Introduction
The development of targeted diagnostic clinical
panels based on next-generation sequencing (NGS) has allowed the
identification of various pathogenic variants in high penetrant
genes associated with different types of cancer (1–3).
This is especially the case in those involving hereditary
syndromes. For example, pathogenic variants in the BRCA
genes (BRCA1 and BRCA2) have been reported to be
associated with a higher lifetime risk of developing breast cancer
in women. They account for ~20% all familial breast cancers and
>10% patients with early-onset triple-negative breast
cancer.
The Evidence-based Network for the Interpretation of
Germline Mutant Alleles (ENIGMA) consortium has received to date
>6,000 submissions of unique variants of unknown significance
(VUS) identified in >13,000 families from >17 countries
(http://www.enigmaconsortium.org/).
These figures are expected to increase with the increased use of
gene sequencing techniques, especially NGS. NGS is capable of also
covering untranslated and deeper intronic regions (4). For certain types of BRCA gene
variants, generation of functional evidence is essential before a
variant can be clearly classified to be pathogenic, VUS or benign.
How the multiple functions of the BRCA1 and BRCA2 proteins are
associated with cancer predisposition remains poorly understood
(5).
Some of the high penetrant genes involved in
hereditary cancers include genes of the DNA damage response (DDR)
pathway. DNA repair serves a critical role in preventing the
development of cancer. A number of genes in the DDR pathway have
been documented to be mutated in hereditary cancers, such as
BRCA1, BRCA2 and ATM. In response to DNA damage,
cells activate the DDR pathway and arrest cell-cycle progression,
allowing time for DNA repair or, depending on the extent of damage,
activation of apoptosis (6–9). The
complex and multi-layered process of DNA repair is critical in
response to DNA damage and subsequent cancer cell survival
(10–13). Double-stranded DNA breaks (DSBs)
are amongst the major threats to genomic integrity. DSBs are
repaired by either one of the following two mechanistically
distinct pathways: Homologous recombination (HR), which is a
conservative form; and non-homologous end-joining (NHEJ), which is
a non-conservative form (13,14).
BRCA1/2 proteins are crucial for HR repair (15,16).
BRCA1 interacts with tumor suppressors, DNA repair proteins and
cell cycle regulators through its numerous functional domains.
Therefore, it can serve a role in a multitude of DNA repair
pathways and checkpoint regulation during DDR (17). Accordingly, cells carrying
BRCA1 mutations are particularly sensitive to DNA-damaging
agents (18). This enhanced
sensitivity to DNA-damaging agents provides an opportunity to
assess the response of BRCA1 variants to genotoxic agents
in vitro. This can then be compared with that of
non-pathogenic BRCA1 variants (18).
Sequence variants that disrupt the interaction of
BRCA1/2 with its binding partners are associated with increased
risks of developing breast and ovarian cancer (17,19).
Although some sequence variants can be pathogenic, such as
non-synonymous variants, other variants can result in amino acid
changes that do not alter the network of BRCA1/2 interactions, even
if they are non-synonymous. However, BRCA1/2 gene products
are involved in multiple processes during various stages of the
cell cycle, each of which serves a specific function (20). Therefore, the simple diagnosis of a
sequence variant by NGS is typically insufficient to directly
predict its putative role in breast cancer. To overcome this
hurdle, functional analysis of VUS could be highly beneficial, even
if it is time-consuming, labor intensive and not necessarily
conclusive (21–23).
During the course of routine NGS assessment of
families at risk for breast cancer, a VUS was identified in the
BRCA1 gene of two women, without information on its
pathogenicity. Therefore, the aim of the present study was to
perform a functional in vitro analysis of the identified
BRCA1 VUS using peripheral blood lymphocytes from these
women. This was performed through the assessment of cellular
responses to genotoxic challenge induced by γ-radiation and the
chemotherapeutic agent doxorubicin.
Material and methods
Patient target population
Women at high risk of familial cancer were genotyped
through NGS with a costum-made panel of high-risk genes, namely
BRCA1, BRCA2, PTEN, TP53, BRCA1-interacting helicase 1, RAD51C,
RAD51D, ATM, partner and localizer of BRCA2, checkpoint kinase
2 and cadherin-1, which are described to be essential in
the clinical guidelines for breast cancer studies (21,23,24).
All exons and exon-intron boundaries had 100% coverage. In
addition, two healthy, non-carrier controls (NC) with no VUS
identified after NGS for the same clinical panel were also included
in this study.
The participants enrolled were selected from a
collaboration with the company Ophiomics Precision Medicine. All
participants were informed about the present study and the
collection of blood samples by venous puncture were preceded by the
signing of an informed consent form agreeing to the use of their
blood samples for research. Detailed family history of oncological
diseases for each patient/participant was also collected. All
personal data was anonymized, and the samples were coded. The
present study was approved by the National Commission of Data
Protection (approval no. 10637/2016) for the use of samples for
research and also by the Ethical Commission of NOVA Medical
School/Faculdade Ciências Médicas (NMS/FCM; approval no.
54/2018/CEFCM).
DNA extraction
Genomic DNA extraction from total peripheral blood
cells was performed with the GeneJET Whole Blood Genomic DNA
Purification Mini kit (Thermo Fisher Scientific, Inc.) according to
manufacturer's instructions, with a minor change: the final elution
volume was 75 µl. The quantification and the quality of the
extracted DNA was evaluated with the electrophoresis system Agilent
2200 TapeStation System (Agilent Technologies, Inc.) using the
Agilent Genomic DNA ScreenTape and Reagents kit (Agilent
Technologies, Inc.) according to manufacturer's instructions.
Next-generation sequencing (NGS) for
variant detection
Genomic DNA extracted from peripheral blood (200 µl)
of patients was evaluated for DNA concentration and integrity; DNA
isolated from each sample was quantified in 2200 TapeStation using
the Genomic DNA ScreenTape (Agilent Technologies, Inc.). Genomic
DNA libraries were prepared using the Ion Ampliseq Library kit
(2.0) using the custom Ion Ampliseq panel described above and
quantified by quantitative PCR with the Ion Library Quantification
kit (Thermo Fisher Scientific, Inc.). The emulsion PCR of amplified
libraries was performed using Ion Chef (Thermo Fisher Scientific,
Inc.). Sequencing runs were performed with Ion personal machine
using 316 Chips (Thermo Fisher Scientific, Inc.) aiming for a mean
sequencing depth coverage of 100×. Variant annotation was performed
in reference to Human Genome version GRCh38 and based on
information contained in the databases ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/),
DGVa (https://www.ebi.ac.uk/dgva/), dbSNP
(https://www.ncbi.nlm.nih.gov/snp/),
HGMD-PUBLIC (https://www.hgmd.cf.ac.uk/ac/index.php), EBI Variation
HomoSapiens (https://www.ebi.ac.uk/eva/). The bioinformatics
algorithms used to predict the functional impact of variants were:
PolyPhen (http://genetics.bwh.harvard.edu/pph2/), SIFT
(https://sift.bii.a-star.edu.sg), LoF
(http://aloft.gersteinlab.org), Condel
(https://bbglab.irbbarcelona.org/fannsdb/help/condel.html),
BLOSUM62 scoring matrix used in BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and CAROL
(https://www.sanger.ac.uk/tool/carol/).
Detection of copy number variants for BRCA1
and BRCA2 genes was performed by MLPA with the panels MLPA®
Salsa® P002-BRCA1 and MLPA® Salsa® P090-BRCA2 (MRC-Holland BV),
respectively. The Portuguese founder mutation (c_156_157 inserção
Alu) BRCA2 (OMIM:600185) was also screened by PCR. All
reported variants classified as pathogenic or unknown significance,
occurring in coding regions and at frequencies >10% were
validated by Sanger sequencing (ABI3100 Avant; Thermo Fisher
Scientific, Inc.).
Sanger sequencing
PCR reactions to prepare samples for Sanger
sequencing were performed according to Platinum® PCR SuperMix High
Fidelity (Invitrogen; Thermo Fisher Scientific, Inc.)
manufacturer's instructions. Briefly, a total volume of 20 µl was
used, containing 18 µl of Platinum® PCR SuperMix High Fidelity
(Invitrogen; Thermo Fisher Scientific, Inc.), 0.2 µM of forward
(5′-AATGATAGGCGGACTCCCAG-3′) and reverse
(5′-GAGGCTTGCCTTCTTCCGAT-3′) primers. High quality genomic DNA
(5–50 ng) extracted from peripheral blood cells was added to the
PCR mix and the PCR reactions were performed in a Veriti™ 96-Well
Thermal Cycler (Thermo Fisher Scientific, Inc.). The cycling
conditions employed were initial denaturation at 94°C, 2 min, 30
cycles of amplification at 94°C, 15 sec, 55°C, 15 sec, 68°C, 1 min
and held at 10°C. The size and quantity of each fragment analyzed
was evaluated with the electrophoresis system Agilent 2200
TapeStation System (Agilent Technologies, Inc.) using the kit DNA
ScreenTape and Reagents (Agilent Technologies, Inc.) according to
manufacturer's instructions. Each sample was sequenced with both
forward and reverse primers in an Applied Biosystems 3130 Genetic
Analyzer (Thermo Fisher Scientific, Inc.) according to
manufacturer's instructions.
In vitro γ-irradiation
Blood samples were irradiated in vitro using
a 60Co radiation source in a Precisa 22 irradiator at
the Ionizing Radiation Installations at Center for Nuclear Sciences
and Technologies-Instituto Superior Técnico (C2TN-IST) in Lisbon.
Each donor sample was irradiated with a dose of 2 Gy and a
non-irradiated control (0 Gy) was included. A total of ~4 ml whole
blood was isolated from each donor and subject to irradiation after
which each assay was performed as described below. To perform the
comet assay, lymphocytes were isolated and then distributed into
4-ml glass tubes for irradiation.
CA assay
After irradiation, blood samples were cultured in
triplicates or quadruplicates for each donor. The experiments were
performed as previously described (25–27)
with minor modifications. Briefly, 500 µl irradiated and
non-irradiated whole blood was added to 4.5 ml RPMI-1640 medium
with L-Glutamine (MilliporeSigma), supplemented with 25% FBS
(MilliporeSigma), 1.5% penicillin-streptomycin (Pen-Strep), 0.5%
sodic heparin (5,000 UI/ml; B. Braun Medical Inc.) and 2.5%
phytohemagglutinin (Gibco; Thermo Fisher Scientific, Inc.).
Cultures were maintained in an incubator at 37°C and 5%
CO2 at an 40° angle for 48 h. After 24 h, colcemid (0.08
µg/ml; Gibco; Thermo Fisher Scientific, Inc.) was added to the
culture. At the end of the 48 h, cultures were centrifuged at 400 ×
g for 5 min at RT (RT). The pellet was then resuspended with mild
stirring before 10 ml KCl solution [0.56% (p/v)] previously warmed
to 37°C was added and homogenization by inversion. The tubes were
incubated at 37°C for 20 min to promote hypotonic shock and then
centrifuged at 400 × g for 5 min at RT. The cells were fixed under
stirring with 5 ml fixative mixture of methanol:acetic acid [3:1
(v/v)] previously cooled at −20°C, before being centrifuged at 400
× g for 5 min at RT. These two steps of fixation and subsequent
centrifugation were repeated two or three times, until the
supernatant became clear. Finally, 10 ml fixative mixture was added
to each tube and the samples were stored at −20°C.
Samples held at −20°C were centrifuged at 400 × g
for 5 min at RT following which the supernatant was removed and the
suspension homogenized by gentle tapping. Glass slides were washed
and immersed in distilled water at 4°C and a few drops of the cell
suspension were spread onto each slide. Once well dried for 24 h at
RT, the slides were stained with Giemsa's solution 4% (v/v) in 0.01
M phosphate buffer (pH 6.8) for 10 min. Excess dye was then washed
off under running water. Once well dried again, permanent slides
were prepared using a mounting medium [Entellan® (MilliporeSigma)].
Slides were then scored using an optical microscope at ×1,000
magnification. Scoring was performed in 200 complete metaphases (46
chromosomes) by two independent evaluators (100 each), according to
the criteria described by Rueff et al (28) and following the recommendations of
the International Atomic Energy Agency (IAEA) (29). Each metaphase was analyzed
according to the following criteria: The presence of chromosomal
aberrations, namely chromatid with gaps or breaks; chromosomes with
gaps or breaks; excess of acentric fragments; dicentric chromosomes
DIC; and rings. The metaphases containing ≥1 chromosome aberration
except gaps were accounted for the frequency (%) of aberrant cells
excluding gaps [chromosomal aberration excluding gaps (CAEG)].
Cytokinesis-blocked micronucleus assay
(CBMN)
For the CBMN assay, blood samples were cultured
after irradiation in triplicate or quadruplicate for each donor
(26,30,31).
Briefly, 0.5 ml irradiated whole blood was added into each tube
containing 4.5 ml RPMI-1640 medium with L-Glutamine, supplemented
with 25% FBS, 1.5% of Pen-Strep, 0.5% of sodic heparin and 2.5% of
phytohemagglutinin. Cultures were maintained at 37°C, 5%
CO2 and at an angle of 40° for ~72 h. After 44 h,
cytochalasin-B (6 µg/ml; MilliporeSigma) was added. At the end of
the 72 h, cultures were centrifuged at 110 × g for 10 min at RT.
After discarding the supernatant, cells were washed twice with 5 ml
washing solution [RPMI-1640 medium with L-Glutamine and
NaHCO3 (0.1 g/l), supplemented with 2% FBS] and
centrifuged at 110 × g for 7 min at RT. Mild hypotonic treatment
was then performed by adding 5 ml 4:1 distilled water: RPMI-1640
medium with L-Glutamine (pH 7.2) and NaHCO3 (0.1 g/l),
supplemented with 2% FBS, followed by centrifugation at 110 × g for
5 min at RT. After concentrating the pellet by discarding most of
the supernatant, a drop of cell suspension was placed onto each
glass slide and a smear was performed.
Once the glass slides were completely dry, they were
fixed with pre-cooled 5 ml methanol:acetic acid solution [3:1
(v/v)] for 20 min at −20°C. The slides were then dried and stained
with Giemsa's solution in 0.01 M phosphate buffer (pH 6.8) for 8
min at RT. The permanent slides were prepared as aforementioned
with Entellan® mounting medium. Slides were imaged and scored using
an optical microscope at ×400 magnification. For each donor and
dose, 2,000 binucleated cells were scored by two independent
scorers (1,000 each) according to the IAEA criteria (29). The number of micronucleated
binucleated cells were recorded.
Single-cell gel electrophoresis (comet
assay)
Peripheral blood mononuclear cells (PBMCs) were
prepared from fresh blood samples and isolated through density
gradient centrifugation using Histopaque-1077 (MilliporeSigma)
according to the manufacturer's protocols. Briefly, blood was
diluted with an equal volume of PBS before 5 ml of this diluted
blood was carefully added to a canonical centrifuge tube, which
contains 3.5 ml Histopaque-1077, before being centrifuged at 700 ×
g for 30 min at RT. PBMCs were then harvested from the interface
and washed with PBS and centrifuged again at 200 × g for 10 min at
RT. The pellet was suspended in RPMI-1640 medium supplemented with
25% FBS and 1.5% Pen-Strep. For irradiation, part of the cell
suspension (~4 ml) was used, whereas the rest was used as control.
Cell suspensions were held on ice until the single-cell gel
electrophoresis assay was performed. For chemical exposure,
1×106 PBMCs were cultured in 12-well plates and exposed
for 2 h with doxorubicin (BioAustralis) at 37°C at 5%
CO2. The samples were then centrifuged at 200 × g for 5
min at RT, washed in 1 ml PBS and centrifuged again. The pellet was
then resuspended in 100 µl 0.5% low-melting point agarose.
The comet assay (SCGE) was performed as previously
described (32) with slight
modifications. Briefly, the cell suspensions were spread on glass
microscope slides previously coated with 1% normal-melting point
agarose and kept at 4°C for 20 min. The slides were then left
overnight in a cold lysis buffer (2.5 M NaCl, 10 mM Tris, 100 mM
EDTA and 1% Triton, pH 10). After the overnight lysis, slides were
washed with previously cooled double-distilled water and remained
immersed for 10 min at 4°C. They were then immersed in cold
electrophoresis buffer (10 M NaOH and 200 mM EDTA, pH >13) for
20 min at 4°C. Electrophoresis was conducted for 20 min at 25 V
(400 mA) before the slides were neutralized three times with
neutralization buffer (0.4 M Tris, pH 7.5) at 5 min each, dried
with ethanol (50, 75 and 100%; 5 min each) and stained with 3X
GelRed (Biotium, Inc.). Slides were scored using a fluorescent
microscope (Zeiss Z2; Carl Zeiss AG) at ×200 magnification, before
~200 cells were selected and images captured. The cell images
captured were then examined using the CometScore V1.5 Software,
which calculated the % DNA in the tail.
Blood cell culture and chemical
treatment
The functional assays through chemical exposure were
performed for all samples carrying the VUS and for NC controls. In
total. three different concentrations of doxorubicin were chosen
(0.1, 1.0 and 5.0 µM). The duration of chemical exposure varied
according to the assay performed. Treated samples were incubated at
37°C for specific periods of time.
Functional assays
γH2A histone family member X (γH2AX)
assay
Following doxorubicin treatment for 2 h at 37°C with
5% CO2, the samples were centrifuged at 200 × g for 5
min at RT. RPMI-1640 medium (1 ml) supplemented with 25% of FBS and
1.5% of Pen-Strep was then added to each sample and incubated for
30 min at 37°C with 5% CO2. Samples were centrifuged at
200 × g at RT and 1 ml PBS and 1 µl violet fluorescent reactive dye
(LIVE/DEAD™ Fixable Violet Dead Cell Stain kit; Thermo Fisher
Scientific, Inc.) was added to the respective sample and incubated
for 30 min at RT protected from light. The samples were then
centrifuged again at 200 × g for 5 min at RT and the pellet was
washed with 1 ml PBS, followed by centrifugation at 200 × g for 5
at RT min again. This was followed by fixation in 500 µl 2%
formaldehyde for 15 min on ice. The samples were centrifuged again
at the same speed and time at 4°C, and each pellet was resuspended
in 500 µl 70% cold ethanol in PBS before being kept overnight at
4°C. The next day, the samples were centrifuged at 200 × g for 5
min at 4°C and each pellet was resuspended in 1 ml blocking buffer
(containing 4% BSA in PBS, 4% goat serum and 0.25% Triton X-100)
and were centrifuged further in the same conditions as before. In
total, 1:500 antibody [Phospho-Histone H2A.X (Ser139) Monoclonal
Antibody (CR55T33), PE, eBioscience; Thermo Fisher Scientific,
Inc.] was added to the respective pellet, followed by 2 h
incubation at RT protected from light. Cells were washed with 1.5
ml 1% BSA and centrifuged at 200 × g for 5 min at RT. Each pellet
was resuspended in 200 µl 0.1% BSA. The samples were analyzed by
flow cytometry using a BD FACSCanto II Cytometer (BD Biosciences),
where 20,000 events were counted. Image analysis were performed
using the FlowJo v10 software (FlowJo LLC).
Caspase activity assay
Caspase assays were performed using commercially
available kits, specifically by CaspaTag™ Caspase-3/7 In
Situ Assay kit and CaspaTag™ Caspase-9 In Situ Assay kit
(Thermo Fisher Scientific, Inc.). The methodology was performed
according to the manufacturer's protocols, with minor alterations.
The methodology used for both assays was the same, with the main
difference being the FLICA concentration specific for each one,
according to the manufacturer's instructions. Briefly, after
treatment for 2 h (or overnight for Caspase-9) at 37°C with 5%
CO2, samples were centrifuged at 200 × g for 5 min at RT
before the pellet was resuspended in 200 µl PBS. In total, 10 µl 6X
FLICA for Caspases 3/7 and 15X FLICA for Caspase 9 were added to
the respective tubes. They were then incubated for 1 h at 37°C with
5% CO2 protected from light. Tubes were gently swirled
three times, before 1 ml 1X wash buffer (10X wash buffer provided
in the kit) was added and centrifuged at 400 × g for 5 min at RT.
The pellet was resuspended in 1 ml 1X wash buffer and centrifuged
again at 400 × g for 5 min at RT. The pellet was resuspended in 400
µl 1X wash buffer and 2 µl propidium iodide (provided in kit) was
added to the respective tubes. Samples were analyzed by flow
cytometry using a BD FACSCanto II Cytometer (BD Biosciences) and
20,000 events were counted. Image analysis were performed using the
FlowJo v10 software (FlowJo LLC).
TUNEL assay
TUNEL assay was performed using the APO-BrdU™ TUNEL
Assay kit (Thermo Fisher Scientific, Inc.). The methodology was
performed according to the manufacturer's protocols with minor
alterations. Briefly, after treatment for 4 h at 37°C with 5%
CO2, samples were centrifuged at 200 × g for 5 min at
RT. In total, 500 µl PBS was added to the samples before PBMCs were
pelleted (300 × g for 5 min at RT) followed by fixation in 500 µl
2% formaldehyde for 15 min on ice. Samples were then centrifuged at
300 × g for 5 min at 4°C and each pellet was resuspended in 1 ml
PBS and centrifuged again at 300 × g for 5 min at 4°C. The pellet
was resuspended in 500 µl PBS and 1 ml 70% cold ethanol in PBS
before being incubated for 30 min on ice. Samples were centrifuged
(300 × g for 5 min at 4°C) and the pellet was resuspended and
centrifuged twice with 1 ml wash buffer (provided in kit). Each
pellet was then resuspended in 50 µl DNA-labeling solution, which
contains reaction buffer, TdT enzyme, BrdUTP and ddH2O,
before being kept overnight at 22–24°C. The next day, samples were
resuspended and centrifuged twice in same conditions as before (300
× g for 5 min at RT) with 1 ml rinse buffer (provided in kit),
106 cells were added to 100 µl diluted solution, which
contains the Alexa Fluor™ 488-conjugated anti-BrdU mouse monoclonal
antibody PRB-1 (provided in kit) and rinse buffer. They were then
incubated for 30 min at RT protected from light. Samples were
analyzed by flow cytometry using a BD FACSCanto II Cytometer (BD
Biosciences), where 20,000 events were counted. Image analysis were
performed using FlowJo v10 software (FlowJo LLC).
Statistical analysis
All graphs were plotted using the GraphPad Prism 9
software (Dotmatics). Data were presented as the means ± standard
deviation. All graphs were obtained for the grouped samples
according to their genetic status: NC carriers or VUS carriers.
Statistical analysis was performed using GraphPad Prism 9 taking
into account the pooled samples. For the CA and MN assays,
χ2 or Fisher's exact tests was applied, where P<0.05
was considered to indicate a statistically significant association.
For the SCGE or comet assays, Kolmogorov-Smirnov normality test was
performed to examine if samples followed a Gaussian distribution.
If this was not observed, then non-parametric tests were used to
analyze the data. To compare controls (0 Gy) and irradiated samples
(2 Gy), Wilcoxon signed rank test was applied. The non-parametric
Mann-Whitney U test was performed to compare the different groups
of samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
Study summary
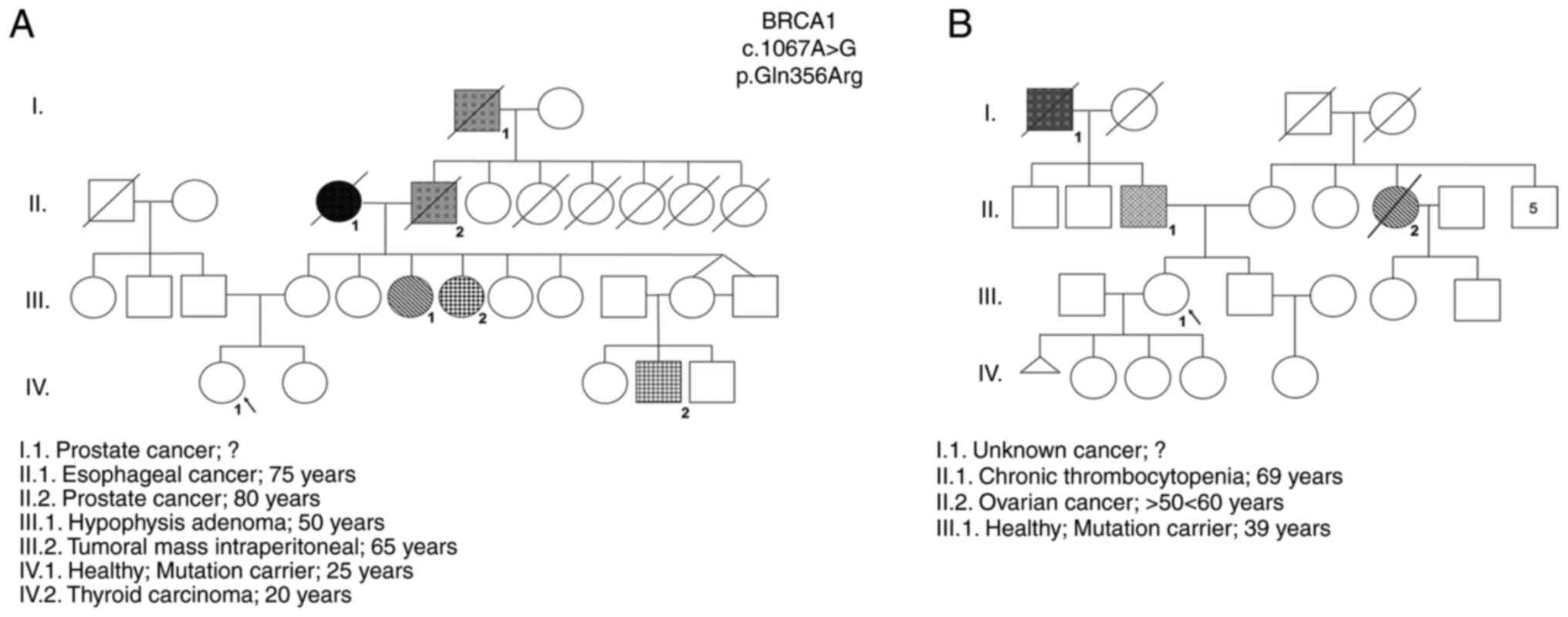
The results obtained by NGS revealed two women with
a VUS in the BRCA1 gene (NM_007294.3:c.1067A>G)
ambiguously defined as probably pathogenic according to the
PolyPhen2 database and as benign in the ClinVar database were
identified (Table I). These
VUS-carriers were female individuals with no identified tumors,
belonging to two distinct families with high incidence of oncologic
diseases and the same VUS (rs1799950). The familial history of each
woman and their pedigrees were constructed and are shown in
Fig. 1.
 | Table I.Characterization of participants
carrying the VUS in the BRCA1 gene. |
Table I.
Characterization of participants
carrying the VUS in the BRCA1 gene.
|
|
Characterization |
|---|
|
|
|
|---|
| Sample ID | Age (years) | Cancer | Gene | Variant ID | EBI
amino/genomic | rs ID | PolyPhen | ClinVar |
|---|
| VUS_BRCA1_ 1 | 25 | Healthy | BRCA1 | NM_007294.3 |
ENSP00000418960.2: | rs1799950 | Probably | Benign |
|
|
|
|
| c.1067A>G | p.Gln356Arg
17:g |
| damaging |
|
|
|
|
|
|
| 43094464T>C |
|
|
|
| VUS_BRCA1_2 | 39 |
|
|
|
|
|
|
|
In order to validate the sensitivity of the
functional assays to verify the pathogenicity of the gene variant,
and since it was not possible to include a pathogenic BRCA1
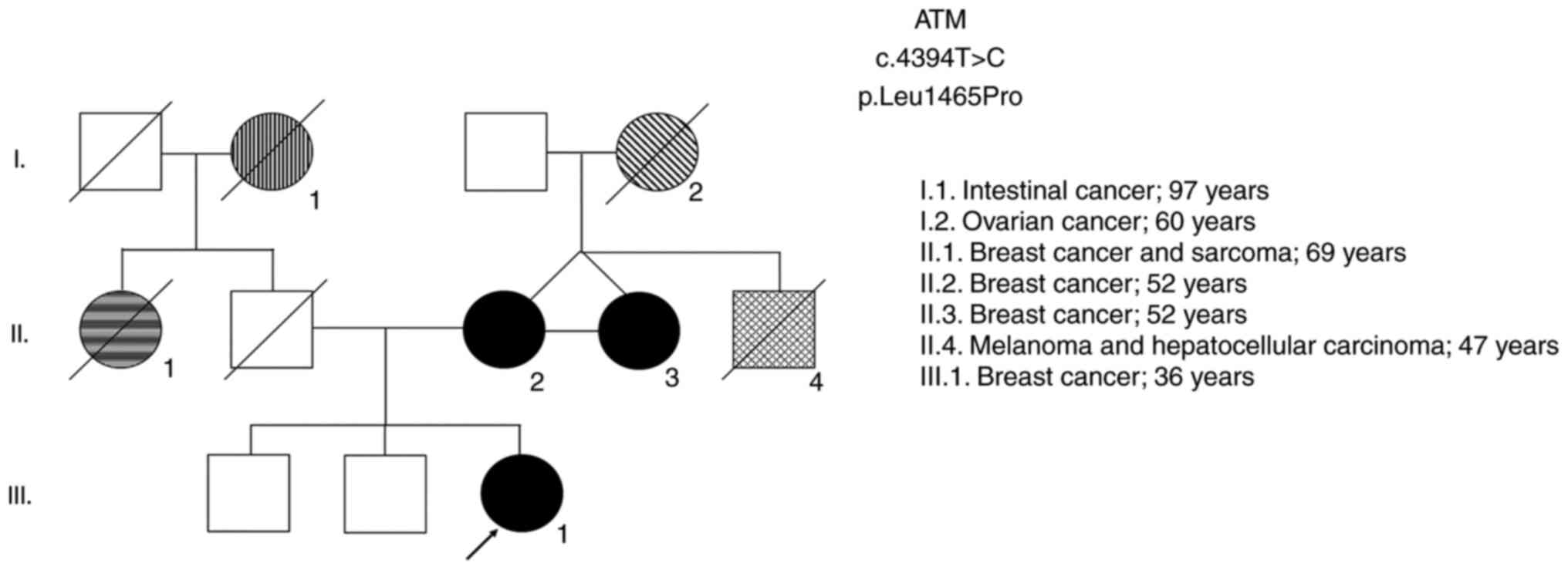
variant selected by NGS in the present study, two women also with
high-risk genealogy harboring variants in the ATM gene that
are likely to be pathogenic were included in the study (Table II). These carriers of ATM
mutations are first degree-relatives (mother and daughter). The two
were diagnosed with breast cancer in a family with a relevant
pedigree history of oncological diseases (Fig. 2). In addition, this variant was
also identified in a third relative in this family (case II.3), who
was also diagnosed with breast cancer along with her twin sister
(case II.2; ATM2) and her niece (case III.1; ATM1), emphasizing the
high probability of this being a pathogenic variant. Together with
the BRCA1 gene, the ATM gene is also involved in the
DDR pathway and is important in the cellular response to genotoxic
agents.
 | Table II.Characterization of participants
carrying the probable pathogenic variant in the ATM
gene. |
Table II.
Characterization of participants
carrying the probable pathogenic variant in the ATM
gene.
|
|
Characterization |
|---|
|
|
|
|---|
| Sample ID | Age | Cancer | Gene | Variant ID | EBI
amino/genomic | rs ID | PolyPhen | ClinVar |
|---|
| ATM 1 | 36 | Breast | ATM | NM_000051.3: |
ENSP00000278616.4: | rs730881391 | Probably | Likely |
|
|
|
|
| c.4394T>C | p.Leu1465Pro |
| damaging | Pathogenic |
|
|
|
|
|
| NC_000011.10:g |
|
|
|
|
|
|
|
|
|
108289759T>C |
|
|
|
| ATM 2 | 52 |
|
|
|
|
|
|
|
DNA damage was induced by γ-radiation and chemical
exposure to doxorubicin, a DNA damaging agent that is also used as
a first line chemotherapeutic for several cancers. The present
study was intended to be exploratory and a proof of concept. The
genotoxic and functional studies performed between NC carriers and
carriers of VUS and ATM mutation are described below and were
chosen with the objective of measuring the extent of DNA damage and
several apoptosis end-points.
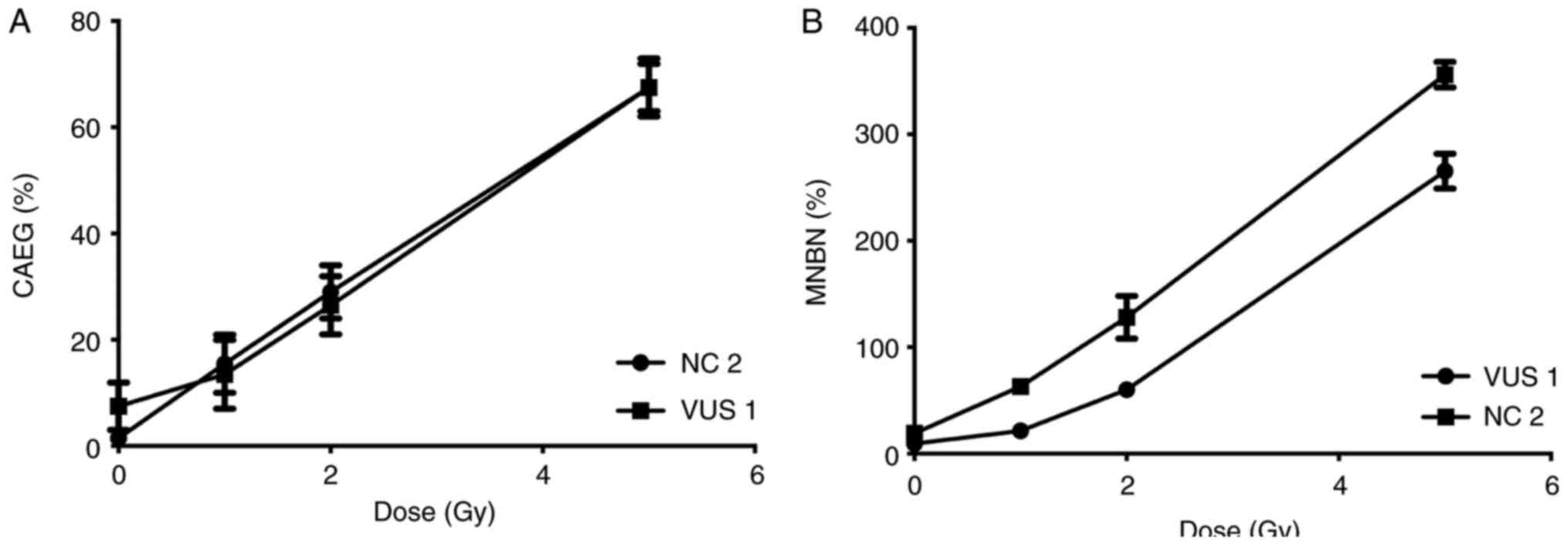
Samples from two participants, VUS_BRCA1_1 and NC 2,
were first used to establish a dose response curve for the present
study. Dose-response curves at 0, 1, 2 and 5 Gy were performed for
the MN and CA assays (Fig. 3). The
radiation dose chosen (2 Gy) was previously described (33) and is used in biological dosimetry
requirements.
CA assay results
Table III shows
the results obtained after analysis, where it is possible to
observe a global increase in the frequency of CAEG globally
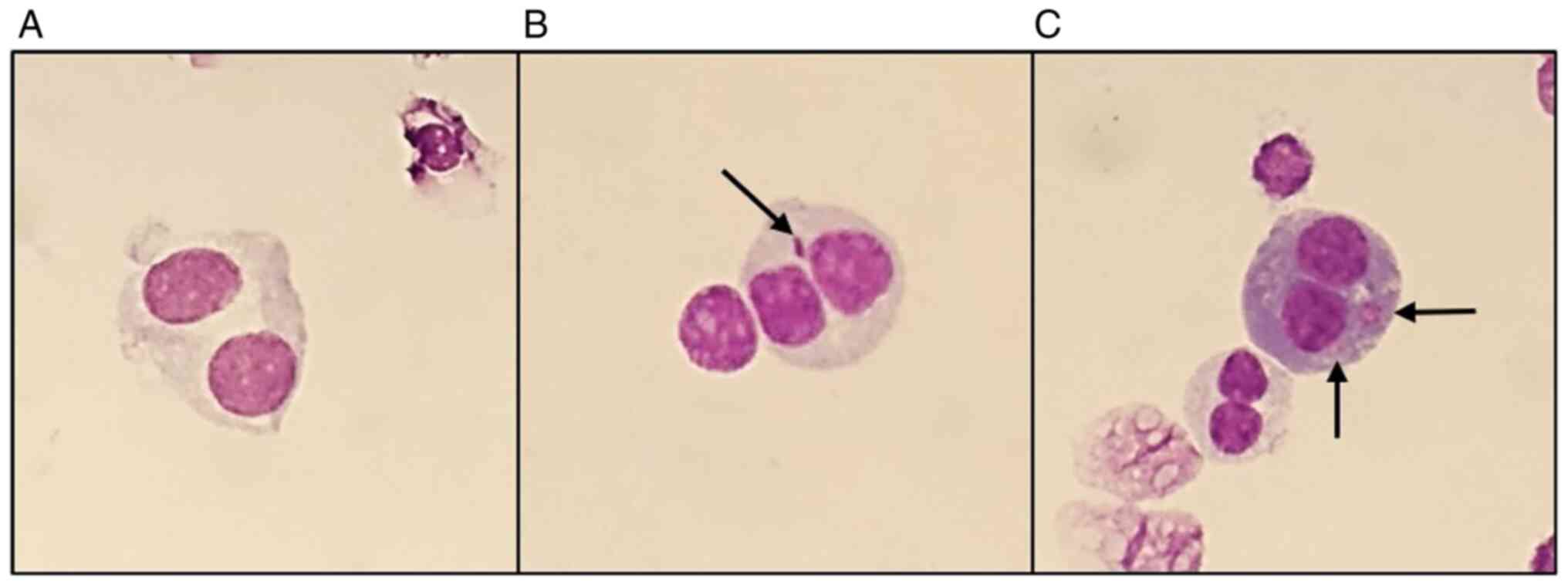
following radiation exposure. The most frequent CA present were
acentric fragments and DIC which, apart from rings, are the main
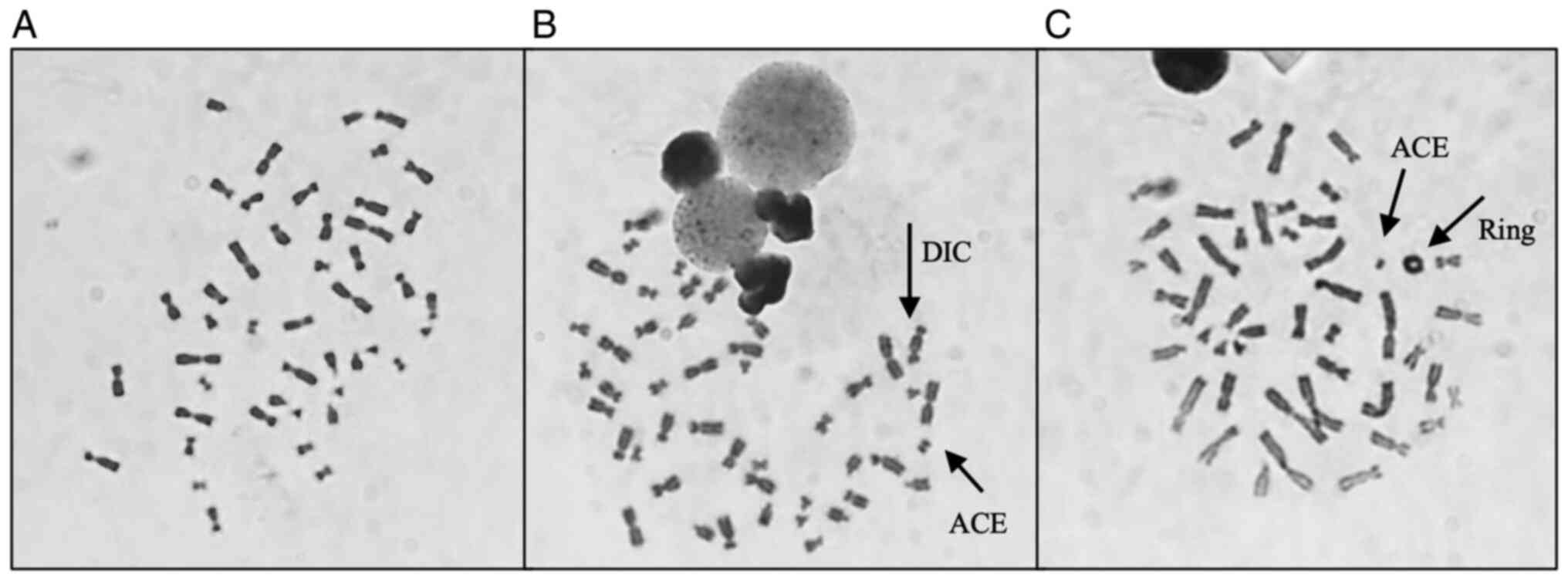
CAs identified following γ-radiation exposure. Fig. 4 shows representative images of
metaphases showing these structures that were observed during
analysis. The results obtained individually for each woman were
then grouped to evaluate the effect attributed to the presence of
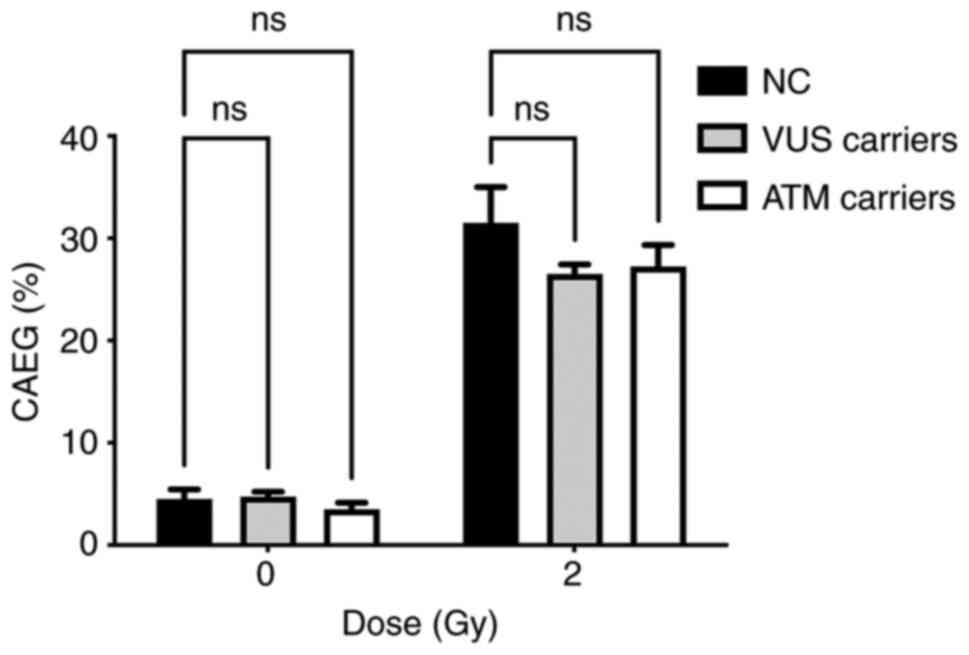
each genetic variant. The frequency of CAEG observed is shown in
Fig. 5. No significant differences
could be observed among the NC carriers, VUS_BRCA1 carriers and ATM
carriers.
 | Table III.Chromosomal aberrations distribution
in cells, and the frequency of cells with at least one chromosomal
aberration excluding gaps (CAEG %). |
Table III.
Chromosomal aberrations distribution
in cells, and the frequency of cells with at least one chromosomal
aberration excluding gaps (CAEG %).
|
|
|
|
|
|
|
|
|
| DIC
Distribution |
|
|
|---|
| Sample name | Dose (Gy) | Total Cells | CTG | CHG | CTB | CHB | Excess ACE | DIC |
|
| CAEG (%) |
|---|
| 0 | 1 | 2 | 3 | 4 | Ring |
|---|
| NC 1 | 0 | 200 | 0 | 0 | 5 | 0 | 2 | 1 | 199 | 1 | 0 | 0 | 0 | 0 | 3.50 |
|
| 2 | 200 | 0 | 0 | 2 | 2 | 34 | 21 | 179 | 21 | 0 | 0 | 0 | 2 | 25.50 |
| NC 2 | 0 | 200 | 0 | 0 | 5 | 1 | 3 | 3 | 197 | 3 | 0 | 0 | 0 | 1 | 6.00 |
|
| 2 | 200 | 1 | 0 | 3 | 3 | 45 | 28 | 172 | 22 | 3 | 0 | 0 | 1 | 32.50 |
| Global | 0 | 400 | 0 | 0 | 10 | 1 | 5 | 4 | 396 | 4 | 0 | 0 | 0 | 1 | 4.75 |
|
| 2 | 400 | 1 | 0 | 5 | 5 | 79 | 49 | 351 | 43 | 3 | 0 | 0 | 3 | 29.00 |
| VUS_BRCA1_1 | 0 | 200 | 5 | 0 | 6 | 1 | 3 | 0 | 200 | 0 | 0 | 0 | 0 | 0 | 4.50 |
|
| 2 | 200 | 0 | 1 | 6 | 0 | 26 | 22 | 178 | 22 | 0 | 0 | 0 | 4 | 25.00 |
| VUS_BRCA1_ 2 | 0 | 200 | 0 | 0 | 4 | 1 | 0 | 1 | 199 | 1 | 0 | 0 | 0 | 0 | 3.00 |
|
| 2 | 200 | 2 | 0 | 7 | 3 | 30 | 30 | 170 | 22 | 4 | 0 | 0 | 3 | 30.50 |
| Global | 0 | 400 | 5 | 0 | 10 | 2 | 3 | 1 | 399 | 1 | 0 | 0 | 0 | 0 | 3.75 |
|
| 2 | 400 | 2 | 1 | 13 | 3 | 56 | 52 | 348 | 44 | 4 | 0 | 0 | 7 | 27.75 |
| ATM 1 | 0 | 200 | 3 | 0 | 5 | 1 | 0 | 0 | 200 | 0 | 0 | 0 | 0 | 1 | 3.50 |
|
| 2 | 200 | 2 | 1 | 3 | 1 | 39 | 33 | 167 | 21 | 6 | 0 | 0 | 2 | 29.00 |
| ATM 2 | 0 | 200 | 0 | 0 | 1 | 1 | 9 | 0 | 200 | 0 | 0 | 0 | 0 | 0 | 5.00 |
|
| 2 | 200 | 2 | 0 | 2 | 3 | 31 | 27 | 173 | 27 | 0 | 0 | 0 | 2 | 28.00 |
| Global | 0 | 400 | 3 | 4 | 6 | 2 | 9 | 0 | 400 | 0 | 0 | 0 | 0 | 1 | 5.50 |
|
| 2 | 400 | 4 | 1 | 5 | 4 | 70 | 60 | 340 | 48 | 6 | 0 | 0 | 4 | 28.50 |
CBMN assay
Micronuclei slides were then analyzed. For each
participant, 1,000 binucleated cells were counted and independently
analyzed by two independent evaluators. The data obtained for the
micronuclei distribution in each participant are shown in Table IV. Fig. 6 shows representative images
captured during the data analysis, with the results observed. An
overall analysis of the results showed an increase in the rates of
binucleated cells with micronuclei (MNBN) and total micronuclei
(TMN) following exposure to a dose of 2 Gy, compared with those in
the control group of 0 Gy. Furthermore, the occurrence of ≥2
micronuclei after radiation exposure (Table III) was observed across all
samples.
 | Table IV.Micronuclei distribution in
binucleated cells, frequency of binucleated cells with micronuclei,
total micronuclei (TMN) and nuclear division index (NDI) for each
volunteer. |
Table IV.
Micronuclei distribution in
binucleated cells, frequency of binucleated cells with micronuclei,
total micronuclei (TMN) and nuclear division index (NDI) for each
volunteer.
|
|
|
| MN
Distribution |
|
|
|
|---|
| Sample name | Dose (Gy) | Total BN |
| MNBN (‰) | TMN (‰) | NDI |
|---|
| 0 MN | 1 MN | 2 MN | 3 MN | 4 MN |
|---|
| NC 1 | 0 | 2,000 | 1,981 | 15 | 2 | 0 | 0 | 8.50 | 9.50 | 1.58 |
|
| 2 | 2,000 | 1,626 | 263 | 43 | 7 | 1 | 157.00 | 187.00 | 1.54 |
| NC 2 | 0 | 2,000 | 1,961 | 35 | 2 | 0 | 0 | 18.50 | 19.50 | 1.72 |
|
| 2 | 2,000 | 1,629 | 288 | 37 | 3 | 0 | 164.00 | 185.50 | 1.60 |
| Global | 0 | 4,000 | 3,942 | 50 | 4 | 0 | 0 | 13.50 | 14.50 |
|
|
| 2 | 4,000 | 3,255 | 551 | 80 | 10 | 1 | 160.50 | 186.25 |
|
| VUS_BRCA1_1 | 0 | 2,000 | 1,984 | 16 | 0 | 0 | 0 | 8.00 | 8.00 | 1.84 |
|
| 2 | 2,000 | 1,753 | 176 | 29 | 3 | 1 | 104.50 | 123.50 | 1.81 |
| VUS_BRCA1_2 | 0 | 2,000 | 1,991 | 9 | 0 | 0 | 0 | 4.50 | 4.50 | 1.82 |
|
| 2 | 2,000 | 1,733 | 209 | 26 | 2 | 0 | 118.50 | 133.50 | 1.71 |
| Global | 0 | 4,000 | 3,975 | 25 | 0 | 0 | 0 | 6.25 | 6.25 |
|
|
| 2 | 4,000 | 3,486 | 385 | 55 | 5 | 1 | 111.50 | 128.50 |
|
| ATM 1 | 0 | 2,000 | 1,992 | 8 | 0 | 0 | 0 | 4.00 | 4.00 | 1.71 |
|
| 2 | 2,000 | 1,834 | 126 | 20 | 0 | 0 | 73.00 | 83.00 | 1.77 |
| ATM 2 | 0 | 2,000 | 1,980 | 18 | 1 | 0 | 0 | 9.50 | 10.00 | 1.23 |
|
| 2 | 2,000 | 1,745 | 192 | 27 | 3 | 0 | 111.00 | 127.50 | 1.17 |
| Global | 0 | 4,000 | 3,972 | 26 | 1 | 0 | 0 | 6.75 | 7.00 |
|
|
| 2 | 4,000 | 3,579 | 318 | 47 | 3 | 0 | 92.00 | 105.25 |
|
The main aim of the present study was to
functionally characterize the genetic variant by assessing the
cellular response to γ-radiation. Given the limited number of
samples analyzed, the data were grouped according to the presence
of each genetic variant: VUS carriers, ATM carriers and NC
carriers. Fig. 7 represents the
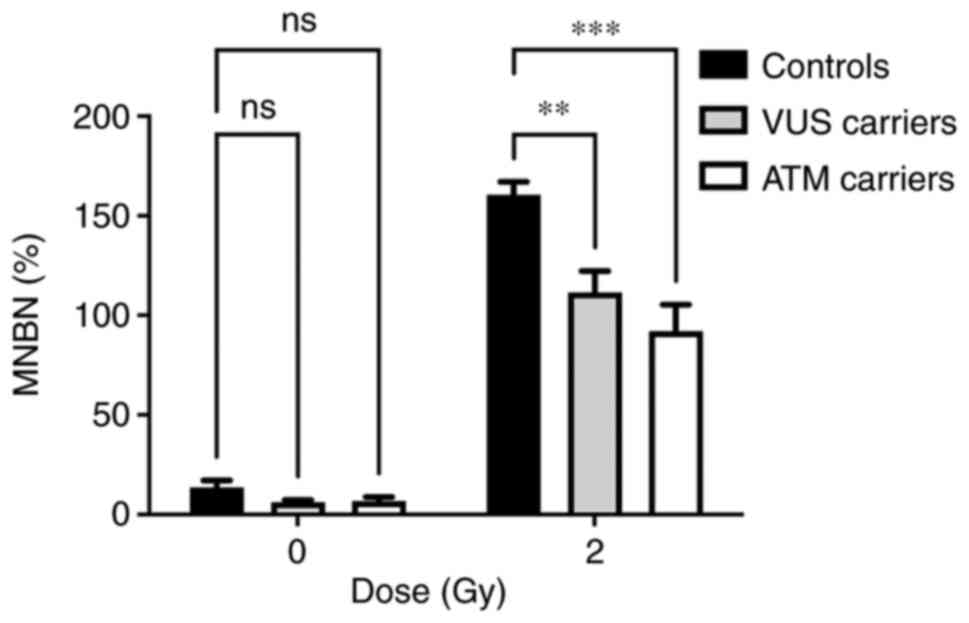
distribution of MNs in each group. Analysis of the MN frequency
distribution revealed a significant decrease in the MNBN frequency
in VUS and ATM carriers compared with NC carriers.
Single-cell gel electrophoresis (comet
assay)
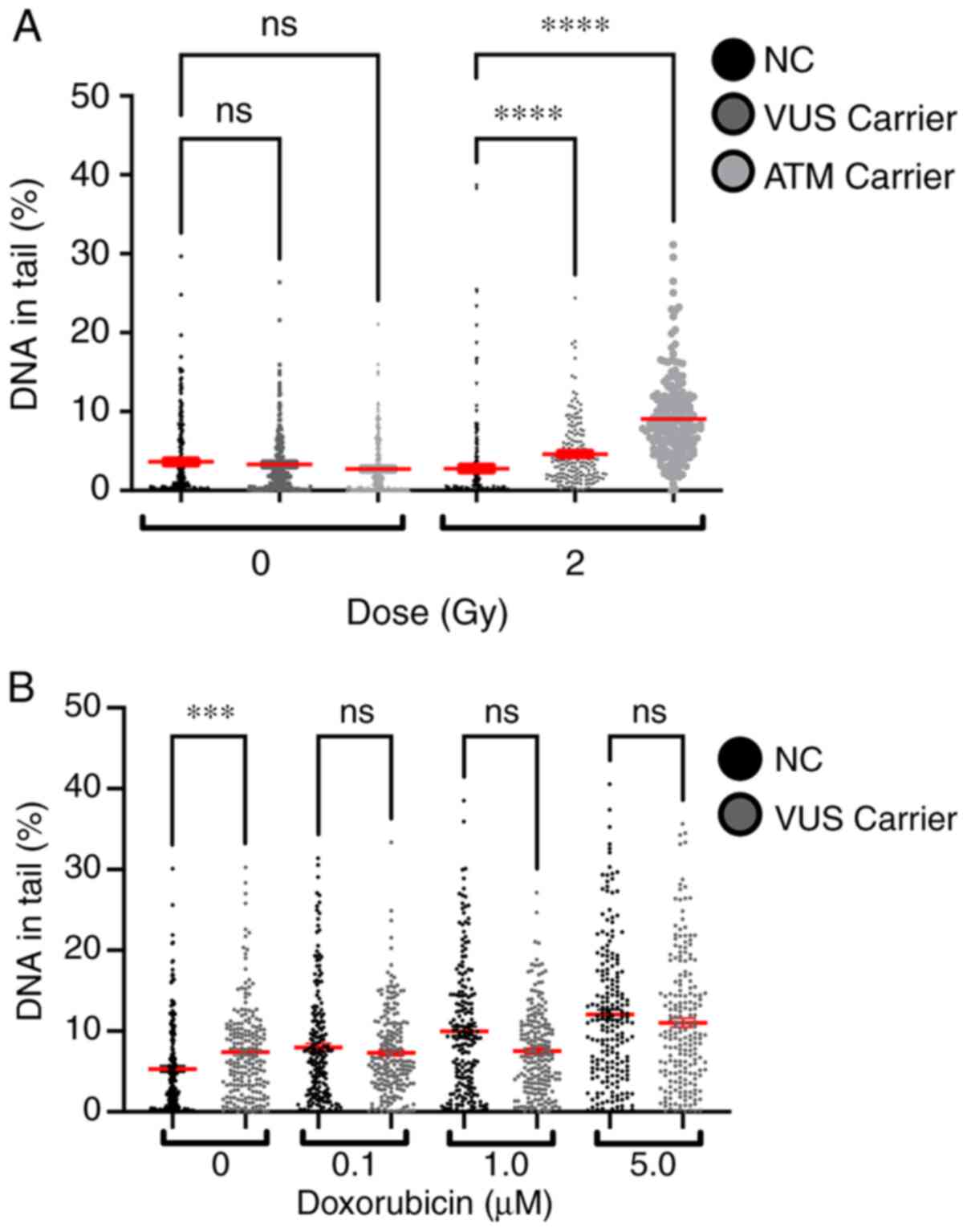
The % DNA in tail for all samples was measured to
evaluate the effect of γ-radiation and chemical exposure to
doxorubicin. A dose-dependent effect was observed in both
experiments, with higher doses inducing more lesions (Fig. 8). Comparing the effects of 2 Gy on
NC and VUS carriers, an increase in the number of DNA lesions was
observed in the VUS carriers, with an even more significant
increase in ATM carriers, compared with the NC carriers. However,
this effect was not observed after doxorubicin exposure except for
the basal level, where VUS carriers have significantly more basal
DNA damage than NC carriers (Fig.
8B).
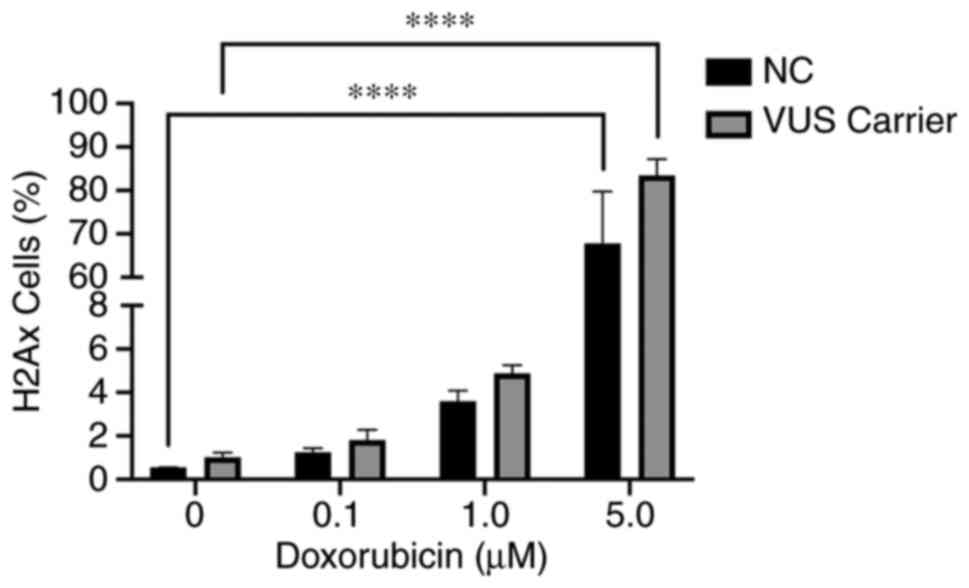
γH2AX assay
The γH2AX assay is one functional method that can be
used to detect DSBs following chemical exposure. The data obtained
was grouped into NC and VUS carriers. The results obtained
demonstrated a dose-dependent effect in both NC and VUS carriers
(Fig. 9). However, the differences
between VUS carriers and NC carriers were non-significant.
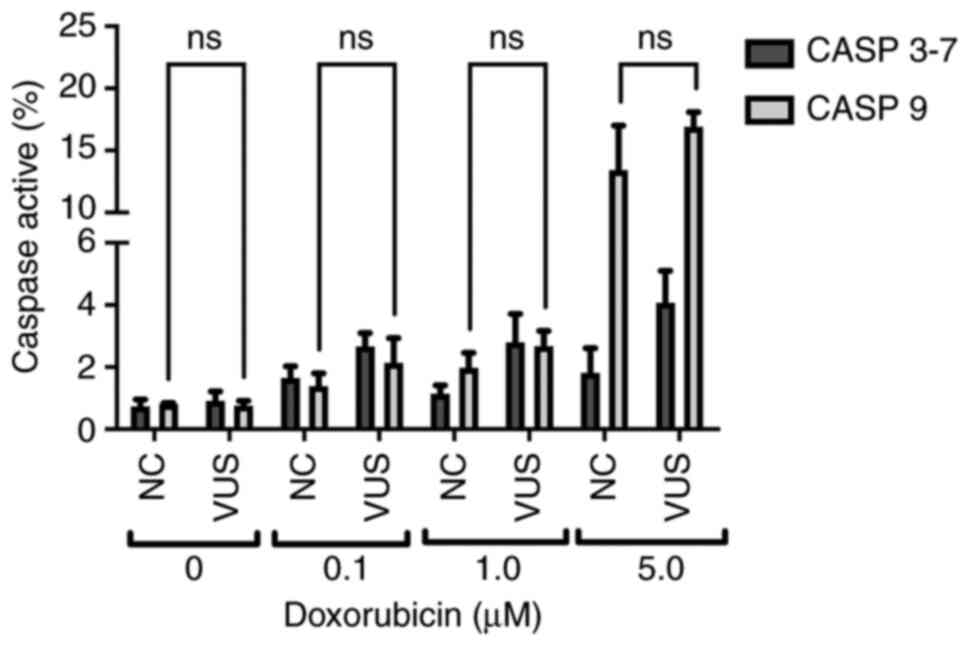
Caspase 3–7 and 9 assays
The caspase signaling cascade is responsible for the
activation of apoptosis and inflammatory processes, which allow
cells to maintain their genomic stability whilst controlling
programed cell death. In the present study, two caspases with two
different functions in the apoptosis pathway were assessed; caspase
9 (initiator caspase) and caspases 3–7 (executioner caspases)
(34). These caspases operate in
the intrinsic pathway, which is triggered in response to death
stimuli generated by DNA damage. Chemical exposure was found to
slightly activate caspase 9, while almost no activation could be
detected for caspase 3–7 (Fig.
10). Caspases 3–7 activity exhibited only slight variations at
the different doxorubicin concentrations. However, the activity of
caspase 9 increased sharply at higher doxorubicin concentrations,
suggesting that activation of the initiator caspase cascade
occurred due to DNA damage.
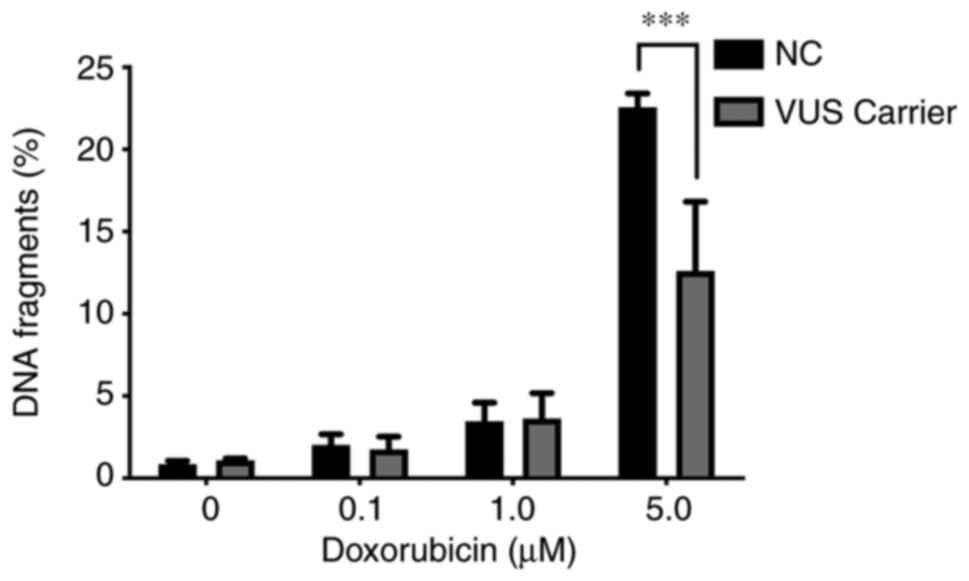
TUNEL assay
To the best of the authors' knowledge, cells
undergoing apoptosis exhibit several changes in nuclear morphology,
especially during the later stages of programmed cell death or
apoptosis. These features include DNA fragmentation and DNA strand
breaks, both of which are relevant when evaluating the biological
role of DNA repair genes. The damage inflicted in PBMCs by
doxorubicin exposure was also evaluated using the TUNEL assay as
quantified by flow cytometry. This assay measures DNA fragments
resulting from the apoptotic process. A significant difference at
higher concentrations of doxorubicin between NC carriers and VUS
carriers was observed (Fig. 11),
with NC carriers being more sensitive to DNA fragmentation,
consistent with the results obtained for MN (Fig. 4).
Discussion
One of the limitations in studying the clinical
significance of VUS in human samples is the rarity of their
occurrence, restricting the number of individuals available for
functional studies which can be performed in peripheral
lymphocytes. BRCA1 plays a major role in DNA repair and is broadly
expressed in a wide variety of cells, including lymphocytes
(https://www.proteinatlas.org/ENSG00000012048-BRCA1/tissue),
which justifies the use of patients' lymphocytes as a surrogate
tissue for breast tissue. The BRCA1 protein is involved in
repairing damaged DNA, produced either endogenously or by exogenous
factors or when chromosomes exchange genetic material in
preparation for cell division, replication fork protection, cell
cycle regulation and gene transcription regulation (35). The BRCA1 protein interacts with
several other proteins to repair DNA strand breaks that also occur
when chromosomes exchange genetic material in preparation for cell
division. Thus, BRCA1 acts as a tumor suppressor. Mutations in
BRCA1 have long been associated with increased risk of breast
cancer in men and women, as well as several other types of cancer
and increased DSB, indicating a defect in DNA repair (36). To date, there is no evidence of
cell-type specific differences in the activity of DSB repair
pathways and in other BRCA1-specific interactions.
Identification of a pathogenic germline variant is
crucial for the correct clinical management of families with
increased risk for hereditary breast cancer. This would enable the
early identification of individuals most at-risk and those who
require increased surveillance and/or prophylactic interventions
(1). The increasingly common
application of NGS has identified a number of variants in genes
suspected to be involved in cancer predisposition, in particular
for breast cancer. Several of these genes are associated with DNA
repair and have been reported in female and male breast cancer
patients (36). However, the
increased identification of variants of high penetrant genes
through NGS has led to considerable difficulties in the adequate
classification of their pathogenicity. This assessment therefore
relies mostly on co-segregation with disease, co-occurrence with
known pathogenic variants and family history of cancer. Therefore,
understanding the impact of VUS on protein function is critical for
understanding the functional consequences and potential therapy
responses (37–39).
The c.1067A>G (rs1799950) BRCA1 missense
variant results in the replacement of glutamine with arginine at
codon 356 of the BRCA1 gene. Missense mutations that do not
lead to the complete disruption of protein function may slightly
alter the structures of domains important for protein function. The
effect of these mutations may be estimated according to their
position and the type of altered amino acid using specific
software, which is measured by the probability of disrupting a
particular protein function increasing the disease risk. For the
present study, analysis using two different in silico
prediction tools revealed two distinct prognostic results for this
VUS. PolyPhen2 identified this VUS as likely damaging (0.998)
(PolyPhen 2, 2020), whereas ClinVar classified this as
benign (ClinVar-NCBI, 2020). Therefore, this substitution
cannot be classified as benign or pathogenic with confidence, since
in silico prediction tools could only provide a theoretical
prediction of the effects of this variant on protein structure and
function. In addition, given its rarity, data on the role of this
variant on cancer risk are scarce.
The role of DNA repair genes in breast cancer has
been extensively studied, to an extent that clinical panels
integrating the most relevant genes for breast cancer progression
have been reported, emphasizing their importance. The challenge in
the present study was to establish a set of assays that allowed the
evaluation and characterization of genetic variants that may affect
the DNA repair mechanisms. Therefore the decision was centered on
approaches that facilitate the measurement of DNA lesions induced
by genotoxic agents (radiation and doxorubicin) with the added
advantage of studying human samples. One of the methodologies
selected is the CA assay, which is considered to be the ‘gold
standard’ for radiation biodosimetry. This approach allows for the
microscopic visualization of features of DNA damage, such as DSB
(40). The most representative
lesion caused by radiation exposure is dicentric chromosomes, as
discussed in previous studies (Fig.
4B) (29,33). High frequencies of CA in peripheral
blood lymphocytes have been associated with significantly elevated
risks of cancer development (40,41).
Results in the present study showed a clear dose-dependent increase
in the rates of CA after radiation exposure. These results are
consistent with those from previous biodosimetry studies (33). However, no statistical difference
could be observed between NC and VUS carriers.
An alternative method to the CA assay that also
allows for the detection and evaluation of DNA damage induced by
genotoxic agents is the CBMN assay (29,42).
The MN results from the present study also revealed a
dose-dependent effect. In particular, increases in the frequency of
MNBN and TMN were observed at higher doses. However, the VUS
carrier group exhibited lower levels of DNA lesions in response to
radiation compared with those in the NC group. Previous studies
have associated higher frequencies of MN with an increased risk of
cancer (43–45). Therefore, if this VUS is a
potentially pathogenic variant in the BRCA1 gene, then
higher levels of DNA damage should have been observed.
The comet assay has also been previously applied to
evaluate both DNA damage and repair. In addition, it is a
well-known technique for assessing DNA damage after radiation and
chemical exposure (46–48). According to this assay, the present
study showed a global increase in DNA damage after exposure. The
differences between the exposure types were also evident, showing
statistical differences, specifically higher sensitivity to DNA
damage in BRCA1 VUS carriers. The effect observed for
irradiated samples demonstrates that this assay is a viable method
for evaluating primary DNA lesions (49), suggesting a potential role of this
genetic variant in hampering the repair mechanisms.
To measure the DSB repair sites induced by chemical
exposure, a H2AX assay was also performed. Although this assay can
also evaluate DNA damage, it could not reveal a significant
difference between NC and VUS (Fig.
9). This assay is likely to be more beneficial for evaluating
primary DNA lesions but showed substantial limitations over longer
time-scales, due to the rapid signal decline (48). Nevertheless, the data suggest that
the VUS carriers displays a higher trend of doxorubicin-induced
γH2AX foci.
The relationship between DNA repair and apoptotic
pathways remains poorly understood. However, it is clear that both
radiation and chemotherapy exposure can activate apoptotic
pathways. Different programed cell death pathways can be activated
depending on the stimulus and the damage level of the cells.
According to the three main activation pathways, results from the
present study appear to indicate activation of the intrinsic
pathway, which mainly involves the formation of apoptosomes,
followed by the activation of caspase-9 (50). Once activated, caspase-9 initiates
a caspase activation cascade by processing caspases-3 and −7
(34). The present study showed a
clear dose-dependent signal in caspase-9 activity, but no
significant difference could be observed in the signals for
caspases-3 and −7, even at increasing doxorubicin concentrations.
However, these data could not be correlated with the presence of
the VUS.
Later stage of apoptosis can be measured through the
detection and quantification of apoptotic DNA fragmentation using
the TUNEL assay (50). In
agreement with the results from MN assay, a significant difference
between the NC and VUS carriers was observed, with the former
displaying less DNA fragmentation. Theoretically, the similarity
between the MN and TUNEL results suggests a benign effect
attributed to the presence of this VUS in the BRCA1
gene.
BRCA1 associates with the MRE11/RAD50/NBS1 complex
of proteins, which acts as a DSB sensor and signals for the
recruitment of downstream DNA repair pathway components. This
process typically favors HR rather than NHEJ (19). This association is mediated by
RAD50 with residues 341–748 of the BRCA1 protein. The VUS
(rs1799950; T>C) analyzed in the present study occurs in residue
356 of the BRCA1 protein, leading to an arginine instead of a
glutamine. Although this change was within the RAD50 interacting
region, it did not show clear divergent results compared with NC
carriers even though glutamine has an uncharged R group and
arginine is a positively charged amino acid. This suggested that
this amino acid change did not affect its interaction with
RAD50.
DNA repair mechanisms serve a crucial role in
maintaining genome stability and integrity. The presence of a
single VUS in the BRCA1 gene may modulate its role in
protecting the cell population from DSBs that lead to chromosomal
rearrangements by decreasing micronuclei formation. This in turn
reduces genomic instability and leads to the activation of
programmed cell death. Thus, the present study suggested that this
VUS is probably benign. Supporting this, recent meta-analyzes
described this VUS to be a low-risk variant for breast cancer,
demonstrating its possible protective behavior (51,52).
However, further studies should be performed to
understand the underlying mechanisms, due to the exploratory nature
of the present study. Genome-wide sequencing technologies will
continue to identify novel VUS that will urgently require
functional characterization. This can be achieved using assaying
techniques applied in the present study. In particular, a clear
classification is of utmost importance in the clinical setting and
for planning resulting actions.
The strategy followed by the present study, which
mainly assessed DNA-damage endpoints as readout for investigating
the effects of putative modifications on the DNA repair capacity of
this BRCA1 VUS, may shed light on the possible functional
consequences of sequence variants. This is because VUS may not
result in pathogenicity through a direct effect on protein-protein
interaction due to amino acid changes. Several regulatory variants
discovered by expression quantitative trait loci mapping have
already been validated. It was assumed that these regulatory
variants may act by affecting transcription factors binding sites
to interfere with the function of the protein. For example, they
may operate through a regulatory mechanism that lowers or even
abort BRCA1 expression (53).
In conclusion, the results obtained suggested that
this VUS is benign or highly likely to be benign. Although the
present study was exploratory, the strategy can be successfully
used to study other variants.
Acknowledgements
The authors would like to do acknowledge in
particular all participants of this study. The authors also
acknowledge all the technical support by Dr Ana Catarina Antunes
and the irradiation of samples by Dr Pedro Santos (Centro de
Ciências e Tecnologias Nucleares).
Funding
The present study was funded by Terry Fox Grant 2017 from Liga
Portuguesa Contra o Cancro and by Fundação de Ciência e Tecnologia
(FCT; grant nos. UID/BIM/0009/2020 and UIDP/00009/2020.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization was mainly performed by SNS, JR,
JBPL and JC. Collection of patient clinical data and family history
was performed by TL. SNS, JBPL and JC confirm the authenticity of
all the raw data. RAL and ML performed the experiments, and OG
designed the methodology and performed the experiments. Validation
of proceedings was performed by SNS and OG. Formal analysis was
performed by SNS. Investigation was mainly performed by RAL and ML.
Resources were acquired in collaboration with Ophiomics-Precision
Medicine by SNS, JR, JBPL and JC. JC and JBPL were responsible for
the recruitment of families to be included in the present study and
performed the NGS sequencing analysis. ASR analyzed and interpreted
data, and critically revised the manuscript. Data curation was
performed by JBPL, JC and SNS. Writing of the original draft was by
SNS and reviewing and editing was by SNS, JR and ASR. Supervision
of the present study was by SNS and JR. Project administration
funding acquisition was performed by SNS. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the National
Commission of Data Protection (approval no. 10637/2016) for the use
of samples for research and also by the Ethical Commission of
NMS/FCM (approval no. 54/2018/CEFCM). All regulations were
respected and were in agreement with the Declaration of Helsinki.
Informed consent was obtained from all participants, after their
being informed about the usage of their blood samples were used for
scientific research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kar SP, Beesley J, Amin Al Olama A,
Michailidou K, Tyrer J, Kote-Jarai Z, Lawrenson K, Lindstrom S,
Ramus SJ, Thompson DJ, et al: Genome-wide meta-analyses of breast,
ovarian, and prostate cancer association studies identify multiple
new susceptibility loci shared by at least two cancer types. Cancer
Discov. 6:1052–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paulo P, Pinto P, Peixoto A, Santos C,
Pinto C, Rocha P, Veiga I, Soares G, Machado C, Ramos F and
Teixeira MR: Validation of a next-generation sequencing pipeline
for the molecular diagnosis of multiple inherited cancer
predisposing syndromes. J Mol Diagn. 19:502–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pinto P, Paulo P, Santos C, Rocha P, Pinto
C, Veiga I, Pinheiro M, Peixoto A and Teixeira MR: Implementation
of next-generation sequencing for molecular diagnosis of hereditary
breast and ovarian cancer highlights its genetic heterogeneity.
Breast Cancer Res Treat. 159:245–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Breast Cancer Association Consortium, .
Dorling L, Carvalho S, Allen J, González-Neira A, Luccarini C,
Wahlström C, Pooley KA, Parsons MT, Fortuno C, et al: Breast cancer
risk genes-association analysis in more than 113,000 women. N Engl
J Med. 384:428–439. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eccles DM, Mitchell G, Monteiro ANA,
Schmutzler R, Couch FJ, Spurdle AB and Gómez-García EB; ENIGMA
Clinical Working Group, : BRCA1 and BRCA2 genetic testing-pitfalls
and recommendations for managing variants of uncertain clinical
significance. Ann Oncol. 26:2057–2065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gasparini P, Lovat F, Fassan M, Casadei L,
Cascione L, Jacob NK, Carasi S, Palmieri D, Costinean S, Shapiro
CL, et al: Protective role of miR-155 in breast cancer through
RAD51 targeting impairs homologous recombination after irradiation.
Proc Natl Acad Sci USA. 111:4536–4541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lord CJ and Ashworth A: The DNA damage
response and cancer therapy. Nature. 481:287–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prasad CB, Prasad SB, Yadav SS, Pandey LK,
Singh S, Pradhan S and Narayan G: Olaparib modulates DNA repair
efficiency, sensitizes cervical cancer cells to cisplatin and
exhibits anti-metastatic property. Sci Rep. 7:128762017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ratanaphan A: A DNA repair BRCA1 estrogen
receptor and targeted therapy in breast cancer. Int J Mol Sci.
13:14898–14916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conde J, Silva SN, Azevedo AP, Teixeira V,
Pina JE, Rueff J and Gaspar JF: Association of common variants in
mismatch repair genes and breast cancer susceptibility: A multigene
study. BMC Cancer. 9:3442009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Silva SN, Costa B, Rueff J and Gaspar JF:
DNA repair perspectives in thyroid and breast cancer: The role of
DNA repair polymorphisms. DNA Repair and Human Health. Vengrova S:
InTech; 2011
|
|
12
|
Silva SN, Moita R, Azevedo AP, Gouveia R,
Manita I, Pina JE, Rueff J and Gaspar J: Menopausal age and XRCC1
gene polymorphisms: Role in breast cancer risk. Cancer Detect Prev.
31:303–309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Silva SN, Tomar M, Paulo C, Gomes BC,
Azevedo AP, Teixeira V, Pina JE, Rueff J and Gaspar JF: Breast
cancer risk and common single nucleotide polymorphisms in
homologous recombination DNA repair pathway genes XRCC2, XRCC3,
NBS1 and RAD51. Cancer Epidemiol. 34:85–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gomes BC, Silva SN, Azevedo AP, Manita I,
Gil OM, Ferreira TC, Limbert E, Rueff J and Gaspar JF: The role of
common variants of non-homologous end-joining repair genes XRCC4,
LIG4 and Ku80 in thyroid cancer risk. Oncol Rep. 24:1079–1085.
2010.PubMed/NCBI
|
|
15
|
Helleday T: The underlying mechanism for
the PARP and BRCA synthetic lethality: Clearing up the
misunderstandings. Mol Oncol. 5:387–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lord CJ and Ashworth A: BRCAness
revisited. Nat Rev Cancer. 16:110–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roy R, Chun J and Powell SN: BRCA1 and
BRCA2: Different roles in a common pathway of genome protection.
Nat Rev Cancer. 12:68–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sonnenblick A, de Azambuja E, Azim HA Jr
and Piccart M: An update on PARP inhibitors-moving to the adjuvant
setting. Nat Rev Clin Oncol. 12:27–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Christou C and Kyriacou K: BRCA1 and Its
network of interacting partners. Biology (Basel). 2:40–63.
2013.PubMed/NCBI
|
|
20
|
Sharma B, Kaur RP, Raut S and Munshi A:
BRCA1 mutation spectrum, functions, and therapeutic strategies: The
story so far. Curr Probl Cancer. 42:189–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Colas C, Golmard L, de Pauw A, Caputo SM
and Stoppa-Lyonnet D: ‘Decoding hereditary breast cancer’ benefits
and questions from multigene panel testing. Breast. 45:29–35. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calò V, Bruno L, La Paglia L, Perez M,
Margarese N, Di Gaudio F and Russo A: The clinical significance of
unknown sequence variants in BRCA genes. Cancers (Basel).
2:1644–1660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beitsch PD, Whitworth PW, Hughes K, Patel
R, Rosen B, Compagnoni G, Baron P, Simmons R, Smith LA, Grady I, et
al: Underdiagnosis of hereditary breast cancer: Are genetic testing
guidelines a tool or an obstacle? J Clin Oncol. 37:453–460. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuchenbaecker KB, Hopper JL, Barnes DR,
Phillips KA, Mooij TM, Roos-Blom MJ, Jervis S, van Leeuwen FE,
Milne RL, Andrieu N, et al: Risks of breast, ovarian, and
contralateral breast cancer for BRCA1 and BRCA2 mutation carriers.
JAMA. 317:24022017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martins V, Antunes AC, Cardoso J, Santos L
and Gil OM: Influence of age and gender in response to γ-radiation
in Portuguese individuals using chromosomal aberration
assay-preliminary findings. Radiat Meas. 46:1000–1003. 2011.
View Article : Google Scholar
|
|
26
|
Gil OM, Oliveira NG, Rodrigues AS, Laires
A, Ferreira TC, Limbert E, Léonard A, Gerber G and Rueff J:
Cytogenetic alterations and oxidative stress in thyroid cancer
patients after iodine-131 therapy. Mutagenesis. 15:69–75. 2000.
View Article : Google Scholar
|
|
27
|
Rodrigues AS, Oliveira NG, Gil OM, Léonard
A and Rueff J: Use of cytogenetic indicators in radiobiology.
Radiat Prot Dosimetry. 115:455–460. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rueff J, Brás A, Cristóvão L, Mexia J, Sá
da Costa M and Pires V: DNA strand breaks and chromosomal
aberrations induced by H2O2 and 60Co gamma-radiation. Mutat Res.
289:197–204. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cytogenetic Dosimetry, . Applications in
preparedness for and response to radiation emergencies.
International Atomic Energy Agency; Vienna: 2011
|
|
30
|
Antunes AC, Martins V, Cardoso J, Santos L
and Monteiro Gil O: The cytokinesis-blocked micronucleus assay:
Dose estimation and inter-individual differences in the response to
γ-radiation. Mutat Res Genet Toxicol Environ Mutagen. 760:17–22.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oliveira NG, Neves M, Rodrigues AS,
Monteiro Gil O, Chaveca T and Rueff J: Assessment of the adaptive
response induced by quercetin using the MNCB peripheral blood human
lymphocytes assay. Mutagenesis. 15:77–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pingarilho M, Oliveira NG, Martins C,
Fernandes AS, de Lima JP, Rueff J and Gaspar JF: Genetic
polymorphisms in detoxification and DNA repair genes and
susceptibility to glycidamide-induced DNA damage. J Toxicol Environ
Health A. 75:920–933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martins V, Antunes AC and Monteiro Gil O:
Implementation of a dose-response curve for γ-radiation in the
Portuguese population by use of the chromosomal aberration assay.
Mutat Res. 750:50–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cullen SP and Martin SJ: Caspase
activation pathways: Some recent progress. Cell Death Differ.
16:935–938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gudmundsdottir K and Ashworth A: The roles
of BRCA1 and BRCA2 and associated proteins in the maintenance of
genomic stability. Oncogene. 25:5864–5874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Silva SN, Gomes BC, André S, Félix A,
Rodrigues AS and Rueff J: Male and female breast cancer: The two
faces of the same genetic susceptibility coin. Breast Cancer Res
Treat. 188:295–305. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guidugli L, Carreira A, Caputo SM, Ehlen
A, Galli A, Monteiro AN, Neuhausen SL, Hansen TV, Couch FJ and
Vreeswijk MP; ENIGMA consortium, : Functional assays for analysis
of variants of uncertain significance in BRCA2. Hum Mutat.
35:151–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boonen RACM, Rodrigue A, Stoepker C,
Wiegant WW, Vroling B, Sharma M, Rother MB, Celosse N, Vreeswijk
MPG, Couch F, et al: Functional analysis of genetic variants in the
high-risk breast cancer susceptibility gene PALB2. Nat Commun.
10:52962019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Millot GA, Carvalho MA, Caputo SM,
Vreeswijk MP, Brown MA, Webb M, Rouleau E, Neuhausen SL, Hansen
TVO, Galli A, et al: A guide for functional analysis of BRCA1
variants of uncertain significance. Hum Mutat. 33:1526–1537. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Obe G, Pfeiffer P, Savage JRK, Johannes C,
Goedecke W, Jeppesen P, Natarajan AT, Martínez-López W, Folle GA
and Drets ME: Chromosomal aberrations: Formation, identification
and distribution. Mutat Res. 504:17–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Terzoudi GI and Pantelias GE: Cytogenetic
methods for biodosimetry and risk individualisation after exposure
to ionising radiation. Radiat Prot Dosimetry. 122:513–520. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sommer S, Buraczewska I and Kruszewski M:
Micronucleus assay: The state of art, and future directions. Int J
Mol Sci. 21:15342020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Murgia E, Ballardin M, Bonassi S, Rossi AM
and Barale R: Validation of micronuclei frequency in peripheral
blood lymphocytes as early cancer risk biomarker in a nested
case-control study. Mutat Res. 639:27–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cardinale F, Bruzzi P and Bolognesi C:
Role of micronucleus test in predicting breast cancer
susceptibility: A systematic review and meta-analysis. Br J Cancer.
106:780–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Scott D, Hu Q and Roberts SA: Dose-rate
sparing for micronucleus induction in lymphocytes of controls and
ataxia-telangiectasia heterozygotes exposed to 60Co
gamma-irradiation in vitro. Int J Radiat Biol. 70:521–527. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gunasekarana V, Raj GV and Chand P: A
comprehensive review on clinical applications of comet assay. J
Clin Diagn Res. 9:GE01–GE05. 2015.PubMed/NCBI
|
|
47
|
Kopjar N, Garaj-Vrhovac V and Milas I:
Assessment of chemotherapy-induced DNA damage in peripheral blood
leukocytes of cancer patients using the alkaline comet assay.
Teratog Carcinog Mutagen. 22:13–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vinnikov V, Hande MP, Wilkins R, Wojcik A,
Zubizarreta E and Belyakov O: Prediction of the acute or late
radiation toxicity effects in radiotherapy patients using ex vivo
induced biodosimetric markers: A review. J Pers Med. 10:2852020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kaur S, Sangeeta, Galhna KK and Gautam N:
Assessment of radiation induced DNA damage in human peripheral
blood lymphocytes using COMET assay. Int J Life Sci Scienti Res.
3:1208–1214. 2017. View Article : Google Scholar
|
|
50
|
Majtnerová P and Roušar T: An overview of
apoptosis assays detecting DNA fragmentation. Mol Biol Rep.
45:1469–1478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Brignoni L, Cappetta M, Colistro V, Sans
M, Artagaveytia N, Bonilla C and Bertoni B: Genomic diversity in
sporadic breast cancer in a Latin American population. Genes
(Basel). 11:12722020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu GP, Zhao Q, Wang D, Xie WY, Zhang LJ,
Zhou H, Chen SZ and Wu LF: The association between BRCA1 gene
polymorphism and cancer risk: A meta-analysis. Oncotarget.
9:8681–8694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Majewski J and Pastinen T: The study of
eQTL variations by RNA-seq: From SNPs to phenotypes. Trends Genet.
27:72–79. 2011. View Article : Google Scholar : PubMed/NCBI
|