Introduction
Peripheral nerve injury causes a loss of sensory and
motor function, and has a significant impact on the quality of life
(1). In the United States alone,
>200,000 nerve repair treatments are performed each year
(2). For short lengths of nerve
damage, the conventional clinical procedure, end-to-end
anastomosis, is appropriate (3).
If the damaged nerve is too long to be repaired with tension-free
sutures, autologous transplantation can be used for nerve
reconstruction, which is considered the 'gold standard' of
contemporary nerve repair treatment (4). In recent decades, several
configurations of potential autografts for nerve conduits have been
investigated (5). Despite recent
advances in the understanding of nerve regeneration and surgical
techniques, complete functional repair of damaged nerves is rarely
achieved (6).
Pericytes, which promote vessel growth and maintain
the neurovascular unit, are thought to be progenitor cells and may
play a role in neurovascular regeneration (7). It has also been proposed that
peripheral nerve pericytes may stimulate axon regeneration and
barrier enhancement, preventing neuronal loss in the central
nervous system (8,9). Degeneration of pericytes has been
observed in diseases related to neurovascular dysfunction, such as
dementia and Alzheimer's disease (10). Our previous study showed that
pericytes enhanced neurovascular regeneration in a mouse model of
cavernous nerve injury (11).
Several researchers have demonstrated that
extracellular vesicles (EVs) incorporate proteins, lipids, and RNA,
which are involved in physiological and pathological communications
between cells (12,13). Previous studies have shown that
EVs from particular cell types and environments can affect tissue
regeneration (14,15). However, one of the most
significant disadvantages of EVs is their poor production yield
(16). Therefore, to overcome
this limitation, a mini extruder system was developed, and
>100-fold greater EV-mimetic NVs were extracted from different
cell types, such as embryonic stem cells (ESCs) and mouse cavernous
pericytes (MCPs). In addition, ESC-derived EV-mimetic NVs (ESC-NVs)
and MCP-derived EV-mimetic NVs (PC-NVs) showed similar qualities to
natural EVs, thus might be helpful for studies on neurovascular
regeneration (17,18). However, the detailed molecular
mechanisms by which PC-NVs promote nerve regeneration remains
largely unknown.
The sciatic nerve is the longest nerve in the human
body. It is composed of motor and sensory fibers, and has been used
as a model of neurovascular regeneration. Regeneration of severed
nerves requires polarized vasculature induced by macrophages in the
hypoxic bridge to guide the collective migration of Schwann cells
(19). Our recent studies showed
that ESC-NVs could rescue erectile function in diabetic mice,
whereas PC-NVs significantly improved erectile dysfunction in a
mouse cavernous nerve injury (CNI) model (17,18). However, there are no studies
assessing the functional evaluation of PC-NVs in the peripheral
nervous system (PNS), to the best of our knowledge. Therefore, it
was hypothesized that PC-NVs may also induce peripheral nervous
regeneration through these aforementioned effects, including
macrophage-induced angiogenesis to guide Schwann cell
migration.
The aim of the present study was to evaluate the
role of PC-NVs in peripheral nerve regeneration. The results showed
that the exogenous delivery of PC-NVs increased the content of
endothelial cells, macrophages, Schwann cells and neuronal cells in
the sciatic nerve transection (SNT) model, thereby inducing
neurovascular regeneration. Therefore, we hypothesize that PC-NVs
may display a protective effect and accelerate nerve recovery,
thereby improving damaged nerve function.
Materials and methods
Ethics statement and animal study
design
In total, 85 adult male C57BL/6J mice (8 weeks old;
weight, 20-25 g; Orient Bio, Inc.) were used in the present study:
10 for the MCP primary culture and PC-NV isolation, 45 for mouse
sciatic nerve neurovascular regeneration and pathway signaling
experiments, and 30 for the motor function recovery test. All
animals' health and behavior were monitored every day and
experiments performed in this study were approved by the
Institutional Animal Care and Use Committee of Inha University
(approval no. 171129-527). Mice were fed with commercial standard
laboratory food and water ad libitum, and maintained at room
temperature (23±2°C) with 40-60% humidity, specific pathogen-free
conditions and 12-h light/dark cycles. The cage, bedding, food and
water were strictly disinfected. All animals were placed under
general anesthesia with intramuscular injections of ketamine (100
mg/kg) and xylazine (5 mg/kg) before preparing the models. All
animals were euthanized by 100% CO2 gas replacement rate
at 10-30% container volume/min, and cessation of the heartbeat and
respiratory arrest were confirmed before harvesting tissues. No
mice were found dead during any of the experimental procedures
MCP culture, preparation and
characterization of PC-NVs
The MCP primary cultures were performed as described
previously (20). Briefly,
8-week-old male C57BL/6J mice were euthanized immediately, the
urethra and dorsal neurovascular bundle were removed from the penis
tissue and only the corpus cavernosum tissues were used. The corpus
cavernosum tissues were sectioned into 1-2 mm sections and cultured
at 37°C with 5% CO2 in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 20% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 1% penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) and 10 nM human pigment epithelium-derived factor
(Sigma-Aldrich; Merck KGaA). The medium was changed every 2 days,
and the cells were sub-cultured after 10 days. Cells from passages
2-4 were used for all of the experiments.
As described in our previous study, a mini extruder
system (Avanti Polar Lipids, Inc.) was used to prepare PC-NVs
(17). Briefly, MCPs were
detached with 0.25% trypsin-EDTA (Invitrogen; Thermo Fisher
Scientific, Inc.) and re-suspended in HEPES buffer solution (HBS;
Gibco; Thermo Fisher Scientific, Inc.). The cell suspension was
extruded 10 times through filters with 10, 5 and 1 µm
polycarbonate membranes (Nuclepore; Whatman plc; Cytiva). Next,
they were subjected to ultracentrifugation at 100,000 × g for 2 h
at 4°C with a step gradient, which was overlapped with 50%
iodixanol (1 ml; Axis-Shield Diagnostics, Ltd.) and 10% iodixanol
(2 ml) and the extruded samples were placed (7 ml) on top. The
PC-NVs were filtered through a 0.45 µm filter (Altmann
Analytik Gmbh & Co. KG) and stored at -80°C until subsequent
analysis. The EXOCET exosome quantitation analysis kit (System
Biosciences, LLC) was used to quantify the PC-NVs, and their
concentration was adjusted to 1 µg/µl.
The morphology of the PC-NVs was detected by
transmission electron microscopy (TEM; Electron Microscopy
Sciences) as described previously (17). The PC-NVs were verified by
determining the expression of one negative and three positive NV
markers by western blotting (according to the western blotting
protocol): Golgi matrix protein 130 (GM130; negative marker; cat.
no. 610822; 1:1,000; BD Biosciences), programmed cell death
6-interacting protein (Alix; positive marker; cat. no. NB100-65678;
1:1,000; Novus Biologicals, LLC), tumor susceptibility 101 (TSG101;
positive marker; cat. no. NB200-112; 1:1,000, Novus Biologicals,
LLC) and CD81 (cat. no. NBP1-77039; positive marker; 1:1,000; Novus
Biologicals, LLC).
Fluorescence dye labelling of the PC-NVs
for the tracking analysis
The PC-NVs were labelled with
1,1′-Dioctadecyl-3,3,3′,3′-Tetramethyl indodicarbo cyanine,
4-Chlorobenzene sulfonate salt (DiD) red-fluorescent dye (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Briefly, 2.5 µl DiD dye solution was added to
100 µg PC-NVs in a total volume of 500 µl HBS,
incubated at room temperature for 10 min and then further diluted
with 9.5 ml cold HBS. The samples were then ultracentrifuged at
100,000 × g for 2 h at 4°C. Next, the PC-NVs pellet in HBS were
centrifuged again at 100,000 × g for 2 h at 4°C to remove the free
DiD dye. The particles containing the DiD-labeled PC-NVs were then
resuspended in HBS and used for tracking analysis.
Establishment of the sciatic nerve
crushing (SNC) and SNT model
In order to evaluate the neurovascular regenerative
ability of the PC-NVs, the SNC and SNT model were prepared as
described previously (21,22). The SNC model was used for the
tracking analysis of the injected PC-NVs. The 8-week-old male
C57BL/6J mice were immediately placed under general anesthesia with
intramuscular injections of ketamine (100 mg/kg) and xylazine (5
mg/kg). The right hind paw sciatic nerves were exposed and crushed
with a non-serrated needle holder under uniform pressure for 30
sec. After suturing the muscle layer, DiD-labeled PC-NVs (5
µg/100 µl) were immediately injected around the
crushed sciatic nerve, and the skin layer was sutured. A total of
24 h after SNC model preparation, the mice from all groups were
euthanized and the nerves were harvested after the confirming a
lack of heartbeat and respiratory arrest. The nerves were fixed in
4% paraformaldehyde overnight at 4°C. After fixing, a longitudinal
section (15 µm) was prepared and the DiD-labeled PC-NVs were
observed under a confocal fluorescence microscope (K1-Fluo;
Nanoscope Systems, Inc.).
The SNT model was used to study the sciatic nerve
function index, as well for immunofluorescence and western blot
experiments. The surgical procedure was the same as that of the SNC
model. The 8-week-old male C57BL/6J mice were immediately
anesthetized, and the right hind paw sciatic nerve was exposed and
cut across the middle of the thigh with scissors. After suturing
the muscle layer, HBS or PC-NVs (1 or 5 µg, respectively)
was immediately injected around the SNT and the skin layer was
sutured. A total of 5 and 14 days after SNT model preparation, the
mice from all of the groups were euthanized, and the nerves were
harvested after confirmation of a lack of heartbeat and respiratory
arrest. The nerves were fixed in 4% paraformaldehyde overnight at
4°C and a longitudinal section (15 µm) was prepared for
subsequent experiments. A phase contrast image of the sciatic
nerves was observed under a confocal fluorescence microscope.
Histological examination
For fluorescence microscopy, the sciatic nerve
tissues were fixed in 4% paraformaldehyde for 24 h at 4°C. After
blocking with 1% BSA (cat. no. A3294-50G; Sigma-Aldrich; Merck
KGaA) for 1 h at room temperature, the frozen tissue sections (15
µm) were incubated with antibodies against CD31 (1:50; cat.
no. MAB1398Z; MilliporeSigma), neurofilaments (NF; 1:100; cat. no.
N-5389; Sigma-Aldrich; Merck KGaA), glial fibrillary acidic protein
(GFAP; 1:100; cat. no. ab53554; Abcam), ionized calcium-binding
adapter molecule 1 (Iba-1; 1:100; cat. no. ab5076; Abcam) and
neural specific 10 (SCG10; 1:100; cat. no. NBP1-49461; Novus
Biologicals, LLC) at 4°C overnight. After several washes with PBS,
the sections were incubated with secondary antibodies against FITC
Affinipure Goat anti-armenian hamster IgG (H+L) (1:100; cat. no.
127-095-160; Jackson ImmunoResearch Laboratories, Inc.), rhodamine
(TRITC) Affinipure Rabbit anti-mouse IgG (H+L) (1:100; cat. no.
315-025-003; Jackson ImmunoResearch Laboratories, Inc.), Alexa
Fluor 488 affinipure Donkey anti-Goat IgG (H+L) (1:100; cat. no.
705-545-147; Jackson ImmunoResearch Laboratories, Inc.), Donkey
anti-Rabbit IgG H&L (DyLight® 550) (1:100; cat. no.
ab98489; Abcam) for 2 h at room temperature. After washing with
PBS, the samples were mounted in a solution containing DAPI (100
µl; cat. no. H-1500; Vector Laboratories, Inc.). DAPI was
used for nuclei labeling for 2 min at room temperature. The signals
were visualized and digital images were obtained using a confocal
fluorescence microscope (K1-Fluo; Nanoscope Systems, Inc.).
Quantitative analysis of the histological examinations was
performed using ImageJ version 1.34 (National Institutes of
Health).
Rotarod test
The rotarod test was performed on a rotarod machine
with automatic timers and falling sensors (cat. no. JD-A-07TS;
Jeung-Do Bio & Plant Co., Ltd.) as described previously
(23). The 8-week-old male
C57BL/6J mice were allowed to acclimate to the test room for 30
min. The mice were trained on the rotarod for 5 min for 3
consecutive days under the following conditions: Initially set the
rod to a low speed of 4 rpm, and then increased to 12 rpm in 1 min.
A total of 3 days later, SNT model preparation and PC-NV injections
were performed. The rotarod test was performed from 2 weeks after
SNT model preparation under the following conditions: The rotarod
was set to accelerate mode at a speed of 4-50 rpm for a maximum of
5 min. The machine automatically recorded the time and latency of
each test mouse before it fell. The longest falling latency of
three test trials was selected to represent the motor function of
each mouse. Rotarod test results were recorded at 2, 4, 6 and 8
weeks after the sciatic nerve injury. At 2 weeks after the final
rotarod test, the mice from all of the groups were euthanized.
Walking track analysis
Motor function recovery was evaluated by calculating
the sciatic nerve function index (SFI). Walking track analysis was
carried out by using open narrow corridors, and the assessment of
gait in this model has been widely used as previously described
(24). The walking track
analysis was performed immediately after the rotarod test (2, 4, 6
and 8 weeks after the sciatic nerve injury). Briefly, mouse
footprints were obtained by painting the hind paws and then letting
the mouse walk along a 5×50 cm narrow corridor covered with paper.
Based on previous a study (25),
the tracks were evaluated according to three different parameters:
i) Toe spread (TS), the distance between the first toe and the
fifth toes; ii) intermediate toe spread (IT), the distance between
the second, third and fourth toes; and iv) print length (PL), the
distance between the third toe and the hind pad. The measurements
were taken on both the experimental (E) and normal control side
(N). SFI was calculated using the following formula:
-38.3[(EPL-NPL)/NPL]+ 109.5[(ETS-NTS)/NTS]+13.3[(EIT-NIT)/NIT]-8.8
(26). An SFI value close to 0
means normal nerve function, and a value close to -100 means
complete dysfunction. All tracks were analyzed by an experimenter
blinded to the group assignments. Pawprints were recorded at 2, 4,
6 and 8 weeks after the sciatic nerve injury. At 2 weeks after the
final pawprint recordings, the mice from all of the groups were
euthanized.
Western blot
The sciatic nerve tissues (from the cut site and at
the area located 5 mm distal or proximal to the cut site) were
harvested for western blot analysis (n=4). The sciatic nerve
tissues were lysed in RIPA buffer (Sigma-Aldrich; Merck KGaA)
supplemented with protease inhibitors (GenDEPOT, LLC) and
phosphatase inhibitors (GenDEPOT, LLC). Equal amounts of protein
(30 µg per lane) were subjected 8-15% SDS-PAGE and then
transferred to PVDF membranes. After blocking with 5% non-fat dry
milk for 1.5 h at room temperature, the membranes were incubated at
room temperature with antibodies against brain-derived nerve growth
factor (BDNF; 1:500; cat. no. sc-546; Santa Cruz Biotechnology,
Inc.), nerve growth factor (NGF; 1:500; cat. no. sc-548; Santa Cruz
Biotechnology, Inc.), neurotrophin-3 (NT-3; 1:500; cat. no. sc-547;
Santa Cruz Biotechnology, Inc.), phosphorylated (p)-PI3K (1:500;
cat. no. 4228; Cell Signaling Technology, Inc.), total (t)-PI3K
(1:1,000; cat. no. 4292; Cell Signaling Technology, Inc.), p-Akt
(1:500; cat. no 9271; Cell Signaling Technology, Inc.), t-Akt
(1:1,000; cat. no. 9272; Cell Signaling Technology, Inc.), p-c-JUN
(1:500; cat. no. 9261; Cell Signaling Technology, Inc.), t-c-JUN
(1:1,000; cat. no. 9165; Cell Signaling Technology, Inc.),
p-SAPK/JNK (1:500; cat. no. 9251; Cell Signaling Technology, Inc.),
t-JNK (1:1,000; cat. no. 9252; Cell Signaling Technology, Inc.) and
β-actin (1:2,000; cat. no. ab16051; Abcam) for 2 h. The membranes
were washed three times for 10 min with PBST (0.1% Tween-20) at
room temperature. Subsequently, the membranes were incubated with
goat anti-rabbit IgG H&L (HRP) (1:1,000; cat. no. ab6721;
Abcam) and goat anti-mouse IgG H&L (HRP) (1:1,000; cat. no.
ab6789; Abcam) secondary antibodies for 2 h at room temperature.
The signals were visualized using an ECL (Amersham Pharmacia
Biotech, Inc.) detection system. The results were quantified by
densitometry analysis with ImageJ.
Statistical analysis
The data are expressed as the mean ± SEM of at least
four independent experiments. Statistical analyses were performed
using one-way ANOVA followed by Tukey's post hoc test (Figs. 1-8) or an unpaired Student's t-test
(Fig. 9) in GraphPad Prism
version 8 (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
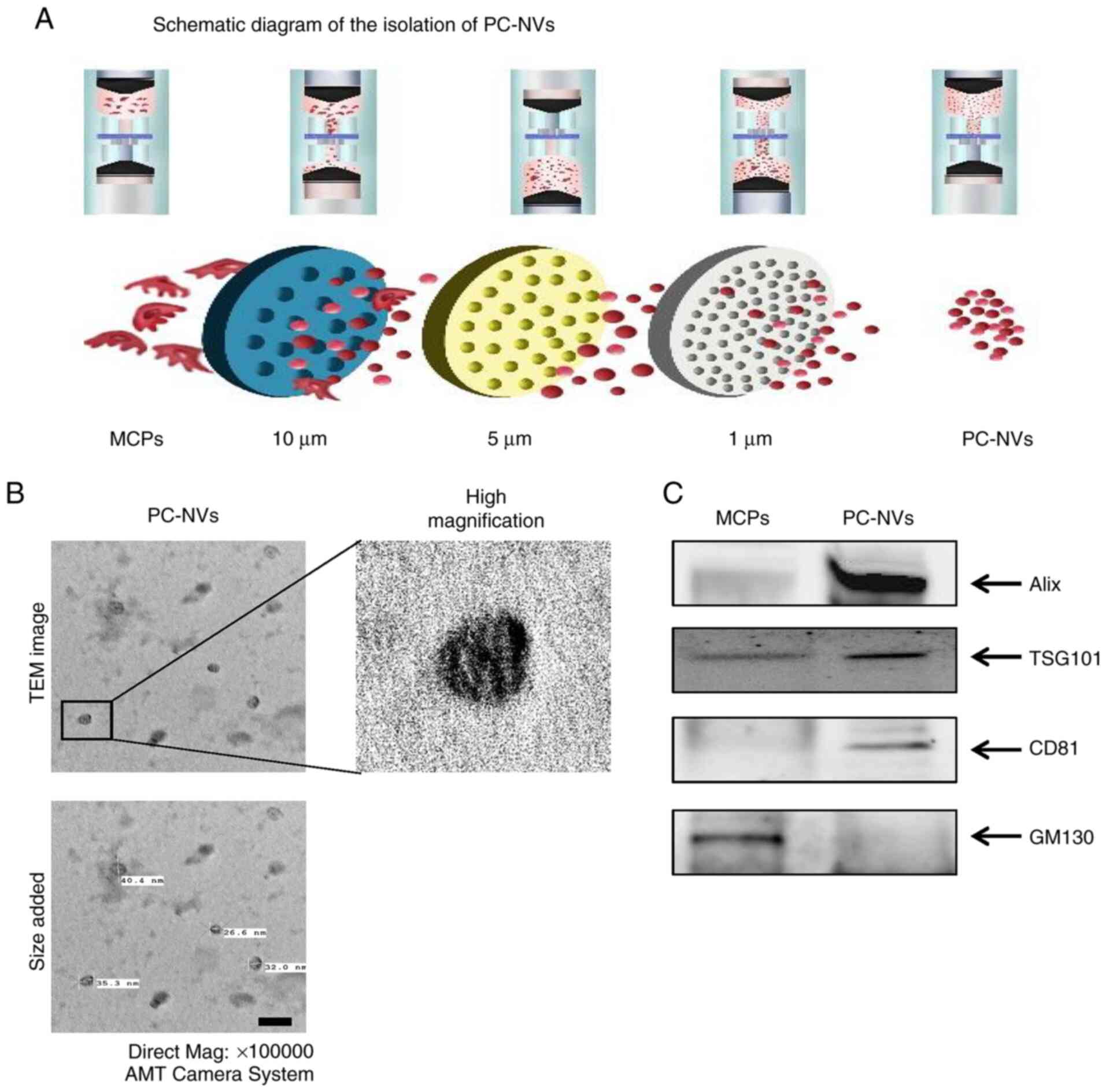 | Figure 1PC-NV isolation and characterization.
(A) Schematic diagram of the isolation of PC-NVs. (B)
Representative TEM phase images of PC-NVs. Magnification, ×100,000.
(C) Representative western blots for EV-positive markers (Alix,
TSG101 and CD81) and an EV-negative marker (GM130) in the MCP cell
lysates and PC-NVs. EV, extracellular vesicle; MCP, mouse cavernous
pericyte; PC-NV, pericyte-derived EV-mimetic nanovesicles; TEM,
transmission electron micrograph; AMT, Advanced Microscopy
Techniques; Alix, programmed cell death 6-interacting protein;
TSG101, tumor susceptibility 101; GM130, golgi matrix protein
130. |
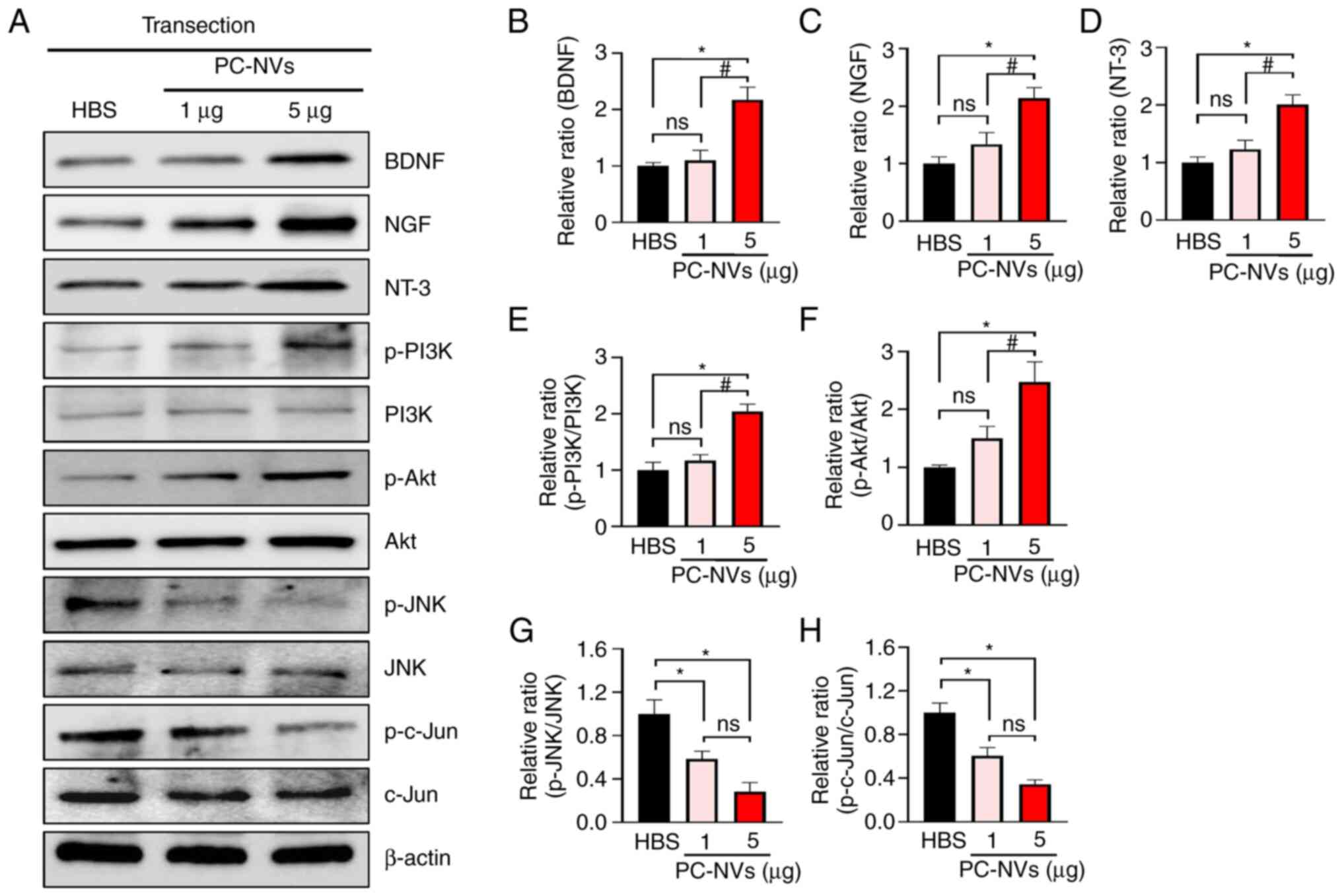 | Figure 8PC-NVs induce neurovascular
regeneration by enhancing NF expression and survival signaling in
SNT model mice. (A) Representative western blot for NFs (BDNF, NT-3
and NGF), survival signaling (activation of PI3K and Akt) and cell
death signaling (activation of JNK and c-Jun) in sciatic nerve
tissue from SNT model mice at 5 days after injection with HBS or
PC-NVs (1 and 5 µg). Data are presented as the relative
density of (B) BDNF, (C) NGF, (D) NT-3, (E) p-PI3K/PI3K, (F)
p-Akt/Akt, (G) p-JNK/JNK and (H) p-c-Jun/c-Jun, with β-actin as the
loading control. Each bar depicts the mean ± SEM of four repeats.
The relative ratio of the HBS group was arbitrarily set to 1.
*P<0.05 vs. HBS group; #P<0.05 vs.
PC-NVs (1 µg) group. ns, not significant. HBS, HEPES buffer
solution; PC-NV, pericyte-derived extracellular vesicle-mimetic
nanovesicle; SNT, sciatic nerve transection; NF, neurotrophic
factor; BDNF, brain-derived nerve growth factor; NT-3,
neurotrophin-3; NGF, nerve growth factor. |
Results
PC-NV measurements
PC-NVs were extracted from MCPs according to
previous methods (17) and a
schematic overview of the experimental procedure is shown in
Fig. 1A. The PC-NVs exhibited a
unique cup-shaped form, similar to the shape of endogenous EVs seen
on TEM (Fig. 1B). The diameter
of the purified PC-NVs was ~30 nm. As previous studies have shown,
the NVs also expressed EV surface markers, such as Alix, TSG101 and
CD81 (17,18). Therefore, the extracted PC-NVs
were characterized by western blot analysis. The expression of
EV-positive markers, such as Alix, TSG101 and CD81, was higher in
PC-NVs than in the PC lysate. Conversely, the expression of the
EV-negative marker, GM130, was lower in the PC-NVs than in the PC
lysates (Fig. 1C).
PC-NVs tracking in the SNC model
In order to determine the distribution of
exogenously injected PC-NVs, DiD was used to label the PC-NVs,
which were then injected into the SNC model mice. After 24 h,
PC-NVs labelled with DiD were primarily detected in the crush site,
and only partially detected in the proximal and distal areas
(Fig. 2). This data indicated
that PC-NVs were more preferably absorbed by injured tissues.
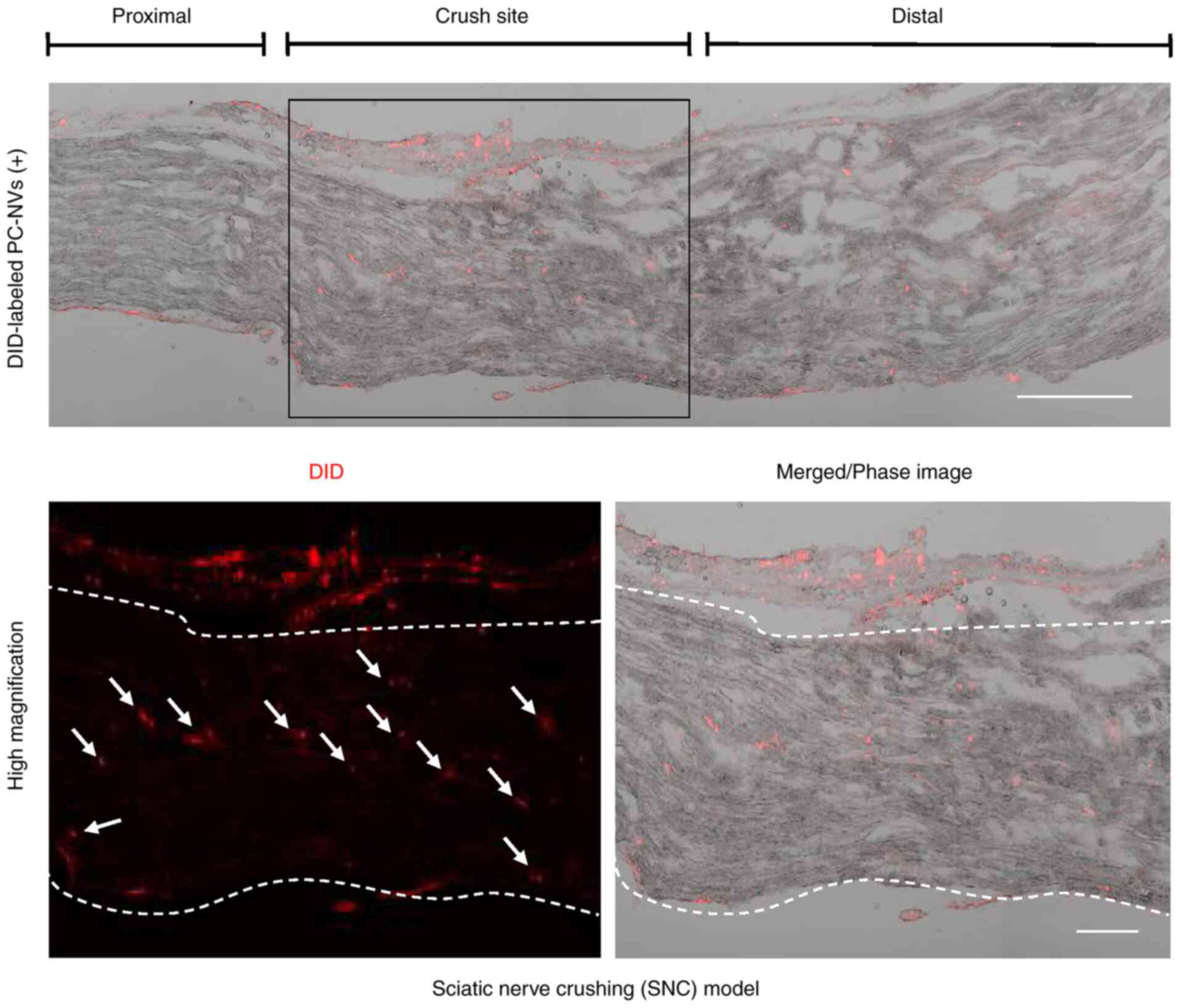 | Figure 2Tracking analysis of DiD-red
fluorescently labeled PC-NVs in SNC model mice. DiD-labeled PC-NVs
were injected around the crushed sciatic nerve for 24 h.
Representative phase images of mice sciatic nerve longitudinal
sections showed that DiD-labeled PC-NVs were detected in the
crushed site of the SNC model mice. White arrows indicate the
absorbed PC-NVs. Scale bar in upper image, 200 µm. Scale bar
in lower image, 100 µm. PC-NV, pericyte-derived
extracellular vesicle-mimetic nanovesicle; SNC, sciatic nerve
crushing; DiD,
1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindodicarbocyanine,
4-Chlorobenzenesulfonate salt. |
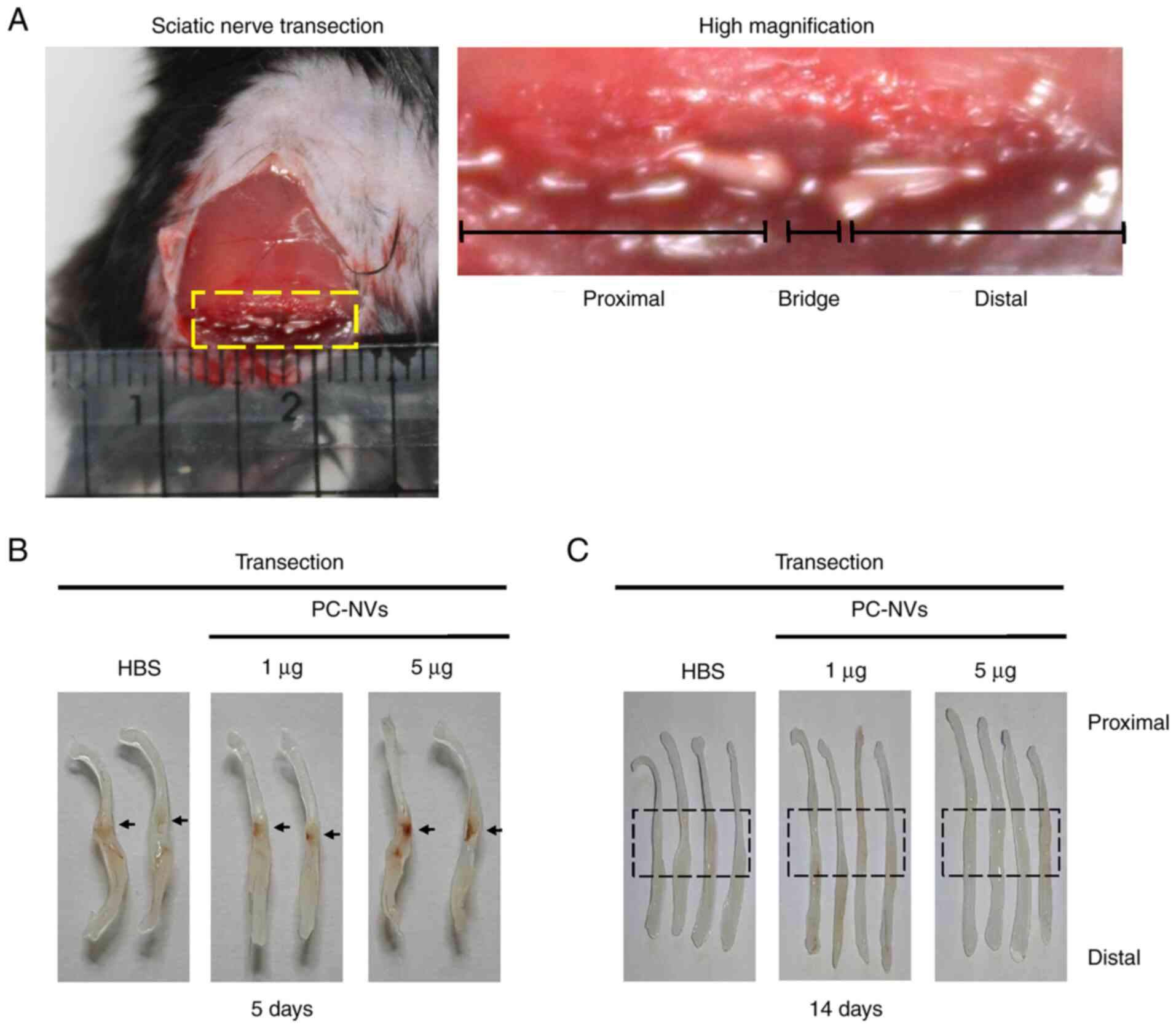
PC-NVs promote neurovascular regeneration
in the SNT model
Given that PC-NVs play critical roles in cavernous
nerve injury induced erectile dysfunction (18), we hypothesized that the PC-NVs
could also influence neurovascular regeneration in peripheral
nerves, such as the sciatic nerve. In order to determine the effect
of PC-NVs on sciatic nerve regeneration, SNT was performed and
PC-NVs (1 and 5 µg) were injected after the transection
surgery (Fig. 3A). At day 5,
phase images of the sciatic nerve showed that the high-dose of
PC-NVs (5 µg) group transection region contained a notable
influx of blood vessels compared with the HBS group. Interestingly,
the number of blood vessels increased markedly at the proximal
stump rather than the distal stump (Fig. 3B). At day 14, phase images of the
sciatic nerves showed that, compared with the HBS group, the
sciatic nerve link in the high-dose of PC-NVs (5 µg) group
appeared to be closer and stronger (Fig. 3C).
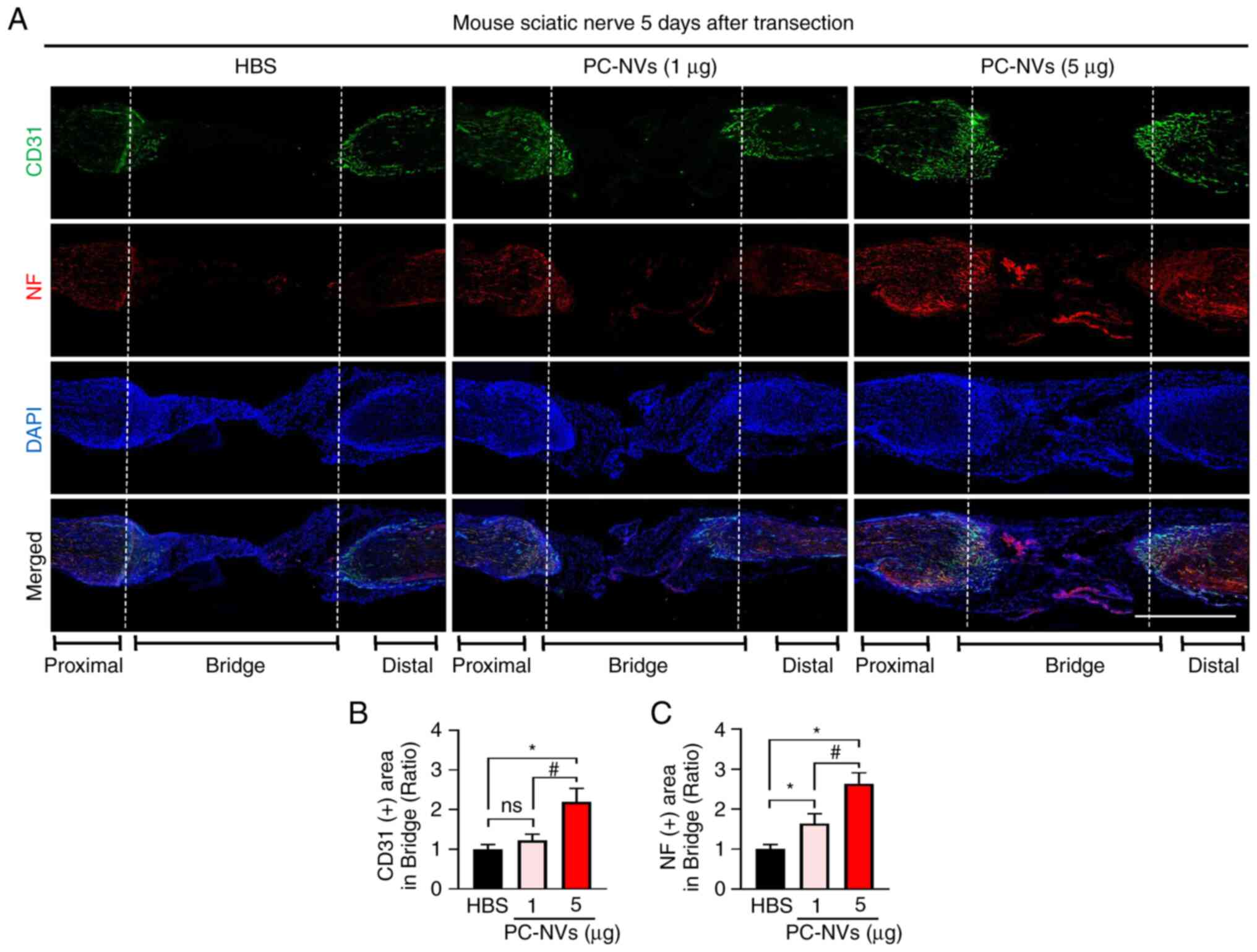
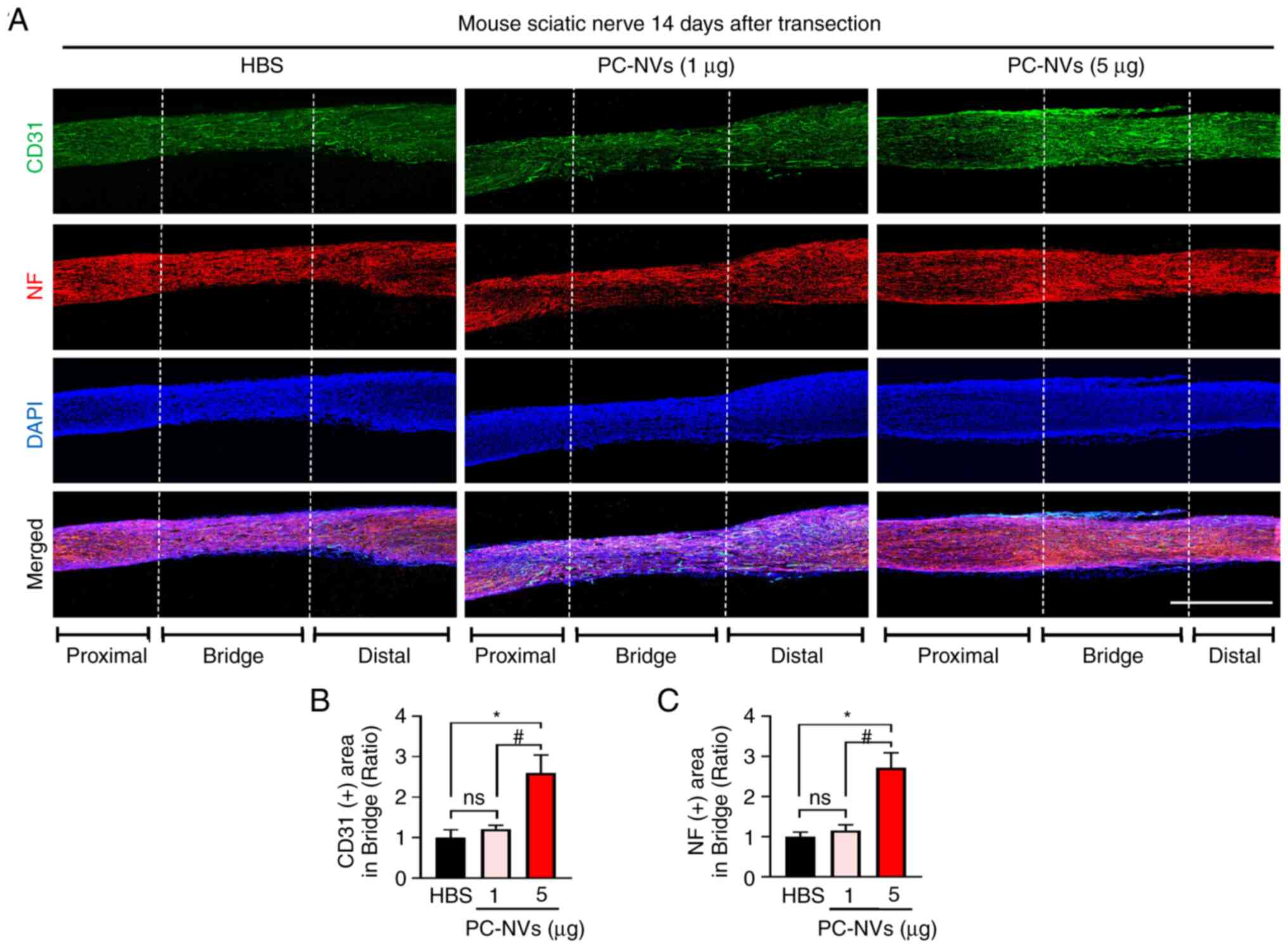
In addition, consistent with the sciatic nerve phase
images, immunofluorescence staining of the sciatic nerve with CD31
(an endothelial cell marker) and NF (an axonal marker) revealed
that the high-dose of PC-NVs significantly induced the endothelial
cells and axonal content compared with the HBS group, which may
have led to the sciatic nerve becoming more vascularized. A low
dose of PC-NVs (1 µg) injection showed a partial effect on
sciatic nerve neurovascular regeneration (Fig. 4). At day 14, the CD31 and NF
immunofluorescence staining showed that the blood vessels and axons
succeeded in crossing the bridged part in the PC-NVs (5 µg)
group (Fig. 5). Taken together,
these data showed that PC-NVs promoted neurovascular regeneration
in the SNT model.
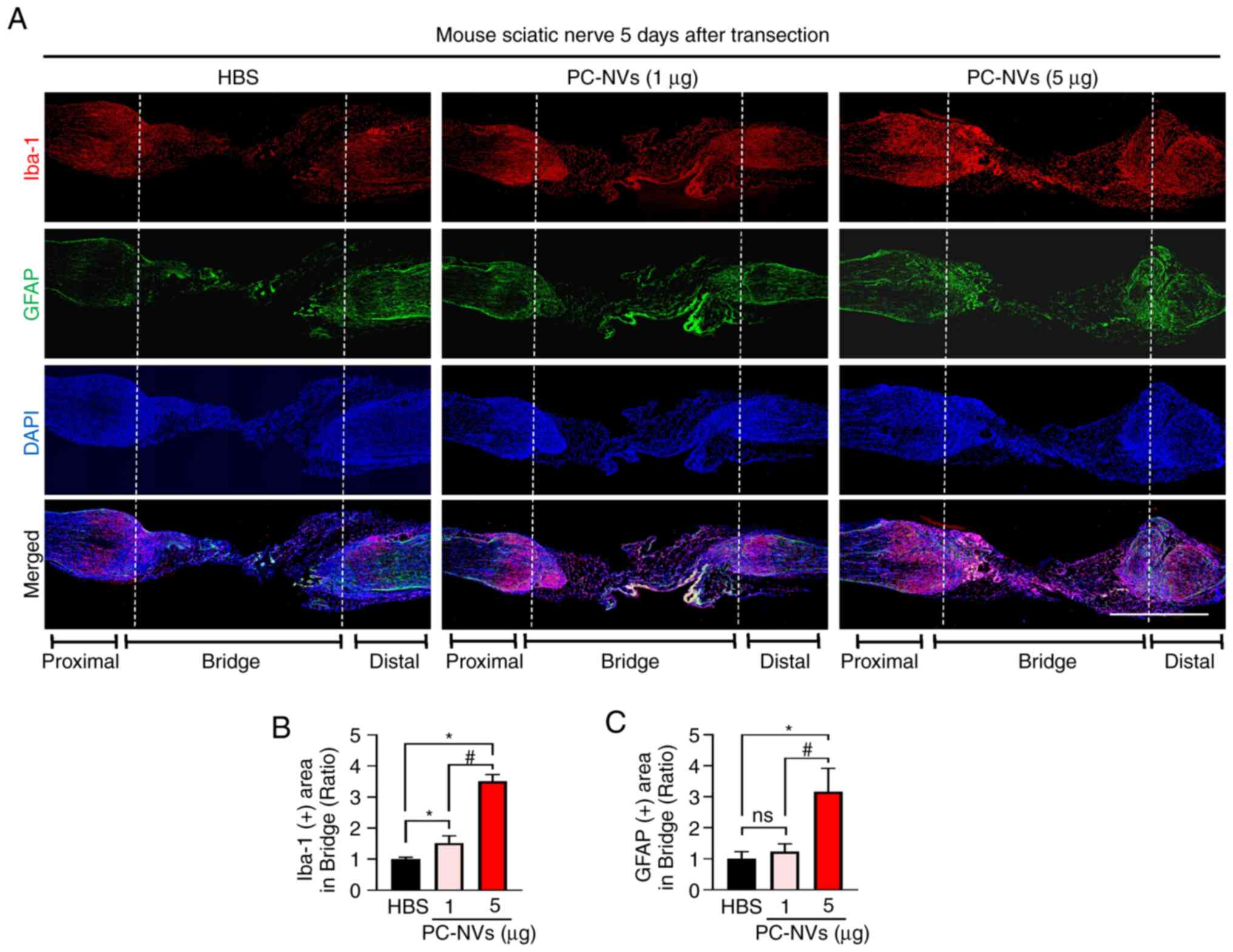
PC-NVs promote macrophage and Schwann
cell content in the SNT model mice
During the regeneration of the sciatic nerve,
macrophages play an important role in inducing the polarized
vasculature in the nerve bridge and in guiding the migration of
Schwann cells during (19,21). To determine whether PC-NVs could
regulate the expression of macrophages to promote sciatic nerve
regeneration, immunofluorescence staining of the sciatic nerve with
Iba-1 (a macrophage marker) and GFAP (a Schwann cell marker) was
performed. The results showed that the high-dose of PC-NVs
significantly increased the presence of macrophages and Schwann
cells compared with the HBS group. Injection of a low dose of
PC-NVs only resulted in a significant increase in the presence of
macrophages, but not Schwann cells. In addition, both Iba-1 and
GFAP expression levels were high in the proximal transected site,
but not the distal site in the high-dose PC-NVs treatment group
(Fig. 6). These data are
consistent with previous studies showing (19,27) that the increased presence of
macrophages may provide signals to direct the movement of Schwann
cells to assist them in crossing the gap left by the wound during
the early stages of sciatic nerve regeneration.
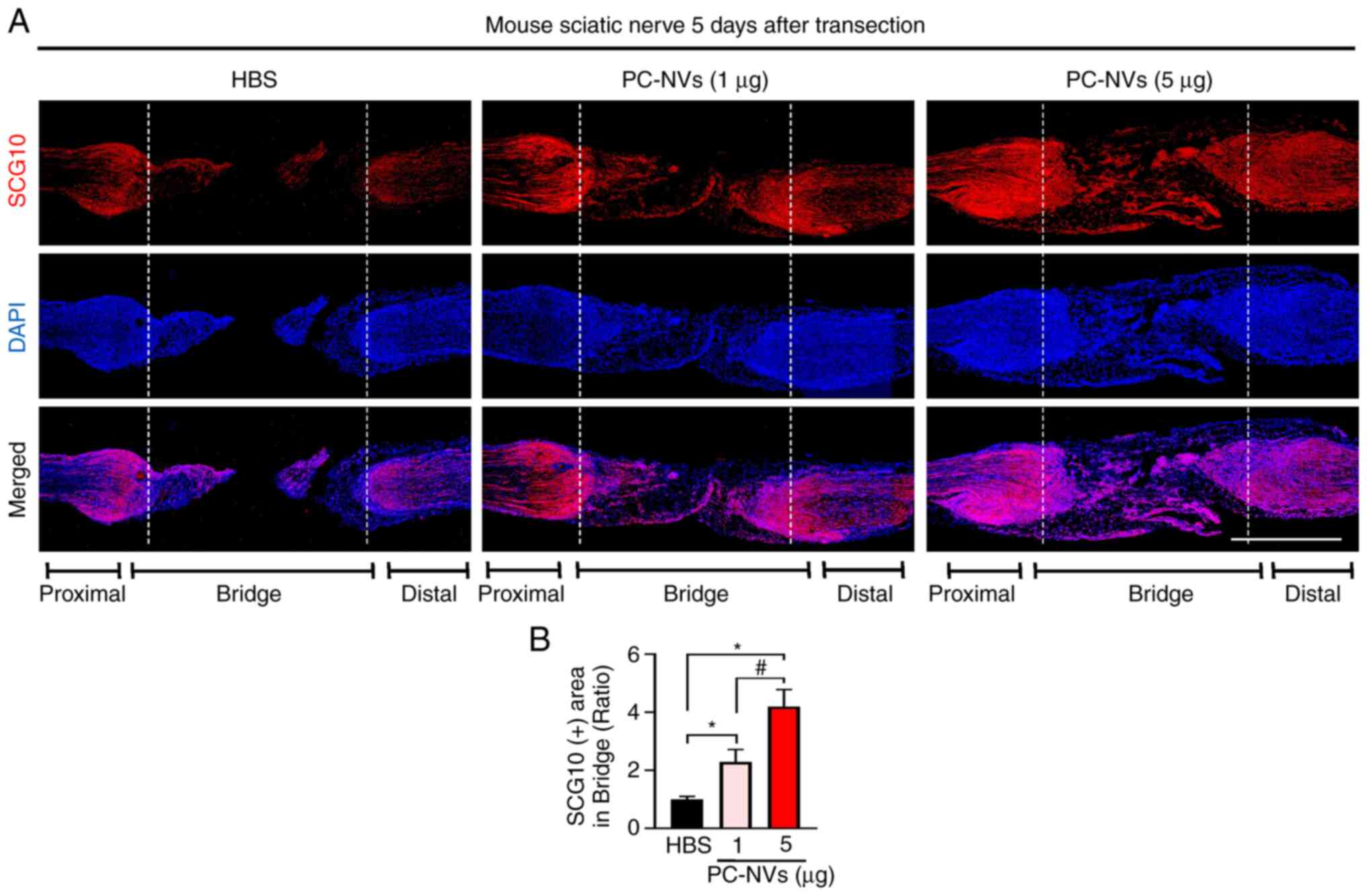
PC-NVs promote nerve regeneration in SNT
model mice
SCG10 is expressed in adult sensory neurons and is
an effective and selective marker for sensory axon regeneration
during the early stages of axon regeneration (28). Therefore, in order to evaluate
the regenerative state in the early stage of axonal regeneration,
immunofluorescence staining was performed for SCG10 in the SNT
tissues after 5 days of treatment with PC-NVs. The results showed
that, compared with the HBS group, low-dose and high-dose PC-NV
treatment significantly increased the levels of SCG10 in the area
of bridge (Fig. 7). This finding
indicated that PC-NVs promoted nerve regeneration by increasing
axon regeneration in the early stages of sciatic nerve
regeneration.
PC-NVs induced neurovascular regeneration
by enhancing expression of NFs and survival signaling in SNT model
mice
In the mammalian PNS, growth factor-mediated
PI3K/Akt signaling is associated with intrinsic neurite outgrowth
and synaptic plasticity (29).
Consistent with previous reports (18,29), it was found that PC-NVs also
significantly upregulated the expression of NFs (BDNF, NT-3 and
NGF) and cell survival signaling-related proteins (PI3K/Akt
signaling), whereas cell death signaling-related proteins, such as
JNK, were significantly attenuated in the SNT model mice compared
with those in the HBS group (Fig.
8). Collectively, these results suggested that regulation of
BDNF, NT-3, NGF, PI3K, Akt, JNK and c-JUN occurred via
PC-NV-mediated neurovascular regeneration in SNT model mice.
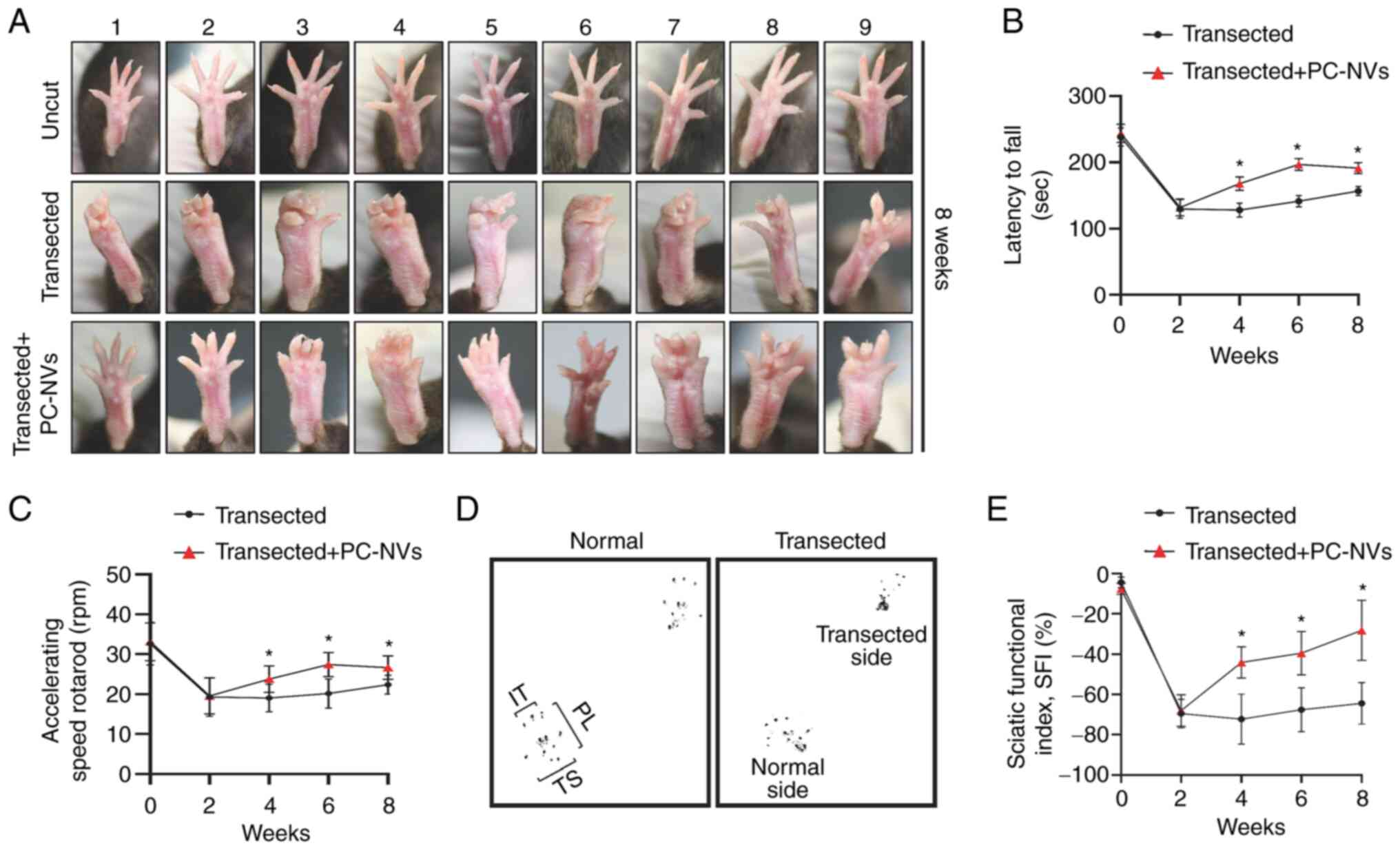
PC-NVs promote the recovery of sciatic
nerve function in SNT model mice
In order to evaluate the motor coordination, balance
and sensory function recovery of the injured sciatic nerve, most
researchers use the rotarod test and walking track analysis
(23). Therefore, SNT was
performed on mice locally treated with a high-dose of PC-NVs (5
µg) around the transected site of the sciatic nerves and
then made to undergo the rotarod test and walking track analysis
every 2 weeks for a total of 8 weeks. The sensory motor
coordination was evaluated by measuring the SFI. The results showed
that the toe extension of SNT model mice was notably reduced
compared with that in the uncut group; however, after 8 weeks of
local application of PC-NVs in SNT model mice, toe extension
abilities were markedly improved (Fig. 9A). In the accelerating rotarod
examination, local application of PC-NVs improved motor functions
(latency and speed to fall) significantly from 4 weeks onwards
compared with those in the transfected group (Fig. 9B and C). In addition, in the
walking track analysis, walking patterns were analyzed to evaluate
sensory and motor functionality during exercise. The pace of a
normal mouse and an SNT mouse is shown in Fig. 9D. The SFI score was measured from
0 to 8 weeks after SNT. At 2 weeks after SNT, mice showed obvious
neurological dysfunction (SFI score, -69±2.35). However, after
PC-NV treatment, the SFI score improved significantly from 4 weeks
(SFI score, -44±2.58) to 8 weeks (SFI score, -28±4.98) after SNT
(Fig. 9E). Taken together, the
functional examinations showed that the local application of PC-NVs
significantly improved the impaired motor and sensory function in
SNT model mice.
Discussion
Compared with the CNS, one of the conspicuous
characteristics of the PNS is its greater regenerative ability
(30). Although peripheral axons
can regenerate and form functional connections, factors such as
age, delay before intervention and type of injury determine the
degree of functional recovery after repair (31,32). Therefore, in order to improve
regeneration, increasing the speed of axonal growth, increasing
neuron survival and preventing neuronal apoptosis are potential
strategies. In the present study, it was demonstrated that
exogenous PC-NVs were capable of improving the injured sciatic
nerve through the increased expression of NFs, promotion of the
cell survival signaling pathway and attenuation of the apoptotic
signaling pathway. Thus, determining the functions of PC-NVs and
identifying its detailed mechanisms may provide important clues to
assist in developing a rapid and effective treatment strategy for
the repair of peripheral nerve damage with limited side
effects.
Pericytes play a crucial role in the early phases of
angiogenesis in damaged tissue, such as disintegration of the
basement membrane and directing endothelial sprouting for
angiogenesis (33). Previously,
a relationship between peripheral nerve regeneration and
angiogenesis following nerve damage was discovered (11,19). Previous studies showed how
various NVs, such as PC-NVs and ESC-derived EV-mimetic NVs
(ESC-NVs), could restore the erectile function of the autonomic
nervous system of diabetic mice (17,18). Therefore, it was hypothesized
that PC-NVs may play a beneficial role in the PNS.
To evaluate this hypothesis, PC-NVs were injected
around the sciatic nerve immediately after transection. According
to previous studies, the time point for sciatic nerve neurovascular
regeneration is 5 days for angiogenesis and 14 days for nerve
regeneration (19,34). In the present study, high-dose
PC-NVs (5 µg) significantly promoted angiogenesis at the
proximal stump in the transected sciatic nerve 5 days after
treatment, and the sciatic nerve connections were closer and
stronger in the PC-NVs (5 µg) treatment group after 14 days
of treatment. In addition, a previous study showed that macrophages
may detect hypoxia within the neural bridge and generate a
polarized vasculature, which provides a scaffold for Schwann cell
migration (19). Schwann cells
have been shown to migrate with blood vessels to damaged tissues,
produce neurotrophic substances and use autophagy to clear the
necrotic tissue produced by Wallerian degeneration, thereby
promoting nerve regeneration (35). Consistently, the present study
showed that PC-NVs increased the content of endothelial cells
(CD31), macrophages (Iba-1) and Schwann cells (GFAP), and enhanced
SCG10 expression in the transected sciatic nerve bridge. Based on
these results, it was hypothesized that the Schwann cells induced
by PC-NVs might be involved in the removal of necrotic tissues
produced by Wallerian degeneration. However, at present, there are
no relevant experimental results to support this hypothesis, thus
more detailed experiments are required to assess. These findings
strongly suggested that PC-NVs may serve an important role in
peripheral nerve neurovascular regeneration.
It has been reported that Schwann cell-derived NVs
could increase the production of NFs (BDNF, NT-3 and NGF), thereby
improving neurite outgrowth in vitro (36). The activation of Akt signaling
has been shown to promote cell proliferation and the migration of
multiple cell types (37). In
addition, previous studies have also shown that PC-NVs could
improve erectile function by enhancing the expression of NFs and
activating survival signaling in a mouse CNI model (18). Consistently, it was also found
that a high-dose of PC-NVs significantly enhanced these
factors.
Finally, in order to evaluate the role of PC-NVs in
PNS functional regeneration, a rotarod test and walking track
analysis were performed in the present study. The results showed
that from 4 weeks after treatment, high-dose PC-NVs significantly
improved the sensory-motor coordination and motor function. These
findings indicated that local injection of PC-NVs may be a
promising strategy for the treatment of peripheral nerve
injury.
To the best of our knowledge, the present study was
the first to evaluate the therapeutic impact of PC-NVs in sciatic
nerve injury models. The current research did have several
limitations. Only the effect of PC-NVs injected immediately after
nerve injury was evaluated, and different time frames between
injury and treatment should be assessed to determine the optimal
time frame. Only male mice were used to prepare SNC and SNT models;
therefore, the use of both female and male mice may be more
meaningful for experimental study. The present study did elucidate
which components of PC-NVs were involved in neurovascular
regeneration and how long PC-NVs remained at the injection site.
Additional studies to evaluate which components of PC-NVs play a
role in neurovascular regeneration may help in understanding the
detailed mechanism of action of PC-NVs in neurovascular
regeneration.
In summary, the present study showed that PC-NVs
significantly enhanced cell survival signaling and the expression
of NFs in neurovascular regeneration in the SNT mouse model. Thus,
PC-NVs may serve as a novel treatment strategy for management of
neurovascular diseases.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GNY, TYS, JO, JKS and JKR conceived and designed the
study. GNY, TYS, JO, MJC, AL, MHK, FYL, SSH, JHK and YSG performed
the experiments, the data collection, statistical analysis, data
interpretation. GNY, TYS, and JO confirm the authenticity of all
the raw data. GNY, TYS, JO and JKR wrote the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The experiments performed with animals were approved
by the Institutional Animal Care and Use Committee of Inha
University (Incheon, Republic of Korea; approval no.
171129-527).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This work was supported by the National Research Foundation of
Korea (grant no. 2019R1A2C2002414), the Medical Research Center
(grant no. NRF-2021R1A5A2031612), the Joint Grant [composed of the
Korean government (Ministry of Science, ICT and Future Planning),
the Bio & Medical Technology Development Program of the
National Research Foundation and the Korean government (MSIT);
grant no. 2019069621] and the National Research Foundation of Korea
(grant no. 2021R1A2C4002133).
References
|
1
|
Thomas S, Ajroud-Driss S, Dimachkie MM,
Gibbons C, Freeman R, Simpson DM, Singleton JR, Smith AG; PNRR
Study Group; Höke A: Peripheral neuropathy research registry: A
prospective cohort. J Peripher Nerv Syst. 24:39–47. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peters BR, Russo SA, West JM and Moore AM:
Schulz SA. Targeted muscle reinnervation for the management of pain
in the setting of major limb amputation. SAGE Open Med.
8:20503121209591802020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Korus L, Ross DC, Doherty CD and Miller
TA: Nerve transfers and neurotization in peripheral nerve injury,
from surgery to rehabilitation. J Neurol Neurosurg Psychiatry.
87:188–197. 2016.
|
|
4
|
Hoshal SG, Solis RN and Bewley AF: Nerve
grafts in head and neck reconstruction. Curr Opin Otolaryngol Head
Neck Surg. 28:346–351. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vijayavenkataraman S: Nerve guide conduits
for peripheral nerve injury repair: A review on design, materials
and fabrication methods. Acta Biomater. 106:54–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Massri KF, Ahmed LA and El-Abhar HS:
Mesenchymal stem cells in chemotherapy-induced peripheral
neuropathy: A new challenging approach that requires further
investigations. J Tissue Eng Regen Med. 14:108–122. 2020.
View Article : Google Scholar
|
|
7
|
Geranmayeh MH, Rahbarghazi R and Farhoudi
M: Targeting pericytes for neurovascular regeneration. Cell Commun
Signal. 17:262019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tamaki T, Hirata M, Nakajima N, Saito K,
Hashimoto H, Soeda S, Uchiyama Y and Watanabe M: A long-gap
peripheral nerve injury therapy using human skeletal muscle-derived
stem cells (Sk-SCs): An achievement of significant morphological,
numerical and functional recovery. PLoS One. 11:e01666392016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rivera FJ, Hinrichsen B and Silva ME:
Pericytes in multiple sclerosis. Adv Exp Med Biol. 1147:167–187.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kisler K, Nelson AR, Rege SV, Ramanathan
A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, et al:
Pericyte degeneration leads to neurovascular uncoupling and limits
oxygen supply to brain. Nat Neurosci. 20:406–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin GN, Jin HR, Choi MJ, Limanjaya A,
Ghatak K, Minh NN, Ock J, Kwon M, Song KM, Park HJ, et al:
Pericyte-derived Dickkopf2 regenerates damaged penile
neurovasculature through an angiopoietin-1-Tie2 pathway. Diabetes.
67:1149–1161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li C, Qin F, Hu F, Xu H, Sun G, Han G,
Wang T and Guo M: Characterization and selective incorporation of
small non-coding RNAs in non-small cell lung cancer extracellular
vesicles. Cell Biosci. 8:22018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Brien K, Breyne K, Ughetto S, Laurent LC
and Breakefield XO: RNA delivery by extracellular vesicles in
mammalian cells and its applications. Nat Rev Mol Cell Biol.
21:585–606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mäe MA, He L, Nordling S, Vazquez-Liebanas
E, Nahar K, Jung B, Li X, Tan BC, Foo JC, Cazenave-Gassiot A, et
al: Single-cell analysis of blood-brain barrier response to
pericyte loss. Circ Res. 128:e46–e62. 2021. View Article : Google Scholar
|
|
15
|
Liu Y and Holmes C: Tissue regeneration
capacity of extracellular vesicles isolated from bone
marrow-derived and adipose-derived mesenchymal stromal/stem cells.
Front Cell Dev Biol. 9:6480982021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim OY, Lee J and Gho YS: Extracellular
vesicle mimetics: Novel alternatives to extracellular vesicle-based
theranostics, drug delivery, and vaccines. Semin Cell Dev Biol.
67:74–82. 2017. View Article : Google Scholar
|
|
17
|
Kwon MH, Song KM, Limanjaya A, Choi MJ,
Ghatak K, Nguyen NM, Ock J, Yin GN, Kang JH, Lee MR, et al:
Embryonic stem cell-derived extracellular vesicle-mimetic
nanovesicles rescue erectile function by enhancing penile
neurovascular regeneration in the streptozotocin-induced diabetic
mouse. Sci Rep. 9:200722019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin GN, Park SH, Ock J, Choi MJ, Limanjaya
A, Ghatak K, Song KM, Kwon MH, Kim DK, Gho YS, et al:
Pericyte-derived extracellular vesicle-mimetic nanovesicles restore
erectile function by enhancing neurovascular regeneration in a
mouse model of cavernous nerve injury. J Sex Med. 17:2118–2128.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cattin AL, Burden JJ, Van Emmenis L,
Mackenzie FE, Hoving JJA, Calavia NG, Guo Y, McLaughlin M,
Rosenberg LH, Quereda V, et al: Macrophage-induced blood vessels
guide schwann cell-mediated regeneration of peripheral nerves.
Cell. 162:1127–1139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin GN, Park SH, Song KM, Limanjaya A,
Ghatak K, Minh NN, Ock J, Ryu JK and Suh JK: Establishment of in
vitro model of erectile dysfunction for the study of
high-glucose-induced angiopathy and neuropathy. Andrology.
5:327–335. 2017. View Article : Google Scholar
|
|
21
|
Meng FW, Jing XN, Song GH, Jie LL and Shen
FF: Prox1 induces new lymphatic vessel formation and promotes nerve
reconstruction in a mouse model of sciatic nerve crush injury. J
Anat. 237:933–940. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen B, Carr L and Dun XP: Dynamic
expression of Slit1-3 and Robo1-2 in the mouse peripheral nervous
system after injury. Neural Regen Res. 15:948–958. 2020. View Article : Google Scholar
|
|
23
|
Lim EF, Nakanishi ST, Hoghooghi V, Eaton
SE, Palmer AL, Frederick A, Stratton JA, Stykel MG, Whelan PJ,
Zochodne DW, et al: AlphaB-crystallin regulates remyelination after
peripheral nerve injury. Proc Natl Acad Sci USA. 114:E1707–E1716.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JI, Hur JM, You J and Lee DH:
Functional recovery with histomorphometric analysis of nerves and
muscles after combination treatment with erythropoietin and
dexamethasone in acute peripheral nerve injury. PLoS One.
15:e02382082020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaka G, Arum J, Sadraie SH, Emamgholi A
and Mohammadi A: Bone marrow stromal cells associated with poly
L-Lactic-Co-Glycolic acid (PLGA) nanofiber scaffold improve
transected sciatic nerve regeneration. Iran J Biotechnol.
15:149–156. 2017. View Article : Google Scholar
|
|
26
|
Moldovan M, Pinchenko V, Dmytriyeva O,
Pankratova S, Fugleholm K, Klingelhofer J, Bock E, Berezin V,
Krarup C and Kiryushko D: Peptide mimetic of the S100A4 protein
modulates peripheral nerve regeneration and attenuates the
progression of neuropathy in myelin protein P0 null mice. Mol Med.
19:43–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stratton JA, Holmes A, Rosin NL, Sinha S,
Vohra M, Burma NE, Trang T, Midha R and Biernaskie J: Macrophages
regulate schwann cell maturation after nerve injury. Cell Rep.
24:2561–2572.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim KJ and Namgung U: Facilitating effects
of Buyang Huanwu decoction on axonal regeneration after peripheral
nerve transection. J Ethnopharmacol. 213:56–64. 2018. View Article : Google Scholar
|
|
29
|
Li R, Li DH, Zhang HY, Wang J, Li XK and
Xiao J: Growth factors-based therapeutic strategies and their
underlying signaling mechanisms for peripheral nerve regeneration.
Acta Pharmacol Sin. 41:1289–1300. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El Seblani N, Welleford AS, Quintero JE,
van Horne CG and Gerhardt GA: Invited review: Utilizing peripheral
nerve regenerative elements to repair damage in the CNS. J Neurosci
Methods. 335:1086232020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuffler DP and Foy C: Restoration of
neurological function following peripheral nerve trauma. Int J Mol
Sci. 21:18082020. View Article : Google Scholar :
|
|
32
|
Alsmadi NZ, Bendale GS, Kanneganti A,
Shihabeddin T, Nguyen AH, Hor E, Dash S, Johnston B, Granja-Vazquez
R and Romero-Ortega MI: Glial-derived growth factor and
pleiotrophin synergistically promote axonal regeneration in
critical nerve injuries. Acta Biomater. 78:165–177. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sweeney M and Foldes G: It takes two:
Endothelial-perivascular cell cross-talk in vascular development
and disease. Front Cardiovasc Med. 5:1542018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Zhu H, Guo Q, Qian T, Zhang P, Li
S, Xue C and Gu X: Overlapping mechanisms of peripheral nerve
regeneration and angiogenesis following sciatic nerve transection.
Front Cell Neurosci. 11:3232017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen G, Luo X and Wang W, Wang Y, Zhu F
and Wang W: Interleukin-1β promotes schwann cells
de-differentiation in wallerian degeneration via the c-JUN/AP-1
pathway. Front Cell Neurosci. 13:3042019. View Article : Google Scholar
|
|
36
|
Rhode SC, Beier JP and Ruhl T: Adipose
tissue stem cells in peripheral nerve regeneration-in vitro and in
vivo. J Neurosci Res. 99:545–560. 2021. View Article : Google Scholar
|
|
37
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|