Introduction
Angiosarcomas is a malignant tumour that
recapitulate the functional and morphologic features of normal
endothelium. Angiosarcomas are rare forms of soft tissue neoplasm,
comprising less than 1% of all soft tissue tumours (1–3). The
majority of these tumours develop as subcutaneous lesions, usually
at head and neck region (1). Also,
these vasoformative soft tissue tumours arise in a number of
well-defined clinical settings such as chronic lymphedema, usually
post mastectomy (Stewart-Treves Syndrome) and rarely congenital
lymphedema (Milroy's disease) or filarial lymphedema (4).
The risk of developing cancer is increased among
persons who already have had cancer previously, which may be caused
by both the joint causal factors for the first primary cancer and
the treatment of the first cancer, as both chemotherapy and
radiotherapy are known to increase the risk (5–9). Excess
risks of developing soft tissue sarcoma have been reported in
relation to radiation treatment for cancers such as of breast,
ovary, cervix, and Hodgkin's and non-Hodgkin lymphoma. For breast
and ovary cancer, during a follow-up of over 10 years, the soft
tissue cancer risk increased to 8–25-fold, and latency time has
varied between 2 and 40 years (5–9).
To our knowledge, we report the first two cases of
intra-abdominal angiosarcoma eight and five years after therapeutic
prostate adenocarcinoma radiation. We discuss previous literature
regarding intra-abdominal angiosarcoma and radiation treatment.
Case reports
Case 1
A 71-year-old man presented in June 2016 with
diffuse abdominal pain. The patient's history was significant for
pT2a prostatic adenocarcinoma (Gleason score 7) in 2011 treated
with external radiation therapy (70 Gy) and hormonal treatment six
months. In 2015 the patient was diagnosed with in-situ
urothelial carcinoma treated with BCG six cycles. He received
regular follow-up, and his latest serum prostate-specific antigen
level (March 2016) was 0.0023 ng/ml (normal 0–0.04 ng/ml). His past
medical history was not relevant. He had no history of exposure to
chemicals. Physical examination revealed ascites and peritoneal
fluid liquid was obtained. Computed tomographic scan of the
abdominal cavity showed peritoneal carcinomatosis. An exploratory
laparoscopy was performed with peritoneal biopsy. After diagnosis
the patient received palliative chemotherapy with Paclitaxel. He
died five months after the diagnostic. No autopsy was
performed.
Case 2
An 82-year-old man presented in July 2016 with
diarrhea and generalized weakness. The patient's history was
significant for pT3a prostatic adenocarcinoma (Gleason score 7) in
2008 treated with external radiation therapy (75 Gy) and hormonal
treatment. In 2012 the patient was diagnosed with colonic
adenocarcinoma pT4aN0 Mx. In 2016 the diagnosis of invasive
high-grade urothelial carcinoma was performed, with posterior
cistoprostatectomy. His past medical history revealed systemic
arterial hypertension, type 2 diabetes mellitus, and chronic renal
failure. He had no history of exposure to chemicals. Physical
examination was unremarkable. After admission, abdominal CT was
performed with compatible diagnosis of local recurrence at the
sigmoid level by urothelial carcinoma, and laparoscopic resection
was performed. He died seven days after the diagnostic. No autopsy
was performed.
Peritoneal fluid liquid was processed by liquid
cytology using ThinPrep 2000 System (Cytyc Corp, Marlborough, MA)
and stained according to the Papanicolau technique and
hematoxylin-eosin staining (H&E). The surgical specimens from
case 1 and 2 were fixed in 10% buffered formalin for at least 24 h.
After fixation, biopsied sections from the peritoneal and
sigmoidectomy were embedded in paraffin, cut at 2 µm, and stained
with H&E. In addition, 2-µm sections were obtained from the
paraffin-embedded samples and were placed in an automatic processor
VENTANA® Benchmark ULTRA/LT immunohistochemistry,
Ventana Medical Systems, USA, using the previously standardized
protocol for CD31 (pre-diluted), CD34 (pre-diluted), FVIII
(pre-diluted), cytokeratin AE1/AE3 (pre-diluted), calretinin
(pre-diluted), Kaposi sarcoma herpes virus (pre-diluted) and c-Myc
(pre-diluted), including retrieval solution pH9 and detection kit
for Immunohistochemistry Optiview® DAB
(VENTANA®). The primary antibodies (Roche
Pharmaceutical, Inc) were incubated for 32 min. Finally, the
immunohistochemical sections were revealed with Diaminobenzidine,
contrasted with Meyer's hematoxylin. For each immunohistochemical
study cecal appendix was used as positive and negatives
control.
Results
Case 1
The cytology sample consisted of 20 ml haemorrhagic
fluid, which was processed for liquid cytology and cell block. The
surgical biopsy specimen consisted of 20×15 mm of tan-yellow soft
tissue mixed with clotted blood. The entire specimen was submitted
for histopathologic examination. Cytological smears were cellular
and showed a population of spindle and pleomorphic cells with
ill-defined cell borders. The nuclei were oval and hyperchromatic
with irregular nuclear membranes. Branching papillary clusters and
pseudoacini or rosettelike groups were also seen. The tumour cells
were positive by immunocytochemistry for cytoplasmic endothelial
markers CD31 (Fig. 1), CD34 and
factor VIII, confirming the endothelial origin of sarcoma (10). The tumor cells were negative for
keratin and Kaposi sarcoma herpes virus. The tumour cells were also
positive for c-Myc (Fig. 1).
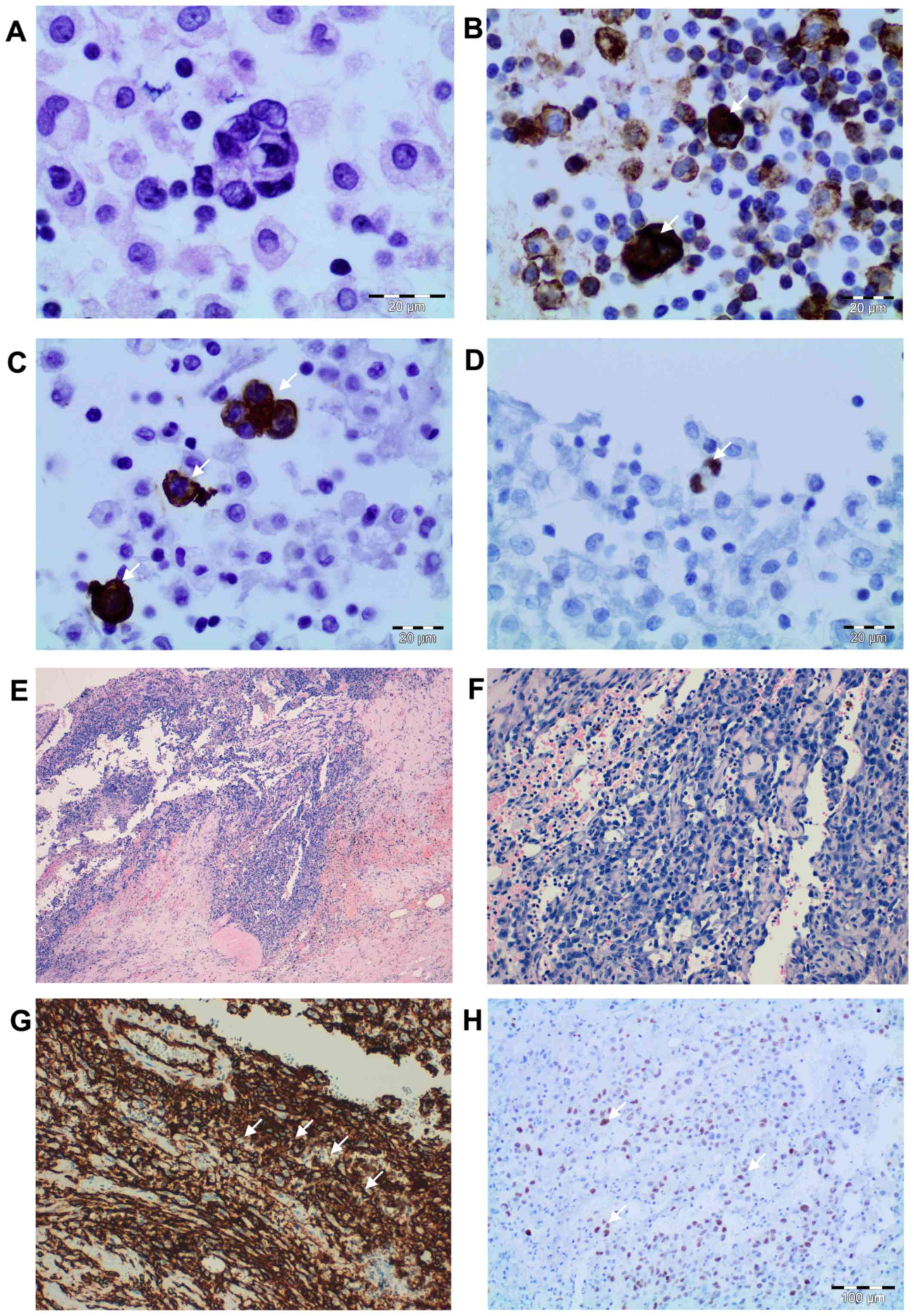 | Figure 1.Cytologic and histopathologic
findings. Peritoneal fluid cytologic smears revealing a population
of atypical and pleomorphic cells with ill-defined cell borders.
(A) The nuclei were oval and hyperchromatic with irregular nuclear
membranes (H&E; magnification, ×200). Tumor cells demonstrating
positive immunocytochemistry for (B) CD34 and (C) CD31 (indicated
by the white arrows) (DAB; magnification, ×200), and (D) nuclear
expression of c-Myc (indicated by the white arrow) (DAB;
magnification, ×200). Irregular proliferating vascular channels,
with nodular appearance, lined by atypical endothelial cells and
epithelioid areas at (E) magnification, ×40 and (F) magnification,
×100 (H&E). (G) The tumor cells were positive for CD31 as
determined by immunocytochemistry (indicated by the white arrows)
(DAB; magnification, ×100) and (H) nuclear expression for c-Myc was
present (indicated by the white arrows) (DAB; magnification, ×100).
H&E, hematoxylin and eosin staining; DAB, 3′-diaminobenzidine
staining; CD, cluster of differentiation. |
Microscopically, the epiplon showed an irregular
proliferating vascular channels, with nodular appearance, lined by
atypical endothelial cells, which in turn were surrounded by
spindle-shaped cells and epithelioid areas. The tumour cells
varying from elongated and spindle-shaped to large and plump. The
epithelioid morphology showed large rounded cells, arranged in
small nests, cords or rudimentary vascular channels. Mitoses were
fairly frequent and some were atypical. The tumour cells were
positive by immunocytochemistry for cytoplasmic endothelial markers
CD31 (Fig. 1), CD34 and factor VIII,
confirming the endothelial origin of sarcoma (10). The tumor cells were negative for
keratin and Kaposi sarcoma herpes virus. The tumour cells were also
positive for c-Myc (Fig. 1).
Case 2
The surgical specimen consisted of a sigmoidectomy
of 280 mm in length. The serosa showed congestive zones,
alternating with necro-haemorrhagic areas and fibrin deposits. The
mucosa presented oedematous aspect. Representative material was
submitted for histopathologic examination. Microscopically, a
neoplastic lesion located on the serous surface and affecting the
colonic muscle layer was observed. Neoplasia consisted of
vasoformative areas consist of ramifying channels lined by atypical
endothelial cells forming intraluminal buds and focal papillar
formations. The tumour cells were pleomorphic, varying from
elongated and spindle-shaped to large and plump. The nuclei were
large and pleomorphic with clumped chromatin and prominent nucleoli
(Fig. 2). The tumour cells were
positive by immunocytochemistry for cytoplasmic endothelial markers
CD31 (Fig. 1), CD34 and factor VIII,
confirming the endothelial origin of sarcoma. The tumor cells were
negative for the Kaposi sarcoma herpes virus, keratin and
calretinin. The tumour cells were also positive for c-Myc (Fig. 2).
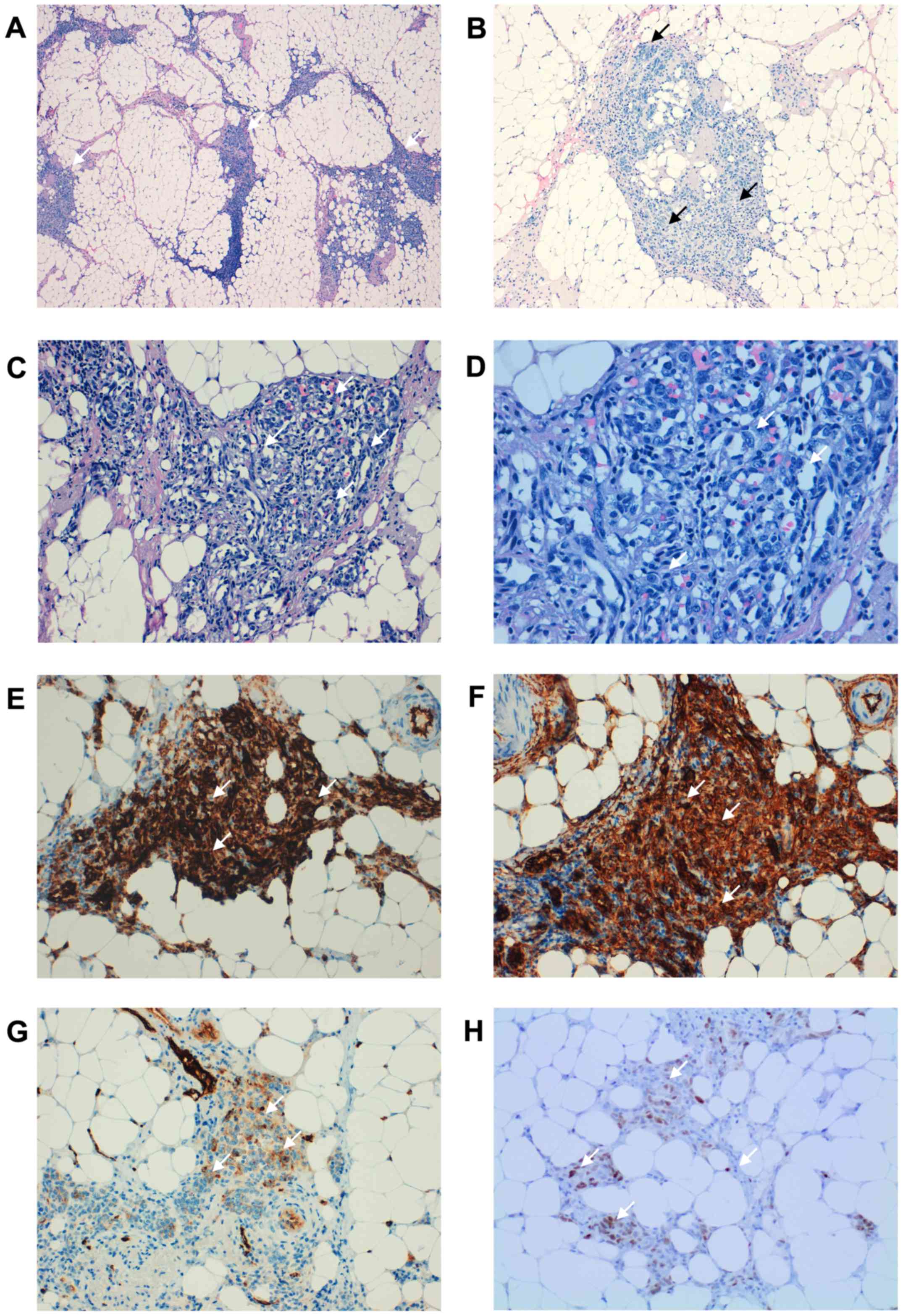 | Figure 2.Histopathological findings. (A) A
neoplastic lesion located on the serous surface, which affected the
colonic muscle layer was observed (white arrows) (H&E;
magnification, ×40). Vasoformative areas consisted of ramifying
channels lined by atypical endothelial cells forming (B)
intraluminal buds (white arrows) and (C) focal papillations (white
arrows) (H&E; magnification, ×100). (D) Tumor cells varying
from elongated and spindle-shaped to large and plump (white arrows)
(H&E; magnification, ×200). The tumor cells were positive for
(E) CD34, (F) CD31 and (G) factor VIII (white arrows) (DAB;
magnification, ×100), supporting an endothelial origin. (H) The
tumor cells were also positive for c-Myc (white arrows) (DAB;
magnification, ×100). H&E, hematoxylin and eosin staining; DAB,
3′-diaminobenzidine staining; CD, cluster of differentiation. |
Discussion
Radiation therapy is a routinely used therapeutic
modality for prostate cancer and may predispose to the appearance
of secondary neoplasms such as bladder, lung and rectum cancer
(10,11). It has also been reported that
sarcomas increase their incidence after radiotherapy for prostate
carcinoma, being 6% within the radiation field and 2% outside the
radiation field (11). In this
regard, few cases of angiosarcoma have been reported following
radiotherapy for carcinoma of the prostate (12–16). To
the best of our knowledge we describe the first two cases of
intra-abdominal angiosarcomas secondary to radiotherapy treatment
for prostate carcinoma.
Our cases met the suggested diagnostic criteria
described by Cahan et al (17) for the development of
radiotherapy-induced sarcomas (Table
I), such as: the sarcoma should arise in the area previously
subjected to irradiation, (2) a
latent period (in years) must exist between the time of irradiation
and development of the sarcoma and (3) the sarcoma must be confirmed
histologically. In both cases, the latency period was 5 to 8 years,
they were developed in the areas where the radiotherapy was
performed and finally, the diagnosis was confirmed from the
cytological and histopathological points of view.
 | Table I.Cahan criteria for the development of
radiotherapy-induced sarcomas and the present cases. |
Table I.
Cahan criteria for the development of
radiotherapy-induced sarcomas and the present cases.
| Cahan
criteriaa | Case 1 | Case 2 |
|---|
| Sarcoma in the area
of irradiation | Hypogastrium | Sigmoid |
| Latent period | 5 years | 8 years |
| Sarcoma
confirmed | Angiosacoma | Angiosacoma |
Additionally, one of the pathways related to the
development of radiotherapy-induced angiosarcomas may be c-Myc.
c-Myc is a well-established proto-oncogene, which when
overexpressed drives cell proliferation, blocks cell
differentiation, promotes angiogenesis and genetic instability
(18–20). Some studies have shown that c-Myc
amplification is a recurrent genetic alteration in secondary
angiosarcomas, but not in primary angiosarcomas, suggesting
distinct pathogenetic mechanisms between them (1,3,6,20–25). In
our cases, the immunohistochemical expression of c-Myc could be
correlated with genetic amplifications and this mechanism of c-myc
c-Myc seems to be key for the development of secondary
angiosarcomas. This finding supports the possibility of relating
the angiosarcomas studied with the radiant pre-effect. To our
knowledge, we describe two cases of intra-abdominal angiosarcoma
with c-myc post-radiotherapy expression for prostatic
adenocarcinoma.
After an exhaustive bibliographic review, we found
no cases of secondary angiosarcomas located in the abdominal region
in patients treated with prostate cancer radiotherapy. This
indicates that the risk for the development of these sarcomas after
radiotherapy in this group of patients with prostate cancer is
practically absent. Thus, given the large number of patients who
can be cured or who receive palliative treatment with radiation
therapy, concern regarding post irradiation sarcoma should not be a
major factor influencing treatment decisions in patients with
cancer (26). Different population
studies have shown that the appearance of secondary angiosarcomas
is frequently observed related to breast or gynaecological cancers
(5–9). This suggests an association between
female hormonal factors and secondary angiosarcoma.
In summary, secondary angiosarcomas of the abdominal
cavity are exceedingly rare. They may pose considerable diagnostic
difficulty in a partial sampling or cytology study. Awareness of
the possibility of angiosarcomas occurring in this location,
particularly with a history of prostate cancer radiation, and use
of appropriate immunohistochemical studies are crucial to establish
a correct diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DP and KP contributed to the cytological diagnosis
and manuscript preparation. DP and KP performed the
immunocytochemical and immunohistochemical staining and the
histopathological diagnosis. DP contributed to the collection of
patient data. The final version of the manuscript has been read and
approved by all authors.
Ethics approval and consent to
participate
The authors obtained consent from the Ethics
Committee of the Sant Joan University Hospital in Reus
(registration no. CEIM: 034/2018) for publication of the case
details.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Doyle LA: Sarcoma classification: An
update based on the 2013 World Health Organization classification
of tumors of soft tissue and bone. Cancer. 120:1763–1774. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mastrangelo G, Coindre JM, Ducimetière F,
Dei Tos AP, Fadda E, Blay JY, Buja A, Fedeli U, Cegolon L, Frasson
A, et al: Incidence of soft tissue sarcoma and beyond: A
population-based prospective study in 3 European regions. Cancer.
118:5339–5348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antonescu C: Malignant vascular tumors-an
update. Mod Pathol. 27 (Suppl 1):S30–S38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolov RB, Sato N, Azumi N and Lack EE:
Intra-abdominal ‘angiosarcomatosis’ report of two cases after
pelvic irradiation. Cancer. 67:2275–2279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar S: Second malignant neoplasms
following radiotherapy. Int J Environ Res Public Health.
9:4744–4759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sholl LM, Barletta JA and Hornick JL:
Radiation-associated neoplasia: Clinical, pathological and genomic
correlates. Histopathology. 70:70–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anzalone CL, Cohen PR, Diwan AH and Prieto
VG: Radiation-induced angiosarcoma. Dermatol Online J.
19:22013.PubMed/NCBI
|
|
8
|
Virtanen A, Pukkala E and Auvinen A:
Incidence of bone and soft tissue sarcoma after radiotherapy: A
cohort study of 295,712 Finnish cancer patients. Int J Cancer.
118:1017–1021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Virtanen A, Pukkala E and Auvinen A:
Angiosarcoma after radiotherapy: A cohort study of 332,163 Finnish
cancer patients. Br J Cancer. 97:115–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhojani N, Capitanio U, Suardi N, Jeldres
C, Isbarn H, Shariat SF, Graefen M, Arjane P, Duclos A, Lattouf JB,
et al: The rate of secondary malignancies after radical
prostatectomy versus external beam radiation therapy for localized
prostate cancer: A population-based study on 17,845 patients. Int J
Radiat Oncol Biol Phys. 76:342–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wallis CJ, Mahar AL, Choo R, Herschorn S,
Kodama RT, Shah PS, Danjoux C, Narod SA and Nam RK: Second
malignancies after radiotherapy for prostate cancer: Systematic
review and meta-analysis. BMJ. 352:i8512016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Black PC, Skinnider BF, Hayes MM
and Jones EC: Post-radiation epithelioid angiosarcoma of the
urinary bladder and prostate. Can Urol Assoc J. 10:E197–E200. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta A, Patnaik MM and Naina HV:
Angiosarcoma of the prostate gland following brachytherapy for
prostatic adenocarcinoma. Curr Urol. 8:109–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ojerholm E, Stripp D, Mamtani R, Van
Arsdalen K and Tripp P: Angiosarcoma of the bladder following
prostate radiotherapy. Am J Med. 128:e11–e12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chandan VS and Wolsh L: Postirradiation
angiosarcoma of the prostate. Arch Pathol Lab Med. 127:876–878.
2003.PubMed/NCBI
|
|
16
|
Sakemi M, Sakemi R, Harada M, So S,
Uchiyama D, Morimitsu Y, Kakiuchi S, Ishihara Y, Kubo Y, Matsugaki
S, et al: A case of postirradiation angiosarcoma of the greater
omentum with hemorrhage. Clin J Gastroenterol. 4:302–306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cahan WG, Woodard HQ, et al: Sarcoma
arising in irradiated bone; report of 11 cases. Cancer. 1:3–29.
1948. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wade MA, May FE, Onel K and Allan JM: Does
radiation-induced c-MYC amplification initiate breast oncogenesis?
Mol Cell Oncol. 3:e10109502015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wade MA, Sunter NJ, Fordham SE, Long A,
Masic D, Russell LJ, Harrison CJ, Rand V, Elstob C, Bown N, et al:
c-MYC is a radiosensitive locus in human breast cells. Oncogene.
34:4985–4994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Styring E, Seinen J, Dominguez-Valentin M,
Domanski HA, Jönsson M, von Steyern FV, Hoekstra HJ, Suurmeijer AJ
and Nilbert M: Key roles for MYC KIT and RET signaling in secondary
angiosarcomas. Br J Cancer. 111:407–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manner J, Radlwimmer B, Hohenberger P,
Mössinger K, Küffer S, Sauer C, Belharazem D, Zettl A, Coindre JM,
Hallermann C, et al: MYC high level gene amplification is a
distinctive feature of angiosarcomas after irradiation or chronic
lymphedema. Am J Pathol. 176:34–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fraga-Guedes C, André S, Mastropasqua MG,
Botteri E, Toesca A, Rocha RM, Peradze N, Rotmensz N, Viale G,
Veronesi P and Gobbi H: Angiosarcoma and atypical vascular lesions
of the breast: Diagnostic and prognostic role of MYC gene
amplification and protein expression. Breast Cancer Res Treat.
151:131–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fernandez AP, Sun Y, Tubbs RR, Goldblum JR
and Billings SD: FISH for MYC amplification and anti-MYC
immunohistochemistry: Useful diagnostic tools in the assessment of
secondary angiosarcoma and atypical vascular proliferations. J
Cutan Pathol. 39:234–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laé M, Lebel A, Hamel-Viard F, Asselain B,
Trassard M, Sastre X and Kirova YM: Can c-myc amplification
reliably discriminate postradiation from primary angiosarcoma of
the breast? Cancer Radiother. 19:168–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mentzel T, Schildhaus HU, Palmedo G,
Büttner R and Kutzner H: Postradiation cutaneous angiosarcoma after
treatment of breast carcinoma is characterized by MYC amplification
in contrast to atypical vascular lesions after radiotherapy and
control cases: clinicopathological, immunohistochemical and
molecular analysis of 66 cases. Mod Pathol. 25:75–85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mark RJ, Poen J, Tran LM, Fu YS, Selch MT
and Parker RG: Postirradiation sarcomas. A single-institution study
and review of the literature. Cancer. 73:2653–2662. 1994.
|
















