Introduction
Spinal perimedullary arteriovenous fistula (PAVF) is
an uncommon arteriovenous shunt of the conus medullaris and cauda
equina (1). PAVFs at this location
are often only supplied by the thoracolumbar radiculomedullary
artery (2). The artery of
Desproges-Gotteron (ADG) may arise from the internal iliac artery
or the iliolumbar artery up to the conus medullaris (3). In rare cases, the ADG can be involved
as the feeding artery of the PAVF (4).
The present study reports the case of a female
patient with PAVF supplied by the ADG. As a PAVF feeding artery,
the ADG can be missed upon angiography. The spinal cord has a
complex origin of the feeding artery (Fig. 1). Therefore, the understanding of
spinal cord vascular disease is often difficult; thus, reports of
such cases are of utmost importance. In addition, the present study
also performed a brief literature review of this condition in order
to shed further light into this rare occurrence.
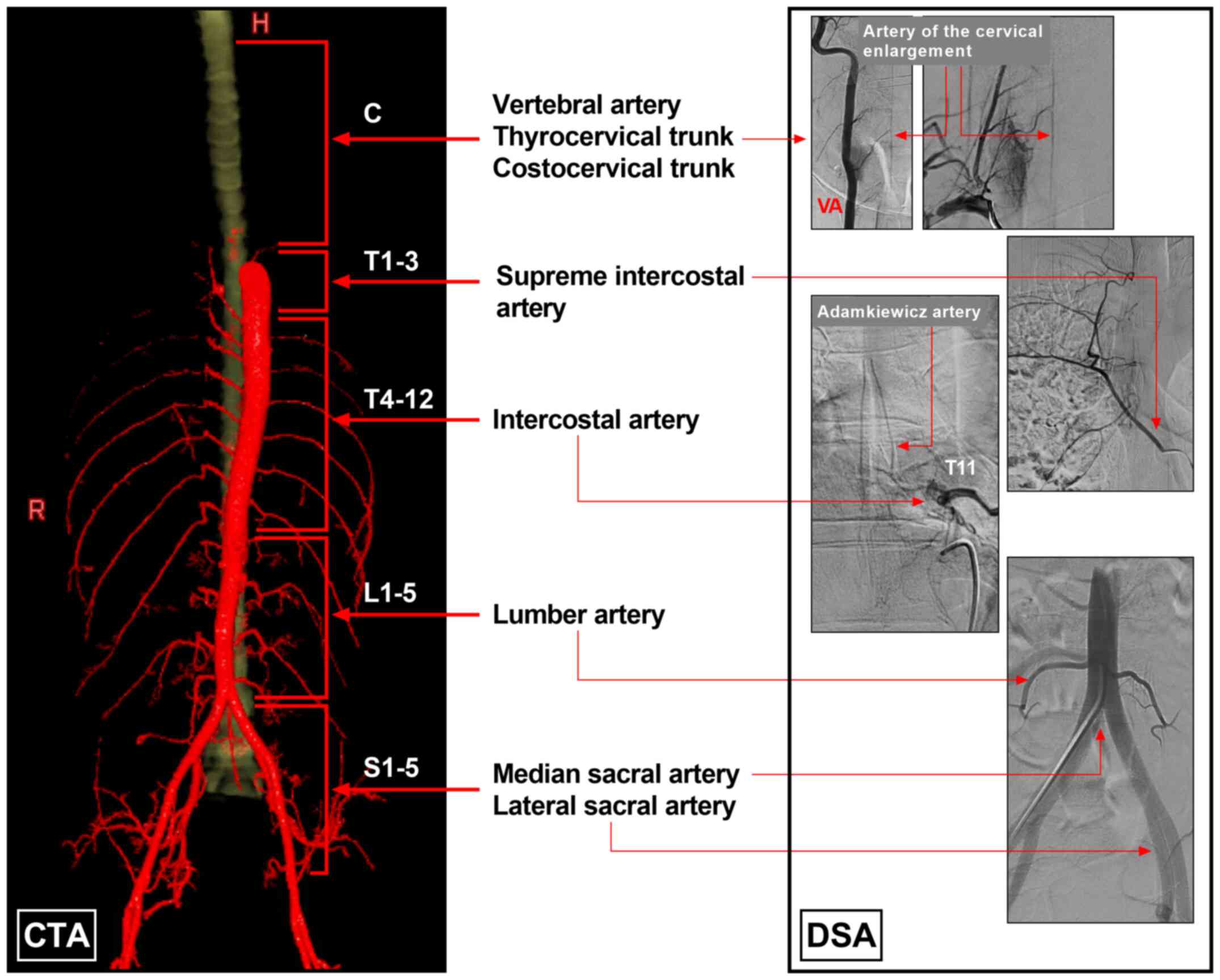 | Figure 1Blood supply system of the spinal
cord. The left section of the image was created using CTA imaging,
and the blood supply of the spinal cord was divided into five parts
as follows: Cervical arteries (vertebral artery, thyrocervical
trunk and costocervical trunk), supreme intercostal artery,
intercostal artery, lumbar artery, and median and lateral sacral
artery. The right section of the image was established using DSA
imaging and illustrates spinal arteries and their origins. C
cervical vertebra; CTA, computed tomography angiography; DSA,
digital subtraction angiography; H, head; L, lumbar vertebra; R,
right; S, sacral vertebra; T, thoracic vertebra. |
Case report
A 30-year-old female was admitted to the First
Hospital of Jilin University (Changchun, China) on September 23,
2019. She was in a good health prior to her admission. At 3 days
prior to her admission, she suffered a sudden weakness of the lower
left limb, and the symptoms gradually became aggravated. At 2 days
following symptom onset, she suffered paraplegia, as well as
difficulties in urination and defecation. A physical examination
revealed that she had a muscle strength of grade 1 in the right
lower limb and grade 2 in the left lower limb (5). Superficial and deep sensations were
reduced in the bilateral lower limbs. The sensory level was at the
T12 and L1 level (6). The Babinski
sign was negative in the left lower limb and positive in the right
lower limb.
Spinal magnetic resonance imaging (MRI) and computed
tomography angiography (CTA) revealed extensive intradural
extramedullary flow void signs in the spinal canal, the dilation of
the spinal vein extending upward to a high cervical level, and the
involvement of the ADG and artery of Adamkiewicz (AKA) as the main
feeding arteries. The location of the vascular lesion was at the
conus medullaris and cauda equina and was filled with the spinal
canal. The length of the whole spinal cord was measured using a GE
Workstation (version 4.7; GE Healthcare; Cytiva) and was 39 cm. The
pre-operative data are presented in Fig.
2. Microsurgical treatment with intraoperative digital
subtraction angiography (DSA) assistance was planned. The
intraoperative DSA revealed a PAVF at the L1 level supplied by the
right AKA and the left ADG (Fig.
3).
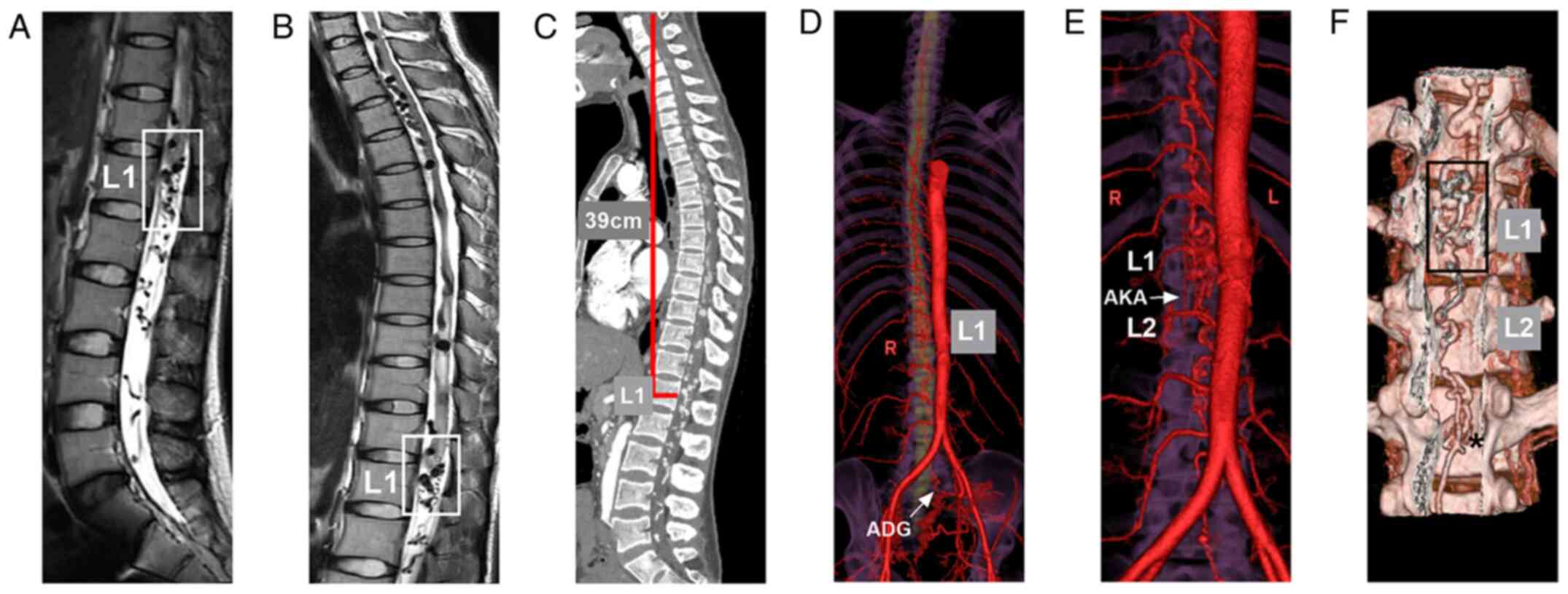 | Figure 2Pre-operative MRI and CTA. (A Lumber
and (B) thoracolumbar T2-weighted MRI illustrating extensive
intradural extramedullary flow void signs. The frames indicate the
vascular lesion located at the conus medullaris and cauda equina
nerves (L1 level). (C) CTA of maximum intensity projection revealed
an extensive contrasted lesion in the whole spinal canal. In the GE
Workstation (version 4.7), the cured three-dimensional length of
the spinal canal from the atlas to the L1 level (the same as the
length of the spinal cord) was 39 cm, which was measured using the
two-click AVA tool. (D) Reconstructive CTA of the spinal cord
arteries illustrating a vascular lesion at the L1 level, with the
dilation of the whole spinal vein extending upward to a high
cervical level from the L1 level, and the involvement of the ADG
from the left internal iliac artery as the main feeding artery
(arrow). (E) Reconstructive CTA of the spinal cord arteries
illustrating the right AKA (arrow) from the lumbar artery at the L2
level also supplied the vascular lesion. (F) Reconstructive CTA
with the bone and vessel illustrating the vascular lesion located
at the L1 level (frame), and the ADG divided into two branches
(asterisk) into the lesion. ADG, artery of Desproges-Gotteron; AKA,
artery of Adamkiewicz; CTA, computed tomography angiography; L,
left; L1, first lumbar vertebra; L2, second lumbar vertebra; MRI,
magnetic resonance imaging; R, right. |
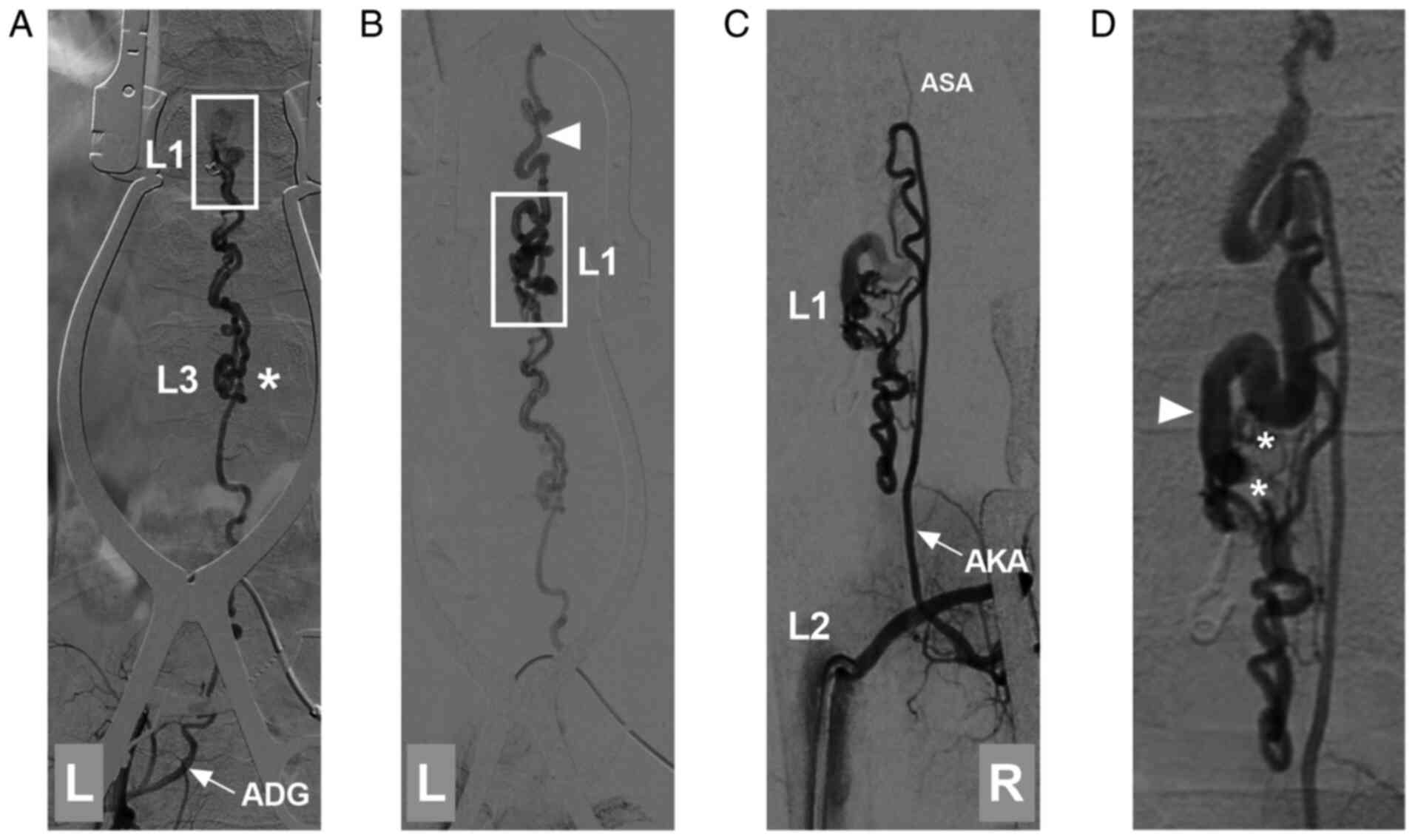 | Figure 3DSA prior to PAVF removal. (A)
Arterial-phase DSA of the left iliac artery illustrating that the
ADG was from the internal iliac artery as the feeding artery to the
PAVF (frame at L1). At the L3 level, the ADG was divided into two
branches (asterisk). (B) Late arterial-phase DSA illustrating that
the PAVF at the L1 level (frame) transferred into the draining vein
(arrowhead). (C) Arterial-phase DSA of the lumbar artery (L2 level)
showing that the PAVF was supplied by the right AKA (arrow); a
hypoplastic ASA was found. (D) Late arterial-phase DSA illustrating
the PAVF architecture, in which the asterisks indicate multiple
slims feeding arteries directly into a large draining vein
(arrowhead). AKA, artery of Adamkiewicz; ADG, artery of
Desproges-Gotteron; ASA, anterior spinal artery; DSA, digital
subtraction angiography; L, left; L1, first lumbar vertebra; L2,
second lumbar vertebra; L3, third lumbar vertebra; PAVF,
perimedullary arteriovenous fistula; R, right. |
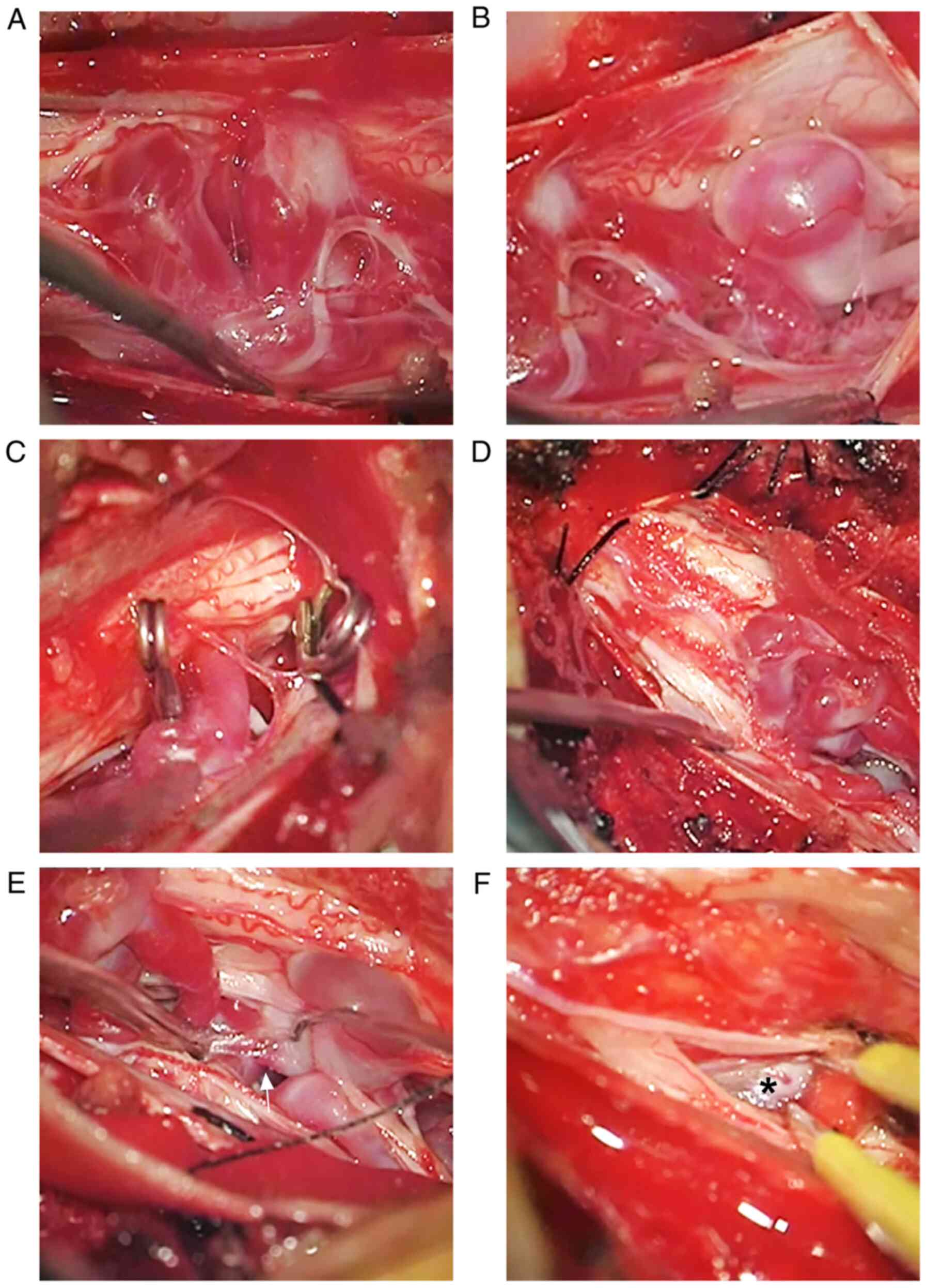
The surgery was centered on the level of the L1
vertebral body, and the spinous processes and lamina of the T12 and
L1-3 segments were removed. After opening the spinal dura, the PAVF
was found, presenting with dilated and tortuous vessels on the
surface of the conus medullaris and cauda equina nerves (Fig. 4A and B). An intraoperative angiography of the
left iliac artery confirmed that two large feeding arteries from
the ADG supplied the PAVF, and they were coagulated and cut
(Fig. 4C and D). Subsequently, the angiography of the
right lumbar artery revealed that the feeding artery from the AKA
supplied the PAVF, and it was coagulated and cut (Fig. 4E). The PAVF was then removed; the
draining vein is illustrated in Fig.
4F. The complete removal of the PAVF was confirmed upon an
intraoperative DSA (Fig. 5A and
B).
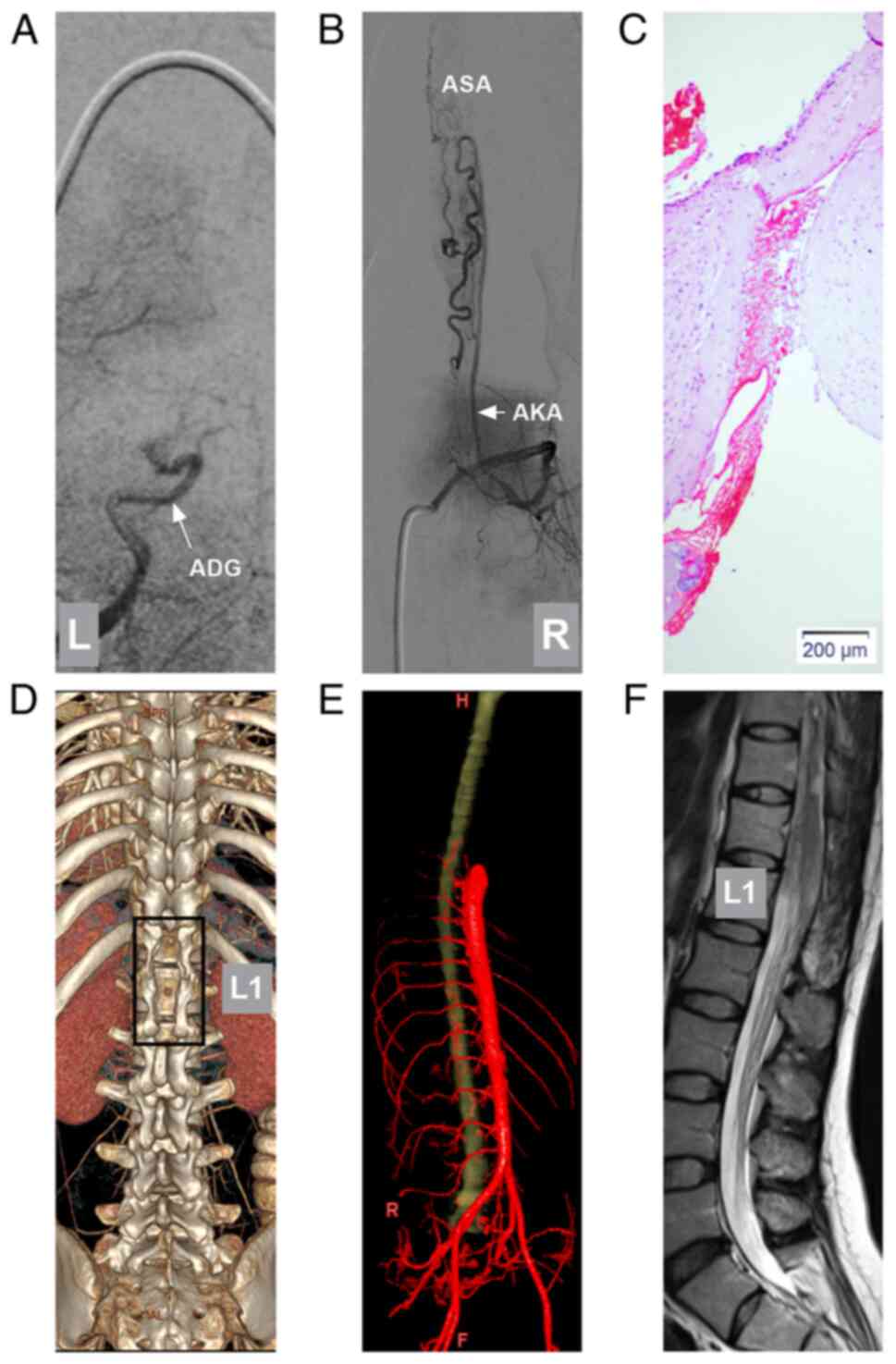 | Figure 5Post-operative DSA, pathology,
follow-up CTA and MRI. (A) Post-operative DSA of the left iliac
artery illustrating that the ADG (arrow) was occluded. (B)
Post-operative DSA of the lumbar artery (L2 level) illustrating
that the AKA (arrow) did not supply the PAVF, and a hypoplastic ASA
was found. (C) Pathological evaluation illustrating that the PAVF
was an irregular vessel with a dilated lumen and thickened wall.
(D) The 3-month follow-up CTA illustrating the post-operative bone
window (frame) centered on the L1 level. (E) The 3-month follow-up
CTA illustrating no dilated draining vein. (F) The 2-year follow-up
T2-weighted MRI illustrating the regression of the PAVF; dilated
vessels were not found. AKA, artery of Adamkiewicz; ADG, artery of
Desproges-Gotteron; CTA, computed tomography angiography; DSA,
digital subtraction angiography; L1, first lumbar vertebra; MRI,
magnetic resonance imaging; PAVF, perimedullary arteriovenous
fistula. |
After the surgery, the patient's symptoms did not
become aggravated, and anticoagulation therapy was administered to
avoid thrombosis of the draining vein. A pathological examination
revealed PAVF changes (Fig. 5C).
Following discharge, she was prescribed rehabilitation exercises,
including functional electrical stimulation and exercise therapy on
motor control and functional ability of the lower extremity, and
she gradually recovered. The 3-month follow-up CTA did not reveal a
PAVF (Fig. 5D and E). At 2-years post-surgery, an MRI did not
reveal a PAVF (Fig. 5F). Her
modified Rankin scale score was 2(7).
Discussion
The ADG is the spinal posterior radiculomedullary or
radiculopial artery, also known as the ‘cone artery’; this
inconstant artery was first described by Desproges-Gotteron in 1955
(4,8). The ADG may arise from the internal
iliac artery or the iliolumbar artery and courses alongside the L5
or S1 nerve roots up to the conus medullaris and anastomoses with
the perimedullary network (conal basket) (1).
The ADG is a rare arterial variation that functions
as a feeder of spinal vascular malformations (1). Therefore, this artery is often missed,
particularly when it is located near the contralateral iliac
artery, and spinal angiography is not performed. If the ADG is
missed, treatment may be incomplete. Due to its rarity, the present
study reports a case of a PAVF supplied by the ADG in an aim to
share the experience with its treatment. At the same time, a search
of the literature was performed on PubMed, which yielded only three
cases (Table I).
 | Table IReports of spinal arteriovenous shunts
supplied by the artery of Desproges-Gotteron. |
Table I
Reports of spinal arteriovenous shunts
supplied by the artery of Desproges-Gotteron.
| Case no. | Author, year | Age/sex | Main symptom | Arteriovenous
shunt | Feeding artery | Draining vein | Treatment | Prognosis | (Refs.) |
|---|
| 1 | Tubbs et al,
2011 | 54/F | Urination difficulty,
lower extremity weakness | AVM of the conus
medullaris | Artery of
Desproges-Gotteron | Thoracolumbar
perimedullary vein | Embolization with
Onyx | Good | (4) |
| 2 | Cohen et al,
2013 | 8/F | Urination difficulty,
paraparesis | PAVF of the conus
medullaris | Artery of
Desproges-Gotteron, thoracic radiculomedullary artery | Thoracolumbar
perimedullary vein | Embolization with
Onyx | Good | (1) |
| 3 | Munich et al,
2019 | 65/F | Urination difficulty,
lower extremity weakness | DAVF of the S1 nerve
root | Artery of
Desproges-Gotteron |
Cervico-thoracic-lumbar perimedullary
vein | Embolization with
n-butyl cyanoacrylate | Good | (9) |
| 4 | Present study | 31/F | Urination difficulty,
paraparesis | PAVF of the conus
medullaris | Artery of
Desproges-Gotteron, artery of Adamkiewicz |
Cervico-thoracic-lumbar perimedullary
vein | Microsurgery under
intraoperative DSA assistance | Good | |
Among these three cases, one was an arteriovenous
malformation (4), one was a dural
AVF (9) and one was a PAVF (1). In the case described herein, the
patient had a PAVF. As shown in Table
I, all patients, including the patient in the present study,
were female, ranging in age from 8-65 years. PAVFs are considered
type IV spinal vascular malformations and are located on the
surface of the spinal cord without an intervening nidus of abnormal
vessels (10). When PAVFs are
ventral and directly fed by anterior spinal arteries, they are
subpial in location, whereas those fed by posterior spinal arteries
are subarachnoid.
When PAVFs occur, the fistula shunts high-pressure
arterial blood into the spinal cord vein system, which leads to
pial venous reflux and congestion, causing myelopathy (11). PAVFs typically cause clinical
symptoms, including a weakness of the lower extremities, urination
difficulties and intradural subarachnoid hemorrhage. Of these
symptoms, hemorrhage occurs in 36% of PAVFs, primarily high-flow
PAVFs (10). The symptoms of PAVFs
vary; in the present case, the high-flow PAVF did not result in
bleeding, although it did result in neurological deficits, and the
symptoms progressed very rapidly. Within 3 days, lower limb
paralysis occurred due to the high-flow shunt, which tends to have
a malignant natural history.
Currently, DSA remains the gold standard for
diagnosis; although CTA and MRI can be used to identify the PAVF
trace, the trace is often the draining vein. In PAVFs, the draining
vein can dilate too thicker than the feeding artery, and due to
insufficient resolution, the feeding artery may not be identified.
When a PAVF creates a high-flow shunt, the affected area of
perimedullary veins over the anterior and posterior surfaces of the
spinal cord often extends over multiple levels; in the present
case, the perimedullary draining vein reached the cervical level
(Fig. 2D). Therefore, when searching
for the fistula point, it is necessary to examine the whole spinal
cord by DSA from the vertebral artery to the iliac artery.
Symptomatic PAVFs should be administered treatment
promptly. The currently available treatments for PAVF include
surgery, endovascular embolization and a combination of the two
methods (12). Regardless of the
method used, the fistula must be completely removed or blocked
during treatment. For small PAVFs with a simple angioarchitecture,
endovascular embolization is considered a beneficial choice. For
instance, Cohen et al (1)
reported the case of a PAVF in an 8-year-old boy that was supplied
by the ADG and a thoracic radiculomedullary artery that joined at
the fistula site in a large partially thrombosed varix, which was
completely occluded with the liquid embolic material Onyx.
When PAVFs are large and exhibit a high flow, simple
surgical removal is associated with certain risks, as it is
difficult to determine the fistula angioarchitecture, and it can be
difficult to completely occlude the fistula via endovascular
embolization (13). Therefore, the
optimal choice is the combination of intraoperative DSA and
microsurgery. In the case described herein, the selective
angiography of the AKA and the ADG was performed, and the feeding
arteries were easily identified and resected. Notably, DSA can
confirm the completion of treatment.
In the majority of cases of PAVFs, satisfactory
outcomes can be achieved after timely treatment, as well as in
cases of other arteriovenous shunts, as presented in Table I. In these cases, timely treatment is
considered critical in order to avoid an excessive amount of
delayed neuronal death. Following surgery, anticoagulation therapy
should be administered to avoid postsurgical retrograde thrombosis
of the spinal artery and the pial venous plexuses along the spinal
cord. Therefore, according to the literature review performed in
the present study, microsurgery with intraoperative DSA assistance
may be an effective treatment strategy for PAVFs involving the
ADG.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YW and JY designed the study and drafted the
manuscript. YW collected the data. JY and YW confirm the
authenticity of all the raw data. Both authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was not required by the authors'
institution, as the present study is a case report. Informed signed
consent to participate was obtained from the patient.
Patient consent for publication
The patient provided consent and agreed for her data
to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cohen JE, Constantini S, Gomori JM,
Benifla M and Itshayek E: Pediatric perimedullary arteriovenous
fistula of the conus medullaris supplied by the artery of
Desproges-Gotteron. J Neurosurg Pediatr. 11:426–430.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mizutani K, Consoli A, Maria FD, Condette
Auliac S, Boulin A, Coskun O, Gratieux J and Rodesch G: Intradural
spinal cord arteriovenous shunts in a personal series of 210
patients: Novel classification with emphasis on anatomical
disposition and angioarchitectonic distribution, related to spinal
cord histogenetic units. J Neurosurg Spine: April 2, 2021 (Epub
ahead of print).
|
|
3
|
Reis C, Rocha JA, Chamadoira C, Pereira P
and Fonseca J: Foraminal L5-S1 disc herniation and conus medullaris
syndrome: A vascular etiology? Acta Neurochir (Wien). 149:533–535;
discussion 535. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tubbs RS, Mortazavi MM, Denardo AJ and
Cohen-Gadol AA: Arteriovenous malformation of the conus supplied by
the artery of Desproges-Gotteron. J Neurosurg Spine. 14:529–531.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Seeder L: Muscle strength grading. Ann
Emerg Med. 12(407)1983.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harrow-Mortelliti M, Reddy V and
Jimsheleishvili G: Physiology, Spinal Cord. In: StatPearls.
StatPearls Publishing, Treasure Island, FL, 2021.
|
|
7
|
Rankin J: Cerebral vascular accidents in
patients over the age of 60. II. Prognosis. Scott Med J. 2:200–215.
1957.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rouques L and David M: Desproges and
Israel. A new case of traumatic segmental arachnoiditis. Rev Neurol
(Paris). 91:124–126. 1954.PubMed/NCBI(In French).
|
|
9
|
Munich SA, Krishna C, Cress MC, Dhillon
GS, Pollina J and Levy EI: Diagnosis and endovascular embolization
of a sacral spinal arteriovenous fistula with ‘Holo-spinal’ venous
drainage. World Neurosurg. 128:328–332. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ji T, Guo Y, Shi L and Yu J: Study and
therapeutic progress on spinal cord perimedullary arteriovenous
fistulas. Biomed Rep. 7:214–220. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gailloud P and Jallo GI: Delayed formation
of a symptomatic de novo low-flow perimedullary arteriovenous
fistula two years after successful treatment of a high-flow
perimedullary arteriovenous fistula. J Neuroradiol. 48:22–24.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ioannidis I, Nasis N, Plakas S,
Chrysicopoulos C and Andreou A: Combined surgical and endovascular
approach to treat a ventrally located perimedullary arteriovenous
fistula. Childs Nerv Syst. 37:645–648. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li J, Zeng G, Zhi X, Bian L, Yang F, Du J,
Ling F and Zhang H: Pediatric perimedullary arteriovenous fistula:
Clinical features and endovascular treatments. J Neurointerv Surg.
11:411–415. 2019.PubMed/NCBI View Article : Google Scholar
|