Introduction
Osteoporotic fracture (OF) is a major public health
concern among the aging population worldwide. The risk of recurrent
OF (ROF) is substantially high among the elderly; for instance, a
previous study found that 26% of elderly community dwellers with an
index OF (background OF at baseline) experienced a ROF during the
16-year follow-up period (1).
Another study also reported that 15% of elderly post-menopausal
women developed an imminent ROF within 2 years following an index
OF (2). However, predicting ROF in
the elderly, particularly their imminent ROF, remains suboptimal in
clinical practice, which poses significant challenges to
osteoporosis management and fracture prevention.
Bone turnover markers (BTMs) have been proposed for
the risk prediction of OFs. For instance, the International
Osteoporosis Foundation suggested the use of BTMs as a potential
surrogate for OF risk prediction, independent of bone mineral
density (BMD) (3). A recent
meta-analysis also demonstrated significant associations between
BTMs and future OF risk after adjusting for clinical risk factors
and BMD (4). Nevertheless, evidence
regarding the role of BTMs in the prediction of imminent ROFs is
limited. Therefore, the present prospective cohort study aimed to
assess the association between BTMs and risk of ROFs in the elderly
who were hospitalized due to an index OF. Clarifying the
association between BTMs and the risk of ROFs may help with
enhanced risk prediction and management among the elderly with an
index fracture.
Patients and methods
Study participants and setting
Details about the study procedures have been
published in a previous study (5).
In brief, a cross-sectional study was conducted to explore sleep
patterns in relation to BMD by enrolling elderly patients from the
Department of Orthopedics in a general hospital in Zhuhai, China
from February, 2020 to September, 2021. The consecutive sampling
method was used for patient enrollment. Patients were included if
they were ≥55 years of age and were hospitalized due to an index
OF, where the index OF was defined as all fragility fractures apart
from those on toes, the face and fingers. All the elderly patients
were admitted to the hospital within 24 h after they developed the
index OF. The data were collected through the hospital information
system, laboratory measures and the face to face interviews with
the research personnel. A total of 100 to 160 patients were
estimated to satisfy the sample size requirement (5).
The present study was a post hoc exploratory study
aiming to assess the BTMs in relation to imminent ROFs in the
elderly. Therefore, follow-up was conducted from December, 2021 to
January, 2022 to collect data on ROFs by contacting the
participants via telephone calls and searching for their medical
records. The Guangdong Second Provincial General Hospital Ethics
Committee approved the study. All participants provided written
informed consent prior to enrollment.
BTMs
Data on bone resorption marker [C-terminal
telopeptide of type I collagen (CTX)] and bone formation markers
[procollagen type I N propeptide (P1NP), osteocalcin (OC) and total
alkaline phosphatase (TALP)] were collected. The selection of these
BTMs was based on the recommendations on the use of BTMs in
clinical studies from some consensus and position statements
(3,6,7). Fasting
blood samples were drawn from all patients on the day or the
following morning when they were admitted to hospital and before
they received any therapy. All the BTMs were measured using
immunoassays on the Roche Cobas e411 analyzer (Roche Diagnostics),
where the coefficients of variation (CVs) ranged from 3.4 to 5.7%
for the BTM measures. The serum P1NP/CTX ratio was calculated by
dividing the P1NP values by the CTX measurements.
Outcome
The outcome in the present study was the time to the
first ROF after the index fracture. The index fracture was defined
as the OF when the patients were hospitalized at baseline, while a
recurrent fracture was defined as the OF that occurred during
follow-up. By contacting the patients and searching their medical
records, the present study documented whether they developed an
imminent ROF, and if so, the time and type of their OF, and whether
this fracture required hospitalization. Patients without an
imminent ROF during follow-up were categorized into the control
group.
Other independent variables
Patient data on sex, age, body mass index (BMI),
smoking status and alcohol consumption were collected at baseline.
Information on whether they had a prior OF in the previous 5 years,
and whether they were administered any anti-osteoporotic drugs
before they were hospitalized, was documented. Patient baseline BMD
T-scores at lumbar spine L1 - L4 were
measured using dual-energy X-ray absorptiometry (GE Prodigy,
HyClone; Cytiva), where the T-scores were calculated based on the
standards for Chinese individuals (8). The CV of spine BMD measures was 1.2%.
Data on other independent variables, including circulating
25-hydroxyvitamin D [25(OH)D], parathyroid hormone (PTH) and growth
hormone (GH) were also obtained. 25(OH)D levels were measured using
the colloidal gold immunochromatography assay with kits from
Pro-Med Technology, Ltd. (cat. no. 20142400066). PTH levels were
measured using enzyme linked immunosorbent assay (sandwich
technique) with kits from Cusabio Technology LLC (cat. no.
CSB-E06934h). GH levels were quantified using Siemens IMMULITE
platforms (1000) with the standardized assay kits (Siemens
Healthcare Diagnostics; cat. no. 20101402367). All the CVs for the
three tests were <10%.
Statistical analysis
Frequency and percentage were used to describe
categorical variables, and median and interquartile was used for
continuous variables due to their violation of normality assumption
(all P-values <0.05 from the Shapiro-Wilk test). The
Mann-Whitney U test was used to compare whether there was a
significant difference in BTMs between patients with and without a
ROF during follow-up.
The Cox proportional hazards model was employed to
evaluate the association between BTMs and the risk of ROFs, with
hazard ratios (HRs) and corresponding 95% confidence intervals
(CIs) for per-SD (standard deviation) increase in BTMs reported.
The results are shown for both the univariate and multivariable
models, in which the multivariable model was adjusted for BMD, age,
sex and BMI. A global statistical test and a graphical assessment
using Schoenfeld residuals were used to test the proportional
hazards assumption in the Cox models. Moreover, a post-hoc
sensitivity analysis was performed in the model by further
adjusting for history of OF, using anti-osteoporotic drugs
(bisphosphonates, selective estrogen receptor modulators,
calcitonin, parathyroid hormone, calcium and/or vitamin D
supplementation, and others), 25(OH)D, PTH and GH, to evaluate
whether the results were similar to those from the main
analysis.
As an exploratory analysis, the present study
assessed whether the BTMs had potential for predicting the risk of
an imminent ROF. The BTMs that had a significant association with
the risk of OF were entered in the basic model to generate
Harrell's C-index, where the basic model included age, sex, BMD and
BMI. The C-index from the model with BTMs was also compared to the
model only including BMD and the basic model, to examine whether
the addition of BMTs can enhance the model discrimination (9). The model only including BMD (BMD-alone)
was used as a reference.
All analyses were conducted using STATA Version 17
(StataCorp LLC) and SAS Version 9.4 (SAS Institute, Inc.) software.
Unless otherwise specified, all the tests were two-sided and a
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
A total of 169 eligible patients were enrolled and
included in the analyses. They had a median age of 72 years and a
median BMI of 22.2 kg/m2. The majority of the patients
were female (87.6%). A small proportion of patients were smokers or
consumed alcohol. The median BMD T-score was -3.70 (Q1 to Q3, -4.30
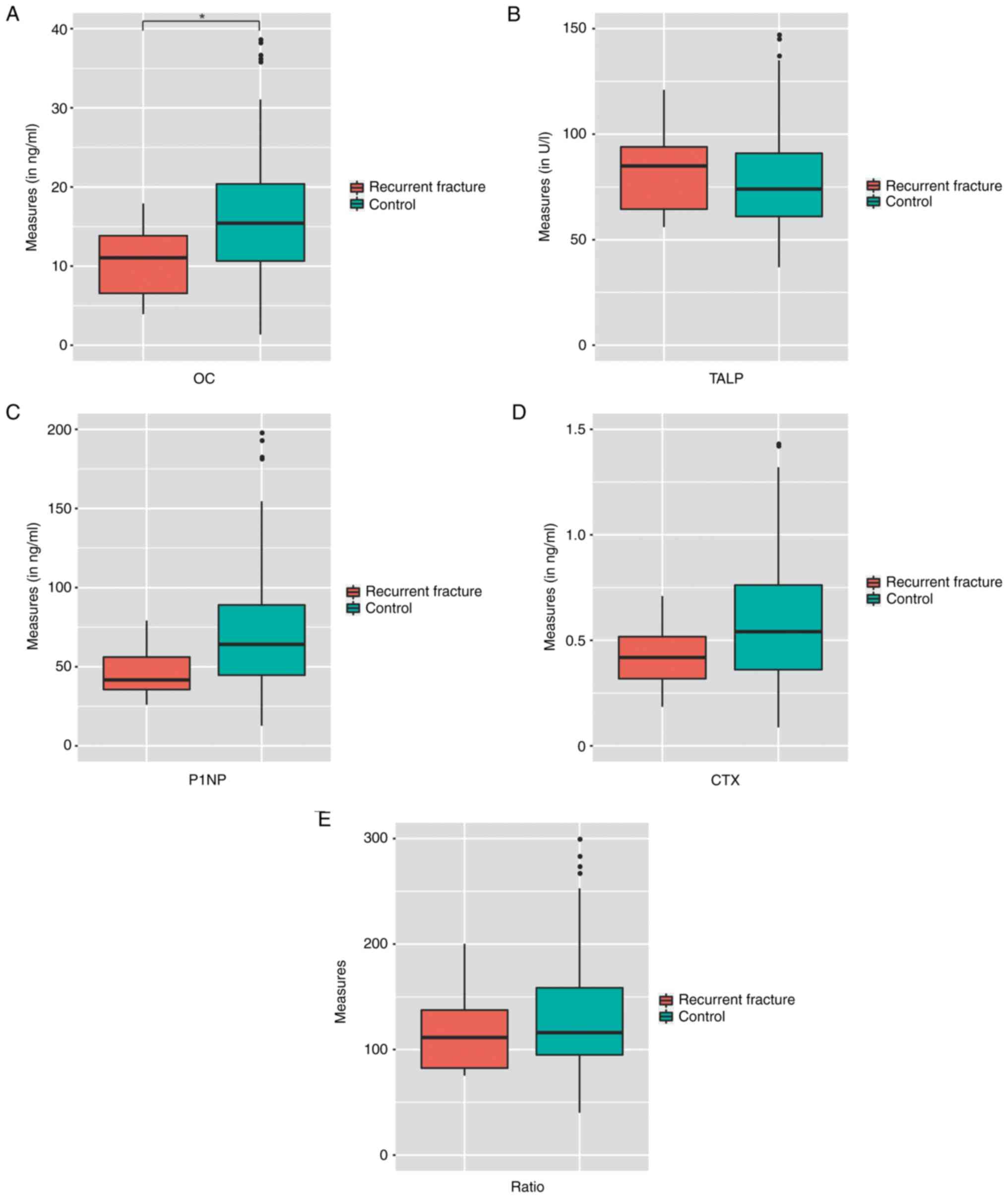
to -2.60). The median measures for OC were 15.33 ng/ml, 75.0 U/l
for TALP, and 61.14 and 0.54 ng/ml for P1NP and CTX, respectively
(Table I).
 | Table IDescription of patient
characteristics. |
Table I
Description of patient
characteristics.
| Characteristics | Overall patients
(n=169) | Patients with ROFs
(n=7) | Patients without ROFs
(n=162) |
|---|
| Age, median (Q1, Q3),
years | 72.0 (64.0,
80.0) | 73.0 (72.0,
85.0) | 71.0 (64.0,
80.0) |
| Female proportion, n
(%) | 148 (87.57) | 5 (71.43) | 143 (88.27) |
| BMI, median (Q1, Q3),
kg/m2 | 22.20 (19.50,
24.55) | 20.90 (20.21,
23.33) | 22.25 (19.20,
24.60) |
| Use of
anti-osteoporotic medication prior to hospitalization, n (%) | 26 (15.38) | 1 (14.29) | 25 (15.43) |
| History of
osteoporotic fracture in the past 5 years, n (%) | 71 (42.01) | 5 (71.43) | 66 (40.74) |
| Smoking status, n
(%) | 15 (8.88) | 1 (14.29) | 14 (8.64) |
| Alcohol consumption,
n (%) | 10 (5.92) | 0 | 10 (6.17) |
| OC, median (Q1, Q3),
ng/ml | 15.33 (10.13,
20.57) | 11.05 (4.41,
14.72) | 15.92 (10.86,
20.65) |
| TALP, median (Q1,
Q3), U/l | 75.0 (61.0,
95.0) | 85.0 (57.0,
96.0) | 74.0 (61.0,
95.0) |
| P1NP, median (Q1,
Q3), ng/ml | 61.14 (43.44,
89.56) | 41.64 (32.74,
70.45) | 64.63 (44.70,
91.12) |
| CTX, median (Q1, Q3),
ng/ml | 0.54 (0.36,
0.75) | 0.42 (0.29,
0.55) | 0.55 (0.36,
0.77) |
| 25(OH)D, median (Q1,
Q3), ng/ml | 23.12 (18.59,
29.72) | 22.97 (18.63,
24.60) | 23.23 (18.55,
30.30) |
| PTH, median (Q1, Q3),
pg/ml | 31.40 (23.21,
43.26) | 31.11 (24.29,
44.63) | 31.43 (22.76,
43.15) |
| GH, median (Q1, Q3),
ng/ml | 0.39 (0.15,
0.90) | 0.38 (0.19,
0.91) | 0.39 (0.14,
0.90) |
| BMD T-score, median
(Q1, Q3) | -3.70 (-4.30,
-2.60) | -3.70 (-4.10,
-3.10) | -3.70 (-4.30,
-2.60) |
During a median follow-up of 10.5 months, there
seven ROFs (4.1%) were observed. All the ROFs were vertebral
fractures requiring hospital admission. The baseline
characteristics of the patients with and without ROFs are presented
in Table I. The comparisons of BTMs
between the patients with and without ROFs are illustrated in
Fig. 1. Patients with ROFs had lower
levels of OC (11.05 vs. 15.92 ng/ml), P1NP (41.64 vs. 64.63 ng/ml),
CTX (0.42 vs. 0.55 ng/ml) and P1NP/CTX ratio (111.5 vs. 116.6)
compared with the controls; however only the difference in OC
levels was significant (P=0.035). By contrast, patients with ROFs
had a non-significantly higher TALP level when compared with the
controls (85.0 vs. 74.0 U/l; P=0.69). The results of the
association between BTMs and the risk of recurrent fracture are
presented in Table II. OC was found
to be significantly associated with the risk of ROFs (HR, 0.13; 95%
CI, 0.018-0.90; P=0.039) for per-SD increase in OC from
multivariable analysis. There was no significant association
between the other BTMs and the risk of ROFs, while a marginally
significant association was observed for P1NP (HR, 0.19; 95% CI,
0.034-1.04; P=0.056 for per-SD change in P1NP). Similar results
were found from the univariate and sensitivity analyses.
 | Table IIResults for associations between BTMs
and risk of ROFs. |
Table II
Results for associations between BTMs
and risk of ROFs.
| | Univariate
analysis | Multivariable
analysisa | Sensitivity
analysisb |
|---|
| BTMs | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| OC | 0.16 (0.031,
0.86) | 0.032 | 0.13 (0.018,
0.90) | 0.039 | 0.14 (0.023,
0.88) | 0.036 |
| TALP | 1.07 (0.51,
2.25) | 0.86 | 1.02 (0.49,
2.14) | 0.95 | 1.00 (0.45,
2.22) | 0.99 |
| P1NP | 0.22 (0.042,
1.16) | 0.075 | 0.19 (0.034,
1.04) | 0.056 | 0.17 (0.026,
1.05) | 0.057 |
| CTX | 0.44 (0.14,
1.35) | 0.15 | 0.35 (0.10,
1.23) | 0.10 | 0.35 (0.10,
1.25) | 0.11 |
| P1NP/CTX ratio | 0.54 (0.094,
3.09) | 0.49 | 0.53 (0.081,
3.45) | 0.51 | 0.50 (0.068,
3.60) | 0.49 |
The results from the exploratory analyses for model
discriminatory performance after taking BTMs into account are
presented in Table III. The
BMD-alone model had a C-index of 0.54, while the basic model
yielded a C-index of 0.75. After incorporating OC into the basic
model, a C-index of 0.83 (95% CI, 0.70-0.96; P<0.001) was found.
This discrimination from the model of OC plus basic model was
observed to significantly outperform the BMD-alone model, with an
improvement for C-index of 0.29 (95% CI, 0.028-0.55; P=0.030)
found.
 | Table IIIResults of model discrimination
between different models. |
Table III
Results of model discrimination
between different models.
| | Discriminatory
performance | |
|---|
| Model | C-index (95%
CI) | P-value | Comparison of model
discrimination |
|---|
| BMD-alone
model | 0.54 (0.35,
0.73) |
<0.001 | Reference | Reference |
| Basic
modela | 0.75 (0.60,
0.91) |
<0.001 | 0.21 (-0.013,
0.44) | 0.065 |
| BTM-alone
modelb | 0.71 (0.51,
0.90) |
<0.001 | 0.17 (-0.18,
0.51) | 0.34 |
| BTM + basic
modelc | 0.83 (0.70,
0.96) |
<0.001 | 0.29 (0.028,
0.55) | 0.030 |
Discussion
In the present prospective cohort study, the
association between BTMs and the risk of imminent ROFs in the
elderly hospitalized with an index fracture was investigated. It
was found that the per-SD increase in the OC measure was
significantly associated with an 87% lower risk of developing ROFs.
Exploratory analysis results revealed that incorporating OC into
the prediction enhanced the predictive accuracy of recurrent
fracture risk when compared with the BMD-alone model.
BTMs have been reported to directly influence some
important elements in the development of OFs, including BMD, bone
matrix, macro-architecture and micro-architecture (10,11).
BTMs reflect the bone turnover process more rapidly than BMD;
therefore, they are increasingly used for the assessment of patient
responses to treatment (3,12,13).
Some observational studies have also revealed an association
between baseline BTMs and the risk of index OFs in community
dwellers (14-19),
with inconclusive results reported. For instance, while BTMs were
found to be significantly associated with OFs in elderly women
(14,17), a non-significant association was
observed in other research on the elderly (19). It has been argued that the use of
BTMs as predictors of OFs may be sensitive to be included in a
fracture prediction model, requiring more exploration for
clarifying the potential of BTMs for risk prediction improvement
(20).
P1NP and CTX have been suggested as preferred BTMs
in bone turnover assessment due to their specificity to bone
health, acceptable performance in clinical research and low
variability for measurement (13,21). A
slight improvement for OF prediction was indicated in models
incorporating P1NP and CTX (4).
However, they were not significantly associated with the risk of
imminent ROFs in the present study. Furthermore, unlike the
majority of published results (14,18,19), the
present study found that patients with ROFs had lower levels of OC,
P1NP and CTX. Likewise, while previous findings reported that high
BTM levels were generally associated with an increased fracture
risk, in the present study all the BTMs, apart from TALP, were
associated with a decreased risk of OFs (Table II). More specifically, for example,
a gradient of risk of ~1.2 per-SD increase in P1NP and CTX was
previously reported regarding the fracture risk (4); by contrast, the present study revealed
a markedly decreased risk of ROFs with per-SD increase in P1NP (HR,
0.19) and CTX (HR, 0.35), although the association was not
significant. While previous studies have focused on fracture-naive
patients or those receiving anti-resorptive or anabolic treatment,
in the present study, the included patients were among those who
were hospitalized within 24 h after they experienced an index
fracture and whose BTMs were measured before they received any
treatment. Therefore, the BTMs explored in the present study may
reflect the acute self-recovery responses of bone turnover after an
OF without medication or surgical intervention in the elderly. The
pathophysiology of OFs in the elderly remains largely unclear,
particularly as regards their substantially high risk of ROFs
within a short period of time (22).
Thus, the findings of the present study may provide some insight
into how BTMs before treatment are associated with an imminent risk
of ROFs in the elderly.
The present exploratory analysis found that after
incorporating OC in the model including clinical risk factors and
BMD, the model yielded an acceptable discrimination (C-index up to
0.83). This result may suggest the potential of adding serum OC
measures before treatment to enhance predictive accuracy for ROF
risk. OC is produced by osteoblasts only and is excreted by the
kidneys; therefore, it has specificity to bone health (23,24). OC
had been found to be broadly associated with bone mineralization,
body metabolism, cognition and reproduction, thereby being proposed
as a bone-derived hormone (23,25).
Moreover, OC has been recognized as a specific biomarker of
osteoblast function in osteoporosis regarding the assessment of
bone formation rate, particularly among elderly women (26). Other potential mechanisms of OC in
relation to ROFs have also been reported, which include the
improvement of bone microenvironment and mineralization, and the
promotion of energy metabolism and hormone downregulation (27-30).
These findings may help interpret why OC could have the potential
to improve the model discrimination for risk of ROF. However,
evidence of whether increased serum OC levels following an OF and
before treatment could react to the acute impaired skeleton
homeostasis and may become involved in physiological processes in
an endocrine manner, remaining sparse and limited. Subsequently,
the serum OC level in relation to an imminent risk of ROFs warrants
further research to clarify its potential to improve the prediction
of fracture risk in the elderly.
While the trajectory, regulation and function of
BTMs in OF remain to be further explored, the present study
provided some preliminary evidence on the association between BTM
measures following an index fracture and the imminent risk of ROFs
in elderly patients. Sound methodology and statistical analysis may
strengthen the study findings. There are some limitations to the
present study. First, the small sample size prevented the authors
from creating further subgroups or performing an exploratory
investigation; likewise, the potential insufficient power may lead
to model instability and fail to identify a significant association
between other BTMs and the risk of ROFs. As an observational study,
confounding effects particularly those of unmeasured variables,
could not be fully precluded, which may weaken the credibility and
strength of the results. For example, little is known about the
lifestyle change, treatment and rehabilitation received for the
patients after they were discharged from hospital, which would
affect the association between BTMs and the imminent risk of ROFs
to an unknown extent. All the ROFs were spine fractures demanding a
hospital admission, which may underestimate the OF recurrence,
particularly when taking the subclinical or undiagnosed fractures
into account. Likewise, it may compromise the generalizability of
the study findings to other OF sites. Thus, these results should be
interpreted with caution and should only be used for hypothesis
generation. The included patients were inpatients with an index OF
who may be in general, frailer than their peers without a need for
hospitalization. Therefore, whether the association between BTMs
and imminent ROFs remains robust in outpatients or community
dwellers who had an index fracture, remains largely unexplored. The
change in or the trajectory of BTMs in relation to ROFs could not
be assessed, due to the unavailability of relevant data. Thus,
further research is required to evaluate the dynamic role of BTMs
in imminent ROF prediction, particularly for the comparisons of
BTMs measured before and after treatment for index fractures.
In conclusion, in the present prospective study, a
significant association was found between serum OC levels and a
decreased risk of imminent ROFs in the elderly with index
fractures. Serum OC levels may have the potential for prediction of
recurrent fractures in the elderly. However, further high-quality
evidence is warranted to further clarify and validate the BTMs in
relation to the risk of imminent ROFs in the elderly.
Acknowledgements
The authors would like to thank Miss Wanlin Wu
(Guangdong Provincial Hospital of Chinese Medicine, Guangzhou,
China) for her assistance with data collection.
Funding
Funding: The authors disclose receipt of the following financial
support for the research, authorship, and/or publication of this
article: The present study was financially supported by the Science
and Technology Program of Guangzhou (grant no. 202002030252) and
the Science Foundation of Guangdong Second Provincial General
Hospital (grant no. YY2018-002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ, LL, MC and GL conceived and designed the study,
and acquired the data, performed the statistical analyses and data
interpretation, and drafted the manuscript. HZ, XX and RW assisted
with the study design and data collection, provided professional
support and assisted with the statistical analyses, and made
several critical revisions to the manuscript. All authors have read
and approved the final manuscript. MC and GL confirm the
authenticity of all the raw data. All authors guaranteed the
research performed.
Ethics approval and consent to
participate
All participants provided written informed consent
prior to enrollment in the study. The Guangdong Second Provincial
General Hospital Ethics Committee approved the study [approval no.
20190717-01(2)-YXKXYJ-KT].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Center JR, Bliuc D, Nguyen TV and Eisman
JA: Risk of subsequent fracture after low-trauma fracture in men
and women. Jama. 297:387–394. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iconaru L, Charles A, Baleanu F, Surquin
M, Benoit F, Mugisha A, Moreau M, Paesmans M, Karmali R, Rubinstein
M, et al: Prediction of an imminent fracture after an index
fracture-models derived from the frisbee cohort. J Bone Miner Res.
37:59–67. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vasikaran S, Eastell R, Bruyère O, Foldes
AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S,
Trenti T, et al: Markers of bone turnover for the prediction of
fracture risk and monitoring of osteoporosis treatment: A need for
international reference standards. Osteoporos Int. 22:391–420.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tian A, Ma J, Feng K, Liu Z, Chen L, Jia H
and Ma X: Reference markers of bone turnover for prediction of
fracture: A meta-analysis. J Orthop Surg Res. 14(68)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zeng H, Li L, Zhang B, Xu X, Li G and Chen
M: Relationship between sleep pattern and bone mineral density in
patients with osteoporotic fracture. Ther Adv Endocrinol Metab.
13(20420188221106884)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu CH, Chang YF, Chen CH, Lewiecki EM,
Wüster C, Reid I, Tsai KS, Matsumoto T, Mercado-Asis LB, Chan DC,
et al: Consensus statement on the use of bone turnover markers for
short-term monitoring of osteoporosis treatment in the asia-pacific
region. J Clin Densitom. 24:3–13. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Park SY, Ahn SH, Yoo JI, Chung YJ, Jeon
YK, Yoon BH, Kim HY, Lee SH, Lee J and Hong S: Position statement
on the use of bone turnover markers for osteoporosis treatment. J
Bone Metab. 26:213–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang ZQ, Ho SC, Chen ZQ, Zhang CX and
Chen YM: Reference values of bone mineral density and prevalence of
osteoporosis in Chinese adults. Osteoporos Int. 25:497–507.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Newson RB: Comparing the predictive powers
of survival models using Harrell's C or Somers' D. Stata J.
10:339–358. 2020.
|
|
10
|
Devogelaer JP, Boutsen Y, Gruson D and
Manicourt D: Is there a place for bone turnover markers in the
assessment of osteoporosis and its treatment? Rheum Dis Clin North
Am. 37:365–386, v-vi. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shetty S, Kapoor N, Bondu JD, Thomas N and
Paul TV: Bone turnover markers: Emerging tool in the management of
osteoporosis. Indian J Endocrinol Metab. 20:846–852.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Naylor KE, Jacques RM, Paggiosi M, Gossiel
F, Peel NF, McCloskey EV, Walsh JS and Eastell R: Response of bone
turnover markers to three oral bisphosphonate therapies in
postmenopausal osteoporosis: the TRIO study. Osteoporos Int.
27:21–31. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lorentzon M, Branco J, Brandi ML, Bruyère
O, Chapurlat R, Cooper C, Cortet B, Diez-Perez A, Ferrari S,
Gasparik A, et al: Algorithm for the use of biochemical markers of
bone turnover in the diagnosis, assessment and follow-up of
treatment for osteoporosis. Adv Ther. 36:2811–2824. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ivaska KK, Gerdhem P, Väänänen HK, Akesson
K and Obrant KJ: Bone turnover markers and prediction of fracture:
A prospective follow-up study of 1040 elderly women for a mean of 9
years. J Bone Miner Res. 25:393–403. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Crandall CJ, Vasan S, LaCroix A, LeBoff
MS, Cauley JA, Robbins JA, Jackson RD and Bauer DC: Bone turnover
markers are not associated with hip fracture risk: A case-control
study in the women's health initiative. J Bone Miner Res.
33:1199–1208. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yao S, Laurent CA, Roh JM, Lo J, Tang L,
Hahn T, Ambrosone CB, Kushi LH and Kwan ML: Serum bone markers and
risk of osteoporosis and fragility fractures in women who received
endocrine therapy for breast cancer: A prospective study. Breast
Cancer Res Treat. 180:187–195. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qu XL, Zheng B, Chen TY, Cao ZR, Qu B and
Jiang T: Bone turnover markers and bone mineral density to predict
osteoporotic fractures in older women: A retrospective comparative
study. Orthop Surg. 12:116–123. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Dai Z, Wang R, Ang LW, Yuan JM and Koh WP:
Bone turnover biomarkers and risk of osteoporotic hip fracture in
an Asian population. Bone. 83:171–177. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bauer DC, Garnero P, Harrison SL, Cauley
JA, Eastell R, Ensrud KE and Orwoll E: Osteoporotic Fractures in
Men (MrOS) Research Group. Biochemical markers of bone turnover,
hip bone loss, and fracture in older men: The MrOS study. J Bone
Miner Res. 24:2032–2038. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vilaca T, Gossiel F and Eastell R: Bone
turnover markers: Use in fracture prediction. J Clin Densitom.
20:346–352. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Eastell R, Pigott T, Gossiel F, Naylor KE,
Walsh JS and Peel NFA: DIAGNOSIS OF ENDOCRINE DISEASE: Bone
turnover markers: Are they clinically useful? Eur J Endocrinol.
178:R19–R31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Migliorini F, Giorgino R, Hildebrand F,
Spiezia F, Peretti GM, Alessandri-Bonetti M, Eschweiler J and
Maffulli N: Fragility fractures: Risk factors and management in the
elderly. Medicina (Kaunas). 57(1119)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Moser SC and van der Eerden BCJ:
Osteocalcin-A versatile bone-derived hormone. Front Endocrinol
(Lausanne). 9(794)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zoch ML, Clemens TL and Riddle RC: New
insights into the biology of osteocalcin. Bone. 82:42–49.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wei J and Karsenty G: An overview of the
metabolic functions of osteocalcin. Curr Osteoporos Rep.
13:180–185. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kuo TR and Chen CH: Bone biomarker for the
clinical assessment of osteoporosis: Recent developments and future
perspectives. Biomark Res. 5(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Manolagas SC: Osteocalcin promotes bone
mineralization but is not a hormone. PLoS Genet.
16(e1008714)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dumitru N, Carsote M, Cocolos A, Petrova
E, Olaru M, Dumitrache C and Ghemigian A: The link between bone
osteocalcin and energy metabolism in a group of postmenopausal
women. Curr Health Sci J. 45:47–51. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Razzaque MS: Osteocalcin: A pivotal
mediator or an innocent bystander in energy metabolism? Nephrol
Dial Transplant. 26:42–45. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Patti A, Gennari L, Merlotti D, Dotta F
and Nuti R: Endocrine actions of osteocalcin. Int J Endocrinol.
2013(846480)2013.PubMed/NCBI View Article : Google Scholar
|