Introduction
Periodontitis is a chronic inflammatory disease
characterized by the loss of periodontal attachment and alveolar
bone due to bacterial infection. Neutrophils are the key immune
cells involved in periodontitis (1). Gingival epithelial cells produce
chemokines, such as interleukin (IL)-8 to recruit neutrophils, and
periodontal pathogens stimulate dendritic cells via Toll-like
receptors, resulting in the induction of the Th1/Th17 subset. Th17
cells are critical mediators of alveolar bone destruction in
periodontitis, and gingival epithelial cells possess IL-17 receptor
(IL-17R), to which IL-17 binds to induce IL-8 production (2-4).
Although rodents lack a direct homologue of IL-8, the C-X-C motif
chemokine ligand 1 (CXCL1) is regarded as a functional homologue of
IL-8(5).
The main approach of current periodontal therapy is
the physical excision of lesions (6). Scaling and root planing are
treatments for removing plaque and calculus from the tooth surface,
while periodontal surgery is debridement for excising infected
granulation tissue. For deep intrabone defects, it is beneficial to
use materials, such as membranes for guided tissue regeneration,
enamel matrix derivatives, and fibroblast growth factor-2 (7,8).
Additionally, pharmacological therapies are used as adjunctive
treatments (7). Chlorhexidine and
sustained-release minocycline are locally applied treatments
(9,10). For systemic treatments, antibiotics
are often used for short periods of time. However, few reports are
available on the development of pharmacological treatments for
mitigating inflammation.
We have previously demonstrated that dopamine
signaling plays a crucial role in Th17 cell differentiation
(11,12). There are five subtypes of dopamine
receptors, D1 to D5, and these subtypes are classified into two
groups: D1-like receptors and D2-like receptors. D1-like receptors,
i.e., D1 and D5, induce an increase in intracellular cyclic
adenosine monophosphate (cAMP), whereas D2-like receptors,
i.e., D2, D3, and D4, induce a decrease in intracellular
cAMP (13). In our previous
studies, D1-like receptor antagonists inhibited Th17
differentiation, and attenuated neutrophilic inflammation caused by
diseases in animal models, such as experimental autoimmune
encephalomyelitis, autoimmune diabetes, and nephrotoxic serum
nephritis models (11,14,15).
It has been reported that Th17 cells markedly produced IL-8, and
that the IL-8 production from activated T cells was suppressed by
D2-like receptor agonists (16,17),
indicating that D2-like receptor agonists can suppress neutrophilic
inflammation that has already developed. Moreover, Parrado et
al demonstrated that dopamine and D2-like receptor agonists
upregulated the expression of IL-8 in keratinocytes (18). Dopamine signaling promotes IL-8
production by stimulating both Th17 cells and epithelial cells via
the dopamine receptor.
We also showed previously that tannic acid is a
D2-like receptor agonist, and that tannic acid suppressed IL-17
production in chemically induced colitis models (19). More recently, we determined that
tannic acid inhibited alveolar bone resorption in a
carrageenan-induced rat model of periodontitis (20). In the present study, we examined
the effect of the D2-like receptor agonist ropinirole, which is
used as a drug for treating Parkinson's disease, on periodontitis
in vivo. To also analyze the action in vitro, we
evaluated whether ropinirole inhibits cytokine production in a
murine gingival epithelial cell line.
Materials and methods
Reagents
We used l-carrageenan (carrageenan; WAKO Pure
Chemical Industries Ltd., Osaka, Japan), ropinirole (Sigma-Aldrich
Japan, Tokyo, Japan) as a D2-like receptor agonist, haloperidol
(WAKO Pure Chemical Industries Ltd.) as D2-like receptor
antagonist, and carrier-free recombinant mouse IL-17A (rmIL-17A;
R&D Systems, Guthrie, MN, USA). In the preliminary in
vitro experiments, we have found that 10 mg/ml is more
effective than 1 mg/ml (data not shown). It is enough to conduct
the in vivo experiment at one dose (20). According to a paper by Hashimoto
et al (15), the endogenous
IL-17A concentration is approximately 1.0 to 1.5 ng/ml, and we used
this concentration as a reference for deciding on the dose of
IL-17A to use in this experiment. We decided on the ropinirole
concentrations by referring to previous reports (17,21).
Cell culture
Cells of the murine gingival epithelial cell line
GE1 (RCB1709; RIKEN BioResource Center Cell Bank, Japan) were
cultured in SFM101 medium (Nissui, Tokyo, Japan) supplemented with
1% fetal bovine serum (Sigma-Aldrich Japan), 100 U/ml penicillin G
(Sigma-Aldrich Japan), 100 µg/ml streptomycin (Sigma-Aldrich
Japan), and 10 ng/ml epithelial growth factor (Sigma-Aldrich Japan)
(22). The cells were incubated in
a humidified atmosphere of 5% CO2 at 33̊C. For the in
vitro assay, we seeded GE1 cells into 24-well plates at
30x104 cells per well. We added carrageenan at the
concentration of 50 or 100 µg/ml and/or rmIL-17A at the
concentration of 0.5 or 2 ng/ml to the wells. Ropinirole was added
to the wells at the concentration of 1, 10, or 50 µg/ml prior to
the addition of carrageenan and/or rmIL-17A. After 24 h of
incubation, the cells and supernatants were harvested.
Quantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis
The harvested cells were rinsed with ice-cold
phosphate-buffered saline (PBS). QIAzol Lysis Reagent (QIAGEN,
Hilden, Germany) was added to the samples, and the total RNA was
extracted. The reverse-transcription reaction was performed with a
High Capacity cDNA Reverse Transcription kit (Thermo Scientific).
TaqMan Gene Expression Assays for CXCL1 (assay identification
number: Mm04207460_m1), IL17-RA (assay identification number:
Mm00434214_m1), and glyceraldehyde-3-phosphate dehydrogenase (assay
identification number: Mm99999915_g1), which was used as an
endogenous control, were obtained from Thermo Scientific. As Taqman
probes do not provide the sequence information, we cannot show the
sequence information. All experiments were performed in
quadruplicate, i.e., each reaction was performed in
quadruplicate on four individual samples. Values were normalized to
those of glyceraldehyde-3-phosphate dehydrogenase using the
2-ΔΔCt method. This experiment was repeated at least
three times.
Measurement of CXCL1 in the culture
supernatants
CXCL1 protein in the harvested supernatants was
measured using a mouse CXCL1 ELISA kit (Proteintech, Rosemont, IL
USA) according to the manufacturer's instructions. All experiments
were performed in quadruplicate, i.e., each reaction was
performed in quadruplicate on four individual samples. This
experiment was repeated at least three times.
Carrageenan-induced rat model of
periodontitis
The bilateral maxillary bone including the second
molar of two rats were used in each group. We used 6 rats in total.
The experiments were approved by the Animal Research Committee of
Saitama Medical University (approval number: 2877), and conducted
according to the institutional guidelines for ethical animal
experiments. The humane endpoint is 20% body-weight reduction and
severe suffering. All rats were housed in appropriate animal care
facilities at Saitama Medical University, Japan. Five-week-old male
Wistar rats (200 to 300 g in weight) were obtained from Japan SLC
(Shizuoka, Japan). The rats had access to food and water ad
libitum, and were maintained on a 12-h light/dark cycle at
23±1˚C with 60±10% humidity. We used a mixture of medetomidine
hydrochloride (0.15 mg/kg; Nippon Zenyaku Kogyo, Fukushima, Japan),
midazolam (2 mg/kg; Astellas Pharma, Tokyo, Japan), and butorphanol
tartrate (2.5 mg/kg; Meiji Seika Pharma, Tokyo, Japan) as an
anesthetic mixture, which was administered intraperitoneally
(23-25).
These drugs do not affect dopamine signaling (26). For euthanasia, sodium pentobarbital
(200 mg/kg; Kyoritsu Seiyaku, Tokyo, Japan) was administered
intraperitoneally. We verified cardiac and respiratory arrest. We
used a previously reported carrageenan-induced rat model of
periodontitis (27). While the
rats were under anesthesia, buccal and lingual gingiva were
exfoliated using an explorer. We divided the rats into three
groups: a PBS group, a carrageenan (CA) group, and a carrageenan
and ropinirole (CA/RP) group. For the PBS group, silk ligature, cut
to the length of the mesiodistal distance of the mandibular second
molar, was immersed in PBS, which is the solvent for carrageenan.
For the CA group, the silk ligature was immersed in 1% carrageenan.
For the CA/RP group, the silk ligature was immersed in 1%
carrageenan and 10 µg/ml ropinirole. These ligatures were inserted
into the periodontal pocket once a week for 4 consecutive weeks.
The ligatures were inserted once a week and removed until the
animal was sacrificed. The ligatures did not affect the animals'
quality of life because 20% body-weight reduction has not been
observed. The experimental protocol is shown in Fig. S1.
Micro-computed tomography (CT)
analysis
The rats were euthanized 4 weeks after the first
operation. The maxillary bone was dissected, and subsequently fixed
with 70% ethanol for analysis on a micro-CT 35 (SCANCO Medical,
Brüttisellen, Switzerland). After preparing three-dimensional
images (Fig. S2), the
perpendicular distance between the cemento-enamel junction and the
alveolar margin on the palatal side of the maxillary second molar
was measured as shown in Fig.
S3(26). This experiment was
repeated at least three times.
Statistical analysis
Differences between more than three groups were
analyzed by one-way analysis of variance with Tukey's post-hoc
tests. P-values <0.05 were considered to indicate statistical
significance. All data are presented as the mean ± standard error
of the mean. The analyses were performed using EZR software version
1.52 which is a graphical user interface for R (The R Foundation
for Statistical Computing, Vienna, Austria) (28). These experiments were repeated at
least three times.
Results
Ropinirole suppresses alveolar bone
loss in an experimental rat model of periodontitis
Previously, we demonstrated that tannic acid, which
is a D2-like receptor agonist, inhibited alveolar bone resorption
in a carrageenan-induced rat model of periodontitis (20). In the present study, we used the
same model to analyze the function of ropinirole, and conducted
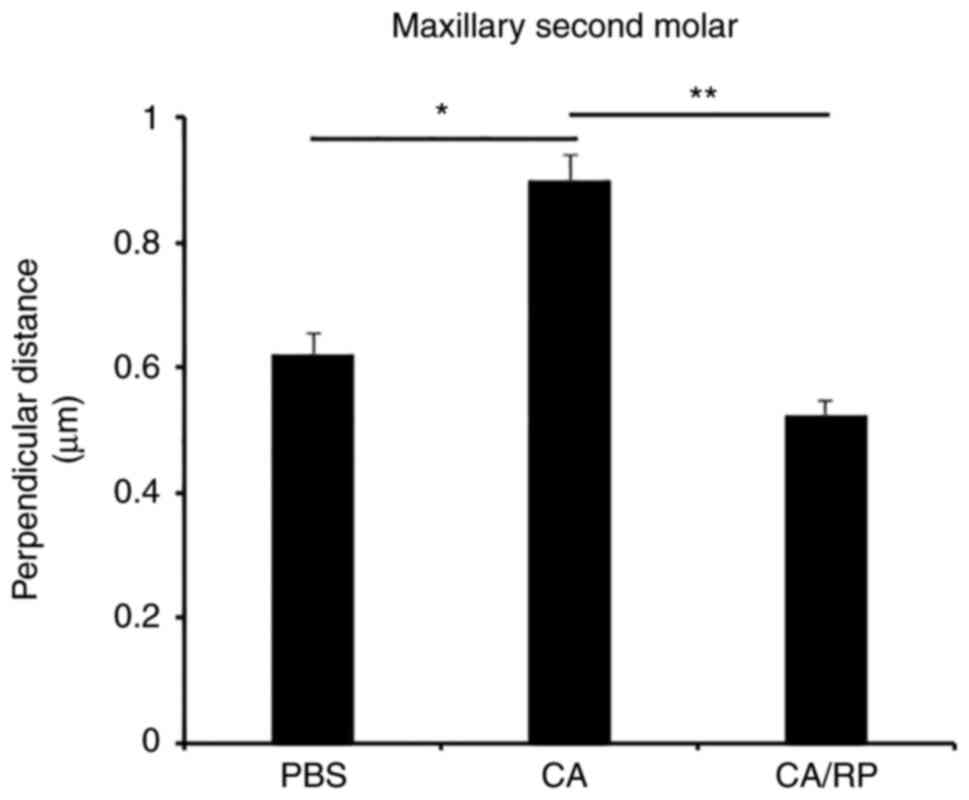
micro-CT analysis. The results revealed that the perpendicular
distance was increased in the CA group when compared to the PBS
group, and the perpendicular distance of the CA/RP group was
significantly shorter than that of the CA group (Fig. 1).
Carrageenan induces CXCL1 expression
in gingival epithelial cells in association with IL-17
modulation
As an inflammatory cytokine, IL-8 plays a key role
for neutrophil migration in human inflammatory diseases (29). Since carrageenan induces IL-8
secretion in human colonic epithelial cells (30), we examined whether carrageenan
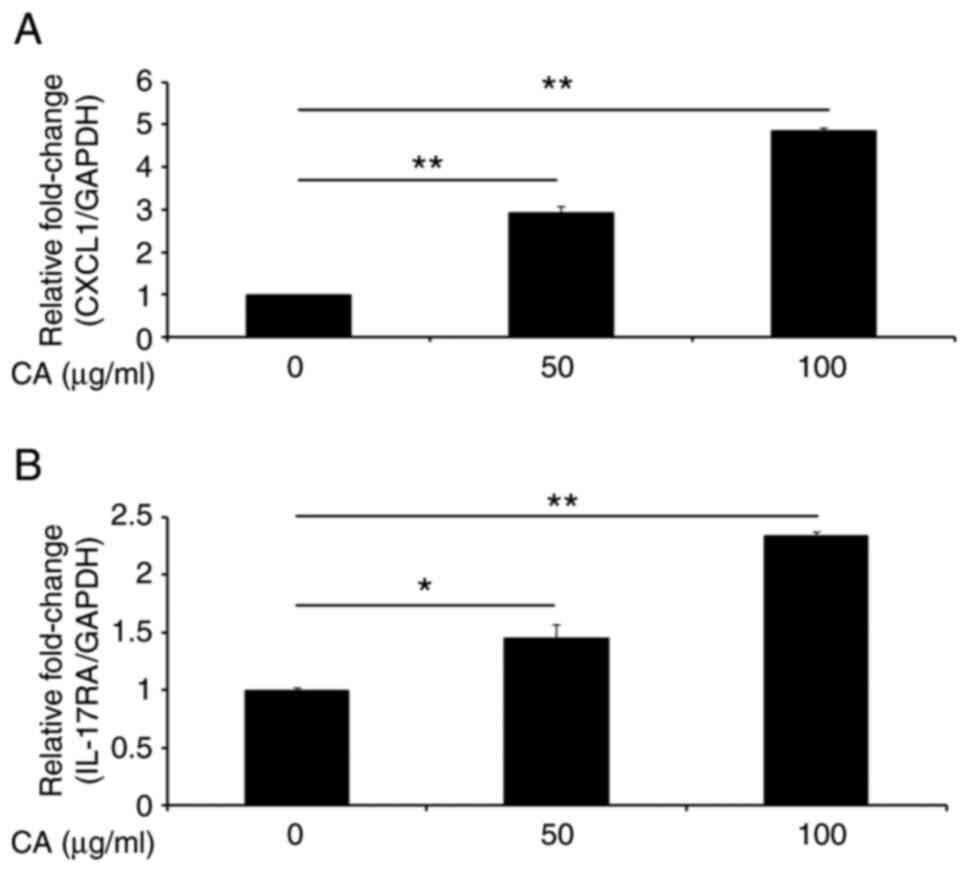
modulates CXCL1 expression in murine gingival epithelial cells. As
shown in Fig. 2A, CXCL1
expression in GE1 cells was enhanced by the addition of carrageenan
in a dose-dependent manner. As the level of IL-17 is significantly
higher in gingival crevicular fluid from patients with
periodontitis than that from healthy participants (31), we evaluated the influence of
carrageenan on IL-17RA mRNA expression in gingival
epithelial cells. We found that carrageenan induced IL-17RA
expression in GE1 cells (Fig.
2B).
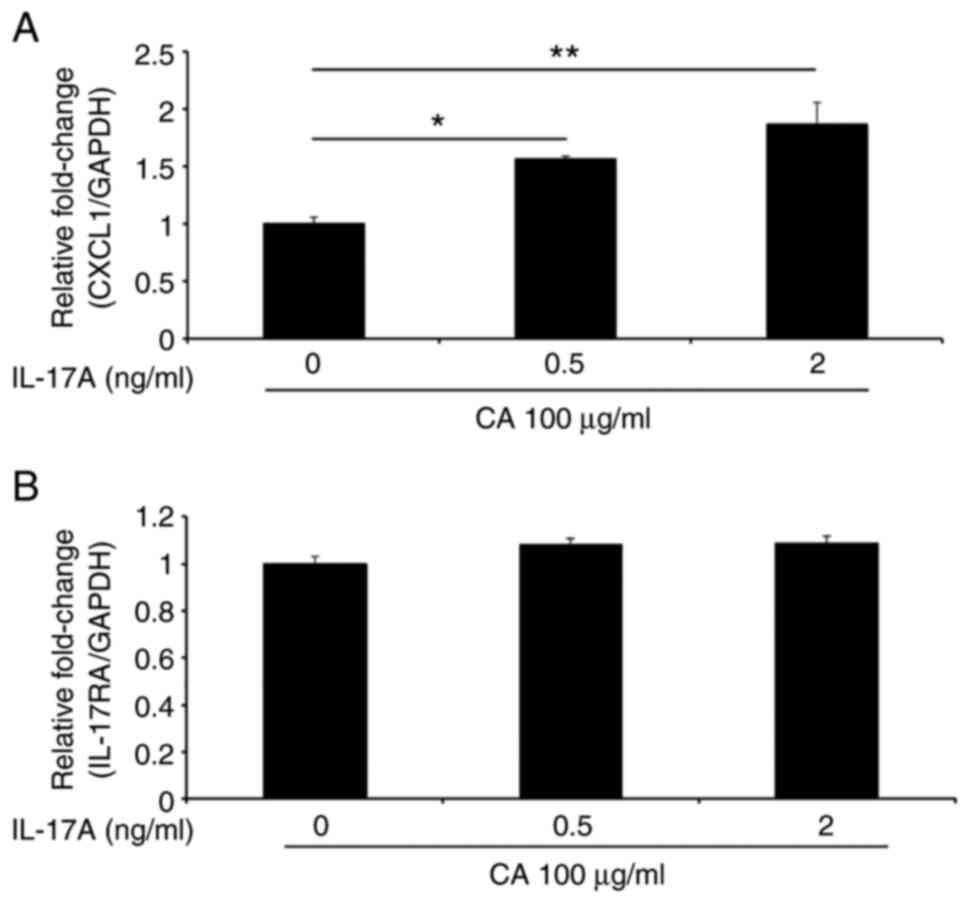
We next examined whether IL-17A, which is the
prototypical member of the IL-17 family, modulates CXCL1 and
IL-17RA mRNA expression in the presence of carrageenan. As
shown in Fig. 3A, CXCL1
expression was increased by carrageenan in a dose-dependent manner.
In contrast, IL-17RA expression was not affected by the
addition of carrageenan (Fig. 3B).
These results suggest that carrageenan modulates IL-17A-mediated
CXCL1 expression in gingival epithelial cells.
Ropinirole inhibits CXCL1 and IL-17RA
expression in gingival epithelial cells in the presence of IL-17A
and carrageenan
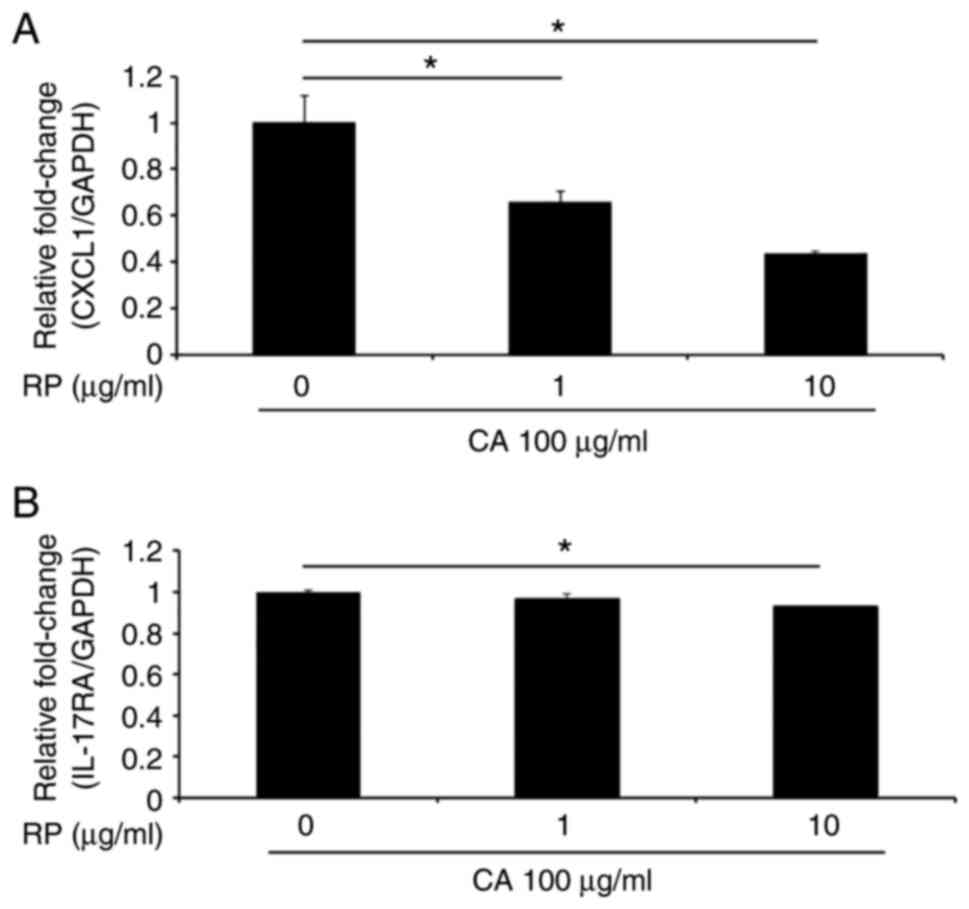
We next examined whether ropinirole, which is a
D2-like receptor agonist, inhibits CXCL1 and IL-17A
mRNA expression in GE1 cells in the presence of carrageenan in
vitro. As expected, CXCL1 expression was significantly
suppressed by the addition of ropinirole in a dose-dependent manner
(Fig. 4A). In addition,
IL-17A expression was significantly suppressed by the
addition of ropinirole at a dose of 10 µg/ml (Fig. 4B).
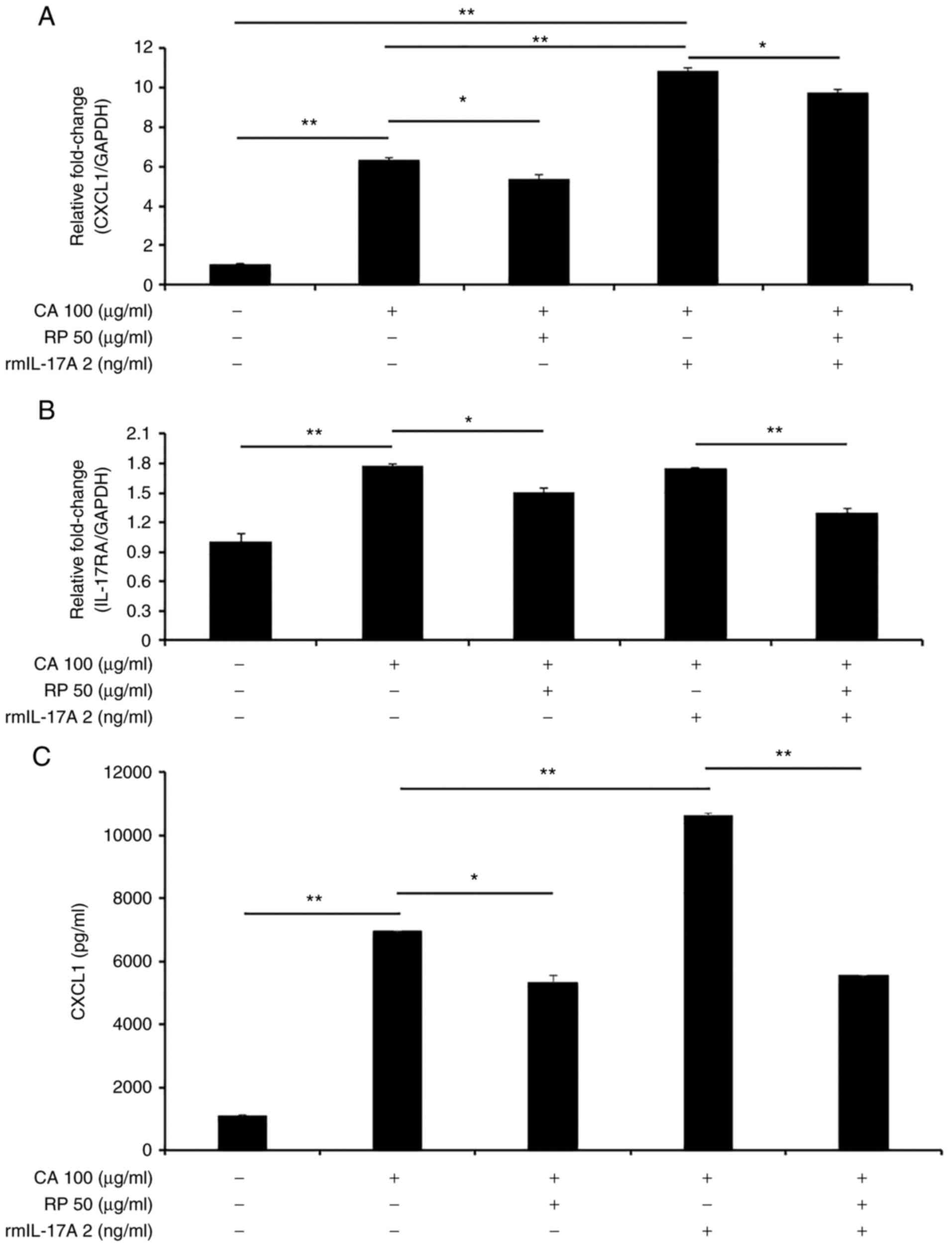
Furthermore, we examined whether ropinirole at a
high dose modulates CXCL1 and IL-17RA mRNA expression
in the presence or absence of IL-17A with or without carrageenan.
Ropinirole at 50 µg/ml in the presence of carrageenan significantly
inhibited the upregulation of CXCL1 and IL-17RA
(Fig. 5A and B). In addition, we confirmed that the
CXCL1 protein level was also suppressed by the addition of
ropinirole in the presence of carrageenan and rmIL-17A (Fig. 5C). These results indicated that
ropinirole inhibits the IL-17A-mediated CXCL1 expression induced by
carrageenan in gingival epithelial cells. We speculate that
carrageenan binds to unknown receptor and subsequently activates
IL-17RA expression, resulting in CXCL1 upregulation (Fig. S4). Thus, carrageenan modulates
IL-17RA as a stimulator.
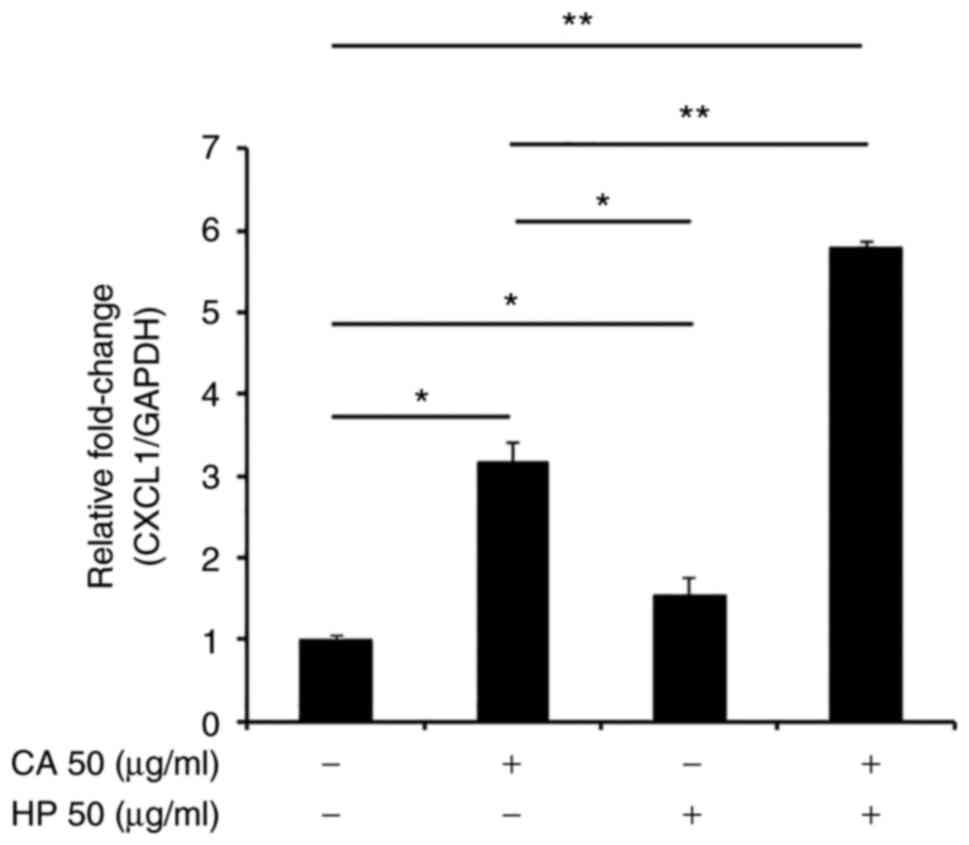
Haloperidol promotes CXCL1 expression
in gingival epithelial cells in the presence of carrageenan
To examine whether the modulation of CXCL1
production by carrageenan is dependent on D2-like receptors, we
lastly explored the effect of haloperidol, which is a D2-like
receptor antagonist, on CXCL1 production. As anticipated,
haloperidol significantly promoted CXCL1 expression
(Fig. 6). This result confirmed
that carrageenan enhances CXCL1 production via D2-like
receptors.
Discussion
Accumulating evidence has suggested that dopamine,
which is produced by dendritic cells, acts on T lymphocytes
(32). We and other researchers
have previously reported the efficacy of agents that target
dopamine receptors via Th17-mediated responses in animal models of
various neutrophilic inflammatory diseases, including experimental
autoimmune encephalomyelitis, type 1 diabetes, nephrotoxic serum
nephritis, colitis, neutrophilic airway inflammation, and
rheumatoid arthritis models (11,14,15,33-35).
Th17-dependent neutrophilic inflammation processes occur in
periodontitis (36). Namely, Th17
cells not only promote IL-8 production in gingival epithelial
cells, but they also play a critical role for bone destruction in
periodontitis (2,37). In the present study, the
inflammatory stimulant IL-17A induced the production of CXCL1 in
gingival epithelial cells, and ropinirole inhibited the
IL-17A-induced CXCL1 production.
Ropinirole has an agonistic effect on D2 receptor.
It is indicated for use in the treatment of early and late
Parkinson's disease (38). The
three cardinal motor signs of Parkinson's disease are akinesia,
rigidity and low-frequency rest tremor, and these symptoms
interfere with the hand movements that control tooth brushing.
According to prior research on periodontitis in patients with
Parkinson's disease, the morbidity of periodontitis is high in
patients with Parkinson's disease, because these patients cannot
brush their teeth well (39-42).
However, little information is available on the prevalence of
periodontitis in patients with Parkinson's disease who are treated
with different medications. For the treatment of Parkinson's
disease, L-dopa and D2 receptor agonists are most commonly
prescribed. Common adverse effects in elderly patients treated with
D2 receptor agonists are delusion and hallucinations. Therefore,
L-dopa is often selected as the first-line drug (43). To clarify the morbidity of
periodontitis in Parkinson's disease, it would be of value to
compare patients who were prescribed L-dopa to those who were
prescribed D2 receptor agonists. We speculate that the application
of a local drug delivery system may be suitable for the treatment
of periodontal tissues and alveolar bone defects.
Two major models for mimicking periodontitis are the
ligature model and the oral gavage with periodontopathogens model.
In the ligature model, ligature induces bacterial colonization and
the accumulation of plaque, leading to epithelial migration and
tissue destruction. In contrast, the oral gavage with
periodontopathogens model involves the inoculation of human
bacterial strains. De Molon et al showed that the ligature
model was more useful than the oral gavage with periodontopathogens
model for long-term experiments (44). However, the ligature model is
technically quite difficult to perform. In contrast, the
carrageenan-induced model, which was proposed by Yamamoto et
al, is technically simple to perform (27). Moreover, the carrageenan-induced
model enables the induction of neutrophilic inflammation, because
carrageenan induces acute inflammation in vivo, and IL-8
production in vitro (30,45).
The present study has several limitations. First,
experiments with the ligature model without carrageenan should be
performed to verify whether ropinirole is effective for
periodontitis. Second, we only tested murine gingival cells in
vitro, and in the future, it will be necessary to perform such
experiments in human gingival epithelial cells to determine whether
similar results can be obtained with human cells. Finally, it
remains unknown whether dopamine receptors are expressed in the
gingival tissues of patients with periodontitis. The significant
issue facing current research is examining dopamine receptors in
human tissues. In addition, it is needed to explore the receptors
which binds to carrageenan.
In conclusion, the results of the present study
suggest that ropinirole suppresses bone destruction in a rat model
of periodontitis. We believe this new finding indicates that
further studies are warranted.
Supplementary Material
Experimental protocol. Rats were
divided into three experimental groups. Each group consisted of two
rats (n=2). PBS group, the silk ligature wire was immersed in PBS
as a control; CA group, the silk ligature wire was immersed in 1%
carrageenan; CA/RP group, the silk ligature wire was immersed in 1%
carrageenan and 10 μg/ml ropinirole. CA, carrageenan; RP,
ropinirole; PBS, phosphate buffered saline.
Three-dimensional micro-CT images. CA,
carrageenan; RP, ropinirole; PBS, phosphate buffered saline.
Method for the measurement of the
perpendicular distance. After making three-dimensional images, the
alveolar bone loss was measured as the length from the connective
line between the cemento-enamel junction (red line) and the
alveolar margin (arrows) on the palatal side of the maxillary
second molar.
Scheme of carrageenan modulation CXCL1
via IL-17RA. CXCL1, C-X-C motif chemokine ligand 1; IL,
interleukin; RA receptor A.
Acknowledgements
The authors thank Mr Rinya Masuko (JEOL Ltd, Tokyo,
Japan.) for guidance regarding micro-CT.
Funding
Funding: This work was supported by a Grant-in-Aid for Young
Researchers from Saitama Medical University Hospital (grant no.
30-E-1-03) and JSPS KAKENHI (grant no. 22K17065).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Author contributions
TS and SM confirm the authenticity of all the raw
data. TS, MU and SM conceived and designed the study. YI, MK and RT
acquired the data. KI and MU contributed to the interpretation of
the results. YI, TS and SM drafted the manuscript. KI, MK and MU
critically revised the manuscript for important intellectual
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Animal
Research Committee of Saitama Medical University (Saitama, Japan;
approval no. 2877).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirschfeld J: Neutrophil subsets in
periodontal health and disease: A mini review. Front Immunol.
10(3001)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Takahashi N, Okui T, Tabeta K and Yamazaki
K: Effect of interleukin-17 on the expression of chemokines in
gingival epithelial cells. Eur J Oral Sci. 119:339–344.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tipton DA, Cho S, Zacharia N and Dabbous
MK: Inhibition of interleukin-17-stimulated interleukin-6 and -8
production by cranberry components in human gingival fibroblasts
and epithelial cells. J Periodontal Res. 48:638–646.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dutzan N and Abusleme L: T helper 17 cells
as pathogenic drivers of periodontitis. Adv Exp Med Biol.
1197:107–117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rot A and von Andrian UH: Chemokines in
innate and adaptive host defense: Basic chemokinese grammar for
immune cells. Ann Rev Immunol. 22:891–928. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lindhe J and Nyman S: Scaling and
granulation tissue removal in periodontal therapy. J Clin
Periodontol. 12:374–388. 1985.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Graziani F, Karapetsa D, Alonso B and
Herrera D: Nonsurgical and surgical treatment of periodontitis: How
many options for one disease? Periodontology. 75:152–188.
2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kitamura M, Akamatsu M, Machigashira M,
Hara Y, Sakagami R, Hirofuji T, Hamachi T, Maeda K, Yokota M, Kido
J, et al: FGF-2 stimulates periodontal regeneration: Results of a
multi-center randomized clinical trial. J Dent Res. 90:35–40.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
da Costa LF, Amaral CD, Barbirato DD, Leão
AT and Fogacci MF: Chlorhexidine mouthwash as an adjunct to
mechanical therapy in chronic periodontitis: A meta-analysis. J Am
Dent Assoc. 148:308–318. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Matesanz-Pérez P, García-Gargallo M,
Figuero E, Bascones-Martínez A, Sanz M and Herrera D: A systematic
review on the effects of local antimicrobials as adjuncts to
subgingival debridement, compared with subgingival debridement
alone, in the treatment of chronic periodontitis. J Clin
Periodontol. 40:227–241. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nakano K, Higashi T, Hashimoto K, Takagi
R, Tanaka Y and Matsushita S: Antagonizing dopamine D1-like
receptor inhibits Th17 cell differentiation: Preventive and
therapeutic effects on experimental autoimmune encephalomyelitis.
Biochem Biophys Res Commun. 373:286–291. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nakano K, Yamaoka K, Hanami K, Saito K,
Sasaguri Y, Yanagihara N, Tanaka S, Katsuki I, Matsushita S and
Tanaka Y: Dopamine induces IL-6-dependent IL-17 production via
D1-like receptor on CD4 naive T cells and D1-like receptor
antagonist SCH-23390 inhibits cartilage destruction in a human
rheumatoid arthritis/SCID mouse chimera model. J Immunol.
186:3745–3752. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Missale C, Nash SR, Robinson SW, Jaber M
and Caron MG: Dopamine receptors: From structure to function.
Physiol Rev. 78:189–225. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Okada H, Inoue T, Hashimoto K, Suzuki H
and Matsushita S: . D1-like receptor antagonist inhibits IL-17
expression and attenuates crescent formation in nephrotoxic serum
nephritis. Am J Nephrol. 30:274–279. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hashimoto K, Inoue T, Higashi T, Takei S,
Awata T, Katayama S, Takagi R, Okada H and Matsushita S: Dopamine
D1-like receptor antagonist, SCH23390, exhibits a preventive effect
on diabetes mellitus that occurs naturally in NOD mice. Biochem
Biophys Res Comm. 383:460–463. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pelletier M, Maggi L, Micheletti A,
Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato
F, Romagnani S and Cassatella MA: Evidence for a cross-talk between
human neutrophils and Th17 cells. Blood. 115:335–343.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matsuyama T, Kawano M, Takagi R, Nakagome
K, Chikamatsu K and Matsushita S: Interleukin-8 produced by T cells
is under the control of dopamine signaling. Clin Exp Neuroimmunol.
9:2571–2257. 2018.
|
|
18
|
Parrado AC, Canellada A, Gentile T and
Rey-Roldán EB: Dopamine agonists upregulate IL-6 and IL-8
production in human keratinocytes. Neuroimmunomodulation.
19:359–366. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kawano M, Saika K, Takagi R, Matsui M and
Matsushita S: Tannic acid acts as an agonist of the dopamine D2L
receptor, regulates immune responses, and ameliorates
experimentally induced colitis in mice. Brain Behav Immun Health.
5(100071)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takagi R, Kawano M, Sato T and Matsushita
S: Tannic acid, a dopamine receptor agonist, ameliorates
periodontitis, atopic dermatitis and psoriasis in animal models.
Curr Trends Immunol. 22:11–17. 2021.
|
|
21
|
Chen S, Zhang X, Yang D, Du Y, Li L, Li X,
Ming M and Le W: D2/D3 receptor agonist ropinirole protects
dopaminergic cell line against rotenone-induced apoptosis through
inhibition of caspase- and JNK-dependent pathways. FEBS Lett.
582:603–610. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hatakeyama S, Ohara-Nemoto Y, Yanai N,
Obinata M, Hayashi S and Satoh M: Establishment of gingival
epithelial cell lines from transgenic mice harboring temperature
sensitive simian virus 40 large T-antigen gene. J Oral Pathol Med.
30:296–304. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kirihara Y, Takechi M, Kurosaki K,
Kobayashi Y, Saito Y and Takeuchi T: Effects of an anesthetic
mixture of medetomidine, midazolam, and butorphanol in rats-strain
difference and antagonism by atipamezole. Exp Anim. 65:27–36.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nakamura T, Ichii O, Irie T, Hosotani M,
Dantsuka A, Nakamura S, Sato S, Sotozaki K, Kouguchi H, Yoshiyasu
T, et al: Usefulness of an anesthetic mixture of medetomidine,
midazolam, and butorphanol in cotton rats (Sigmodn hispidus). Jpn J
Vet Res. 64:273–276. 2016.PubMed/NCBI
|
|
25
|
Bellini L, Banzato T, Contiero B and Zotti
A: Evaluation of three medetomidine-based protocols for chemical
restraint and sedation for non-painful procedures in companion rats
(Rattus norvegicus). Vet J. 200:456–458. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yoshida K, Murakawa M and Hosono A:
Effects of anesthetics on expression of dopamine and acetylcholine
receptors in the rat brain in vivo. J Anesth. 36:436–440.
2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yamamoto H, Yokoyama M, Tamura H, Okumura
S, Kawada E and Kuboyama N: Carrageenin-induced periodontitis as an
experimental model in rats analyzed by micro-computerized
tomography. J Hard Tissue Biol. 20:231–236. 2011.
|
|
28
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mukaida N, Harada A and Matsushima K:
Interleukin-8 (IL-8) and monocyte chemotactic and activating factor
(MCAF/MCP-1), chemokines essentially involved in inflammatory and
immune reactions. Cytokine Growth Factor Rev. 9:9–23.
1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Borthakur A, Bhattacharyya S, Dudeja PK
and Tobacman JK: Carrageenan induces interleukin-8 production
through distinct Bcl10 pathway in normal human colonic epithelial
cells. Am J Physiol Gastrointest Liver Physiol. 292:G829–G838.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mitani A, Niedbala W, Fujimura T, Mogi M,
Miyamae S, Higuchi N, Abe A, Hishikawa T, Mizutani M, Ishihara Y,
et al: Increased expression of interleukin (IL)-35 and IL-17, but
not IL-27, in gingival tissues with chronic periodontitis. J
Periodontol. 86:301–309. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Franco R, Reyes-Resina I and Navarro G:
Dopamine in health and disease: Much more than a neurotransmitter.
Biomedicines. 9(109)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nakagome K, Imamura M, Okada H, Kawahata
K, Inoue T, Hashimoto K, Harada H, Higashi T, Takagi R, Nakano K,
et al: Dopamine D1-like receptor antagonist attenuates
Th17-mediated immune response and ovalbumin antigen-induced
neutrophilic airway inflammation. J Immunol. 186:5975–5982.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu JH, Liu YQ, Deng QW, Peng YP and Qiu
YH: Dopamine D2 receptor is involved in alleviation of type II
collagen-induced arthritis in mice. BioMed Res Int.
2015(496759)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lieberknecht V, Junqueira SC, Cunha MP,
Barbosa TA, de Souza LF, Coelho IS, Santos AR, Rodrigues AL, Dafré
AL and Dutra RC: Pramipexole, a dopamine D2/D3 receptor-preferring
agonist, prevents experimental autoimmune encephalomyelitis
development in mice. Mol Neurobiol. 54:1033–1045. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cheng WC, Hughes FJ and Taams LS: The
presence, function and regulation of IL-17 and Th17 cells in
periodontitis. J Clin Periodontol. 41:541–549. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tsukasaki M, Komatsu N, Nagashima K, Nitta
T, Pluemsakunthai W, Shukunami C, Iwakura Y, Nakashima T, Okamoto K
and Takayanagi H: Host defense against oral microbiota by
bone-damaging T cells. Nat Commun. 9(701)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kuzel MD: Ropinirole: A dopamine agonist
for the treatment of parkinson's disease. Am J Health Syst Pharm.
56:217–224. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pradeep AR, Singh SP, Martande SS, Raju
AP, Rustagi T, Suke DK and Naik SB: Clinical evaluation of the
periodontal health condition and oral health awareness in
parkinson's disease patients. Gerodontology. 32:100–106.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schwarz J, Heimhilger E and Storch A:
Increased periodontal pathology in parkinson's disease. J Neurol.
253:608–611. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Einarsdóttir ER, Gunnsteinsdóttir H,
Hallsdóttir MH, Sveinsson S, Jónsdóttir SR, Olafsson VG, Bragason
TH, Saemundsson SR and Holbrook WP: Dental health of patients with
parkinson's disease in Iceland. Spec Care Dentist. 29:123–127.
2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hanaoka A and Kashihara K: Increased
frequencies of caries, periodontal disease and tooth loss in
patients with parkinson's disease. J Clin Neurosci. 16:1279–1282.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Armstrong MJ and Okun MS: Diagnosis and
treatment of parkinson disease: A review. JAMA. 323:548–560.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
de Molon RS, Mascarenhas VI, de Avila ED,
Finoti LS, Toffoli GB, Spolidorio DM, Scarel-Caminaga RM, Tetradis
S and Cirelli JA: Long-term evaluation of oral gavage with
periodontopathogens or ligature induction of experimental
periodontal disease in mice. Clin Oral Invest. 20:1203–1216.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Barth CR, Funchal GA, Luft C, de Oliveira
JR, Porto BN and Donadio MV: Carrageenan-induced inflammation
promotes ROS generation and neutrophil extracellular trap formation
in a mouse model of peritonitis. Eur J Immunol. 46:964–970.
2016.PubMed/NCBI View Article : Google Scholar
|