceridemia; rs10771894 of
KIAA1551 with DM and hypo-HDL-cholesterolemia; rs954750 of
PLUT with DM and CKD; rs34052647 of LIPE with
hypertriglyceridemia and hyperuricemia; rs145121731 of MKI67
with hypo-HDL-cholesterolemia and CKD; rs41288947 of USP45
and rs9414827 of PRKG1 with hypo-HDL-cholesterolemia and
obesity. The seven SNPs were significantly related to one of the
eight intermediate phenotypes. The rs73053944 of GFY was
associated with hypertension; rs145161932 of ADAT1 with DM;
rs76174573 of YEATS2 with hypertriglyceridemia; rs80015015
of ITGB8 with hypo-HDL-cholesterolemia; rs4907518 of
LINC00354 and rs6537384 at 4q31.2 with
hyper-LDL-cholesterolemia; rs17558532 of KRT27 with CKD.
Given that these intermediate phenotypes are risk factors for CAD
(4), the association between these
loci and CAD may be attributable, at least in part, to their
effects on intermediate phenotypes. By contrast, five SNPs in
TNS3, FAM170B-AS1, BTRC, STIM1 and TRPM1 were not
associated with intermediate phenotypes. The underlying molecular
mechanisms of the association between these loci and CAD remain to
be elucidated.
Recent GWASs have identified potential biological
pathways underlying the association between genetic loci and CAD,
including metabolism of LDL-cholesterol, triglycerides and
lipoprotein (a); insulin resistance; thrombosis; inflammation, cell
adhesion and transendothelial migration; cellular proliferation,
vascular remodeling and extracellular matrix metabolism; and
vascular tone and nitric oxide signaling (50,51).
Network analysis of functional gene-gene interactions may be
informative to clarify biological process of CAD and to identify
therapeutic targets for this condition (52). Therefore, the present study performed
gene ontology and network analyses to predict biological processes
of the identified genes and interactions between these genes and
those previously revealed to be associated with CAD. Gene ontology
analysis revealed that biological functions of ITGB8
(integrin-mediated signaling pathway), PRKG1 (relaxation of
vascular smooth muscle), STIM1 (activation of store-operated
calcium channel activity) and LIPE (cholesterol and
triglyceride metabolism) may serve roles in the development of CAD.
However, the roles of the remaining 17 genes in CAD remain unclear.
The network analysis revealed that the 18 genes identified in the
present study had direct or indirect interactions with the 30 genes
selected from the DisGeNET database (47,48), as
well as complex networks with 228 genes previously identified by
the GWASs (7). However, the
underlying molecular mechanisms of these interactions remain to be
elucidated.
It was previously demonstrated that six SNPs were
associated with CAD (P<0.01), as determined by multivariable
logistic regression analysis with adjustment for covariates
following an initial EWAS screening of allele frequencies among
subjects with early-onset and late-onset forms of this condition
(33). The associations between three
of the six SNPs [rs202069030 (P=2.58×10−6), rs7188
(P=0.0098) and rs2271395 (P=0.0042)] and CAD were replicated
(P<0.05) in the present study. These results suggested that
genetic variants associated with CAD differ, in part, between
early-onset and late-onset patients with this condition. We also
examined nine SNPs associated with MI (P<0.01) in a previous
study (33). Associations between
five of the nine SNPs [rs202103723 (P=0.0033), rs188212047
(P=0.0034), rs1265110 (P=2.69×10−5), rs9258102
(P=0.0374) and rs439121 (P=0.0108)] and CAD (P<0.05) were
identified in the present study.
There are several limitations to the present study:
i) Given that the results were not replicated, their validation
will be necessary in independent study populations or in other
ethnic groups; ii) it is possible that SNPs identified in the
present study are in LD with other genetic variants in the same
gene or in other nearby genes that are actually responsible for the
development of CAD; and iii) the functional relevance of identified
SNPs to the pathogenesis of CAD remains to be elucidated.
In conclusion, the present study identified the 54
SNPs as significant and independent determinants of CAD. Among
these loci, 21 genes (RNF2, YEATS2, USP45, ITGB8, TNS3,
FAM170B-AS1, PRKG1, BTRC, MKI67, STIM1, OR52E4, KIAA1551, MON2,
PLUT, LINC00354, TRPM1, ADAT1, KRT27, LIPE, GFY and
EIF3L) and 5 chromosomal regions (2p13, 4q31.2, 5q12, 13q34
and 20q13.2) that confer susceptibility to CAD were newly
identified in the present study. Determination of genotypes for the
SNPs at these loci may prove informative for assessment of the
genetic risk for CAD in Japanese patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by CREST, Japan
Science and Technology Agency, Kawaguchi, Japan (grant no.
JPMJCR1302).
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY contributed to the conception and design of the
study; to acquisition, analysis and interpretation of the data; and
to drafting of the manuscript. KK, MO, HH and TF all contributed to
the acquisition of the data and to the revision of the manuscript.
YY, IT and JS contributed to the analysis and interpretation of the
data, as well as to the revision of the manuscript.
Ethics approval and consent to
participate
The study protocol complied with the Declaration of
Helsinki and was approved by the Committees on the Ethics of Human
Research of Mie University Graduate School of Medicine, Hirosaki
University Graduate School of Medicine, and participating hospitals
(Gifu Prefectural Tajimi Hospital, Gifu Prefectural General Medical
Center, Japanese Red Cross Nagoya First Hospital, Northern Mie
Medical Center Inabe General Hospital, and Hirosaki Stroke and
Rehabilitation Center). Written informed consent was obtained from
all subjects.
Patient consent for publication
All authors approved submission of the final version
of the article for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libby P: Mechanisms of acute coronary
syndromes and their implications for therapy. N Engl J Med.
368:2004–2013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: American Heart Association Council on
Epidemiology and Prevention Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics-2018
update: A report from the American Heart Association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kathiresan S and Srivastava D: Genetics of
human cardiovascular disease. Cell. 148:1242–1257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McPherson R and Tybjaerg-Hansen A:
Genetics of coronary artery disease. Circ Res. 118:564–578. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erdmann J, Kessler T, Venegas Munoz L and
Schunkert H: A decade of genome-wide association studies for
coronary artery disease: The challenges ahead. Cardiovasc Res.
114:1241–1257. 2018.PubMed/NCBI
|
|
8
|
Dai X, Wiernek S, Evans JP and Runge MS:
Genetics of coronary artery disease and myocardial infarction.
World J Cardiol. 8:1–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soutar AK and Naoumova RP: Mechanisms of
disease: Genetic causes of familial hypercholesterolemia. Nat Clin
Pract Cardiovasc Med. 4:214–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paththinige CS, Sirisena ND and
Dissanayake V: Genetic determinants of inherited susceptibility to
hypercholesterolemia - a comprehensive literature review. Lipids
Health Dis. 16:1032017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bodzioch M, Orsó E, Klucken J, Langmann T,
Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C,
Porsch-Ozcürümez M, et al: The gene encoding ATP-binding cassette
transporter 1 is mutated in Tangier disease. Nat Genet. 22:347–351.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brooks-Wilson A, Marcil M, Clee SM, Zhang
LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO,
et al: Mutations in ABC1 in Tangier disease and familial
high-density lipoprotein deficiency. Nat Genet. 22:336–345. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rust S, Rosier M, Funke H, Real J, Amoura
Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P, et al:
Tangier disease is caused by mutations in the gene encoding
ATP-binding cassette transporter 1. Nat Genet. 22:352–355. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peden JF and Farrall M: Thirty-five common
variants for coronary artery disease: The fruits of much
collaborative labour. Hum Mol Genet. 20(R2): R198–R205. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McPherson R, Pertsemlidis A, Kavaslar N,
Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA,
Tybjaerg-Hansen A, Folsom AR, et al: A common allele on chromosome
9 associated with coronary heart disease. Science. 316:1488–1491.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Helgadottir A, Thorleifsson G, Manolescu
A, Gretarsdottir S, Blondal T, Jonasdottir A, Jonasdottir A,
Sigurdsson A, Baker A, Palsson A, et al: A common variant on
chromosome 9p21 affects the risk of myocardial infarction. Science.
316:1491–1493. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wellcome Trust Case Control Consortium, .
Genome-wide association study of 14,000 cases of seven common
diseases and 3,000 shared controls. Nature. 447:661–678. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samani NJ, Erdmann J, Hall AS,
Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund
P, Wichmann HE, et al: WTCCC and the Cardiogenics Consortium:
Genomewide association analysis of coronary artery disease. N Engl
J Med. 357:443–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schunkert H, König IR, Kathiresan S,
Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M,
Gieger C, et al: Cardiogenics; CARDIoGRAM Consortium: Large-scale
association analysis identifies 13 new susceptibility loci for
coronary artery disease. Nat Genet. 43:333–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
CARDIoGRAMplusC4D Consortium, . Deloukas
P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR,
Ingelsson E, Saleheen D, Erdmann J, et al: Large-scale association
analysis identifies new risk loci for coronary artery disease. Nat
Genet. 45:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van der Harst P and Verweij N:
Identification of 64 novel genetic loci provides an expanded view
on the genetic architecture of coronary artery disease. Circ Res.
122:433–443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lettre G, Palmer CD, Young T, Ejebe KG,
Allayee H, Benjamin EJ, Bennett F, Bowden DW, Chakravarti A,
Dreisbach A, et al: Genome-wide association study of coronary heart
disease and its risk factors in 8,090 African Americans: The NHLBI
CARe Project. PLoS Genet. 7:e10013002011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang F, Xu CQ, He Q, Cai JP, Li XC, Wang
D, Xiong X, Liao YH, Zeng QT, Yang YZ, et al: Genome-wide
association identifies a susceptibility locus for coronary artery
disease in the Chinese Han population. Nat Genet. 43:345–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu X, Wang L, Chen S, He L, Yang X, Shi Y,
Cheng J, Zhang L, Gu CC, Huang J, et al: Coronary ARtery DIsease
Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) Consortium:
Genome-wide association study in Han Chinese identifies four new
susceptibility loci for coronary artery disease. Nat Genet.
44:890–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nikpay M, Goel A, Won HH, Hall LM,
Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell
JC, et al: A comprehensive 1,000 Genomes-based genome-wide
association meta-analysis of coronary artery disease. Nat Genet.
47:1121–1130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nelson CP, Goel A, Butterworth AS, Kanoni
S, Webb TR, Marouli E, Zeng L, Ntalla I, Lai FY, Hopewell JC, et
al: EPIC-CVD Consortium; CARDIoGRAMplusC4D; UK Biobank
CardioMetabolic Consortium CHD working group: Association analyses
based on false discovery rate implicate new loci for coronary
artery disease. Nat Genet. 49:1385–1391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takeuchi F, Yokota M, Yamamoto K,
Nakashima E, Katsuya T, Asano H, Isono M, Nabika T, Sugiyama T,
Fujioka A, et al: Genome-wide association study of coronary artery
disease in the Japanese. Eur J Hum Genet. 20:333–340. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirokawa M, Morita H, Tajima T, Takahashi
A, Ashikawa K, Miya F, Shigemizu D, Ozaki K, Sakata Y, Nakatani D,
et al: A genome-wide association study identifies PLCL2 and
AP3D1-DOT1L-SF3A2 as new susceptibility loci for myocardial
infarction in Japanese. Eur J Hum Genet. 23:374–380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marenberg ME, Risch N, Berkman LF,
Floderus B and de Faire U: Genetic susceptibility to death from
coronary heart disease in a study of twins. N Engl J Med.
330:1041–1046. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zdravkovic S, Wienke A, Pedersen NL,
Marenberg ME, Yashin AI and De Faire U: Heritability of death from
coronary heart disease: A 36-year follow-up of 20 966 Swedish
twins. J Intern Med. 252:247–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nora JJ, Lortscher RH, Spangler RD, Nora
AH and Kimberling WJ: Genetic-epidemiologic study of early-onset
ischemic heart disease. Circulation. 61:503–508. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roncaglioni MC, Santoro L, D'Avanzo B,
Negri E, Nobili A, Ledda A, Pietropaolo F, Franzosi MG, La Vecchia
C and Feruglio GA; GISSI-EFRIM Investigators, : Role of family
history in patients with myocardial infarction. An Italian
case-control study. Circulation. 85:2065–2072. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamada Y, Sakuma J, Takeuchi I, Yasukochi
Y, Kato K, Oguri M, Fujimaki T, Horibe H, Muramatsu M, Sawabe M, et
al: Identification of STXBP2 as a novel susceptibility locus for
myocardial infarction in Japanese individuals by an exome-wide
association study. Oncotarget. 8:33527–33535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamada Y, Matsui K, Takeuchi I, Oguri M
and Fujimaki T: Association of genetic variants with hypertension
in a longitudinal population-based genetic epidemiological study.
Int J Mol Med. 35:1189–1198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grove ML, Yu B, Cochran BJ, Haritunians T,
Bis JC, Taylor KD, Hansen M, Borecki IB, Cupples LA, Fornage M, et
al: Best practices and joint calling of the HumanExome BeadChip:
The CHARGE Consortium. PLoS One. 8:e680952013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anderson CA, Pettersson FH, Clarke GM,
Cardon LR, Morris AP and Zondervan KT: Data quality control in
genetic case-control association studies. Nat Protoc. 5:1564–1573.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Price AL, Patterson NJ, Plenge RM,
Weinblatt ME, Shadick NA and Reich D: Principal components analysis
corrects for stratification in genome-wide association studies. Nat
Genet. 38:904–909. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J R Stat Soc B. 57:289–300. 1995.
|
|
39
|
Leslie R, O'Donnell CJ and Johnson AD:
GRASP: Analysis of genotype-phenotype results from 1390 genome-wide
association studies and corresponding open access database.
Bioinformatics. 30:i185–i194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eicher JD, Landowski C, Stackhouse B,
Sloan A, Chen W, Jensen N, Lien JP, Leslie R and Johnson AD: GRASP
v2.0: An update on the Genome-Wide Repository of Associations
between SNPs and phenotypes. Nucleic Acids Res. 43(D1): D799–D804.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Binns D, Dimmer E, Huntley R, Barrell D,
O'Donovan C and Apweiler R: QuickGO: A web-based tool for Gene
Ontology searching. Bioinformatics. 25:3045–3046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huntley RP, Binns D, Dimmer E, Barrell D,
O'Donovan C and Apweiler R: QuickGO: A user tutorial for the
web-based Gene Ontology browser. Database (Oxford).
2009:bap0102009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
et al: The GeneMANIA prediction server: Biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res. 38 suppl_2:W214–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Montojo J, Zuberi K, Rodriguez H, Kazi F,
Wright G, Donaldson SL, Morris Q and Bader GD: GeneMANIA Cytoscape
plugin: Fast gene function predictions on the desktop.
Bioinformatics. 26:2927–2928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Montojo J, Zuberi K, Rodriguez H, Bader GD
and Morris Q: GeneMANIA: Fast gene network construction and
function prediction for Cytoscape. F1000Res. 3:1532014.PubMed/NCBI
|
|
46
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Piñero J, Queralt-Rosinach N, Bravo À,
Deu-Pons J, Bauer-Mehren A, Baron M, Sanz F and Furlong LI:
DisGeNET: A discovery platform for the dynamical exploration of
human diseases and their genes. Database (Oxford). 2015:bav0282015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Piñero J, Bravo À, Queralt-Rosinach N,
Gutiérrez-Sacristán A, Deu-Pons J, Centeno E, García-García J, Sanz
F and Furlong LI: DisGeNET: A comprehensive platform integrating
information on human disease-associated genes and variants. Nucleic
Acids Res. 45(D1): D833–D839. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bønaa KH, Mannsverk J, Wiseth R, Aaberge
L, Myreng Y, Nygård O, Nilsen DW, Kløw NE, Uchto M, Trovik T, et
al: NORSTENT Investigators: Drug-eluting or bare-metal stents for
coronary artery disease. N Engl J Med. 375:1242–1252. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Klarin D, Zhu QM, Emdin CA, Chaffin M,
Horner S, McMillan BJ, Leed A, Weale ME, Spencer CCA, Aguet F, et
al: CARDIoGRAMplusC4D Consortium: Genetic analysis in UK Biobank
links insulin resistance and transendothelial migration pathways to
coronary artery disease. Nat Genet. 49:1392–1397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Howson JMM, Zhao W, Barnes DR, Ho WK,
Young R, Paul DS, Waite LL, Freitag DF, Fauman EB, Salfati EL, et
al: CARDIoGRAMplusC4D; EPIC-CVD: Fifteen new risk loci for coronary
artery disease highlight arterial-wall-specific mechanisms. Nat
Genet. 49:1113–1119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lempiäinen H, Brænne I, Michoel T,
Tragante V, Vilne B, Webb TR, Kyriakou T, Eichner J, Zeng L,
Willenborg C, et al: CVgenes@target consortium: Network analysis of
coronary artery disease risk genes elucidates disease mechanisms
and druggable targets. Sci Rep. 8:34342018. View Article : Google Scholar : PubMed/NCBI
|