Introduction
Previous clinical trials have demonstrated the
efficacy of certain chemotherapeutic agents against gastric cancer
(1,2). S-1 or S-1 plus cisplatin (CDDP)
combination chemotherapy have gradually been established as the
front-line chemotherapeutic agent in Japan for the treatment of
unresectable, resected but not cured, or recurrent gastric cancer
(3). However, certain types of
gastric cancer do not respond to this agent. Therefore, in the
cases where S-1 therapy was unsuccessful, a second-line regimen was
administered using or adding other agents such as CDDP, paclitaxel
(PTX), docetaxel (DTX) and irinotecan (CPT-11). When a patient
administered S-1 exhibited progressive disease, treatment with
CDDP, PTX, DTX or CPT-11 without cross-tolerance of S-1 resulted in
a good outcome.
However, an effective second-line chemotherapy for
advanced and recurrent gastric cancer has yet to be established.
Previously, we administered S-1 alone or S-1 plus CDDP combination
chemotherapy as the first-line and CPT-11 plus CDDP as the
second-line chemotherapy regimen. The CPT-11 plus CDDP regimen had
a high response rate (RR) of >53.5% in patients without prior
chemotherapy and it was also reported that neutropenia grade 3 or
higher accounted for <40% of the cases (4). Therefore, it was concluded that
curative effects may be expected even after the administration of
S-1 or S-1 plus CDDP. However, there is no evidence that clearly
demonstrates the effectiveness of the CPT-11 plus CDDP regimen
following the failure of S-1 or S-1 plus CDDP.
We conducted a phase II study to evaluate the
effectiveness of CPT-11 plus CDDP as a second-line chemotherapeutic
regimen, following S-1 or S-1 plus CDDP therapy, in the treatment
of advanced gastric cancer, by measuring the objective RR, the time
to progression, the overall survival (OS) and the safety
profile.
Patients and methods
Patient eligibility
A total of 18 patients with unresectable advanced or
recurrent gastric cancer were enrolled in this study between
November, 2006 and May, 2009. Eligibility criteria included
histologically or cytologically confirmed gastric adenocarcinoma
that was either unresectable (n=7) or recurrent (n=11) and the
presence of measurable lesions. The patients were required to have
received prior S-1 (n=17) or S-1 plus CDDP (n=1) chemotherapy
(Table I).
 | Table I.Characteristics of enrolled
patients. |
Table I.
Characteristics of enrolled
patients.
| Characteristics | No. of patients | Percentage |
|---|
| Total number of
patients | 18 | 100 |
| Age (years) | 73 | |
| Median (range) | (53–79) | |
| Gender | | |
| Male | 16 | 88.9 |
| Female | 2 | 11.1 |
| Karnofsky performance
status 80–100 | 18 | 100 |
| Histological type
(Japanese classification) | | |
| Differentiated | 12 | 66.7 |
|
Undifferentiated | 6 | 33.3 |
| Target lesion | | |
| Unresectable | 7 | 38.9 |
| Recurrent | 11 | 61.1 |
| First-line
chemotherapy | | |
| S-1 | 17 | 94.4 |
| S-1 + CDDP | 1 | 5.6 |
Recurrent patients were included if at least 24
weeks had elapsed after the last postoperative S-1 or S-1 plus CDDP
adjuvant chemotherapy. The patients were also required to meet the
following criteria: age <75 years, amenability to oral
administration of drugs, a Karnofsky performance score of ≥60, a
life expectancy of ≥3 months and an adequate hematological status
(defined as a total leukocyte count of >3,500/mm3,
neutrophil count of >1,500/mm3, platelet count of
>100,000/mm3, serum creatinine <1.5 mg/dl, total
serum bilirubin <1.5 mg/dl, AST and ALT levels <2 times the
upper limit of the normal range). Patients were excluded from the
study in the case of concurrent or prior malignancies, active
uncontrolled infections or other diseases, or a neurological or
mental disease that prevented adequate comprehension of
information. The pretreatment evaluation consisted of a complete
history and physical examination, blood count, serum biochemistry
and computed tomography (CT) of the chest and abdomen. The patients
provided informed consent prior to the initiation of the treatment.
This study was approved by the Ethics Committee of each
participating institution.
Study design
The S-1 first-line chemotherapy regimen was as
follows: S-1 was administered orally twice daily following
breakfast or dinner, at 80 mg/m2/day for 4 weeks,
followed by 2 weeks of rest. During the course of the treatment,
the patients had a complete blood count (CBC), biochemical and
physical examinations every 2 weeks and the presence of tumor
markers (CEA, CA19-9, STn and SLX) was assessed every 4 weeks. The
treatment response was then evaluated by CT every 2 months.
The S-1 plus CDDP first-line chemotherapy regimen
was as follows: S-1 was administered at the same dose as described
above for 3 weeks, followed by 2 weeks of rest. On day 8, S-1 was
combined with CDDP at a dose of 60 mg/m2. The patients
were premedicated with 8 mg dexamethasone and 10 mg azasetron
hydrochloride diluted in 50 ml of saline, given intravenously
(i.v.) 30 min prior to treatment. Subsequently, 60 mg/m2
of CDDP was administered by i.v. infusion over 120 min. During the
course of the treatment, the patients had a CBC, biochemical and
physical examinations every 2–3 weeks and were assessed for tumor
markers every 4 weeks. The treatment response was evaluated by CT
every 2 months.
The regimen was modified in the case of >grade 3
toxicity, disease progression and elevated tumor markers. However,
if grade 3–4 toxicity was observed after the first-line
chemo-therapy, the second-line chemotherapy was administered after
the patient recovered from the toxicity.
The second-line chemotherapy regimen was as follows:
CPT-11 and CDDP were administered at a dose of 60 and 30
mg/m2, respectively, on days 1 and 15 of a 4-week
treatment cycle. Prior to the administration, the patients were
administered 10 mg azasetron hydrochloride and 8 mg dexamethasone
i.v., with 100 ml saline water over 30 min. CPT-11 was administered
by i.v. infusion at a dose of 60 mg/m2 with 500 ml
saline water over 90 min and CDDP was administered by i.v. infusion
at a dose of 30 mg/m2 with 500 ml saline water over 90
min.
The i.v. treatments were performed on an outpatient
basis. The treatment was discontinued in the case of any ≥grade 3
hematological or non-hematological toxicity or at the request of
the patients and an alternative third-line chemotherapy was then
performed.
Study evaluations
The responses were assessed by physical examination,
direct visualization, examination of the upper gastrointestinal
tract following a barium meal, gastrofibroscopy and CT. Tumor
evaluation was performed every 2 months according to the World
Health Organization criteria and the responses were confirmed by
radiography within 2 weeks. A complete response (CR) was defined as
remission of the disease for a minimum of 4 weeks. A partial
response (PR) was defined as a >50% reduction in the product of
the perpendicular diameters of the indicator lesions, without the
appearance of new lesions. Progressive disease (PD) was defined as
an enlargement of >25% in an indicator lesion or the development
of new lesions and stable disease (SD) was defined as failure to
meet the criteria for response or progression. The adverse events
were graded during each treatment cycle using the CTCAE version
4.0. In the event of toxicity, chemotherapy was postponed until the
symptoms resolved.
Survival analysis
The lengths of OS and progression-free survival
(PFS) were measured from the initiation of the second-line
treatment to death and progression, respectively. The Kaplan-Meier
method was used to calculate survival rates.
The primary endpoint of this study was RR and the
secondary endpoints were OS, PFS, adverse effects and third-line
chemotherapy performance rate. P≤0.05 was considered to indicate a
statistically significant difference. A statistical calculation was
conducted using the Dr. SPSS II software for Windows.
Results
Patient characteristics
The demographic characteristics of the 18 patients
enrolled in this study are shown in Table I. The patients were assessed for
response and toxicity. The median patient age was 73 years (range,
53–75 years); 16 patients were male (88.9%) and 2 were female
(11.1%), with a good overall general condition (Karnofsky
performance status: 80–100). The patients had histologically
confirmed adenocarcinoma (11 differentiated and 7
undifferentiated).
The first-line treatment was administered in all 18
patients (17 received S-1 and 1 received S-1 plus CDDP), followed
in all cases by the second-line treatment, with an average of 3.2
courses (range, 1–7). A third-line treatment was performed in 13
cases (72.2%).
Efficacy
Assessable lesions were present in all cases. During
the second-line treatment, 2 cases of CR, 1 case of PR and 7 cases
of SD were identified. The RR was 16.7% and the disease control
rate (DCR) was 55.6% (Table
II).
 | Table II.Treatment efficacy. |
Table II.
Treatment efficacy.
| No. of patients | CR | PR | SD | PD | RR (%) | DCR (%) |
|---|
| 18 | 2 | 1 | 7 | 8 | 16.7 | 55.6 |
Survival
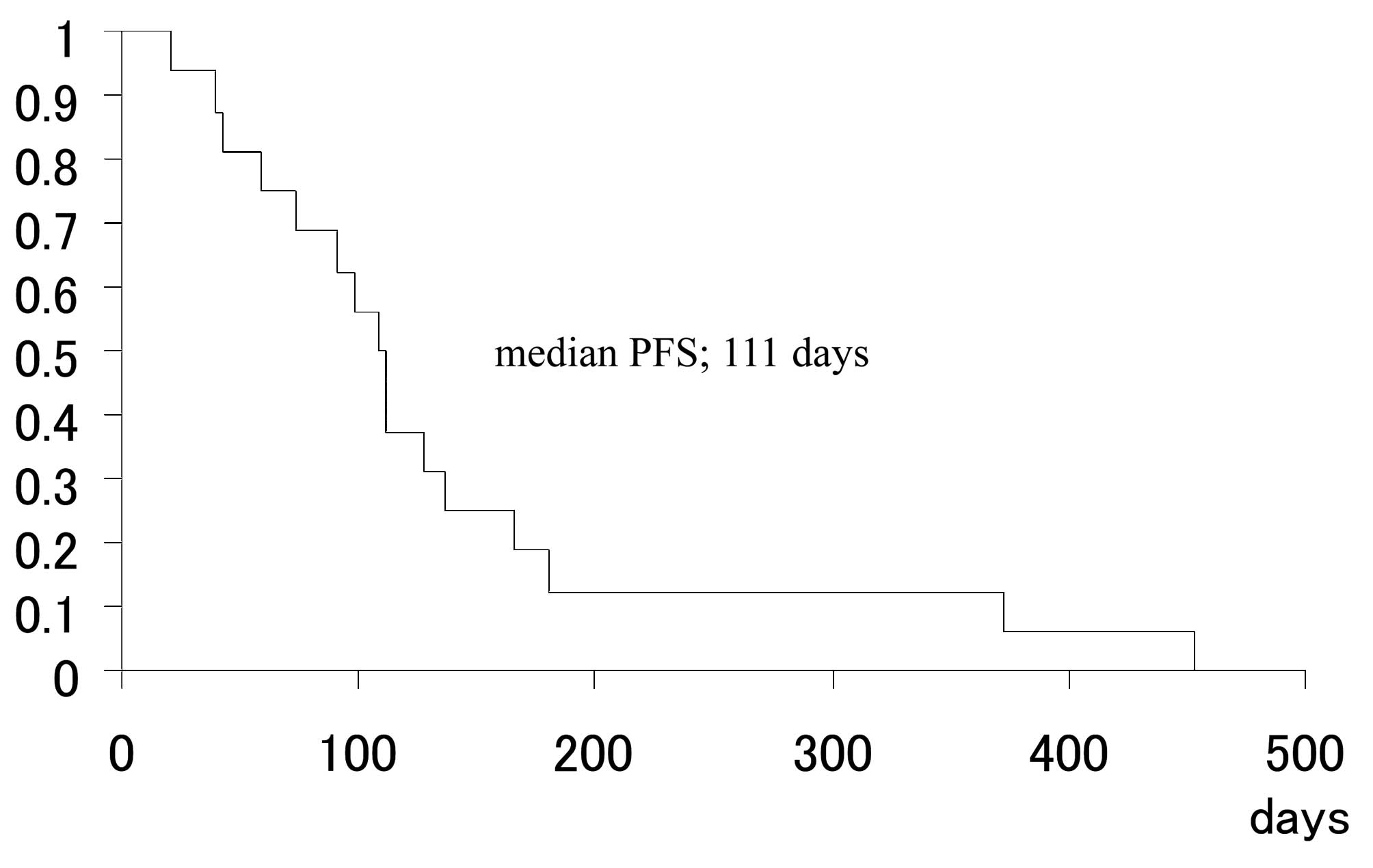
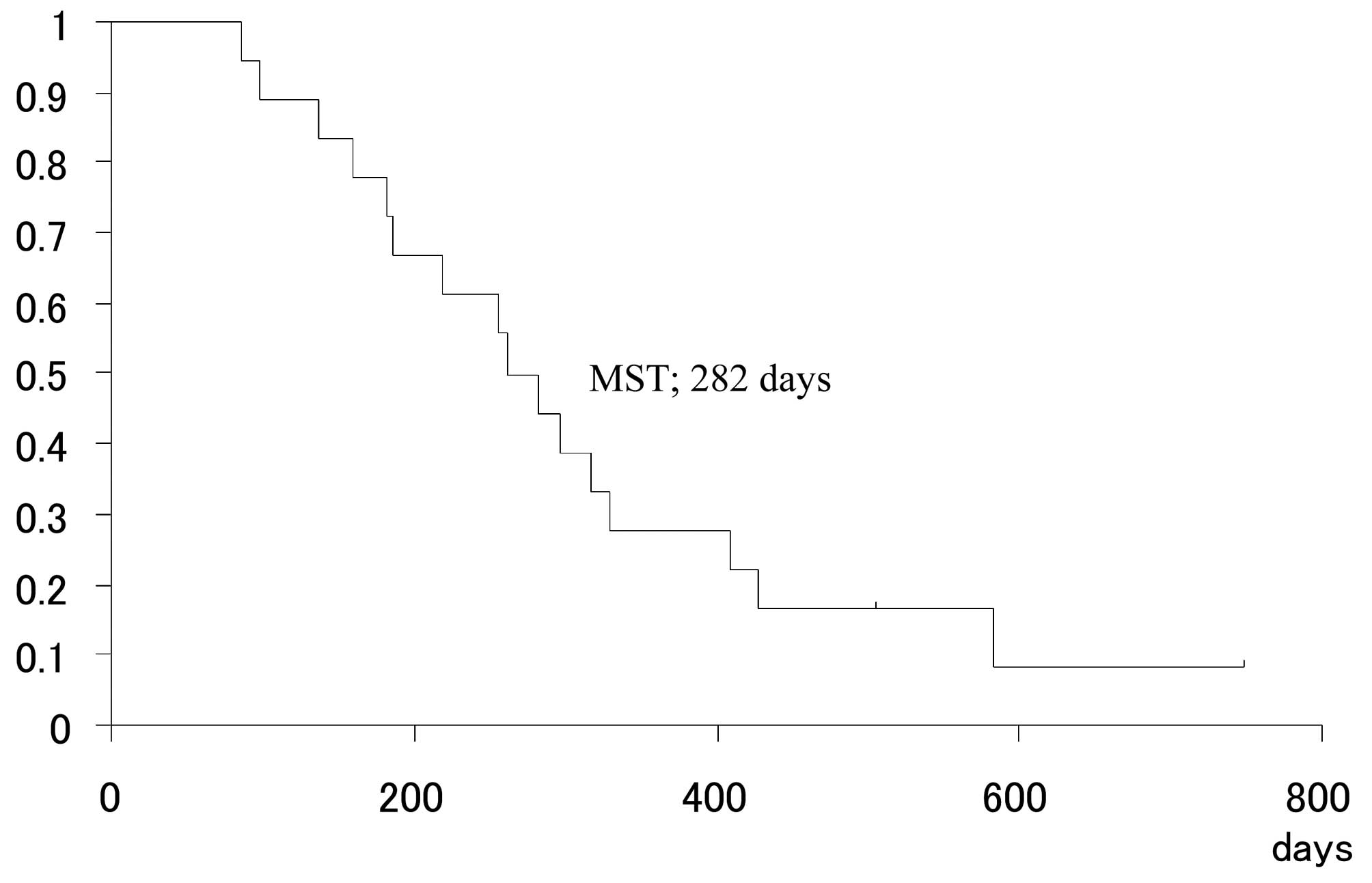
The mean follow-up time was 271.5 days (range,
85–749 days) and the median PFS was 111 days (range, 21–749 days)
(Fig. 1). The median survival time
(MST) was 282 days (Fig. 2).
Toxicity
With regards to hematological toxicity, leukopenia
and neutropenia, a side-effect of ≥grade 3 severity, were the most
frequently encountered, followed by anemia. Four cases of
leukopenia and neutropenia (22.2%) and 2 cases of anemia (11.1%)
were identified. With regards to non-hematological toxicity,
diarrhea and loss of appetite were the most frequently observed,
followed by fatigue. During the second-line treatment, there were 2
cases of diarrhea and loss of appetite (11.1%) and 1 case of
fatigue (5.6%) (Table III).
 | Table III.Occurrence of adverse events. |
Table III.
Occurrence of adverse events.
| Regimen | Grade
|
|---|
| 3 | 4 | 3 and 4 (%) |
|---|
| CPT-11 + CDDP
(n=18) | | | |
| Leukopenia | 3 | 1 | 22.2 |
| Neutropenia | 3 | 1 | 22.2 |
| Anemia | 2 | 0 | 11.1 |
| Diarrhea | 2 | 0 | 11.1 |
| Loss of
appetite | 2 | 0 | 11.1 |
| Fatigue | 1 | 0 | 5.6 |
Discussion
Previous studies have demonstrated a survival
benefit for the treatment of advanced gastric cancer in a
first-line setting. The SPIRITS trial reported an OS and PFS of 13
and 6 months, respectively, in the S-1 plus CDDP arm in a
first-line setting. Subsequently, 74% of the patients who received
S-1 plus CDDP and 75% of those who received S-1 alone, were
administered second-line chemotherapy (1). The JCOG9912 trial reported an OS and
PFS of 11.4 and 4.2 months, respectively, in the S-1 alone arm and
74% of those patients received second-line chemotherapy (2). Furthermore, the FLAGS global trial
reported an OS and PFS of 8.6 and 4.8 months, respectively, in the
S-1 plus CDDP arm and only 29.6% of those patients received
second-line chemotherapy (5). We
reported the results from the first-line chemotherapy with S-1
alone, second-line chemotherapy with S-1 plus CDDP and third-line
chemo-therapy with weekly paclitaxel. In this therapy, the RRs were
not high; however, satisfactory survival rates were observed and
the side-effects were minor. We considered this therapy to be
effective due to the smooth transition to the subsequent regimen
(6). This suggests that, in
certain cases, optimal second-line chemotherapy contributed to the
favorable OS observed with first-line chemotherapy.
A previous study by Koizumi et al (4) reported that biweekly coadministration
of 60 mg/m2 CPT-11 and 30 mg/m2 CDDP is safe
and effective for the management of unresectable advanced or
recurrent gastric cancer. The overall RR was 32.5% and it was 53.3%
in patients who had not received prior chemotherapy (4). We used this CPT-11 and CDDP regimen
as a second-line chemotherapy.
Previous studies have reported that CPT-11 exhibited
effectiveness as second-line chemotherapy and that the combination
of CPT-11 and CDDP at the outpatient setting appeared promising
(7–9).
The Osaka Gastrointestinal Cancer Chemotherapy Study
Group conducted a phase II study on the biweekly administration of
CPT-11 and CDDP to patients with gastric cancer refractory to S-1
(OGSG 0504 study). According to the intention-to-treat analysis,
the overall RR was 28.6%, including 4 cases of CR and 6 cases of
PR. The DCR was 65.7%. The most common grade 3/4 toxicities were
neutropenia (22.4%), anorexia (14.3%), fatigue (8.6%) and diarrhea
(2.9%). The median OS was 450 days. The combination treatment of
CPT-11 and CDDP was proven to be feasible and effective.
Accordingly, this regimen may be considered as one of the standard
second-line treatments for gastric cancer (9). Our RR and DCR rates were 16.7 and
55.6%, respectively, and the median OS was 287 days. These results
were lower compared to those of the OGSG 0504 trial. However, our
grade 3/4 toxicities were comparable. The discrepancies in the
results may be attributed to our limited patient sample compared to
that of the OGSG 0504 trial.
Furthermore, Oba et al (7) reported that CPT-11 mono-therapy (150
mg/m2 on days 1 and 15) offered an advantage over the
combination therapy with CPT-11 (70 mg/m2 on days 1 and
15) plus CDDP (80 mg/m2 on day 1) in the second-line
setting for the treatment of advanced gastric cancer, following
failure of a fluoropyrimidine-based regimen (7). However, this combination therapy was
ultimately proven not to be superior to fluorouracil (2).
Our results suggested that CPT-11 and CDDP
combination therapy in a second-line setting is an effective
regimen in the treatment of advanced gastric cancer. Additional
prospective clinical trials may be useful in developing
individualized optimal treatments, providing evidence on the
efficacy of molecular-targeted agents and the utility of biological
markers for the treatment of advanced gastric cancer in a
second-line setting.
Abbreviations:
|
CPT-11
|
irinotecan;
|
|
CDDP
|
cisplatin;
|
|
5-FU
|
5-fluorouracil;
|
|
PTX
|
paclitaxel;
|
|
DTX
|
docetaxel;
|
|
AST
|
aspartate transaminase;
|
|
ALT
|
alanine transaminase;
|
|
CTAE
|
Common Terminology Criteria for
Adverse Events;
|
|
CT
|
computed tomography;
|
|
CBC
|
complete blood count;
|
|
CR
|
complete response;
|
|
PR
|
partial response;
|
|
PD
|
progressive disease;
|
|
SD
|
stable disease;
|
|
OS
|
overall survival;
|
|
PFS
|
progression-free survival;
|
|
RR
|
response rate;
|
|
DCR
|
disease control rate;
|
|
MST
|
median survival time
|
References
|
1.
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Boku N, Yamamoto S, Fukuda H, et al:
Fluorouracil versus combination of irinotecan plus cisplatin versus
S-1 in metastatic gastric cancer: a randomised phase 3 study.
Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Koizumi W, Kurihara M, Satoh A, et al:
Phase I/II study of bi-weekly irinotecan plus cisplatin in the
treatment of advanced gastric cancer. Anticancer Res. 25:1257–1262.
2005.PubMed/NCBI
|
|
5.
|
Ajani JA, Rodriguez W, Bodoky G, et al:
Multicenter phase III comparison of cisplatin/S-1 with
cisplatin/infusional fluorouracil in advanced gastric or
gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin
Oncol. 28:1547–1553. 2010. View Article : Google Scholar
|
|
6.
|
Rino Y, Yukawa N, Murakami H, et al: A
phase II study of S-1 monotherapy as a first-line combination
therapy of S-1 plus cisplatin as a second-line therapy, and weekly
paclitaxel monotherapy as a third-line therapy in patients with
advanced gastric carcinoma: a second report. Clin Med Insights
Oncol. 4:1–10. 2010. View Article : Google Scholar
|
|
7.
|
Oba M, Chin K, Kawazoe Y, et al:
Irinotecan monotherapy offers advantage over combination therapy
with irinotecan plus cisplatin in second-line setting for treatment
of advanced gastric cancer following failure of
fluoropyrimidine-based regimens. Oncol Lett. 2:241–245. 2011.
|
|
8.
|
Oba M, Chin K, Kawazoe Y, et al:
Availability of irinotecan in second-line setting confers survival
benefit to patients with advanced gastric cancer refractory to
fluoropyrimidine-based regimens. Oncol Lett. 2:247–251.
2011.PubMed/NCBI
|
|
9.
|
Nakae S, Hirao M, Kishimoto T, et al:
Phase II study of bi-weekly CPT-11+CDDP for patients with gastric
cancer refractory to S-1 (OGSG 0504 study). J Clin Oncol. 26:(May
20 suppl; abstr 4571),. 2008.
|