1. Introduction
Cancer immunotherapy is a type of cancer treatment
that uses artificial stimulations to trigger the immune system's
inherent ability to fight cancer. Following surgery, radiation
therapy, chemotherapy and targeted therapy, cancer immunotherapy
has emerged as the ‘fifth pillar’ for cancer treatment (1,2).
There are generally two types of cancer immunotherapy: Passive and
active. Passive immunotherapy is the use of immune system
components, such as monoclonal antibodies (mAbs) generated outside
the body, to stimulate the immune response, immunological memory
and long-term response. By contrast, active immunotherapy,
including the use of cancer vaccines and engineered cell
treatments, comprises the direct activation of the immune response,
immunological memory and long-term response utilizing components of
the patient's immune system to stimulate an immune response
(3,4).
Over the past few decades, numerous
immunotherapeutic strategies have become established pillars of
cancer treatment, seeking to boost the immune system to recognize
specific antigens of cancer cells for their selective elimination,
including cytokines, immune checkpoint inhibitors, engineered T
cells such as T cell receptor (TCR) T cells and chimeric antigen
receptor (CAR) T cells and cancer vaccines. Several of these have
demonstrated promising effects with respect to gastrointestinal
cancer. Immune checkpoint blockade therapies use antagonists to
block immune-inhibitory pathways, such as the cytotoxic
T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell
death protein-1 (PD-1)/programmed death-ligand-1 (PD-L1) pathways,
and this type of therapy has been demonstrated to be one of the
most effective strategies for treating various cancers in the
clinic, including gastric cancer and esophageal cancer (5,6).
Cancers may become treatable, or even curable, in the future,
courtesy of immunotherapy (6,7).
The present review offers a brief introduction to
several types of tumor immunotherapies, highlighting their clinical
status, benefits and drawbacks. Subsequently, the review examines
several of the new delivery systems that have been created to help
with the clinical translation of immunotherapies. The overall
purpose of the present review is to provide novel insight into the
current status of immunotherapies in the treatment of various types
of digestive cancer.
2. Therapeutic strategies of immunotherapy
for cancer
It is well documented that the occurrence of cancer
is due to a loss of the capability of the immune system to
recognize and kill malignant cells (8). Cancer immunotherapy refers to a
series of processes that are able to enhance the immune system,
inducing or restoring the function of cytotoxic T cells or other
immune effectors to kill malignant cells (Fig. 1). Cancer immunotherapy, a
ground-breaking treatment method, attempts to stimulate or increase
the body's own immune systems to detect and kill cancer cells
(9). As a result, cancer
immunotherapy has attracted a lot of interest due to its proven
efficacy and lower toxicity compared with standard types of
chemotherapy or other treatments that kill cancer cells directly
(9). In general, immunotherapy is
widely acknowledged as a promising approach for treating, or
perhaps curing, certain types of cancer (10).
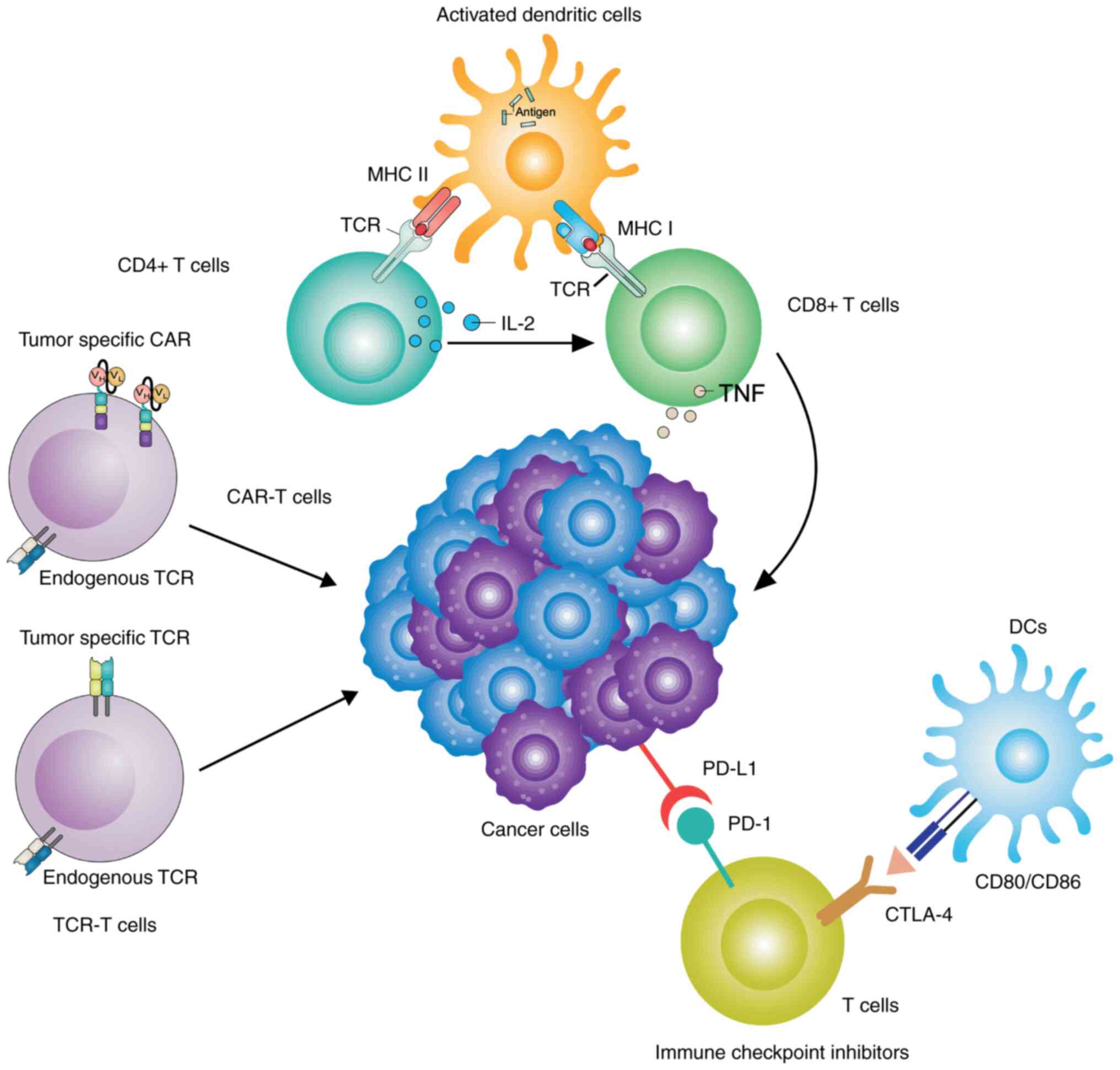 | Figure 1Major immunotherapeutic strategies
for gastrointestinal cancer. Neoantigens, including
proteins/peptides and tumor lysates derived from gastrointestinal
cancer, may be loaded into DCs, and neoantigen-specific genes may
also be transduced into DCs by using viral and non-viral vectors.
DCs with loaded neoantigens may be cultured in vitro for
maturation by providing additional signals and the APC function of
these DCs results in enhanced CTL effector function, not only in
terms of the number of cells, but also in terms of their activity.
The optimizing APC function of DCs may induce CD4+ T
cells to become T helper cells, which results in further killing of
the tumor. Tumor-specific CD8+ T cells are directly
harvested from gastrointestinal cancer and then transfected with
CAR. Subsequently, the engineered CD8+ T cells are
allowed to proliferate in vitro, prior to being re-injected
into patients for antitumor activity. ICIs function in terms of
blocking T-cell inhibitory pathways through reactivating immune
system-targeting cancer cells. The two major checkpoint inhibitors
on T cells that block effector function are PD-1 and CTLA-4, which
interact with PD-L1 expressed by cancer cells and CD80/86 expressed
by APCs, respectively. Recent immunotherapeutic approaches using
monoclonal antibodies that block the inhibitory interactions
between cancer cells and other cells to improve CTL function have
also been used in clinical practice. DC, dendritic cell; APC,
antigen processing cell; CTL, cytotoxic T-lymphocyte; CTLA-4,
CTL-associated antigen 4; ICI, immune checkpoint inhibitor; CAR,
chimeric antigen receptor; PD-1, programmed cell death protein-1;
PD-L1, programmed death-ligand-1. |
On the one hand, the immune system may shield the
human body against the occurrence of cancer. However, the immune
system is not able to exert any scavenging effects on
low-immunogenicity tumor cells. The monitoring function of the
immune system on tumor formation is a dynamically balanced process,
which is called immune monitoring or immune surveillance (11-14).
Herein, the immune system monitors the body for tissue damage,
pathogen invasion and foreign substances. The immune system
initiates a complex inflammatory cascade to eliminate damaged
cells, to re-establish tissue homeostasis and to provide a memory
of the invasion when a subsequent threat is recognized (5,12,14).
Resulting from immune surveillance, the mechanisms of
immunosuppression and immune activation are triggered
simultaneously. In general, the innate immune system is limited to
releasing cytokines and recruiting immune cells to initiate
non-specific immune responses, whereas the adaptive immunity system
directly recognizes and kills cancer cells due to its ability to
specifically identify antigens present on the cancer cells
(14,15). Numerous immunotherapeutic
strategies have become important choices for cancer treatments,
including cytokines, immune checkpoint inhibitors, engineered T
cells such as TCR-T and CAR-T cells, and cancer vaccines (3,13,15).
3. Immunotherapy in esophageal cancer
In recent years, cancer immunotherapy has been
indicated to be a potential new therapeutic option for esophageal
cancer. Various preclinical or clinical trials of esophageal cancer
immunotherapy, including immune checkpoint blockade, tumor
vaccination and adoptive T cell treatment, are in the process of
being conducted (16).
Furthermore, immune checkpoint inhibitors, including an anti-CTLA-4
mAb (iplimumab) and anti-PD-1 mAbs (nivolumab and pembrolizumab),
have been indicated to produce substantial tumor shrinkage and to
increase the overall survival (OS) rates of patients with diverse
types of cancer, findings that have aroused a new enthusiasm for
immunotherapy for cancer (17).
Immune checkpoint inhibition has already been
employed in the treatment of melanoma, and its efficacy in other
types of cancer, including gastrointestinal malignancies, is
currently being investigated (18). High PD-L1 expression levels are
documented to be associated with a prolonged survival rate in
patients with esophageal squamous cell carcinoma (ESCC; low, 41.9%;
high, 84.5%). However, another study suggested that the
membranous/cytoplasm PD-L1 expression was associated with tumor
invasion depth and was an indicator of poor OS in patients with
esophageal cancer; the P-vale of the association of the expression
of PD-L1 with patients' OS was at the statistically significant
threshold (0.0452), indicating the conclusion should be validated
in large cohorts of patients (19,20).
The expression of PD-L1 has been reported to promote the exhaustion
of T cells and drugs that target PD-L1 may provide an effective
approach for treating patients with ESCC who have high expression
levels of PD-L1(21).
Pembrolizumab, a PD-1 inhibitor, is the first immune checkpoint
blockade drug to have been approved by the Food and Drug
Administration (FDA) for the treatment of advanced or unresectable
melanoma (22). Recent studies
have indicated that pembrolizumab may be used as the second-line
treatment of chemotherapy for refractory PD-L1-positive
gastric/gastroesophageal junction cancer. Ipilimumab, an immune
checkpoint inhibitor, was demonstrated to boost the immune system
via targeting CTLA-4 (23-25).
In a pre-clinical model, the combination of ipilimumab and
nivolumab has been indicated to elicit a synergistic effect.
Several studies have demonstrated the safety and effectiveness of
using a combination of nivolumab and ipilimumab in patients with
advanced ESCC, confirming that combination treatment is more
effective than nivolumab monotherapy. Numerous clinical trials are
currently in progress to combine the inhibitory effects of CTLA-4
and PD-1; however, compared with PD-1/PD-L1, the side effects of
CTLA-4 blockade are generally more common and severe. In view of
this, novel strategies are being developed to mitigate these
serious adverse events (26).
In spite of the fact that numerous clinical cancer
vaccine trials have been performed in ESCC (27-31),
regrettably, clinical trials of peptide-based cancer vaccines for
ESCC have yet to be licensed for clinical use. However, in previous
studies, researchers have identified that TTK protein kinase (TTK),
lymphocyte antigen 6 family member K (LY6K), insulin-like growth
factor 2 mRNA-binding protein 3 (IGF2BP3) and NUF2 component of
NDC80 kinetochore complex are able to serve as novel immunogenic
cancer antigens (ICAs) (30,31).
These ICAs are highly and frequently expressed among different
esophageal cancer antigens, and as they have been demonstrated to
be associated with the survival and cell proliferation of ESCC,
they may be anticipated to be used as cancer vaccine targets for
ESCC (30,31). Accordingly, there are three human
leukocyte antigen (HLA)-A24-restricted immune-dominant peptides
that were derived from TTK, IGF2BP3 and LY6K. In an HLA-A24 phase
I/II clinical trial, the prognosis of patients with advanced ESCC
who experienced a vaccination-induced immune response was improved
compared with that of patients without an immune response (30).
For adoptive cell therapy (ACT), activated T cells
are usually collected from cancer tissues or peripheral blood and
the isolated T cells are subsequently activated via in vitro
incubation with interleukin-2 before being reinjected into the
patient. Genetically modified T cells that deliver CAR or TCR into
other T cells are another type of cell treatment. The goal of this
therapy is to boost tumor-specific immunity (32). In 2000, the first ACT clinical
trial for ESCC was conducted, wherein each patient was injected
with 0.8x109 cells once every 2 weeks. Of note, four of
the eleven patients exhibited considerable tumor shrinkage and few
side effects were observed (33).
The first human clinical trial of TCR-T cell treatment was
performed in patients with ESCC who expressed melanoma-associated
antigen 4. Of these patients with ESCC, seven exhibited significant
disease progression after having received treatment for 2 months;
however, three patients with the smallest lesions lived for over 27
months following therapy, indicating the potential effectiveness of
TCR-T cell therapy (34).
Clinical trials have confirmed the great potential
of immunotherapy in esophageal cancer. The combination of
immunotherapy with existing or new treatment methods is expected to
offer the best treatment strategy for esophageal cancer (35). The high incidence of neoantigens
and radiosensitive tumors, and detection of numerous ICAs in ESCC,
are factors that improve the prospects for immunotherapy. Combining
immunotherapy with other therapies (including chemoradiotherapy and
targeted therapy) may prove to be an appropriate and realistic
therapeutic approach for ESCC (32).
4. Immunotherapy in gastric cancer
Tremelimumab is a humanized CTLA-4 mAb that was
found to be effective in treating patients with advanced gastric
cancer (36). However, patients
with advanced gastric cancer who underwent treatment with
ipilimumab, another inhibitor of CTLA-4, did not reach the expected
end-point (clinical trial no. NCT01585987). Ipilimumab treatment
did not improve the progression-free survival (PFS) or OS rates of
patients with gastric cancer following first-line therapy (37). To date, the FDA has approved three
PD-1 inhibitors: Pembrolizumab, atezolizumab and nivolumab. The
phase I clinical trial of patients with gastric cancer with
nivolumab (clinical trial no. NCT01928394) has been completed and
the preliminary results suggested that patients had an objective
response, regardless of the status of PD-L1(38). A randomized phase III trial
reported on the outcomes of patients with advanced gastric cancer
who underwent nivolumab treatment. The results demonstrated that
nivolumab treatment led to improvements in the OS rate, PFS rate
and objective response rate (ORR) in patients with advanced gastric
cancer undergoing multi-line treatment; however, the differences
were found not to be significant (39). Several clinical trials with
anti-PD-L1 inhibitors such as durvalumab, atezolizumab and
BMS936559 have also been conducted to evaluate their effectiveness
in patients with gastric cancer (40) (Table
I).
 | Table IClinical outcomes of immunotherapies
of clinical trials on gastrointestinal cancer. |
Table I
Clinical outcomes of immunotherapies
of clinical trials on gastrointestinal cancer.
| Drug class/study
drug | Trial identifier
no. | Investigator,
year | Clinical
setting | Therapeutic
protocol | AJCC stage | Phase | Patients, n | Outcomes | (Refs.) |
|---|
| Anti-CTLA-4
antibodies | | | | | | | | | |
|
Ipilimumab | NCT01585987 | BMS, 2012 | AGC/GJA | Ipilimumab vs.
BSC | IV | II | 57+57 | PFS: 2.73 vs. 4.90
mo | (37) |
| Anti-PD-1
antibodies | | | | | | | | | |
|
Pembrolizumab | NCT02178722 | Mark Jones,
2014 | MSI-H CRC/GC | Pembrolizumab | NA | II | 16+27 | ORR: 44/22% for
MSI-H CRC/GC | (68) |
|
Pembrolizumab | NCT02370498 | Merck Sharp,
2015 | AGC/GJA | Pembrolizumab vs.
Paclitaxel | IV | III | 296+296 | OS: 9.1 vs. 8.3 mo;
PFS: 1.5 vs. 4.1 mo | (42) |
|
Pembrolizumab | NCT02494583 | Merck Sharp,
2015 | AGC/GJA | Pembrolizumab +SOC
vs. SOC | IV | III | 257+250 | OS: 12.5 vs. 11.1
mo; PFS: 6.9 mo vs. 6.4 mo | (43) |
| Vaccine-based
immunotherapy | | | | | | | | | |
|
GI-6301 | NCT01519817 | James Gulley,
2012 | CRC | Yeast-Brachyury
vaccine (4, 16, 40, 80 YU dose) | NA | I | 3, 3, 16, 9 for
each dose | 1, 1, 8, 7 patients
with Brachyury-specific T-cell responses | (69) |
Gastric cancer may be classified into four molecular
subgroups based on its genomic and transcriptomic characteristics
according to The Cancer Genome Atlas, which exhibit different
therapeutic responses to immune checkpoint inhibitors (41). The KEYNOTE-061 clinical trial
(42) indicated that patients with
gastric cancer with a combined positive score (CPS) ≥10 (i.e., CPS
of PD-L1 expression) benefited most from second-line treatment with
pembrolizumab. The KEYNOTE-061 study also suggested that patients
with a high tumor mutation burden (TMB-H), i.e., TMB-H ≥10 mut/Mb,
exhibited a higher ORR (40 vs. 13%) and a longer OS rate (not
reached vs. 8.1 months) (42). The
phase III KEYNOTE-062 trial (43)
demonstrated that the median OS rate of patients with gastric
cancer with mismatch repair or high microsatellite instability
(MMR/MSI-H) who were treated with pembrolizumab plus chemotherapy
reflected improved survival benefits compared with those who
received chemotherapy alone. An analysis of patients with
metastatic gastric cancer treated with pembrolizumab revealed that
patients with gastric cancer who also had Epstein-Barr virus (EBV)
infection had an ORR of 100% and the EBV infection status appeared
to be a better biomarker for predicting response to pembrolizumab
compared with MSI-H (ORR, 85.7%) or PD-L1 expression (ORR, 50%)
(44). Considered altogether, it
appears that patients with gastric cancer with positive PD-L1
expression, EBV infection, TMB-H or MMR/MSI-H may respond well to
immune checkpoint inhibitor therapy.
Previous clinical trials have assessed the safety
and effectiveness of cancer vaccines in patients with gastric
cancer (45,46). Two vaccines using peptides derived
from vascular endothelial growth factor receptors-1 and -2 were
tested in patients with advanced gastric cancer. This vaccination,
in conjunction with chemotherapy (S-1 combined with cisplatin), was
able to successfully prevent vascular endothelial growth, resulting
in prolonged OS times (47).
However, in spite of these findings, numerous issues in the
development of effective cancer vaccines remain unsolved, such as
the identification of tumor-specific antigens and the development
of vaccine delivery methods. A number of ACT clinical trials have
demonstrated inhibition of gastric cancer progression (48,49).
In addition, human epidermal growth factor receptor 2 (HER2)-based
CAR-T cell therapy was investigated in pre-clinical trials, wherein
human T cells were genetically modified to express CAR, which
targeted the gastric cancer cell antigen HER2; i.e. the T cells
were designed to target HER2-positive gastric cells (50). However, the safety and
effectiveness of this new type of therapy require further
investigation.
In conclusion, given that gastric cancer is
characterized by high heterogeneity in complex host genetic and
immunological settings, and given the high occurrence of somatic
mutations in patients with gastric cancer, which has led to the
suggestion that gastric cancer may be an attractive candidate for
immunotherapy, gastric cancer is continuing to receive considerable
attention in this regard. Specifically, numerous clinical trials
have demonstrated promising results in terms of gastric cancer
treatment, using either immune monotherapies or combination
therapies including immune checkpoint blockade therapies, CAR-T
cells and cancer vaccines, the latter of which have provided the
most promising results in the treatment of gastric cancer (51).
5. Immunotherapy in colorectal cancer
Several therapeutic strategies have transformed the
general strategy for treating patients with colorectal cancer in
recent years, thereby markedly improving patient survival rates. Of
note, novel immunotherapies may change the colorectal cancer
landscape (52). Similarly to the
situation with gastric cancer, at present, three mAbs
(pembrolizumab, nivolumab and ipilimumab) have been approved by the
FDA for patients with metastatic colorectal cancer with MSI-H or
deficiency of (d)MMR (53).
Ipilimumab was the first drug designed to interfere with immune
checkpoints (54). Other promising
checkpoint inhibitors, such as anti-PD-1 mAb or PD-L1 mAb, are able
to boost the immune response to recognize and kill cancer cells.
These drugs are currently being evaluated in clinical studies,
either alone or in combination. The European Society for Medical
Oncology consensus guidelines (55) recommend the use of the MSI test, as
this has high predictive value for checkpoint inhibitor usage in
patients with colorectal cancer, implying that pembrolizumab may be
used in patients with colorectal cancer with MSI-H. A previous
clinical trial indicated that 11 patients with colorectal cancer
with dMMR had relatively high ORRs and PFS rates, compared with 21
patients with proficient (p)MMR (ORR: 40 vs. 0%; and PFS: 78 vs.
11%). These results support the predictive value of the patients'
MMR status in terms of making the most appropriate choice of immune
checkpoint blockade therapy (56).
Currently, cancer vaccines, cytokines and androgen
deprivation therapy are also being evaluated in different clinical
trials. The majority of these immunotherapies are still at the pre-
or early clinical trial stages, although their efficacy in other
types of cancer has already provided some optimism in terms of
their potential for colorectal cancer treatment. Deficiencies in
DNA MMR protein may cause insertion or deletion mutations, which
leads to MSI, resulting in the mutated peptide antigen. These
antigens derived from mutations of the coding region of genes are
thought to be highly immunogenic stimulants, making them ideal
targets for developing cancer vaccines (57). However, clinical trials of
therapeutic vaccines for the treatment of colorectal cancer based
on different delivery approaches have elicited inconsistent results
(58). For instance, 254 patients
with colorectal cancer who underwent surgical resection were
treated with active specific immunotherapy (ASI), comprising
irradiated autologous tumor cells (59). The original study concluded that
patients with stage II colorectal cancer had a high recurrence-free
survival (RFS) rate, whereas no benefits in RFS were observed in
patients with stage III colorectal cancer. However, in a recent
retrospective analysis, 196 well-preserved tumor specimens were
re-evaluated to assess the results associated with the MSI status
[34/196 dMMR/MSI (17.3%)] (60).
Patients who received ASI therapy were observed to have a high
15-year RFS rate compared with those who underwent surgery alone
and this was independent of the MSI status and the American Joint
Committee on Cancer stage. Compared with the patients with
pMMR/MSS, the 15-year RFS rate of the patients with dMMR/MSI-H
colorectal cancer was significantly higher (dMMR/MSI-H, 85% vs.
pMMR/MSS, 64%).
The effectiveness and safety of adoptive T cell
therapy was first assessed in three patients with refractory
colorectal cancer (61). The
patients were injected with TCR-T cells that targeted the
carcinoembryonic antigen (CEA) epitope. A response was observed in
terms of decreasing serum CEA levels in one of three patients, and
the objective tumors were observed to have dissipated liver and
lung metastasis. It is worth noting that all three patients
exhibited severe transient inflammatory colitis. Furthermore, with
the development of CAR-T cells, this novel technology has been
expanded to genetically modify T cells such that they express
target proteins presented on the cancer cells, thereby allowing the
CAR-T cells to recognize and kill cancer cells (62).
Overall, the use of immunotherapy for colorectal
cancer is developing rapidly. As our understanding of the immune
system and its complexity grows, so does our ability to exploit its
potential. Recognizing that one unique approach may not be usefully
applied for each patient, efforts are being made to develop the
immune system using traditional and novel therapies, thereby
providing reasons for optimism for the future immunotherapy of
patients with colorectal cancer.
6. Similarities and differences of
immunotherapy in gastrointestinal cancer
Currently, immunotherapy for gastrointestinal cancer
comprises immune checkpoint inhibitors, TCR-T cells, CAR-T cells,
cytokines and cancer vaccines. Of these, the checkpoint inhibitors
are the drugs that have been the most studied and approved for the
treatment of gastrointestinal cancer. Vaccines, cytokines and
adaptive cell transfer therapies have yet to be approved by the FDA
for gastrointestinal cancer, although they are being investigated
in clinical trials (63). The
FDA-approved drug pembrolizumab has been used for the first-line
treatment of patients with unresectable or metastatic dMMR/MSI-H
colorectal cancer since 2020(64).
Subsequently, the FDA-approved drug nivolumab, in combination with
certain types of chemotherapy, has been used for the initial
treatment of patients with advanced or metastatic gastric cancer,
gastroesophageal junction cancer and esophageal adenocarcinoma
since 2021(65). The immune
checkpoint inhibitors show promising therapeutic effects in all
three types of gastrointestinal cancer that have been discussed in
the present review.
However, the use of immunotherapy has been limited
in the treatment of gastroesophageal cancer, compared with
colorectal cancer, due to high tumor heterogeneity and the complex
underlying immunosuppressive mechanisms (63). Immune checkpoint inhibition therapy
has achieved certain successes in gastric cancer with high mutation
burden, including EBV-positive gastric cancer (66). Furthermore, immunotherapy has been
mainly limited to the treatment of either the advanced stage of the
malignancy or the treatment of refractory gastric or esophageal
cancers (67). For the treatment
of gastric or esophageal malignancies, immunotherapy has also been
combined with certain types of chemotherapy to obtain a better
prognosis for patients with gastric or esophageal cancer (65). Novel biomarkers, and the rational
threshold of current biomarkers, for immunotherapy remain to be
investigated in order to expand the scope of their applicability in
gastric and esophageal cancer.
7. Conclusion
Gastrointestinal cancer is the most commonly
occurring digestive system cancer type and associated with
relatively low survival rates due to inadequate therapies and
aggressive features. Immunotherapeutic methods, including immune
check point inhibitors, CAR-T cells, as well as cancer vaccines,
have demonstrated great promise in terms of eliminating cancer
cells through reactivating the immune system. Promising results of
immunotherapy have been observed in melanoma, lung cancer and
hematological malignant tumors. Anti-PD-1 antibodies have also
achieved promising results with respect to gastrointestinal cancer,
particularly in the case of ESCC. Combination therapies with other
immunotherapeutic drugs, targeted therapies, chemotherapy and
stroma-regulating drugs may provide treatment opportunities for
patients with advanced gastrointestinal cancer. We remain hopeful
that aggressive gastrointestinal cancer will become one type of
chronic disease that is curable by different types of therapies in
the future.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by a grant from the
Outstanding Talents Start-up Grant of Xuzhou Medical University
(grant no. D2021021).
Availability of data and materials
Not applicable.
Authors' contributions
YL, JC and QS conceived the idea for this review. QS
directed the work. YL, JC and QS drafted the manuscript. YX
participated in the discussion and QS revised the manuscript. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Q, Wang C, Chen G, Hu Q and Gu Z:
Delivery strategies for immune checkpoint blockade. Adv Healthc
Mater. 7(e1800424)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Demaria O, Cornen S, Daeron M, Morel Y,
Medzhitov R and Vivier E: Harnessing innate immunity in cancer
therapy. Nature. 574:45–56. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kakimi K, Karasaki T, Matsushita H and
Sugie T: Advances in personalized cancer immunotherapy. Breast
Cancer. 24:16–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang YY and Zhang ZM: The history and
advances in cancer immunotherapy: Understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Abbott M and Ustoyev Y: Cancer and the
immune system: The history and background of immunotherapy. Semin
Oncol Nurs. 35(150923)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen Q, Chen MC and Liu Z: Local
biomaterials-assisted cancer immunotherapy to trigger systemic
antitumor responses. Chem Soc Rev. 48:5506–5526. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Murciano-Goroff YR, Warner AB and Wolchok
JD: The future of cancer immunotherapy: Microenvironment-targeting
combinations. Cell Res. 30:507–519. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tang S, Ning Q, Yang L, Mo Z and Tang S:
Mechanisms of immune escape in the cancer immune cycle. Int
Immunopharmacol. 86(106700)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barbari C, Fontaine T, Parajuli P,
Lamichhane N, Jakubski S, Lamichhane P and Deshmukh RR:
Immunotherapies and combination strategies for immuno-oncology. Int
J Mol Sci. 21(5009)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kumar A, Swain CA and Shevde LA: Informing
the new developments and future of cancer immunotherapy future of
cancer immunotherapy. Cancer Metast Rev. 40:549–562.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Finn OJ: A believer's overview of cancer
immunosurveillance and immunotherapy. J Immunol. 200:385–391.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Helmy KY, Patel SA, Nahas GR and Rameshwar
P: Cancer immunotherapy: Accomplishments to date and future
promise. Ther Deliv. 4:1307–1320. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gubin MM and Vesely MD: Cancer
immunoediting in the era of immuno-oncology. Clin Cancer Res.
20:3917–3928. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kelly RJ: The emerging role of
immunotherapy for esophageal cancer. Curr Opin Gastroenterol.
35:337–343. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gide TN, Quek C, Menzies AM, Tasker AT,
Shang P, Holst J, Madore J, Lim SY, Velickovic R, Wongchenko M, et
al: Distinct immune cell populations define response to anti-PD-1
monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer
Cell. 35:238–255.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hsu A, Mendelson L and Almhanna K: Immune
checkpoint inhibitors in the treatment of gastrointestinal
malignancies: A review of current and future therapies. R I Med J
(2013). 103:33–37. 2020.PubMed/NCBI
|
|
19
|
Chen K, Cheng G, Zhang F, Zhang N, Li D,
Jin J, Wu J, Ying L, Mao W and Su D: Prognostic significance of
programmed death-1 and programmed death-ligand 1 expression in
patients with esophageal squamous cell carcinoma. Oncotarget.
7:30772–30780. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang
J and Wu C: B7-H1 expression associates with tumor invasion and
predicts patient's survival in human esophageal cancer. Int J Clin
Exp Pathol. 7:6015–6023. 2014.PubMed/NCBI
|
|
21
|
Ostrand-Rosenberg S, Horn LA and Haile ST:
The programmed death-1 immune-suppressive pathway: Barrier to
antitumor immunity. J Immunol. 193:3835–3841. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pham F and Dalle S: Safety of
pembrolizumab for resected stage III melanoma. Expert Opin Drug
Saf. 19:1221–1227. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol.
4(e180013)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Seiwert TY, Burtness B, Mehra R, Weiss J,
Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et
al: Safety and clinical activity of pembrolizumab for treatment of
recurrent or metastatic squamous cell carcinoma of the head and
neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial.
Lancet Oncol. 17:956–965. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kato R, Yamasaki M, Urakawa S, Nishida K,
Makino T, Morimoto-Okazawa A, Kawashima A, Iwahori K, Suzuki S,
Ueda R, et al: Increased Tim-3(+) T cells in PBMCs during nivolumab
therapy correlate with responses and prognosis of advanced
esophageal squamous cell carcinoma patients. Cancer Immunol
Immunother. 67:1673–1683. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao Y, Yang W, Huang Y, Cui R, Li X and
Li B: Evolving roles for targeting CTLA-4 in cancer immunotherapy.
Cell Physiol Biochem. 47:721–734. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kojima T and Doi T: Immunotherapy for
esophageal squamous cell carcinoma. Curr Oncol Rep.
19(33)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Saito T, Wada H, Yamasaki M, Miyata H,
Nishikawa H, Sato E, Kageyama S, Shiku H, Mori M and Doki Y: High
expression of MAGE-A4 and MHC class I antigens in tumor cells and
induction of MAGE-A4 immune responses are prognostic markers of
CHP-MAGE-A4 cancer vaccine. Vaccine. 32:5901–5907. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Iinuma H, Fukushima R, Inaba T, Tamura J,
Inoue T, Ogawa E, Horikawa M, Ikeda Y, Matsutani N, Takeda K, et
al: Phase I clinical study of multiple epitope peptide vaccine
combined with chemoradiation therapy in esophageal cancer patients.
J Transl Med. 12(84)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kono K, Iinuma H, Akutsu Y, Tanaka H,
Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R,
et al: Multicenter, phase II clinical trial of cancer vaccination
for advanced esophageal cancer with three peptides derived from
novel cancer-testis antigens. J Transl Med. 10(141)2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kono K, Mizukami Y, Daigo Y, Takano A,
Masuda K, Yoshida K, Tsunoda T, Kawaguchi Y, Nakamura Y and Fujii
H: Vaccination with multiple peptides derived from novel
cancer-testis antigens can induce specific T-cell responses and
clinical responses in advanced esophageal cancer. Cancer Sci.
100:1502–1509. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alsina M, Moehler M and Lorenzen S:
Immunotherapy of esophageal cancer: Current status, many trials and
innovative strategies. Oncol Res Treat. 41:266–271. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Toh U, Yamana H, Sueyoshi S, Tanaka T,
Niiya F, Katagiri K, Fujita H, Shirozou K and Itoh K: Locoregional
cellular immunotherapy for patients with advanced esophageal
cancer. Clin Cancer Res. 6:4663–4673. 2000.PubMed/NCBI
|
|
34
|
Kageyama S, Ikeda H, Miyahara Y, Imai N,
Ishihara M, Saito K, Sugino S, Ueda S, Ishikawa T, Kokura S, et al:
Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced
lymphocytes in patients with recurrent esophageal cancer. Clin
Cancer Res. 21:2268–2277. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schizas D, Charalampakis N, Kole C,
Mylonas KS, Katsaros I, Zhao M, Ajani JA, Psyrri A, Karamouzis MV
and Liakako T: Immunotherapy for esophageal cancer: A 2019 update.
Immunotherapy. 12:203–218. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ralph C, Elkord E, Burt DJ, O'Dwyer JF,
Austin EB, Stern PL, Hawkins RE and Thistlethwaite FC: Modulation
of lymphocyte regulation for cancer therapy: A phase II trial of
tremelimumab in advanced gastric and esophageal adenocarcinoma.
Clin Cancer Res. 16:1662–1672. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
De Mello RA, Lordick F, Muro K and
Janjigian YY: Current and future aspects of immunotherapy for
esophageal and gastric malignancies. Am Soc Clin Oncol Educ Book.
39:237–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Le DT, Bendell JC, Calvo E, Kim JW,
Ascierto PA, Ott PS, Bono P, Jaeger D, Evans TR, Braud FG, et al:
Safety and activity of nivolumab monotherapy in advanced and
metastatic (A/M) gastric or gastroesophageal junction cancer
(GC/GEC): Results from the CheckMate-032 study. J Clin Oncol.
34(79157577)2016.
|
|
39
|
Kang YK, Satoh T, Ryu MH, Chao Y, Kato K,
Chung HC, Chen JS, Muro K, Kang WK, Yoshikawa T, et al: Nivolumab
(ONO-4538/BMS-936558) as salvage treatment after second or
later-line chemotherapy for advanced gastric or gastroesophageal
junction cancer (AGC): A double-blinded, randomized, phase III
trial. J Clin Oncol 35: DOI:10.1200/JCO.2017.35.4_SUPPL.2,
2017.
|
|
40
|
de Guillebon E, Roussille P, Frouin E and
Tougeron D: Anti program death-1/anti program death-ligand 1 in
digestive cancers. World J Gastrointest Oncol. 7:95–101.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cancer Genome Atlas Research Network.
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fuchs CS, Ozguroglu M, Bang YJ, Di
Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated PD-L1-positive advanced gastric or gastroesophageal
junction cancer: 2-year update of the randomized phase 3
KEYNOTE-061 trial. Gastric Cancer. 25:197–206. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shitara K, Van Cutsem E, Bang YJ, Fuchs C,
Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, et al:
Efficacy and safety of pembrolizumab or pembrolizumab plus
chemotherapy vs chemotherapy alone for patients with first-line,
advanced gastric cancer: The KEYNOTE-062 phase 3 randomized
clinical trial. JAMA Oncol. 6:1571–1580. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim ST, Cristescu R, Bass AJ, Kim KM,
Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, et al:
Comprehensive molecular characterization of clinical responses to
PD-1 inhibition in metastatic gastric cancer. Nat Med.
24:1449–1458. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen Y, Guo ZQ, Shi CM, Zhou ZF, Ye YB and
Chen Q: Efficacy of adjuvant chemotherapy combined with
immunotherapy with cytokine-induced killer cells for gastric cancer
after d2 gastrectomy. Int J Clin Exp Med. 8:7728–7736.
2015.PubMed/NCBI
|
|
46
|
Yoneda A, Kuroki T and Eguchi S:
Immunotherapeutic advances in gastric cancer. Surg Today.
51:1727–1735. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Masuzawa T, Fujiwara Y, Okada K, Nakamura
A, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Osawa
R, et al: Phase I/II study of S-1 plus cisplatin combined with
peptide vaccines for human vascular endothelial growth factor
receptor 1 and 2 in patients with advanced gastric cancer. Int J
Oncol. 41:1297–1304. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ishikawa H, Imano M, Shiraishi O, Yasuda
A, Peng YF, Shinkai M, Yasuda T, Imamoto H and Shiozaki H: Phase I
clinical trial of vaccination with LY6K-derived peptide in patients
with advanced gastric cancer. Gastric. 17:173–180. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang GQ, Zhao H, Wu JY, Li JY, Yan X,
Wang G, Wu LL, Zhang XG, Shao Y, Wang Y and Jiao SC: Prolonged
overall survival in gastric cancer patients after adoptive
immunotherapy. World J Gastroenterol. 21:2777–2785. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo
Y, Zhao X, Wang Y, Wang Z, Han W and Chen L: Effective and
persistent antitumor activity of HER2-directed CAR-T cells against
gastric cancer cells in vitro and xenotransplanted tumors in vivo.
Protein Cell. 9:867–878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xie J, Fu L and Jin L: Immunotherapy of
gastric cancer: Past, future perspective and challenges. Pathol Res
Pract. 218(153322)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Johdi NA and Sukor NF: Colorectal cancer
immunotherapy: Options and strategies. Front Immunol.
11(1624)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Marcus L, Lemery SJ, Keegan P and Pazdur
R: FDA approval summary: Pembrolizumab for the treatment of
microsatellite instability-high solid tumors. Clin Cancer Res.
25:3753–3758. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ardolino L and Joshua A: Immune checkpoint
inhibitors in malignancy. Aust Prescr. 42:62–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aguilar EA, Bardelli A, Benson A,
Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Anna Oncol.
27:1386–1422. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Eng
J Med. 372:2509–2520. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Leoni G, D'Alise AM, Cotugno G, Langone F,
Garzia I, De Lucia M, Fichera I, Vitale R, Bignone V, Tucci FG, et
al: A genetic vaccine encoding shared cancer neoantigens to treat
tumors with microsatellite instability. Cancer Res. 80:3972–3982.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Quiroga D, Lyerly HK and Morse MA:
Deficient mismatch repair and the role of immunotherapy in
metastatic colorectal cancer. Curr Treat Options Oncol.
17(41)2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Vermorken JB, Claessen AM, van Tinteren H,
Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E,
Ransom JH, et al: Active specific immunotherapy for stage II and
stage III human colon cancer: A randomised trial. Lancet.
353:345–350. 1999.PubMed/NCBI View Article : Google Scholar
|
|
60
|
de Weger VA, Turksma AW, Voorham QJ, Euler
Z, Bril H, van den Eertwegh AJ, Bloemena E, Pinedo HM, Vermorken
JB, van Tinteren H, et al: Clinical effects of adjuvant active
specific immunotherapy differ between patients with
microsatellite-stable and microsatellite-instable colon cancer.
Clin Cancer Res. 18:882–889. 2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Parkhurst MR, Yang JC, Langan RC, Dudley
ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry
RM, et al: T cells targeting carcinoembryonic antigen can mediate
regression of metastatic colorectal cancer but induce severe
transient colitis. Mol Ther. 19:620–626. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pegram HJ, Park JH and Brentjens RJ: CD28z
CARs and armored CARs. Cancer J. 20:127–133. 2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang DK, Zuo Q, He QY and Li B: Targeted
immunotherapies in gastrointestinal cancer: From molecular
mechanisms to implications. Front Immunol.
12(705999)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Casak SJ, Marcus L, Fashoyin-Aje L, Mushti
SL, Cheng J, Shen YL, Pierce WF, Her L, Goldberg KB, Theoret MR, et
al: FDA approval summary: Pembrolizumab for the first-line
treatment of patients with MSI-H/dMMR advanced unresectable or
metastatic colorectal carcinoma. Clin Cancer Res. 27:4680–4684.
2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Bragagnoli AC, et al: First-line nivolumab plus chemotherapy versus
chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40.
2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Saito M and Kono K: Landscape of
EBV-positive gastric cancer. Gastric Cancer. 24:983–989.
2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Li K, Zhang A, Li X, Zhang H and Zhao L:
Advances in clinical immunotherapy for gastric cancer. Biochim
Biophys Acta Rev Cancer. 1876(188615)2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Mitchell TC, Hamid O, Smith DC, Bauer TM,
Wasser JS, Olszanski AJ, Luke JJ, Balmanoukian AS, Schmidt EV, Zhao
Y, et al: Epacadostat plus pembrolizumab in patients with advanced
solid tumors: Phase I results from a multicenter, open-label phase
I/II Trial (ECHO-202/KEYNOTE-037). J Clin Oncol. 36:3223–3230.
2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Harpreet S, Christopher H, Jennifer M, et
al: A phase I study of a yeast-based therapeutic cancer vaccine,
GI-6301, targeting brachyury in patients with metastatic carcinoma.
J Clin Oncol. 32: 15_suppl(e14026-e14026)2014.
|















