Introduction
The immune system plays a role in controlling tumor
growth and tumor cell metastasis. Human leukocyte antigen (HLA)
class I molecules are transmembrane glycoproteins comprising a
heavy chain (HC-10) and a β2-microglobulin (β2-m) light chain.
These antigens are expressed on the surface of the tumor cells and
their presence is considered to be a prerequisite for an effective
T-cell immune response (1,2). Therefore, tumor cells with downregulated
HLA class I expression escape this immune response, resulting in an
increase in growth or an increased ability to invade other organs
(3).
Downregulation of HLA class I expression occurs in a
variety of cancers and is associated with a poor prognosis
(4–11). Determining HLA class I expression may
prove useful for the prediction of tumor progression and recurrence
risk via the antitumor immune system (6,7). However,
only a limited number of studies have investigated the clinical
implication of HLA class I expression in endometrial cancer. The
degree of HLA class I expression was found to be correlated with
disease stage (12), but that study
lacked a multivariate analysis. Patients in whom HLA class I
expression was downregulated had a lower disease-specific survival
compared with patients exhibiting normal expression (13). On the other hand, HLA class I
expression was not found to be associated with clinicopathological
parameters or survival (10). Further
studies are required to elucidate whether HLA class I expression is
of value as a predictive prognostic factor in endometrial cancer.
The present study was undertaken to investigate the association of
immunohistochemical HLA class I expression patterns with
clinicopathological factors and prognosis in endometrial
cancer.
Materials and methods
Patients and specimens
We investigated 96 patients who underwent
hysterectomy with pelvic and paraaortic lymphadenectomy for
endometrial cancer at the Yamaguchi University Hospital (Ube,
Japan). The pathological diagnosis of the samples was performed by
specialized gynecological pathologists. None of the patients had
received anticancer chemotherapy or radiation therapy prior to
surgery. All the tumor tissues were obtained from surgically
resected specimens.
This study was reviewed and approved by the
Institutional Review Board of Yamaguchi University Graduate School
of Medicine.
Immunohistochemistry
The streptavidin-biotin-peroxidase complex technique
was used for immunohistochemistry, as previously described
(14). In brief, the specimens were
fixed in 10% buffered formalin and embedded in paraffin. All the
paraffin blocks were cut into 3-µm sections for
immunohistochemistry. A series of sections were deparaffinized in
xylene and rehydrated in a graded ethanol series. The samples were
washed with cold phosphate-buffered saline (PBS). Endogenous
peroxidase was blocked by incubating the sections with 0.5%
hydrogen peroxide in methanol for 50 min at room temperature. The
sections were then washed three times in cold PBS. Antigen
retrieval was performed by incubating the sections in heated
antigen retrieval solution (Nacalai Tesque Inc., Kyoto, Japan) for
30 min at 100°C using a water bath, followed by washing three times
in cold PBS. Following incubation with either 10% normal rabbit
serum for HC-10 or 10% normal goat serum for β2-m to block
non-specific binding, the specimens were sequentially incubated
with the primary antibody at 4°C overnight. The following primary
antibodies were used: Mouse anti-human monoclonal antibody to HC-10
(EMR 8–5; Hokudo, Sapporo, Japan) and rabbit anti-human polyclonal
antibody to β2-m (EMR B-6; Dako, Copenhagen, Denmark) (15). Following incubation, the sections were
rinsed in cold PBS and incubated for 10 min at room temperature
with biotinylated anti-mouse IgG + IgA + IgM to HC-10 or
anti-rabbit IgG to β2-m using the Histofine SAB-PO kit (Nichirei,
Tokyo, Japan), followed by the streptavidin-peroxidase complex. The
reaction was visualized by adding diaminobenzidine
tetrahydrochloride chromogen mixture (Sigma, St. Louis, MO, USA).
Following hematoxylin counterstaining, the slides were permanently
mounted and analyzed for the presence and distribution of
immunostaining. The results were assessed in a blinded fashion by
two independent observers.
Evaluation of HLA class I
expression
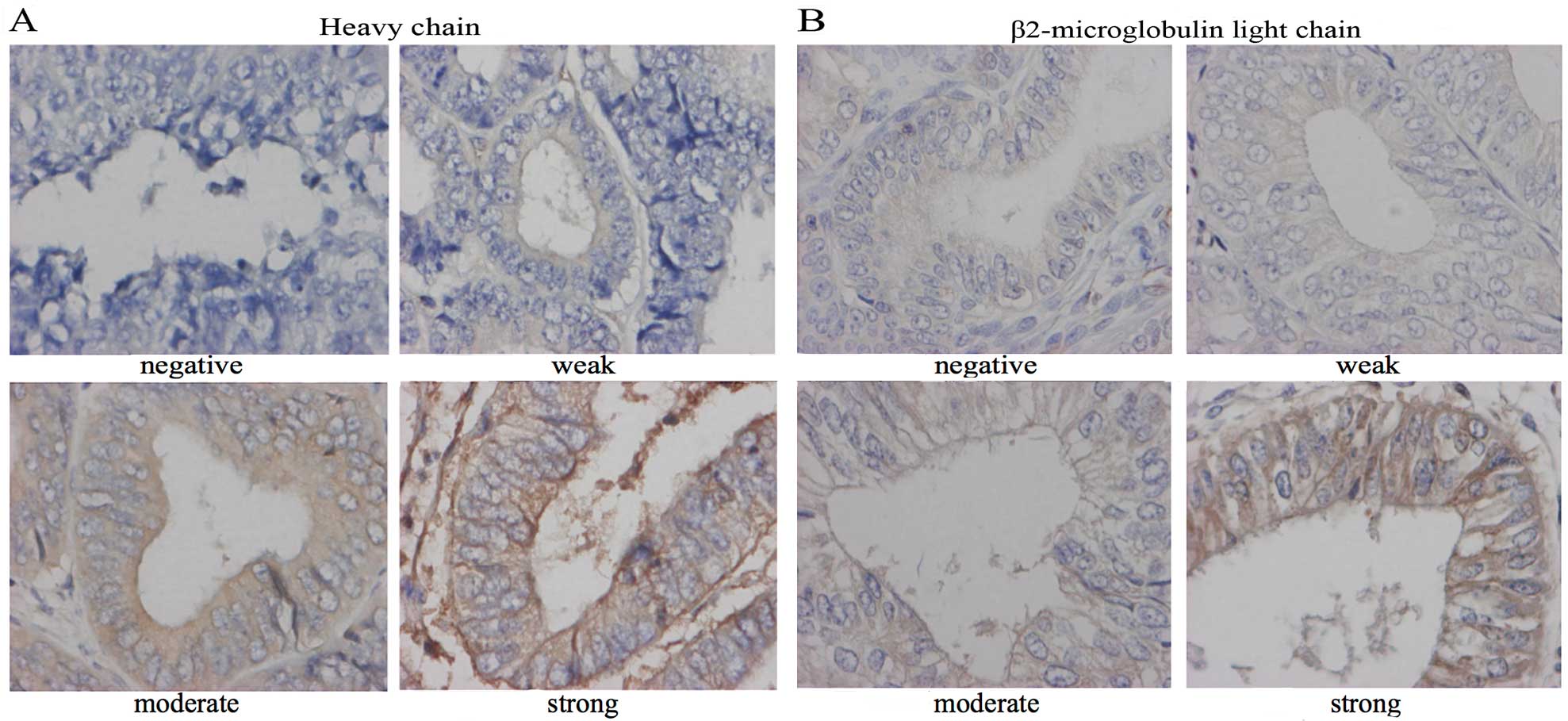
HC-10 and β2-m expression was evaluated according to
the method described by Murakami et al (16). Since HLA class I is usually expressed
on the surface of cancer cells, the staining intensity on cancer
cell membranes was evaluated. A score was established corresponding
to the sum of i) the percentage of positive cells (0, 0%
immunopositive cells; 1, <50% positive cells; and 2, >50%
positive cells); and ii) the staining intensity (0, absent; 1,
weak; 2, moderate; and 3, strong). Representative examples of
staining intensity are shown in Fig.
1. The sum of assigned values of the positive cell percentage
and the staining intensity was calculated and used to identify
three categories of expression: Scores of 0 were considered as
loss, scores of 2 and 3 as weak staining and scores of 4 and 5 as
strong staining. The HLA class I expression patterns were
classified into two groups (positive and negative) according to the
method described by Rolland et al(4). Since HLA class I expression is composed
of HC-10 and β2-m expression, the cases in which both HC-10 and
β2-m were strongly stained were defined as HLA class I-positive;
any deviation from that pattern was defined as HLA class
I-negative.
Statistical analysis
The association between HLA class I positivity and
negativity and clinicopathological parameters was analyzed by the
Chi-square distribution. Progression-free survival (PFS) was
defined as the time between the date of the operation and the date
of recurrence or the last date of follow-up. Overall survival (OS)
was defined as the time between the date of the operation and the
date of death or the last date of follow-up. The analyses of PFS
and OS were based on the Kaplan-Meier method. The PFS and OS curves
were compared with the log-rank test. The Cox proportional hazard
analysis was used for multivariate analysis to determine the
relative risk and independent significance of individual factors.
P<0.05 was considered to indicate statistically significant
differences. The statistical analyses of the study data were
performed using IBM SPSS statistics for Windows software version 11
(SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological
characteristics
The clinicopathological characteristics of the
patients are summarized in Table I.
The median age at diagnosis was 56 years and the median follow-up
duration was 45 months (range, 1–178 months). Of the 96 patients,
77 had stage I (80.2%), 3 had stage II (3.1%), 12 had stage III
(12.5%) and 4 had stage IV (4.2%) disease, as determined by the
International Federation of Gynecology and Obstetrics (FIGO). In 64
(66.7%) of the patients, the tumor invaded less than half of the
thickness of the myometrium (depth a). Lymphovascular space
involvement (LVSI) was detected in 36 patients (37.5%). A total of
9 patients (9.4%) had grade 3, 41 (42.7%) had grade 2 and 46
(47.9%) had grade 1 tumors. Positive lymph node metastasis was
identified in 9 patients (9.4%) and the peritoneal cytology was
positive in 13 patients (13.6%).
 | Table I.Association between
clinicopathological factors and HLA class I expression pattern
(n=96). |
Table I.
Association between
clinicopathological factors and HLA class I expression pattern
(n=96).
| Variables | Positive (n=51) | Negative (n=45) | Total no. (%) | P-value |
|---|
| Age (years) |
|
|
| 0.072 |
|
<50 | 13 | 5 | 18 (18.7) |
|
|
>50 | 38 | 40 | 78 (81.3) |
|
| FIGOa stage |
|
|
| <0.001 |
| I | 48 | 29 | 77 (80.2) |
|
| II | 1 | 2 | 3 (3.1) |
|
| III | 0 | 12 | 12 (12.5) |
|
| IV | 2 | 2 | 4 (4.2) |
|
| Myometrial
invasion |
|
|
| 1.000 |
| Depth
aa | 34 | 30 | 64 (66.7) |
|
| Depth
bb | 17 | 15 | 32 (33.3) |
|
| LVSI |
|
|
| 0.003 |
|
Positive | 12 | 24 | 36 (37.5) |
|
|
Negative | 39 | 21 | 60 (62.5) |
|
| Tumor grade |
|
|
| 0.051 |
| 1 | 30 | 16 | 46 (47.9) |
|
| 2 | 19 | 22 | 41 (42.7) |
|
| 3 | 2 | 7 | 9 (9.4) |
|
| Lymph node
metastasis |
|
|
| 0.005 |
|
Positive | 1 | 8 | 9 (9.4) |
|
|
Negative | 50 | 33 | 83 (86.4) |
|
|
Missing |
|
| 4 (4.2) |
|
| Peritoneal
cytology |
|
|
| 0.211 |
|
Positive | 9 | 4 | 13 (13.6) |
|
|
Negative | 42 | 41 | 83 (86.4) |
|
Expression and evaluation of HLA class
I molecules
As shown in Table II,
51 cases were HLA class I-positive, with strong staining for both
HC-10 and β2-m, while 45 cases were HLA class I-negative. The
latter included weak staining (or loss) of either HC-10 or β2-m, or
both. A univariate analysis using the Chi-square distribution
revealed a significant correlation between strongly stained HC-10
and strongly stained β2-m (P<0.001).
 | Table II.Evaluation of HLA class I
expression. |
Table II.
Evaluation of HLA class I
expression.
| HLA class I
expression | HC-10 | β2-m | No. |
|---|
| Positive | + | + | 51 |
|
| + | - | 13 |
| Negative | - | + | 9 |
|
| - | - | 23 |
Association between HLA class I
expression pattern and clinicopathological factors
The HLA class I-negative pattern was significantly
associated with advanced FIGO stage (P<0.001), LVSI (P=0.003)
and lymph node metastasis (P=0.005) (Table I). However, there was no significant
association between HLA class I expression and age, tumor grade,
myometrial invasion or peritoneal cytology.
Association between HLA class I
expression pattern and prognosis
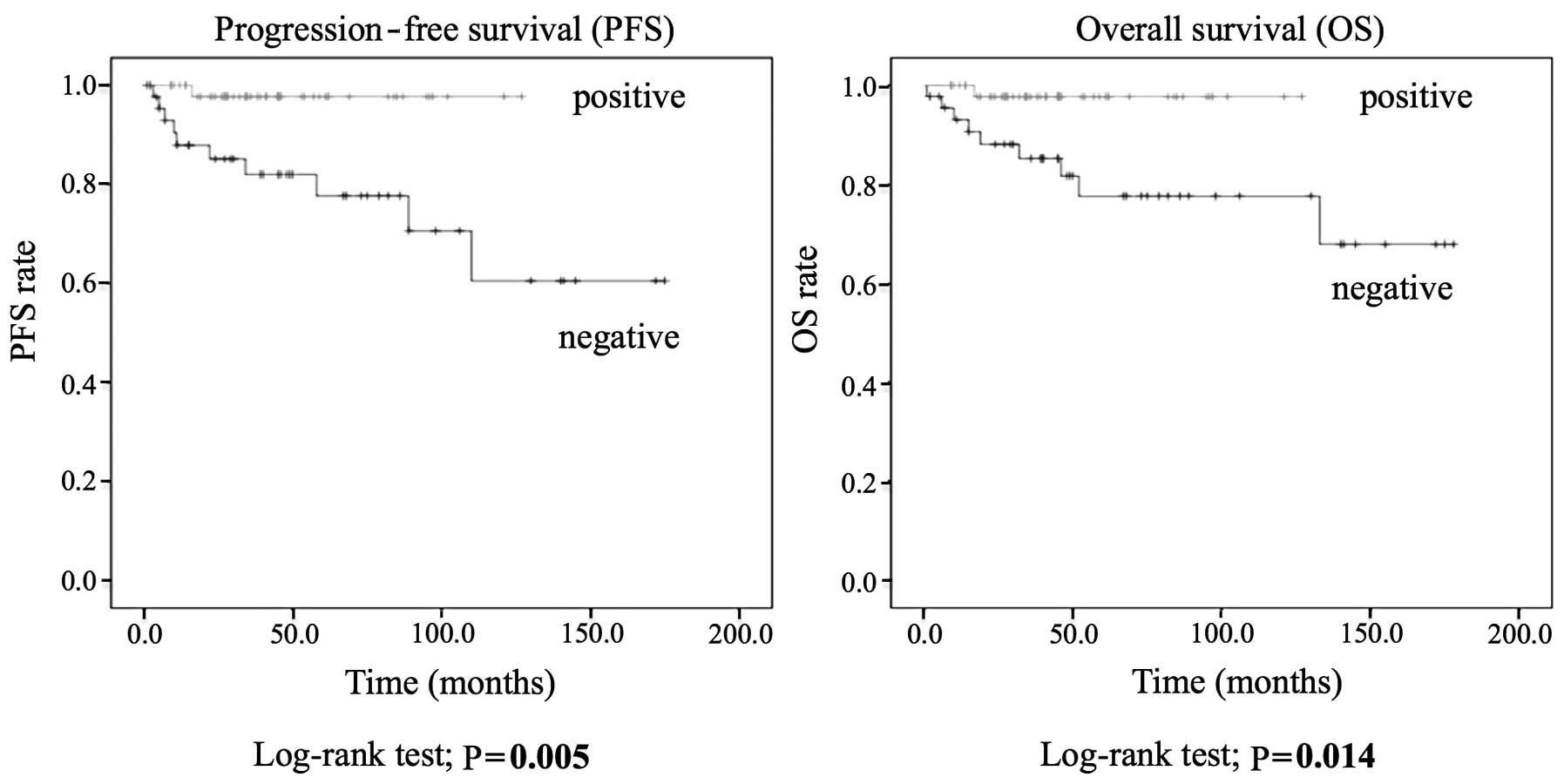
A Kaplan-Meier analysis using the log-rank test
revealed that patients with the HLA class I-positive pattern had
significantly higher PFS (P=0.005) and OS (P=0.014) rates compared
with patients who had a negative pattern (Fig. 2).
Univariate and multivariate analyses
for PFS and OS
In the univariate Cox regression analysis, a poor
PFS was associated with advanced FIGO stage, invasion of more than
half of the thickness of the myometrium (depth b), LVSI, tumor
grade III, positive peritoneal cytology and HLA class I-negative
pattern (Table III). In the
multivariate analysis, only advanced FIGO stage retained its
significance as an independent prognostic factor of poor PFS
(Table III).
 | Table III.Cox regression analysis of
progression-free survival. |
Table III.
Cox regression analysis of
progression-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) |
|
|
|
|
| >50
vs. <50 | 2.329
(0.296–18.317) | 0.422 | 1.103
(0.129–9.425) | 0.928 |
| FIGO stage |
|
|
|
|
| III/IV
vs. I/II | 26.192
(5.613–122.21) |
<0.001 | 31.294
(3.728–262.69) | 0.002 |
| Myometrial
invasion |
|
|
|
|
| Depth
aa vs. bb | 3.586
(1.047–12.279) | 0.042 | - | - |
| LVSI |
|
|
|
|
|
Positive vs. negative | 4.579
(1.205–17.401) | 0.026 | 0.444
(0.081–2.443) | 0.351 |
| Tumor grade |
|
|
|
|
| 3 vs.
1/2 | 5.839
(1.498–22.764) | 0.011 | 3.446
(0.734–16.179) | 0.117 |
| Lymph node
metastasis |
|
|
|
|
|
Positive vs. negative | 9.629
(2.301–40.294) | 0.002 | - | - |
| Peritoneal
cytology |
|
|
|
|
|
Positive vs. negative | 0.505
(0.064–4.009) | 0.518 | 3.381
(0.278–41.099 | 0.339 |
| HLA class I
expression pattern |
|
|
|
|
|
Positive vs. negative | 0.094
(0.012–0.742) | 0.025 | 0.361
(0.033–3.920) | 0.403 |
In the univariate Cox regression analysis, poor OS
was associated with advanced FIGO stage, positive LVSI, tumor grade
3, positive lymph node metastasis and HLA class I-negative pattern
(Table IV). In the multivariate
analyses, none of the parameters examined was an independent
prognostic factor for OS (Table
IV).
 | Table IV.Cox regression analysis of overall
survival. |
Table IV.
Cox regression analysis of overall
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Categories | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) |
|
|
|
|
| >50
vs. <50 | 2.329
(0.296–18.317) | 0.424 | 0.984
(0.108–8.946) | 0.989 |
| FIGO stage |
|
|
|
|
| III/IV
vs. I/II | 8.518
(2.447–29.655) | 0.001 | 2.134
(0.415–10.971) | 0.364 |
| Myometrial
invasion |
|
|
|
|
| Depth
aa vs. bb | 3.387
(0.990–11.588) | 0.052 | - | - |
| LVSI |
|
|
|
|
|
Positive vs. negative | 14.963
(1.886–118.73) | 0.009 | 6.720
(0.661–68.313) | 0.107 |
| Tumor grade |
|
|
|
|
| 3 vs.
1/2 | 7.792
(1.647–35.760) | 0.002 | 2.928
(0.71818.363) | 0.134 |
| Lymph node
metastasis |
|
|
|
|
|
Positive vs. negative | 6.544
(1.709–25.054) | 0.006 | - | - |
| Peritoneal
cytology |
|
|
|
|
|
Positive vs. negative |
0.035
(<0.001–62.661) | 0.381 | - | 0.985 |
| HLA class I
expression pattern |
|
|
|
|
|
Positive vs. negative | 0.114
(0.014–0.913) | 0.041 | 0.482
(0.044–5.254) | 0.466 |
Discussion
In this study, downregulation of HLA class I
expression in endometrial cancer was found in 46.9% of the cases,
which is similar to percentages previously reported in endometrial
cancer patients [41% (12) and 48%
(10)]. This frequency was also
similar to several other types of cancer, including esophageal
cancer (43%), but lower compared with breast (67%), lung (70%),
gastric (75%), ovarian (65%) and uterine cervical cancer (81%)
(4–11). The difference in frequency of the
downregulation of HLA class I expression among several types of
cancer may be due to the differences in organ-specific factors
affecting HLA class I expression, such as immune responses
(13,17,18).
In the present study, downregulation of HLA class I
expression was associated with advanced FIGO stage, LVSI and lymph
node metastasis, which was consistent with previously reported
results (10). It was also observed
that HLA class I-negative patients exhibited lower PFS and OS
compared with HLA class I-positive patients; this is consistent
with previous findings suggesting that downregulation of HLA class
I expression is associated with poor prognosis in a variety of
cancers (4–11). The poor prognosis of patients with
downregulation of HLA class I expression is considered to be due to
a reduced host antitumor immune response, i.e., to the escape of
tumor cells from T lymphocytes, as the presence of HLA class I
antigens on the surface of tumor cells is considered a prerequisite
for cytotoxic T-lymphocyte action (13,18). In
addition, the number of T lymphocytes was found to be significantly
lower in tumors exhibiting downregulation of HLA class I expression
compared with endometrial cancers with normal expression,
suggesting that HLA class I molecules play an important role in the
recruitment of T lymphocytes (13).
An immunosuppressive enzyme, indoleamine 2,3-deoxygenase, has also
been reported to be another prognostic factor in endometrial cancer
(19). Although the detailed
mechanism regulating tumor progression in endometrial cancer
remains unclear, these results suggest that the immune system plays
an important role in the progression of endometrial cancer.
Although the downregulation of HLA class I
expression was found to be associated with poor OS in the
univariate analysis, it was not identified as an independent factor
in the multivariate analysis (Table
IV). In addition, advanced FIGO stage was an independent
predictive factor for worse PFS, but not for OS, in the
multivariate analysis (Tables III
and IV), which is consistent with
the study of Bijen et al(10).
These observations may be a consequence of the significant
percentage of stage I patients in this study (80%). This
explanation is supported by the fact that the group exhibiting
downregulation of HLA class I expression included more
advanced-stage patients (10). It
remains controversial whether HLA class I expression is a
significant predictive prognostic factor in endometrial cancer.
Downregulation of HLA class I expression was reported to be
associated with worse disease-specific survival (13), whereas in other studies HLA class I
expression was not found to be associated with clinicopathological
parameters or survival (10). These
different results, including our data, may be due to the different
definitions of negative HLA class I expression in
immunohistochemistry. Bijen et al (10) and the present study adopted strict
criteria to classify negative HLA class I expression (defined as
negative for both HC10 and β2-m). Further studies including a
larger sample size and applying the same criteria to define HLA
class I expression are required to elucidate the clinical
implications of HLA class I expression in endometrial cancer.
In conclusion, downregulation of HLA class I
expression was observed in half of endometrial cancers in the
present study, similar to what has been reported in several other
types of cancer. The downregulation of HLA class I expression was
associated with tumor progression and poor PFS and OS. Therefore,
HLA class I expression may be useful for predicting postoperative
outcome in endometrial cancer and, thus, may be added to the
existing well-known predictive prognostic factors, such as lymph
node metastasis and LVSI.
Acknowledgements
This study was supported in part by the JSPS KAKENHI
grants 23592425, 23791845 and 26462525 for Scientific Research from
the Ministry of Education, Science and Culture, Japan.
References
|
1
|
Garrido F, Ruiz-Cabello F, Cabrera T,
Pérez-Villar JJ, López-Botet M, Duggan-Keen M and Stern PL:
Implications for immunosurveillance of altered HLA class I
phenotypes in human tumours. Immunol Today. 18:89–95. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hicklin DJ, Marincola FM and Ferrone S:
HLA class I antigen downregulation in human cancers: T-cell
immunotherapy revives an old story. Mol Med Today. 5:178–186. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gabrilovich D and Pisarev V: Tumor escape
from immune response: Mechanisms and targets of activity. Curr Drug
Targets. 4:525–536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rolland P, Deen S, Scott I, Durrant L and
Spendlove I: Human leukocyte antigen class I antigen expression is
an independent prognostic factor in ovarian cancer. Clin Cancer
Res. 13:3591–3596. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mehta AM, Jordanova ES, Kenter GG, Ferrone
S and Fleuren GJ: Association of antigen processing machinery and
HLA class I defects with clinicopathological outcome in cervical
carcinoma. Cancer Immunol Immunother. 57:197–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Speetjens FM, de Bruin EC, Morreau H, et
al: Clinical impact of HLA class I expression in rectal cancer.
Cancer Immunol Immunother. 57:601–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueda Y, Ishikawa K, Shiraishi N, Yokoyama
S and Kitano S: Clinical significance of HLA class I heavy chain
expression in patients with gastric cancer. J Surg Oncol.
97:451–455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizukami Y, Kono K, Maruyama T, Watanabe
M, Kawaguchi Y, Kamimura K and Fujii H: Downregulation of HLA class
I molecules in the tumour is associated with a poor prognosis in
patients with oesophageal squamous cell carcinoma. Br J Cancer.
99:1462–1467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kikuchi E, Yamazaki K, Torigoe T, Cho Y,
Miyamoto M, Oizumi S, Hommura F, Dosaka-Akita H and Nishimura M:
HLA class I antigen expression is associated with a favorable
prognosis in early stage non-small cell lung cancer. Cancer Sci.
98:1424–1430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bijen CB, Bantema-Joppe EJ, de Jong RA,
Leffers N, Mourits MJ, Eggink HF, van der Zee AG, Hollema H, de
Bock GH and Nijman HW: The prognostic role of classical and
nonclassical MHC class I expression in endometrial cancer. Int J
Cancer. 126:1417–1427. 2010.PubMed/NCBI
|
|
11
|
Kaneko K, Ishigami S, Kijima Y, et al:
Clinical implication of HLA class I expression in breast cancer.
BMC Cancer. 11:4542011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barrier BF, Kendall BS, Sharpe-Timms KL
and Kost ER: Characterization of human leukocyte antigen-G (HLA-G)
expression in endometrial adenocarcinoma. Gynecol Oncol. 103:25–30.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Jong RA, Boerma A, Boezen HM, Mourits
MJ, Hollema H and Nijman HW: Loss of HLA class I and mismatch
repair protein expression in sporadic endometrioid endometrial
carcinomas. Int J Cancer. 131:1828–1836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murakami A, Fukushima C, Yoshidomi K,
Sueoka K, Nawata S, Yokoyama Y, Tsuchida S, Ismail E, Al-Mulla F
and Sugino N: Suppression of carbonyl reductase expression enhances
malignant behaviour in uterine cervical squamous cell carcinoma:
Carbonyl reductase predicts prognosis and lymph node metastasis.
Cancer Lett. 311:77–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Torigoe T, Asanuma H, Nakazawa E, Tamura
Y, Hirohashi Y, Yamamoto E, Kanaseki T, Hasegawa T and Sato N:
Establishment of a monoclonal anti-pan HLA class I antibody
suitable for immunostaining of formalin-fixed tissue: Unusually
high frequency of down-regulation in breast cancer tissues. Pathol
Int. 62:303–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murakami A, Yakabe K, Yoshidomi K, Sueoka
K, Nawata S, Yokoyama Y, Tsuchida S, Al-Mulla F and Sugino N:
Decreased carbonyl reductase 1 expression promotes malignant
behaviours by induction of epithelial mesenchymal transition and
its clinical significance. Cancer Lett. 323:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seliger B: Molecular mechanisms of MHC
class I abnormalities and APM components in human tumors. Cancer
Immunol Immunother. 57:1719–1726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kondratiev S, Sabo E, Yakirevich E, Lavie
O and Resnick MB: Intratumoral CD8+ Tlymphocytes as a prognostic
factor of survival in endometrial carcinoma. Clin Cancer Res.
10:4450–4456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ino K, Yamamoto E, Shibata K, Kajiyama H,
Yoshida N, Terauchi M, Nawa A, Nagasaka T, Takikawa O and Kikkawa
F: Inverse correlation between tumoral indoleamine 2,3-dioxygenase
expression and tumor-infiltrating lymphocytes in endometrial
cancer: Its association with disease progression and survival. Clin
Cancer Res. 14:2310–2317. 2008. View Article : Google Scholar : PubMed/NCBI
|