Introduction
Cholangiocarcinoma is a malignant tumor originating
in the biliary tract, with an extremely poor prognosis at the
advanced stages. An estimated 40–70% of cholangiocarcinomas arise
near the bifurcation of the right and left hepatic ducts at the
liver hilum, whereas ~5–20% of the cases develop within the liver
(1). The incidence of
cholangiocarcinoma increases in association with the higher
incidence of chronic inflammatory liver diseases (2,3).
Cholangiocarcinoma comprises ~15% of all primary liver tumors;
however, only a small proportion of patients with early-stage
cholangiocarcinoma are considered suitable for curative surgical
treatment (4). Thus, prolonging the
survival of patients with cholangiocarcinoma remains challenging
and new treatment approaches are urgently required.
Gene therapy is partially effective in the treatment
of certain types of diseases, including cancer (5,6). The
application of gene therapy has been somewhat limited; however,
with the advances in molecular biology, the clinical application of
gene therapy may move forward. The advent of adenoviral vectors
provided a potential tool for the application of gene-based therapy
for treating solid tumors. A major disadvantage was that the
adenoviral vector also infects other replicating cells besides
tumor cells. To minimize the side effects in tumor gene therapy, a
selective adenoviral vector that only infects tumor cells is the
research focus of interest in this field. Tumor-specific promoters
(TSPs) play important roles in viral replication in tumors and they
may be used to restrict gene expression in tumorous lesions.
Attempts to use TSPs of known hepatobiliary biomarker genes, such
as cyclooxygenase-2 (COX-2), midkine (MK), mucin-1 (MUC1), human
telomerase reverse transcriptase (hTERT) and the bile duct marker
cytokeratin-19 (CK19), offer a novel approach to restricting gene
expression only in cholangiocarcinoma cells (7–11). In
addition, the expression of transduced genes does not occur in
non-proliferating primary liver cells when adenoviral vector is
implicated in gene therapy (12).
Other studies have demonstrated the effectiveness of potential TSPs
constructed in adenoviral vectors used in gene therapy in
cholangiocarcinoma; however, the selection of different TSPs may
yield inconsistent results (13,14). The
aim of the present study was to investigate
cholangiocarcinoma-specific biomarker genes and their promoters,
which are functional in cholangiocarcinoma cells but remain silent
in non-tumorous primary human liver cells, in order to determine
the value of the potential application of
cholangiocarcinoma-specific TSP in gene therapy.
Materials and methods
Cell lines and primary cells
The human cholangiocarcinoma cell line QBC939 was
provided by Hanbio Biotechnology Co., Ltd. (Shanghai, China). The
normal human hepatic cell line LO2 was obtained from the American
Type Culture Collection (Manassas, VA, USA). The cells were
cultured in 10% fetal calf serum Dulbecco's modified Eagle's medium
with 5% CO2 at 37°C. Human primary cholangiocytes were
isolated from normal liver tissue from patients undergoing liver
transplantation, and are commercially available from Wuhan PriCells
Biomedical Technology Co., Ltd. (Wuhan, China). The cells were
maintained in culture according to the manufacturer's
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Carlsbad, CA, USA) and
first-strand cDNA was synthesized using the Omniscript RT kit
(Qiagen, Inc., Valencia, CA, USA). RT-qPCR was performed with SYBR
dye on LightCycler 480 (Roche, Ltd., Basel, Switzerland) in
triplicate, using the following cycling conditions: Denaturation at
95°C for 5 min, followed by 50 cycles of amplification at 95°C for
10 sec and extension at 60°C for 20 sec, melting at 95°C for 10
sec, followed by 10 sec at 60°C, and cooling at 40°C for 30 sec.
The sequences of the primers of the genes of interest are listed in
Table I. The ΔΔCq method was used
for data analysis and the results were expressed in arbitrary units
and normalized with GAPDH.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Genes | Forward primers (5′
to 3′) | Reverse primers (5′
to 3′) |
|---|
| COX-2 |
CCAGTATAAGTGCGATTGTACCC |
TCAAAAATTCCGGTGTTGAGCA |
| MK |
CGCGGTCGCCAAAAAGAAAG |
TACTTGCAGTCGGCTCCAAAC |
| MUC1 |
TCAGTGCCGCCGAAAGAAC |
GCTCATAGGATGGTAGGTATCCC |
| hTERT |
ATTGGAATCAGACAGCACTTGAA |
TCCCACGACGTAGTCCATGTT |
| CK19 |
ACCAAGTTTGAGACGGAACAG |
CCCTCAGCGTACTGATTTCCT |
Recombinant adenoviral vectors
Wild-type adenoviral vector pAdeno and shuttle
plasmid pShuttle2-Basic were provided by Hanbio Biotechnology Co.,
Ltd. (Shanghai, China). Plasmid pLuc-MCS containing firefly
luciferase was purchased from Agilent Technologies (Santa Clara,
CA, USA). The human CK19 promoter (−740-1-bp; GenBank accession no.
AB045973) was amplified from human genomic DNA using the following
primers: Forward: 5′-AAACCGCTCGAGCCTGTAATCCCAGCACTTTG-3′; reverse:
5′-CGGGGTACCGGCGAGGCGGAGCACGGAC-3′.
Ad-CK19-Luc, a recombinant adenoviral vector
containing a luciferase gene driven by the CK19 promoter, was
constructed as follows: The 2,700-bp BamHI fragment from
pLuc-MCS, which contains cDNA for Luc, was ligated to the
BamHI site of pShuttle2-Basic to yield pShuttle2-MCS-Luc;
the 740-bp XhoI/KpnI fragment from the CK19 promoter
was ligated to the XhoI/KpnI site of
pShuttle2-MCS-Luc to yield pShuttle-CK19-Luc; the
I-CeuI/I-SceI fragment from the CK19 promoter and Luc
from pShuttle-CK19-Luc were inserted at the
I-CeuI/I-SceI site of pAdeno to yield the
Ad-CK19-Luc. Adenoviruses were propagated in HEK293 cells and the
viral titer (PFU/ml) was determined. The propagated viruses were
purified using CsCl gradient centrifugation, dialyzed against 10%
glycerol PBS and stored at −80°C.
Promoter activity assay
Cells (2×105) were cultured in 6-well
plates. After 12 h, the cells were infected with Ad-cytomegalovirus
(CMV)-Luc and Ad-CK19-Luc at a multiplicity of infection rate of 1
and 10, respectively. The medium was replaced with fresh medium 2 h
after infection. Luciferase activity was determined with the
Dual-Luciferase Reporter Assay system (Promega, Madison, WI, USA)
48 h after transduction, according to the manufacturer's protocol.
The efficiency of transduction was evaluated in all cell types with
Ad-CMV-Luc to ensure non-saturating multiplicity of infection. The
data were presented as value relative to that of the constitutively
active CMV promoter.
Statistical analysis
The statistical analysis was performed using the
SPSS v14.0 statistical software package (SPSS, Inc., Chicago, IL,
USA). Statistical differences were analyzed with the Student's
t-test (unpaired, two-tailed). A P-value of <0.05 was considered
to indicate statistically significant differences between groups
for all experiments.
Results
Analysis of mRNA levels of biomarkers
for high expression in cholangiocarcinoma
To assist with the selection of
cholangiocarcinoma-specific promoters, the best specific biomarker
for cholangiocarcinoma were first screened out. The mRNA expression
of the COX-2, CK19, MK, MUC1 and hTERT genes in human
cholangiocarcinoma cell lines, primary human hepatocytes and
cholangiocytes were measured. The results demonstrated that CK19
exhibited the best tumor specificity, with median mRNA expression
ratios of 128.9100±27.18, 0.0052±0.0013 and 1.0020±0.073 in
cholangiocarcinoma cell lines, normal primary human hepatocytes and
cholangiocytes, respectively (P<0.001; Fig. 1). The median expression in
cholangiocarcinoma cell lines was 24,600-fold higher compared with
that in normal primary human hepatocytes, and 130-fold higher
compared with that in normal primary human cholangiocytes. However,
the mRNA expression of COX-2, MK, MUC1 and hTERT did not show a
relevance to cholangiocarcinoma. The mRNA expression levels of
MUC1, hTERT and COX-2 were significantly higher in primary human
hepatocytes compared with those in the cholangiocarcinoma cell line
(P<0.01). The mRNA expression of MK was high in primary human
cholangiocytes and cholangiocarcinoma cell lines, but without
significant differences between the two types of cells (P>0.05).
Since the mRNA expression of CK19 was cholangiocarcinoma-specific,
the CK19 promoter was selected for further analyses.
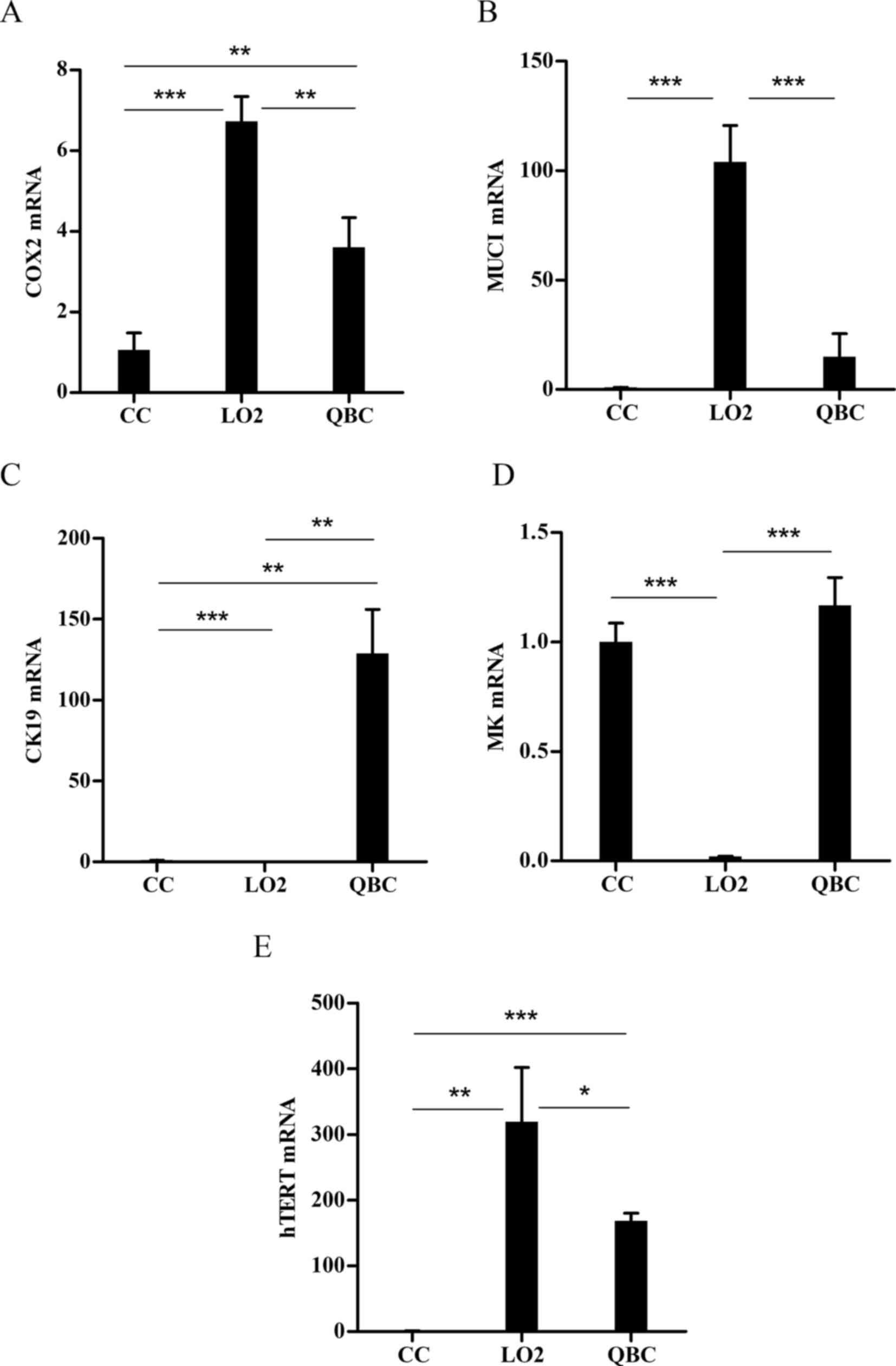 | Figure 1.Analysis of mRNA expression of tumor
biomarker genes in primary human cholangiocytes, primary human
hepatocytes and cholangiocarcinoma cells. All mRNA expression was
determined by reverse transcription-quantitative polymerase chain
reaction and normalized to GAPDH. The expression level was
calculated from Cquantification cycle (Cq) values using
the 2−ΔΔCq method and the expression levels were
presented as the mRNA ratios of the cells analyzed to the primary
human cholangiocytes. Data are expressed as mean ± standard
deviation. ***P<0.001, **P<0.01 and *P<0.05. COX-2,
cyclooxygenase-2; MUC1, mucin-1; CK19, cytokeratin-19; MK, midkine;
hTERT, human telomerase reverse transcriptase; CC, primary human
cholangiocyte; LO2, primary human hepatocyte; QBC, QBC939
cholangiocarcinoma cell line. |
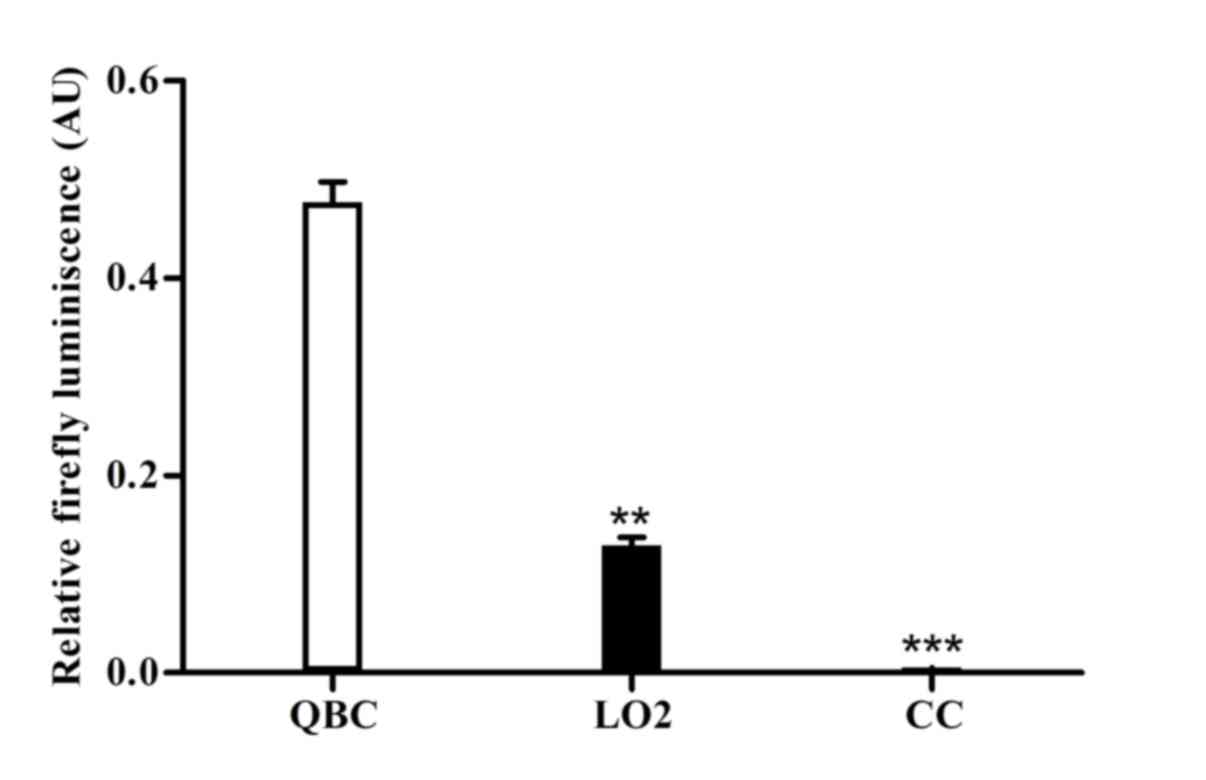
TSP activity assays
To implement tumor promoters in gene therapy, we
investigated whether the CK19 promoter retained tumor specificity
when constructed in an adenovirus vector. Therefore, the adenoviral
reporter vector driving luciferase from the CK19 promoter fragment
(−740-1-bp) was constructed. The activities of the adenoviral
promoter constructs were determined in the infected
cholangiocarcinoma cells, primary human hepatocytes and
cholangiocytes. The data were presented as the value relative to
that of the constitutively active CMV promoter. As shown in
Fig. 2, transduction with
Ad-CK19-Luc resulted in the highest luciferase activity in
cholangiocarcinoma cells as compared with that in primary human
hepatocytes and cholangiocytes. The median activity in QBC939 cells
was 0.473±0.017, which was significantly higher compared with that
in primary human hepatocytes (0.123±0.007) and cholangiocytes
(0.006±0.001) (P<0.001). Unexpectedly, the Ad-CK19-Luc activity
in primary human hepatocytes was higher compared with that in
primary human cholangiocytes, and did not correlate with its lowest
mRNA expression. The results indicated that the CK19 promoter
fragment was highly active in human cholangiocarcinoma cells and
remained inactive in primary human hepatocytes and cholangiocytes;
therefore, it may be a candidate TSP of choice in gene therapy for
cholangiocarcinoma.
Discussion
Cholangiocarcinoma is associated with high
mortality, rapidly increasing incidence and limited effectiveness
of surgical therapy (15).
Gene-based therapy appears to be a viable option for the treatment
of certain highly malignant tumors, such as cholangiocarcinoma
(5). However, a major disadvantage
lies in the hepatotropism of the delivered vector, which is
associated with side effects and limits its potential clinical
application. The adenoviral vectors constructed with TSPs can
effectively restrict gene expression in tumor cells, as they
conditionally replicate in these cells, and this may shed light to
cancer gene therapy (16). It was
reported that the use of survivin promoter in adenovector-mediated
gene delivery restricted gene expression of the delivered gene only
in melanoma cells (17). The results
from another study demonstrated that prostate-specific antigen
promoter-driven gene delivery demonstrated selective expression of
the delivered genes only in prostate cancer cells (18). However, transcriptional targeting is
only one of the approaches that specifically deliver genes in tumor
cells. Other targeting approaches, such as cell cycle-specific
promoters, treatment-responsive promoters, or dual-specificity
promoters selective to both tumor and tissue, were also tested in
gene therapy (19). In this case,
the promoters of the genes of cholangiocarcinoma-specific
biomarkers serve as potential TSPs to be used for
cholangiocarcinoma gene therapy. In this regard, certain
established biomarkers, such as COX-2, MK, MUC1, hTERT and the bile
duct marker CK19 appear to be promising candidates, as there were
tumor-specifically upregulated (13). However, the results on
cholangiocarcinoma-specific promoters have been inconsistent.
Therefore, the aim of the present study was to identify TSPs which
may be used for the development of adenoviral gene therapy for
cholangiocarcinoma.
Our results demonstrated that CK19 gene expression
displayed the best specificity to cholangiocarcinoma, as indicated
by the significant difference in the mRNA expression of CK19 in
cholangiocarcinoma cells, primary human hepatocytes and
cholangiocytes. Although the mRNA expression of COX-2, hTERT and
MUC1 was pronounced in cholangiocarcinoma cells, it was markedly
lower compared with that in primary human hepatocytes, excluding
the use of these genes for the selection of
cholangiocarcinoma-specific promoters. The mRNA expression of MK
was high in both cholangiocarcinoma cells and cholangiocytes;
therefore, it was not suitable as a candidate TSP for
cholangiocarcinoma. It should be noted that our results are
partially inconsistent with those from a previous study, which
reported that the mRNA expression of both hTERT and CK19 genes in
cholangiocarcinoma cells was significantly higher compared with
that in normal primary human hepatocytes (13). This inconsistency may be explained by
the fact that the previous study used a different
cholangiocarcinoma cell line, CC-LP-1.
As the vector components of the adenovirus may
affect promoter activity, it is necessary to evaluate the promoter
activity of the CK19 fragment constructed in the Ad5 vector. The
results revealed that, not only did the vector retain the
substantial promoter activity of CK19, but it also displayed
excellent tumor specificity compared with the corresponding
luciferase activity in hepatocytes and cholangiocytes. The results
from mRNA expression and promoter activity measurements suggested
that the promoter of the CK19 gene can be used for adenovirus-based
gene delivery in cholangiocarcinoma cells to effectively minimize
adenoviral replication in normal liver and bile duct cells. It may
be inferred that the low expression of Ad-CK19-Luc activity in
primary hepatocytes and cholangiocytes is most likely due to a lack
of specific promoters. By contrast, CK19 is a known bile duct
marker. However, in certain cholestatic liver diseases, such as
primary biliary cirrhosis or primary sclerosing cholangitis,
cholangiocytes may have an activated (pro-proliferative) phenotype,
which displays a higher than normal CK19 activity. Under these
conditions, when the patients receive TSP-directed gene therapy,
whether the activity of CK19 is sufficiently high to interfere with
the physiology of normal cholangiocytes requires further
investigation. It should be emphasized that there were distinct
differences in CK19 promoter activity among different cells, so
that it was considered adequate for the development of adenoviral
gene therapy for cholangiocarcinoma, as suggested by similar
previously reported findings (20,21). The
data presented herein suggest that the promoter of the CK19 gene is
a promising tumor-specific regulatory element and may be used to
restrict the transcription of delivered genes in the treatment of
cholangiocarcinoma.
Acknowledgements
We thank the National Natural Science Foundation of
China for its support.
Funding
The present study was supported by the Major Project
of National Natural Science Foundation of China (grant no.
81370561).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JQ designed the study, analyzed the data and was a
major contributor in writing the manuscript. MW performed cell
culture experiments. Jun Qin worked on the transfection of
plasmids. QC performed PCR experiments. ZP analyzed the data and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests related
to the publication of this study.
References
|
1
|
Sirica AE: Cholangiocarcinoma: Molecular
targeting strategies for chemoprevention and therapy. Hepatology.
41:5–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel T: Increasing incidence and
mortality of primary intrahepatic cholangiocarcinoma in the United
States. Hepatology. 33:1353–1357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taylor-Robinson SD, Foster GR, Arora S,
Hargreaves S and Thomas HC: Increase in primary liver cancer in the
UK, 1979–94. Lancet. 350:1142–1143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seo E, Abei M, Wakayama M, Fukuda K, Ugai
H, Murata T, Todoroki T, Matsuzaki Y, Tanaka N, Hamada H and
Yokoyama KK: Effective gene therapy of biliary tract cancers by a
conditionally replicative adenovirus expressing uracil
phosphoribosyltransferase: Significance of timing of 5-fluorouracil
administration. Cancer Res. 65:546–552. 2005.PubMed/NCBI
|
|
5
|
Nakano K, Todo T, Chijiiwa K and Tanaka M:
Therapeutic efficacy of G207, a conditionally replicating herpes
simplex virus type 1 mutant, for gallbladder carcinoma in
immunocompetent hamsters. Mol Ther. 3:431–437. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schauer RJ, Meyer G, Baretton G,
Schildberg FW and Rau HG: Prognostic factors and long-term results
after surgery for gallbladder carcinoma: A retrospective study of
127 patients. Langenbecks Arch Surg. 386:110–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayashi N, Yamamoto H, Hiraoka N, Dono K,
Ito Y, Okami J, Kondo M, Nagano H, Umeshita K, Sakon M, et al:
Differential expression of cyclooxygenase-2 (COX-2) in human bile
duct epithelial cells and bile duct neoplasm. Hepatology.
34:638–650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Itoi T, Shinohara Y, Takeda K, Nakamura K,
Shimizu M, Ohyashiki K, Hisatomi H, Nakano H and Moriyasu F:
Detection of telomerase reverse transcriptase mRNA in biopsy
specimens and bile for diagnosis of biliary tract cancers. Int J
Mol Med. 7:281–287. 2001.PubMed/NCBI
|
|
9
|
Kagaya M, Kaneko S, Ohno H, Inamura K and
Kobayashi K: Cloning and characterization of the 5′-flanking region
of human cytokeratin 19 gene in human cholangiocarcinoma cell line.
J Hepatol. 35:504–511. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato M, Shinozawa T, Kato S, Endo K and
Terada T: Increased midkine expression in intrahepatic
cholangiocarcinoma: Immunohistochemical and in situ hybridization
analyses. Liver. 20:216–221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamada S, Goto M, Nomoto M, Nagata K,
Shimizu T, Tanaka S, Sakoda K, Imai K and Yonezawa S: Expression of
MUC1 and MUC2 mucins in extrahepatic bile duct carcinomas: Its
relationship with tumor progression and prognosis. Pathol Int.
52:713–723. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Afford SC and Young LS: Gene therapy for
hepatocellular carcinoma-teaching old dogs new tricks. Hepatology.
34:207–209. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lie-A-Ling M, Bakker CT, Deurholt T,
Hoekstra R, Wesseling JG, Afford SC and Bosma PJ: Selection of
tumour specific promoters for adenoviral gene therapy of
cholangiocarcinoma. J Hepatol. 44:126–133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagi P, Vickers SM, Davydova J, Adachi Y,
Takayama K, Barker S, Krasnykh V, Curiel DT and Yamamoto M:
Development of a therapeutic adenoviral vector for
cholangiocarcinoma combining tumor-restricted gene expression and
infectivity enhancement. J Gastrointest Surg. 7:364–371. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jarnagin WR: Cholangiocarcinoma of the
extrahepatic bile ducts. Semin Surg Oncol. 19:156–176. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saukkonen K and Hemminki A:
Tissue-specific promoters for cancer gene therapy. Expert Opin Biol
Ther. 4:683–696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu B, Makhija SK, Nettelbeck DM, Rivera
AA, Wang M, Komarova S, Zhou F, Yamamoto M, Haisma HJ, Alvarez RD,
et al: Evaluation of tumor-specific promoter activities in
melanoma. Gene Ther. 12:330–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Latham JP, Searle PF, Mautner V and James
ND: Prostate-specific antigen promoter/enhancer driven gene therapy
for prostate cancer: Construction and testing of a tissue-specific
adenovirus vector. Cancer Res. 60:334–341. 2000.PubMed/NCBI
|
|
19
|
Nettelbeck DM, Jérôme V and Müller R: Gene
therapy: Designer promoters for tumour targeting. Trends Genet.
16:174–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurihara T, Brough DE, Kovesdi I and Kufe
DW: Selectivity of a replication-competent adenovirus for human
breast carcinoma cells expressing the MUC1 antigen. J Clin Invest.
106:763–771. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wirth T, Zender L, Schulte B, Mundt B,
Plentz R, Rudolph KL, Manns M, Kubicka S and Kühnel F: A
telomerase-dependent conditionally replicating adenovirus for
selective treatment of cancer. Cancer Res. 63:3181–3188.
2003.PubMed/NCBI
|