Introduction
The Crk-associated substrate (Cas) family comprises
four non-catalytic scaffolding proteins (NEDD9/HEF1/CAS-L,
BCAR1/p130Cas, EFS/Sin, and HEPL/CASS4) that mediate the cell
cycle, survival, migration/chemotaxis, apoptosis, differentiation
and cell attachment (1–6). The Cas proteins have been thoroughly
investigated, and even mildly overexpressed levels of these
proteins have been found to be correlated with poor survival,
resistance to chemotherapy and metastasis in malignancies such as
melanoma, lung cancer, glioblastoma, and breast cancer. These
proteins have not only been associated with cancer, but they have
also been reported to be associated with other non-malignant
conditions, including polycystic kidney disease (7).
Neural precursor cell-expressed developmentally
downregulated protein (NEDD9) interacts with novel SH2-containing
protein family scaffold proteins and the adaptor proteins SHC and
GRB2 via its C-terminal domain, and mediates the communication
between receptor tyrosine kinases and integrins, so that receptors,
such as T-cell, B-cell and integrin receptors, send upstream
activation signals. Subsequently, focal adhesion kinase (FAK) and
the Src and ABL families of kinases are activated and they, in
turn, phosphorylate NEDD9 substrate domain even more extensively,
which provides multiple binding sites, i.e., Y189, Y317 and Y279,
for downstream effectors. FAK phosphorylation of the DYDY motif in
the NEDD9 C-terminal generates a binding site for Src kinase, which
enables NEDD9 to operate in migration and other signaling functions
(7). Furthermore, Y189
phosphorylation by FAK and Src kinases is involved in focal
adhesion. Aurora-A kinase phosphorylates S296; thus, proteasomal
degradation of NEDD9 ensues, and cell dissemination and the cell
cycle are regulated.
NEDD9 is not only activated by FAK and Src kinases,
but also maintains incessant activation of these kinases. NEDD9
connects tumor growth factor-β/SMAD and Rho-actin-SRF signals, thus
participating in tumorigenesis by coordinating the expression of
relevant genes. It also activates matrix metalloproteinases (MMPs)
and mediates actin branching and lamellipodia formation. NEDD9
downregulates E-cadherin expression by upregulating certain
transcription factors, such as SLUG and SNAIL, modulates the
Src-dependent E-cadherin removal from junctions and furthers
invasion by degradation of the basal membrane through active MMP2
production (7).
In brief, NEDD9 brings protein complexes together to
promote various cellular functions that result in tumorigenesis and
stimulation of tumor cell proliferation, migration, and genomic
instability. The aim of the present study was to analyse the level
of NEDD9 in the serum of melanoma patients in order to evaluate its
prognostic, predictive and diagnostic value in melanoma.
Patients and methods
Patients and treatment
The data of 112 melanoma patients, who had been
treated and followed up between November 2013 and March 2015, were
included in the present study. Neither chemotherapy nor
radiotherapy were administered to the patients over the last 6
months prior to inclusion. The American Joint Committee on Cancer
staging system was used to determine the stage of the disease
(8). Patients were assessed using
clinical history, physical examination and a series of blood tests,
such as lactate dehydrogenase and complete blood count, prior to
the onset of treatment. To patients with an Eastern Cooperative
Oncology Group performance status score of ≤2 and good blood
chemistry test results, treatment comprising interferon-α,
temozolamide, dacarbazine and cisplatin was administered in the
outpatient clinic. In accordance with the stage of their disease,
the patients received radiotherapy. Immunotherapy agents, such as
pembrolizumab and nivolumab, and targeted therapy agents, such as
vemurafenib/cobimetinib and dabrafenib/trametinib, were used for
metastatic or unresectable disease. Clinical, laboratory and
radiological assessments were performed every 8 weeks during
chemotherapy and every 12 weeks after treatment completion. The
revised Response Evaluation Criteria In Solid Tumors, version 1.1.,
were used to determine response to treatment (9). A total of 43 age- and sex-matched
healthy controls were also included in the analysis. Informed
consent was obtained from all patients and the study was reviewed
and approved by the local ethics committee.
Sample collection
Serum samples were collected from treatment-naïve
patients on first admission and after centrifugation they were
stored at −20°C. A double antibody sandwich ELISA kit was used to
determine the level of NEDD9 (cat. no. YHB3351; Shanghai YeHua
Biological Technology Co, Ltd., Shanghai, China) in the samples,
according to the manufacturer's instructions. Serum samples and
standards were added to the wells that had been pre-coated with
human NEDD9 monoclonal antibody. Streptavidin-horseradish
peroxidase (HRP) and biotinylated-Fab monoclonal capture antibody
conjugates were applied to form immune complexes and were then left
to incubate at 37°C for 1 h. Unbound streptavidin-HRP was washed
away, and then a colorless chromogen solution was added and
incubated at 37°C for 10 min (protected from light). The colorless
solution turned blue, and the intensity of this color change was
proportional to the amount of NEDD9 in the sample. The reaction was
terminated by an acidic stop-solution and the color turned yellow.
The end product was measured by an automated ELISA reader
(ChroMate® 4300 microplate reader; Awareness Technology
Inc., Palm City, FL, USA) at 450 nm. The results were expressed as
ng/ml.
Statistical analysis
The statistical calculations were performed using
SPSS software, version 21.0 (IBM Corp., Armonk, NY, USA).
Continuous variables were divided using median values as cut-offs.
The Mann Whitney U-test was used to analyze differences between
groups with non-parametric data distribution. Survival was
calculated from the date of first admission to the hospital to
death from any cause or to the last contact with the patient or any
family member. The survival time was analyzed by the Kaplan-Meier
method and the differences in survival were assessed using log-rank
statistics. A P-value ≤0.05 was considered to indicate
statistically significant differences.
Results
Patient characteristics
The median age at the diagnosis of the 112 patients
was 52 years (range, 16–85 years), with a male predominance (62%).
Truncal lesions were observed in 55% and metastatic disease in 61%
of the patients, with M1c disease in 72% of the cases. The baseline
serum NEDD9 levels of the patients were significantly higher
compared with those of the healthy controls (median values:
3,784.02 vs. 2,149.03 ng/ml, respectively; P<0.001) (Table I). None of the known clinical
parameters, such as age, site of lesion, lymph node involvement,
stage, lactate dehydrogenase level, sex, histology, Breslow
thickness, Clark invasion level, presence of ulceration or
regression, and response to therapy, were found to be correlated
with serum NEDD9 levels (P>0.05) (Table II).
 | Table I.Values of serum assay NEDD9 levels in
melanoma patients and healthy controls. |
Table I.
Values of serum assay NEDD9 levels in
melanoma patients and healthy controls.
|
| Patients (n=112) | Controls (n=43) |
|
|---|
|
|
|
|
|
|---|
| Assay | Median | Range | Median | Range | P-value |
|---|
| NEDD9 (ng/ml) | 3,784.02 |
1,528.09–7,367.17 | 2,149.03 | 54.88–7,505.40 | <0.001 |
 | Table II.Distribution and survival comparisons
of serum NEDD9 levels on various patient/clinical parameters in
patients with melanoma. |
Table II.
Distribution and survival comparisons
of serum NEDD9 levels on various patient/clinical parameters in
patients with melanoma.
| Parameters | NEDD9 distribution
P-value | Survival P-value |
|---|
| Age, years |
|
|
|
<50/≥50 | 0.21 | 0.77 |
| Sex |
|
|
|
Male/female | 0.88 | 0.76 |
| Site of lesion |
|
|
|
Axial/extremity | 0.41 | 0.027 |
| Histology |
|
|
|
Nodular/non-nodular | 0.71 | 0.41 |
| Breslow thickness,
mm |
|
|
|
≤4/>4 | 0.71 | 0.74 |
| Clark invasion
level |
|
|
|
I–III/IV–V | 0.25 | 0.88 |
| Ulceration |
|
|
|
Yes/no | 0.31 | 0.33 |
| Mitotic rate (no. of
mitoses/mm2) |
|
|
|
0-2/≥3 | 0.32 | 0.11 |
| Regression |
|
|
|
Yes/no | 0.71 | 0.62 |
| TIL |
|
|
|
Yes/no | 0.48 | 0.19 |
| Nodal
involvement |
|
|
|
Yes/no | 0.27 | 0.08 |
| Type of nodal
involvement |
|
|
|
Single/multiple | 0.28 | 0.047 |
| Metastasis |
|
|
|
Yes/no | 0.70 | 0.001 |
| M1 status |
|
|
| ab/c | 0.70 | 0.001 |
| Serum LDH level |
|
|
|
High/normal | 0.89 | 0.8 |
| Anemia |
|
|
|
Yes/no | 0.77 | 0.001 |
| ESR |
|
|
|
High/normal | 0.76 | 0.003 |
| Response to
chemotherapy |
|
|
|
Yes/no | 0.49 | 0.006 |
| NEDD9
expression |
|
|
|
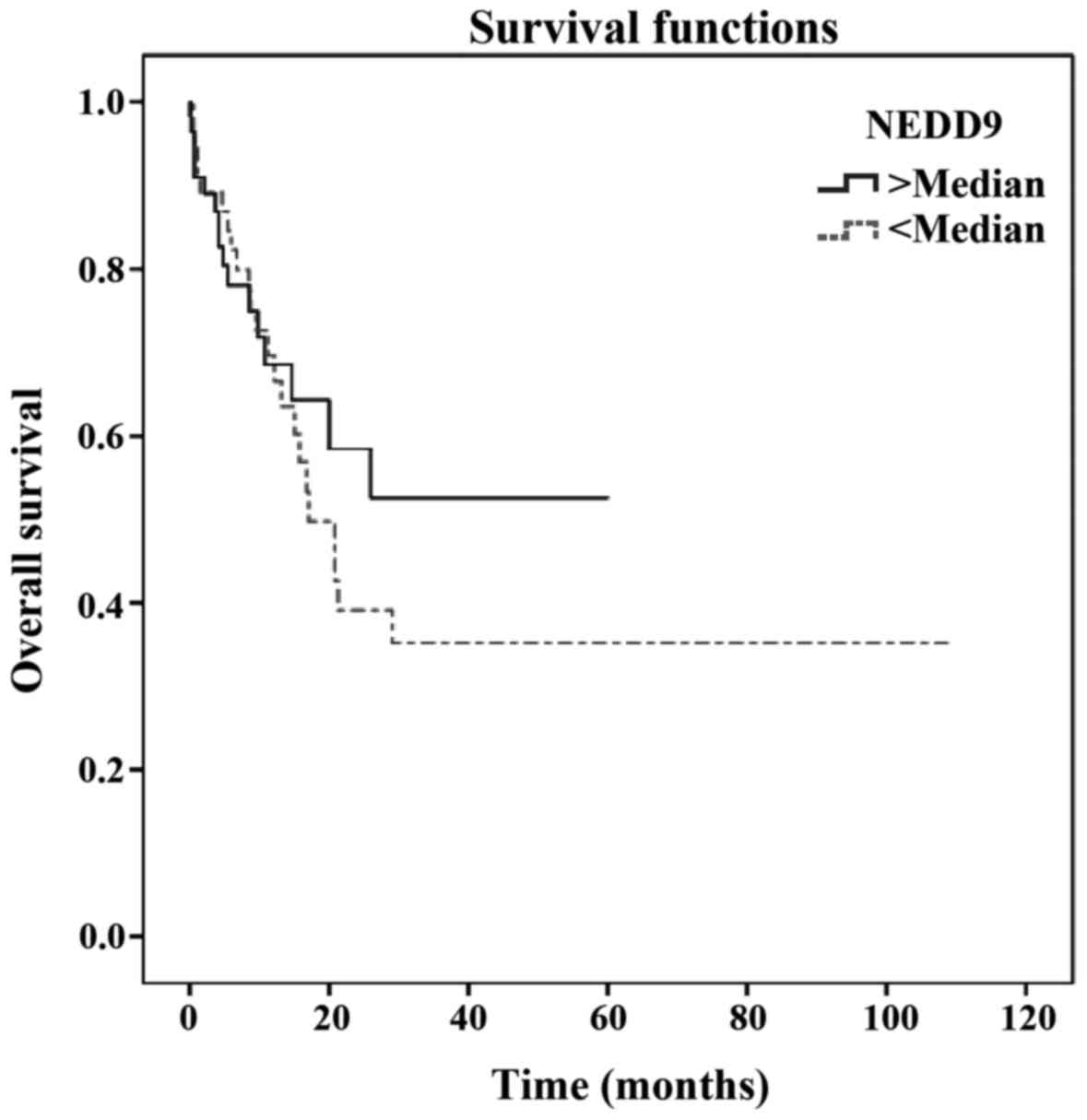
Low<median>high | – | 0.495 |
Factors affecting survival
The median survival of all patients was 20.8 months
(95% CI: 10.7–30.9). The 1- and 2-year overall survival rates were
67.3 and 44.4%, respectively. Truncal lesions (P=0.027), nodal
involvement (P=0.08), multiple nodal involvement (P=0.047),
metastasis (P<0.001), advanced metastasis (P<0.001), anemia
(P<0.001), elevated erythrocyte sedimentation rate (ESR)
(P=0.003) and failure to respond to chemotherapy (P=0.006) were
found to be correlated with poorer survival (Table II). However, serum NEDD9 level did
not appear to be of prognostic value for melanoma survival [hazard
ratio (HR)=1.142; 95% CI: 0.588–2.217; P=0.495] (Table II; Fig.
1).
Discussion
The serum NEDD9 (also referred to as HEF1 and CAS-L)
concentration in the 112 melanoma patients was found to be
significantly higher compared with that in the healthy controls
(median values: 3,784.02 vs. 2,149.03 ng/ml, respectively;
P<0.001). NEDD9 concentration was not found to be correlated any
of the following clinicopathological parameters: Site of lesion,
lymph node involvement, stage, lactate dehydrogenase level, sex,
histology, Breslow thiskness, Clark invasion level, presence of
ulceration or regression and response to therapy (P>0.05).
Truncal lesions (P=0.027), multiple nodal involvement (P=0.047),
metastasis (P<0.001), advanced metastasis (P<0.001), anemia
(P<0.001), elevated ESR (P=0.003) and failure to respond to
chemotherapy (P=0.006) were correlated with poor survival. However,
serum NEDD9 level had no prognostic effect on melanoma survival
(HR=1.142; 95% CI: 0.588–2.217; P=0.495).
Cas scaffolding proteins (NEDD9/HEF1/CAS-L,
BCAR1/p130Cas, EFS/Sin and HEPL/CASS4) play important roles in cell
functions such as migration, proliferation and survival (1). Among these, BCAR1 has been associated
with promotion of tumorigenesis, invasive behavior of the tumor and
enhanced metastasis and, thus, unfavorable prognosis in breast
cancer (10), whereas overexpression
of NEDD9 has been correlated with glioblastoma and melanoma
(11,12). Epithelial-to-mesenchymal transition
(EMT) is necessary for the invasive behavior of tumors, i.e., cells
move more readily once their lateral attachments with adjacent
cells are broken (1). E-cadherin
normally acts as a cell-to-cell adhesion molecule, but it is
downregulated during EMT, so the adherens junctions lose their
stability and, thence, cells disconnect from one another (13). It has been demonstrated that NEDD9 or
BCAR1 overexpression downregulate E-cadherin protein expression in
cells, and conversely, E-cadherin expression is augmented when
either NEDD9 or BCAR1, or both, are knocked down (1). This has been explained by Cas
activation of lysosomal breakdown of E-cadherin through Src kinase.
Similarly, NEDD9 depletion re-boosted E-cadherin expression, which
was previously decreased by dioxin treatment that originally
upregulated NEDD9 expression (14).
E-cadherin loss from the cell surface by Cas proteins appears to be
a plausible explanation for the invasiveness of the tumors that
express high levels of NEDD9.
The metastatic tendency of melanoma has already been
associated with overexpression of NEDD9. Genomic modifications,
such as chromosomal gain and loss, account for the development
and/or invasiveness of several cancer types. Among these events,
chromosome 6p gain has been particularly associated with several
malignancies and their prognosis, including lymphoma,
retinoblastoma, multiple myeloma and non-small-cell lung cancer
(NSCLC), and the Nedd9 gene was found to be constantly
upregulated in this amplified region in metastatic, but not in
primary, melanoma cells excessively expressing the NEDD9 protein,
which promotes metastasis in melanoma (7,12).
Furthermore, NEDD9 knockdown resulted in inhibition of
proliferation and invasion of melanoma cells; thus, continued NEDD9
expression was found to be necessary for melanoma cells to invade
and metastasize (12). These studies
demonstrated that NEDD9 in association with the RAS-RAF pathways
increased the metastatic potential of primary non-transformed
melanocytes and dormant melanoma cells (12).
Similarly, Rozenberg et al discovered
overexpression of NEDD9 in metastatic melanoma cells in a murine
model; however, interestingly, they also stated that NEDD9
lentiviral overexpression did not confer a metastatic ability on
non-metastatic primary cells (15).
This result supports the hypothesis that NEDD9 overexpression alone
is not sufficient for tumorigenesis and invasiveness, but rather
cooperation with other mechanisms impairing either checkpoints or
apoptosis is required. However, downregulation of the Nedd9
gene (rather than its overexpression) was found in several studies,
under suitable conditions, to be associated with increased
invasiveness and metastasis (16,17);
furthermore, when significantly overexpressed, NEDD9 facilitates
apoptosis and mitotic defects that activate checkpoints resulting
in cell cycle dysregulation (18).
All these data support the hypothesis that cells must be exposed to
some genetic pre-alterations in conjunction with NEDD9
overexpression for proliferation, invasiveness and metastasis
(3). Another study, although the
underlying mechanism was not fully elucidated, revealed that
increased activity of the inhibitor of β-catenin and T-cell
factor/lymphoid enhancer factor caused a reduction of the NEDD9
level; this, in turn, resulted in less Rac1-GTP signaling, which is
a positive regulator of mesenchymal movement, and it concurrently
produced more Rho/ROCK-driven amoeboid movement of melanoma cells,
which displayed an enhanced capacity for invasion and metastasis as
a result of transformation to rounder and more motile shapes
(19).
In their study, Lee et al suggested that the
N-terminal truncated protein stimulated tumor growth and it may be
used as a biomarker to predict the metastatic potential of various
cancers, such as hepatocellular carcinoma and neuroendocrine
cancers (20). That study
demonstrated that, after being transported into the nucleus, the
N-terminal truncated protein co-functions with histone deacetylase
1/2 to increase Nedd9 gene expression; in addition, by
referring to the study by Kim et al (12), the significant role of NEDD9 in
promoting melanoma invasiveness and metastasis was stressed
(20).
The interaction between NEDD9 expression and cancer
has been also investigated in other cancer types, including lung,
breast and gastrointestinal cancer and glioblastoma. Since
epidermal growth factor receptor (EGFR) expression and activation
in NSCLC have long been reported and EGFR has already been affirmed
as a treatment target, studies have been focused on possible
molecular associations between EGFR and integrins regarding
cellular invasion and metastasis. Since NEDD9 is a key protein of
β1-integrins and operates under a stringent association with EGFR,
their association was specifically investigated. It was observed
that tyrosine phosphorylation of NEDD9 was affected by
overexpression of active EGFR without requiring integrin
stimulation, and NEDD9 promoted migration and invasion of cells,
thus facilitating NSCLC metastasis, whereas its expression in the
primary tumor was found to be strongly associated with poor
recurrence-free and overall survival (21). As reported by prior studies, the
pro-metastatic role of NEDD9 in lung cancer was explained by its
ability to induce EMT through FAK activation and the inverse
correlation between NEDD9 and E-cadherin expression in lung cancer
was also pointed out (22). It was
successfully demonstrated that NEDD9 knockdown resulted in
inhibition of migration, invasiveness and metastasis of lung
cancer.
In agreement with the studies on other types of
cancer, gastrointestinal cancers were also reported to be affected
by elevated expression of NEDD9. Several studies reported the
association between elevated NEDD9 expression and increased
metastasis and poor prognosis in gastric cancer (23–26),
pancreatic ductal adenocarcinoma (27), hepatocellular carcinoma (also in
patients with early-stage disease and normal α-fetoprotein levels)
(28), and colorectal cancer
(29).
The present study, conversely, demonstrated that
serum NEDD9 levels were not associated with any of the poor
prognostic variables for melanoma, and did not affect metastasis or
survival. This lack of effect of NEDD9 on the prognosis of our
patients may be attributed to the small number of the patients and
the retrospective design of the study, and the results may have
also been affected by the fact that we analyzed data that were
collected over a short period of time. However, serum NEDD9 level
was found to be a diagnostic factor for melanoma. Based on these
results, taken together with the results reported by other studies,
it is strongly believed that serum NEDD9 is of predictive and
prognostic value in melanoma, as well as in other malignancies, and
serum NEDD9 expression may be proven to be one of the predictive
factors and a potential therapeutic target in melanoma. However,
further investigation is required to prove this hypothesis.
References
|
1
|
Tikhmyanova N and Golemis EA: NEDD9 and
BCAR1 negatively regulate E-cadherin membrane localization, and
promote E-cadherin degradation. PLoS One. 6:e221022011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Defilippi P, Di Stefano P and Cabodi S:
p130Cas: A versatile scaffold in signaling networks. Trends Cell
Biol. 16:257–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh M, Cowell L, Seo S, O'Neill G and
Golemis E: Molecular basis for HEF1/NEDD9/Cas-L action as a
multifunctional co-ordinator of invasion, apoptosis and cell cycle.
Cell Biochem Biophys. 48:54–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tikhmyanova N, Little JL and Golemis EA:
CAS proteins in normal and pathological cell growth control. Cell
Mol Life Sci. 67:1025–1048. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Neill GM, Seo S, Serebriiskii IG, Lessin
SR and Golemis EA: A new central scaffold for metastasis: Parsing
HEF1/Cas-L/NEDD9. Cancer Res. 67:8975–8979. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Izumchenko E, Singh MK, Plotnikova OV,
Tikhmyanova N, Little JL, Serebriiskii IG, Seo S, Kurokawa M,
Egleston BL, Klein-Szanto A, et al: NEDD9 promotes oncogenic
signaling in mammary tumor development. Cancer Res. 69:7198–7206.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shagisultanova E, Gaponova AV, Gabbasov R,
Nicolas E and Golemis EA: Preclinical and clinical studies of the
NEDD9 scaffold protein in cancer and other diseases. Gene.
567:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balch CM, Gershenwald JE, Soong S,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van der Flier S, Brinkman A, Look MP, Kok
EM, Meijer-van Gelder ME, Klijn JG, Dorssers LC and Foekens JA:
Bcar1/p130Cas protein and primary breast cancer: Prognosis and
response to tamoxifen treatment. J Natl Cancer Inst. 92:120–127.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Natarajan M, Stewart JE, Golemis EA,
Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR and
Gladson CL: HEF1 is a necessary and specific downstream effector of
FAK that promotes the migration of glioblastoma cells. Oncogene.
25:1721–1732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim M, Gans JD, Nogueira C, Wang A, Paik
JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, et al:
Comparative oncogenomics identifies NEDD9 as a melanoma metastasis
gene. Cell. 125:1269–1281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bui LC, Tomkiewicz C, Chevallier A, Pierre
S, Bats AS, Mota S, Raingeaud J, Pierre J, Diry M, Transy C, et al:
Nedd9/Hef1/Cas-L mediates the effects of environmental pollutants
on cell migration and plasticity. Oncogene. 28:3642–3651. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rozenberg GI, Monahan KB, Torrice C, Bear
JE and Sharpless NE: Metastasis in an orthotopic murine model of
melanoma is independent of RAS/RAF mutation. Melanoma Res.
20:361–371. 2010.PubMed/NCBI
|
|
16
|
Singh MK, Izumchenko E, Klein-Szanto AJ,
Egleston BL, Wolfson M and Golemis EA: Enhanced genetic instability
and dasatinib sensitivity in mammary tumor cells lacking NEDD9.
Cancer Res. 70:8907–8916. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dadke D, Jarnik M, Pugacheva EN, Singh MK
and Golemis EA: Deregulation of HEF1 impairs M-phase progression by
disrupting the RhoA activation cycle. Mol Biol Cell. 17:1204–1217.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Domingues MJ, Rambow F, Job B, Papon L,
Liu W, Larue L and Bonaventure J: β-catenin inhibitor ICAT
modulates the invasive motility of melanoma cells. Cancer Res.
74:1983–1995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee TK, Murthy SR, Cawley NX, Dhanvantari
S, Hewitt SM, Lou H, Lau T, Ma S, Huynh T, Wesley RA, et al: An
N-terminal truncated carboxypeptidase E splice isoform induces
tumor growth and is a biomarker for predicting future metastasis in
human cancers. J Clin Invest. 121:880–892. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo S, Iwata S, Yamada T, Inoue Y,
Ichihara H, Kichikawa Y, Katayose T, Souta-Kuribara A, Yamazaki H,
Hosono O, et al: Impact of the integrin signaling adaptor protein
NEDD9 on prognosis and metastatic behavior of human lung cancer.
Clin Cancer Res. 18:6326–6338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin Y, Li F, Zheng C, Wang Y, Fang Z, Guo
C, Wang X, Liu H, Deng L, Li C, et al: NEDD9 promotes lung cancer
metastasis through epithelial-mesenchymal transition. Int J Cancer.
134:2294–2304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang SS, Wu LH, Liu Q, Chen KS and Zhang
XF: Elevated expression of NEDD9 is associated with metastatic
activity in gastric cancer. Onco Targets Ther. 8:633–640. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Wang D, Zhao KL, Zhu JW, Yin HB,
Wei YZ, Wu ZJ, Cheng GJ, Wang F, Ni F, et al: NEDD9 overexpression
correlates with poor prognosis in gastric cancer. Tumour Biol.
35:6351–6356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi R, Wang L, Wang T, Xu J, Wang F and Xu
M: NEDD9 overexpression correlates with the progression and
prognosis in gastric carcinoma. Med Oncol. 31:8522014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karabulut M, Alis H, Afsar CU, Karabulut
S, Kocatas A, Oguz H and Aykan NF: Serum neural precursor
cell-expressed, developmentally down regulated 9 (NEDD9) level may
have a prognostic role in patients with gastric cancer. Biomed
Pharmacother. 73:140–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue YZ, Sheng YY, Liu ZL, Wei ZQ, Cao HY,
Wu YM, Lu YF, Yu LH, Li JP and Li ZS: Expression of NEDD9 in
pancreatic ductal adenocarcinoma and its clinical significance.
Tumour Biol. 34:895–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu P, Wang ZP, Dang Z, Zheng ZG, Li X,
Zhou L, Ding R, Yue SQ and Dou KF: Expression of NEDD9 in
hepatocellular carcinoma and its clinical significance. Oncol Rep.
33:2375–2383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li P, Zhou H, Zhu X, Ma G, Liu C, Lin B
and Mao W: High expression of NEDD9 predicts adverse outcomes of
colorectal cancer patients. Int J Clin Exp Pathol. 7:2565–2570.
2014.PubMed/NCBI
|