Spandidos Publications style
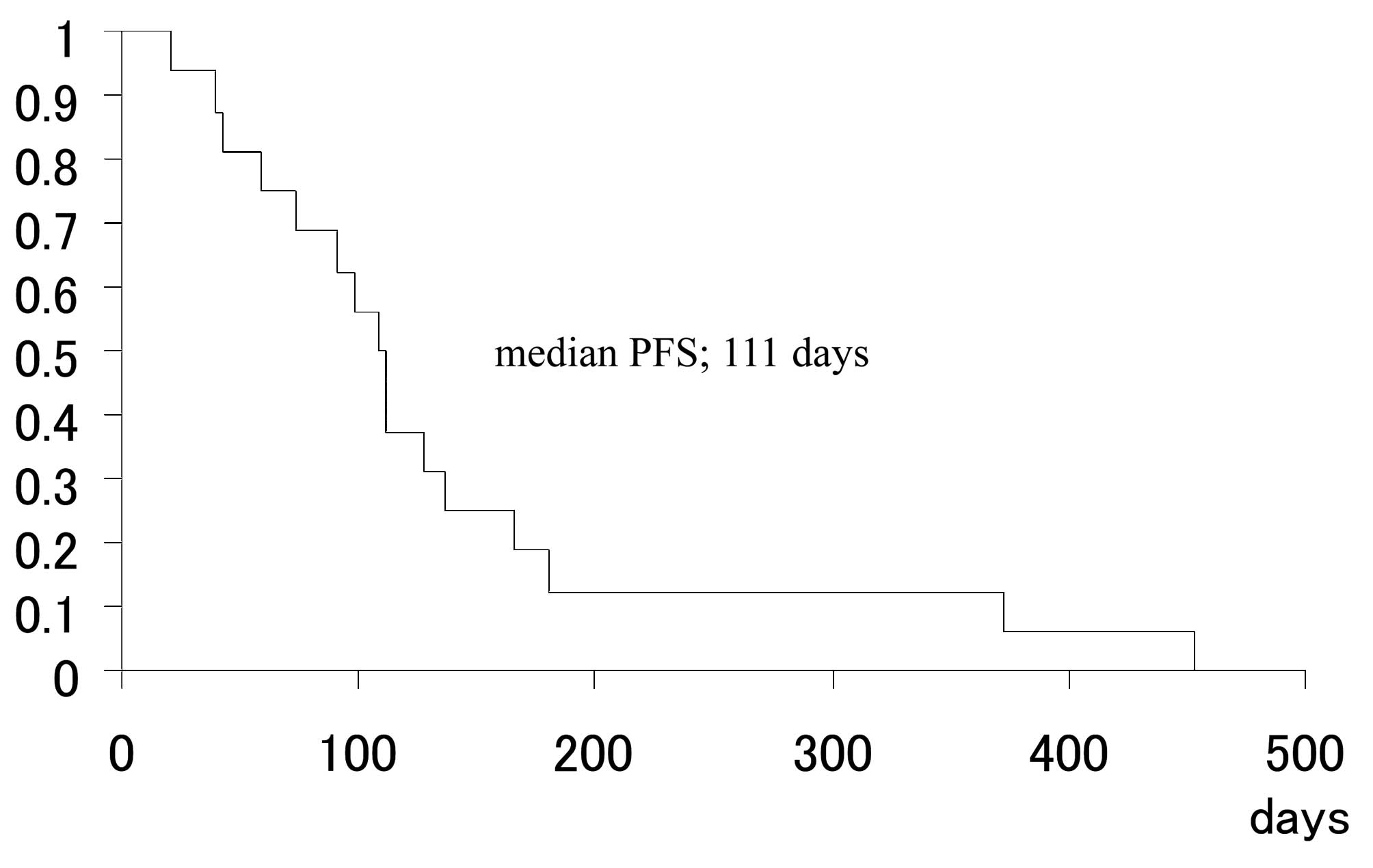
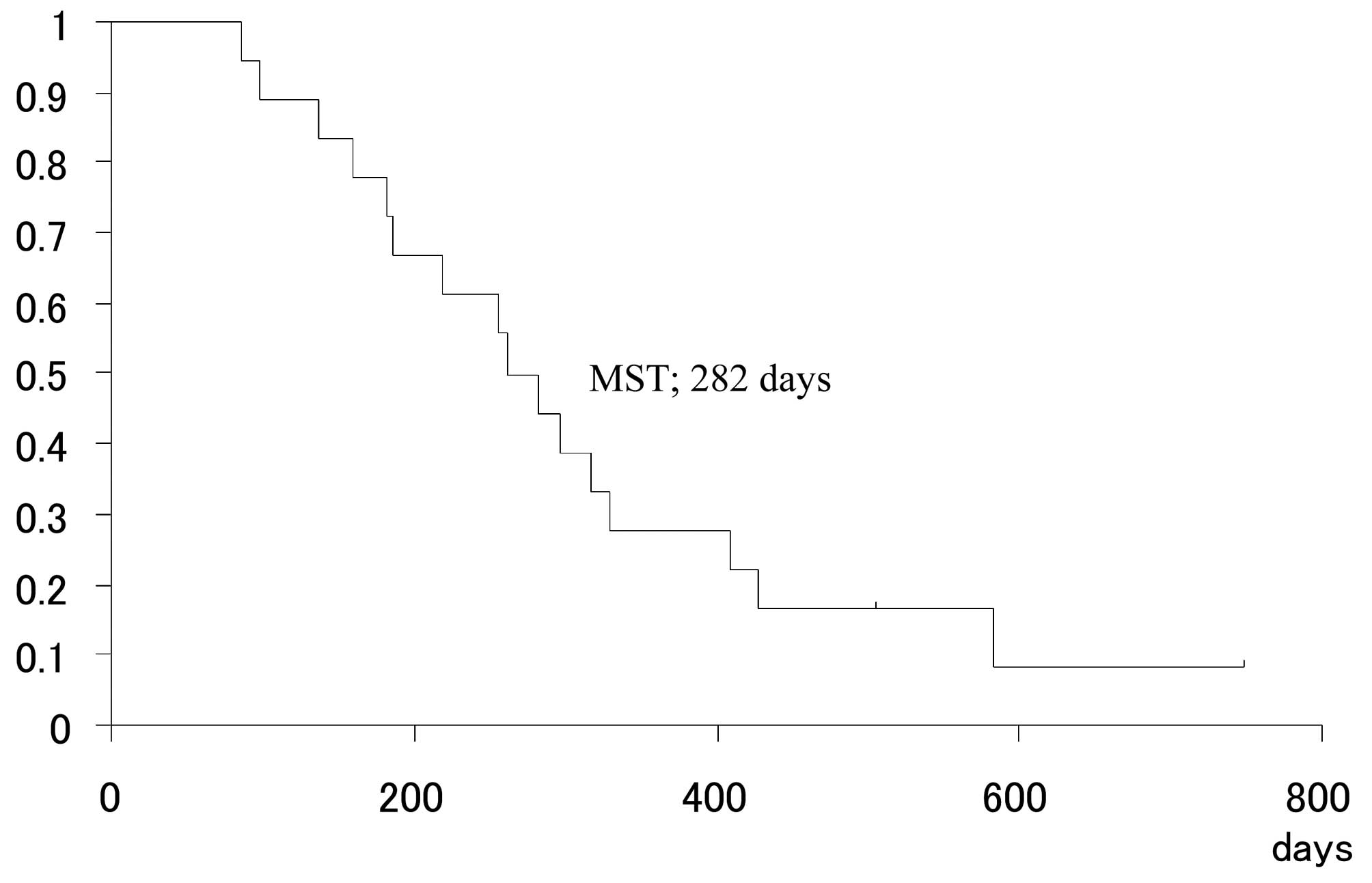
Rino Y, Yukawa N, Sato T, Oshima T, Tanabe H, Yamamoto Y, Matsukawa H, Shiraishi R, Imada T, Masuda M, Masuda M, et al: Phase II study on the combination of irinotecan plus cisplatin as a second‑line therapy in patients with advanced or recurrent gastric cancer. Mol Clin Oncol 1: 749-752, 2013.
APA
Rino, Y., Yukawa, N., Sato, T., Oshima, T., Tanabe, H., Yamamoto, Y. ... Masuda, M. (2013). Phase II study on the combination of irinotecan plus cisplatin as a second‑line therapy in patients with advanced or recurrent gastric cancer. Molecular and Clinical Oncology, 1, 749-752. https://doi.org/10.3892/mco.2013.115
MLA
Rino, Y., Yukawa, N., Sato, T., Oshima, T., Tanabe, H., Yamamoto, Y., Matsukawa, H., Shiraishi, R., Imada, T., Masuda, M."Phase II study on the combination of irinotecan plus cisplatin as a second‑line therapy in patients with advanced or recurrent gastric cancer". Molecular and Clinical Oncology 1.4 (2013): 749-752.
Chicago
Rino, Y., Yukawa, N., Sato, T., Oshima, T., Tanabe, H., Yamamoto, Y., Matsukawa, H., Shiraishi, R., Imada, T., Masuda, M."Phase II study on the combination of irinotecan plus cisplatin as a second‑line therapy in patients with advanced or recurrent gastric cancer". Molecular and Clinical Oncology 1, no. 4 (2013): 749-752. https://doi.org/10.3892/mco.2013.115