Introduction
Renal cell carcinoma (RCC) accounts for ~3–5% of all
carcinomas. According to the World Health Organization, >140,000
individuals succumb to the disease annually (1). Systemic treatment is required for 25% of
patients with RCC with metastases detected at the time of initial
diagnosis and for >10% of patients with RCC experiencing
recurrence following the surgical resection of the tumor (2). The personalized selection of the most
effective drugs is essential to improve the survival of patients
with advanced RCC. In recent years, the selection of treatment
strategies for metastatic RCC has become increasingly complex with
the advent of targeted therapeutics and immune checkpoint
inhibitors (ICIs). Currently, only 10% of all cancer patients have
actionable targets (detected by next-generation sequencing,
immunohistochemical staining and other bioanalytical methods) that
can be used to select targeted therapeutics (3). Owing to tumor heterogeneity and the
complexity of cancer signaling networks, the personalized
functional assessment of each tumor may be more valuable than the
analysis of tumor molecular profiles. Recent advances in the
development of patient-derived tumor organoids (TOs) have provided
new opportunities for the growth and analysis of patient tumors
ex vivo. TOs are three-dimensional (3D) tissue-like cancer
cell clusters derived from tumor tissue that mimic the in
vivo characteristics and cellular heterogeneity of the original
tumor (4–6). Two-dimensional (2D) cancer cell lines
have been used in cancer research for decades. However, the
generation of 2D cell lines from human tumors is highly
inefficient, as it requires the lengthy selection of cancer cells
for 2D culture conditions and numerous passages of rare clones in
2D cultures, with potential genetic changes leading to the
establishment of genetically monoclonal cell lines that do not
represent the genetic heterogeneity of the original tumor (7). On the other hand, the 3D growth of TOs
resembles clinical tumors; moreover, TOs preserve the heterogeneity
of the parental tumors. Thus, TOs have emerged as a potential ex
vivo tumor model for the development of personalized therapy
(8,9).
Clinical tumors and patient-derived TO cultures share a number of
features, including morphology, cell-cell interaction, signal
transduction, gene and protein expression (10,11).
Recent studies have demonstrated that the therapeutic response of
patient-derived TOs to antitumor drugs ex vivo may be
promising for the development of personalized treatment (10–12).
The present study reports the development of a novel
method for the efficient establishment of patient-derived TO
cultures from RCC tumor samples. It is demonstrated that RCC TOs
recapitulate the histological features of the clinical tumor ex
vivo and maintain the genomic features of their originating
tumors during long-term culturing. Using a panel of RCC standard of
care (SOC) tyrosine kinase inhibitors (TKIs) and patient-derived
RCC TOs, an ex vivo testing method was developed for
potential use in customizing and tailoring RCC treatment for
individual patients.
Materials and methods
Development of patient-derived
TOs
The present study was approved by the Institutional
Review Board of Niigata University (approval no. 2018-0254).
Written informed consent was obtained from all patients. Tumor
samples were collected from RCC tissues obtained via radical
nephrectomy or nephron-sparing surgery from 20 patients at Niigata
University Hospital between 2018 and 2020. The patient
characteristics are presented in Table
I.
 | Table I.Analysis of RCC clinicopathological
parameters of RCC cases and corresponding tumor organoids. |
Table I.
Analysis of RCC clinicopathological
parameters of RCC cases and corresponding tumor organoids.
| Case | Age, years | Sex | Pathology | ISUP grade | Fuhrman grade | T stage | TO Growth | Pre Tx | Post Tx |
|---|
| OR007 | 65 | M | Clear cell | G2 | G2 | pT1a | +/+ | – | – |
| OR008 | 46 | M | Clear cell +
spindle cell component | G4 | G4 | pT1b | +/+ | – | Ipi + Nivo |
| OR009 | 29 | M | Chromophobe +
sarcomatoid diff | G4 | G4 | pT3a | +/+ | Ipi + Nivo | – |
| OR010 | 77 | M | Clear cell +
sarcomatoid | G2 | G2 | pT1a | +/+ | – | – |
| OR011 | 68 | F | Clear cell +
sarcomatoid + rhabdoid diff | G4 | G4 | pT3a | +/+ | Axi | Axi |
| OR012 | 73 | M | Clear cell | G2 | G2 | pT1b | +/+ | – | – |
| OR013 | 72 | M | Clear cell | G2 | G2 | pT1b | +/+ | – | – |
| OR014 | 53 | M | Clear cell | G2 | G2 | pT1b | −/− | – | – |
| OR015 | 71 | M | Clear cell | G4 | G4 | pT1a | +/− | – | – |
| OR016 | 81 | M | Cear cell +
sarcomatoid + rhabdoid diff | G4 | G4 | pT3a | +/− | – | – |
| OR017 | 50 | F | Clear cell | G2 | G2 | pT1a | −/− | – | – |
| OR018 | 52 | M | Clear cell +
sarcomatoid + rhabdoid diff | G4 | G4 | pT4 | +/+ | Pem + Axi | Paz → Nivo |
| OR019 | 48 | F | Clear cell | G2 | G2 | pT1a | +/+ | – | – |
| OR020 | 78 | M | Clear cell | G2 | G2 | pT2b | +/+ | – | – |
| OR021 | 39 | M | Clear cell | G2 | G2 | pT1a | +/+ | – | – |
| OR022 | 69 | M | Clear cell +
rhabdoid diff | G4 | G4 | pT3 | +/+ | – | – |
| OR023 | 76 | M | Clear cell | G4 | G4 | pT4 | +/+ | – | – |
| OR024 | 76 | M | Clear cell | G2 | G2 | pT1b | +/+ | – | – |
| OR025 | 77 | F | Clear cell +
sarcomatoid | G3>G4 | G3>G4 | pT1b | +/− | – | – |
| OR026 | 72 | M | Clear cell | G1 | G2 | pT3a | +/+ | – | – |
The TO culture conditions were based on those
previously reported in the study by Lee et al (13) in prostate and bladder cancer models,
including Matrigel (#354230, Corning, Inc.) to support 3D TO
culture, hepatocyte medium and Rho-associated protein kinase (ROCK)
inhibitors. The step-by-step procedure of tissue processing and
development of TO culture is illustrated in Fig. 1. The tumor samples were 1×1 cm tissue
blocks obtained from the portion of the kidney tumor removed in the
operating room, which were promptly cooled and transported to the
laboratory for processing. A portion of the sample was preserved as
a frozen specimen and used for DNA collection. For tissue
dissociation, RCC tissues were placed in cold phosphate-buffered
saline (PBS) and minced using scalpels. The tumor tissue was
incubated in 2 ml of collagenase solution (#07912, STEMCELL
Technologies) at 37°C for 30 min. The dissociated tissue was spun
down (200 × g at room temperature for 5 min), resuspended in PBS,
and passed through a 100-µm cell strainer (#435010003, Funakoshi
Co., Ltd.). The cells were then spun down (200 × g at room
temperature for 5 min), gently mixed with Matrigel on ice, and
placed in a 24-well plate. Following a 20-min at 37°C incubation to
solidify the Matrigel, the TO culture medium was added and the
cells were incubated at 37°C.
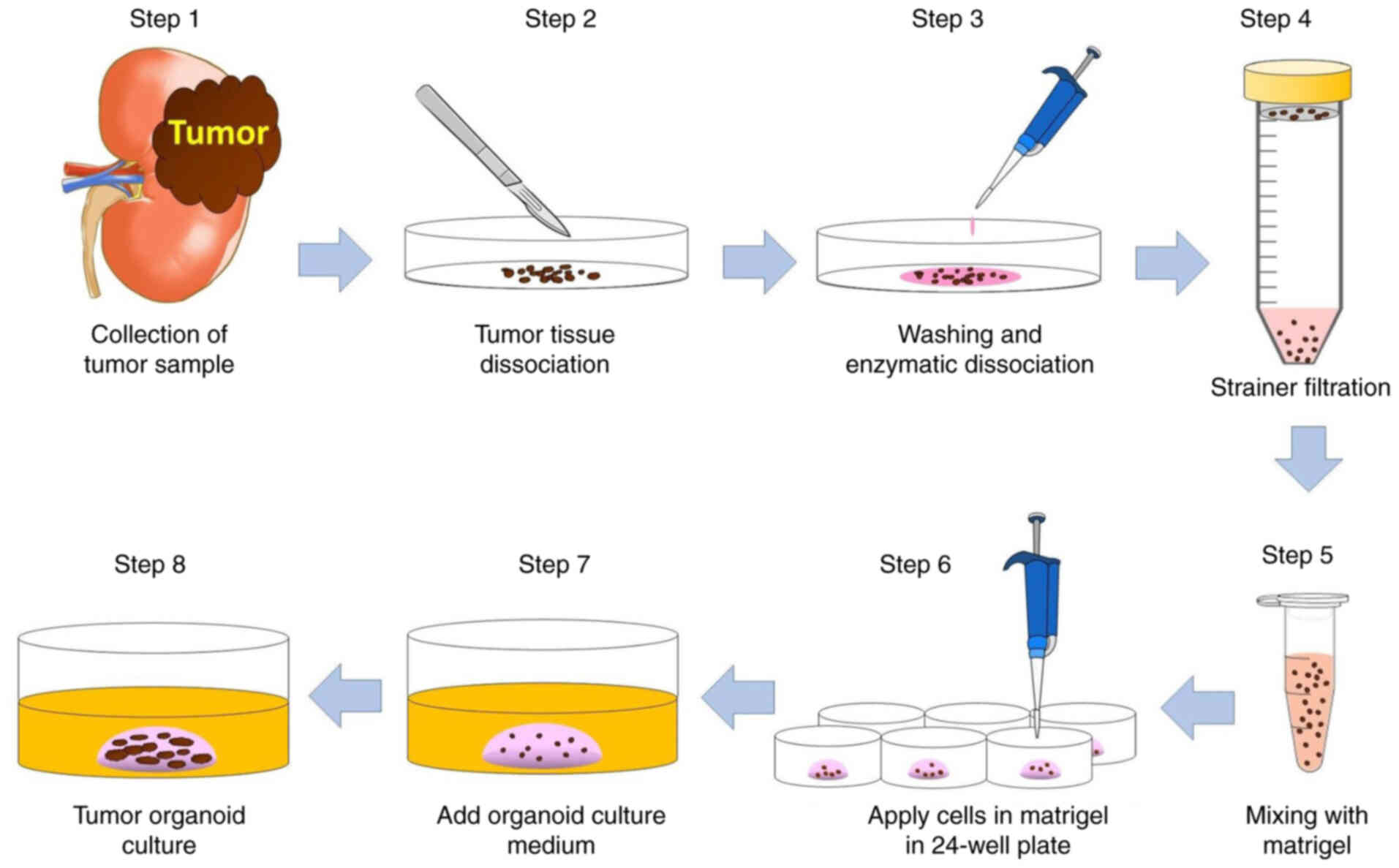 | Figure 1.Method for the development of
patient-derived tumor organoids. Step 1, tumor samples were
collected from RCC tissue removed during radical nephrectomy or
nephron-sparing surgery. Step 2, freshly resected tumor samples
were placed in cold PBS and minced with scalpels. Step 3, the tumor
tissue was incubated in collagenase solution at 37°C for 30 min.
Step 4, the dissociated tumor was passed through a 100-µm cell
strainer. Step 5, tumor cells were gently mixed with Matrigel. Step
6, the suspension of tumor cells in 20 µl Matrigel was added to
each well of a 24-well plate and solidified at 37°C for 30 min.
Steps 7 and 8, TO culture medium was added and tumor organoids were
cultured at 37°C. RCC, renal cell carcinoma; PBS,
phosphate-buffered saline; TO, tumor organoid. |
Histological analysis and
immunohistochemical (IHC) staining
The TOs were fixed in 10% formalin and embedded in
paraffin. The paraffin-embedded sections (4-µm-thick) were prepared
and stained with hematoxylin and eosin using standard protocols.
IHC staining was performed as previously described (14). 1/100 dilution anti-B-cell lymphoma 2
(Bcl-2) mouse monoclonal antibody (#MAB11332, Abnova) was used for
IHC staining (60 min at room temperature). After washing with PBS,
the slides were incubated with horseradish peroxidase (HRP)-labeled
secondary antibody for 30 min at room temperature (Histofine simple
stain MAX-PO, #424152, Nichirei). The staining reaction was
developed using 3,3′-diaminobenzidine (DAB), and nuclear
counterstaining was performed with hematoxylin (Mayer's Hematoxylin
Solution, #131-09665, FUJIFILM Wako Pure Chemical Corporation) for
5 min at room temperature. The sections were analyzed under an
Olympus DP72 microscope (Olympus Corporation).
Whole-exome sequencing (WES)
To identify somatic mutations in patient-derived TOs
and parental tumor tissues, WES was performed. Genomic DNA from
each sample was used to construct a paired-end sequencing library.
The DNA quality was evaluated using 1% agarose gel electrophoresis
and a NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc.).
The samples were prepared using the SureSelect Human All Exon 50Mb
kit (Agilent Technologies, Inc.). WES analysis was performed using
an Illumina HiSeq2500 instrument (Illumina). The informatics
analysis, mainly including quality control, read mapping, variant
calling, filtering and annotation, was conducted using BWA software
(http://bio-bwa.sourceforge.net/bwa.shtml), GATK
(https://www.broadinstitute.org/gatk/)
and the SnpEff tool (http://snpeff.sourceforge.net/SnpEff.html),
respectively.
Cell viability assay and TKIs
The TOs were grown in flat-bottom 96-well plates
(Corning, Inc.) at 37°C in a 5% CO2 atmosphere and
treated with various concentrations (0.1, 0.5, 1, 5, 10, 50, 100
and 500 µM) sunitinib (#PZ0012, Sigma-Aldrich; Merck KGaA),
pazopanib (#12097, Cayman Chemical Company), cabozantinib (#C8999,
LC Laboratories), axitinib (#PZ0193, Sigma-Aldrich; Merck KGaA) and
sorafenib (#CS0164, Chemscene) for 72 h. TO proliferation was
measured using a CellTiter 96® AQueous One Solution Cell
Proliferation assay (#93582, Promega Corporation). The experiments
were repeated three times, and the results were read using an
iMark™ 96-well microplate reader (Bio-Rad Laboratories, Inc.). The
absorbance was measured at 490 nm. The drug concentration that
inhibited the growth of cancer cells by 50% (GI50) for
each drug was calculated using GraphPad Prism software (version
8.0; GraphPad Software, Inc.).
Results
Histopathological features of RCC
tumors and tumor-derived TOs
The present study developed TO organoid cultures
from freshly resected human tumors in 15 of 20 RCC cases (Table I). The histopathological diagnosis was
clear cell carcinoma in all tumors, apart from one case of
chromophobe RCC. Approximately half of the RCC cases (9/20) had a
Fuhrman grade ≥3. The established RCC TOs were propagated for three
or more passages and cryopreserved. Although TO culture was
established in the majority of cases, a cessation of TO growth was
observed after several passages in 5 of the 20 RCC cases (Table I). The established TOs were cultured
for up to 15 passages over a 12-month period. No apparent loss of
growth capacity was observed following multiple passages, and all
15 established TO cultures were successfully restarted following
cryopreservation. The cultured TOs were passaged every 3–4 weeks.
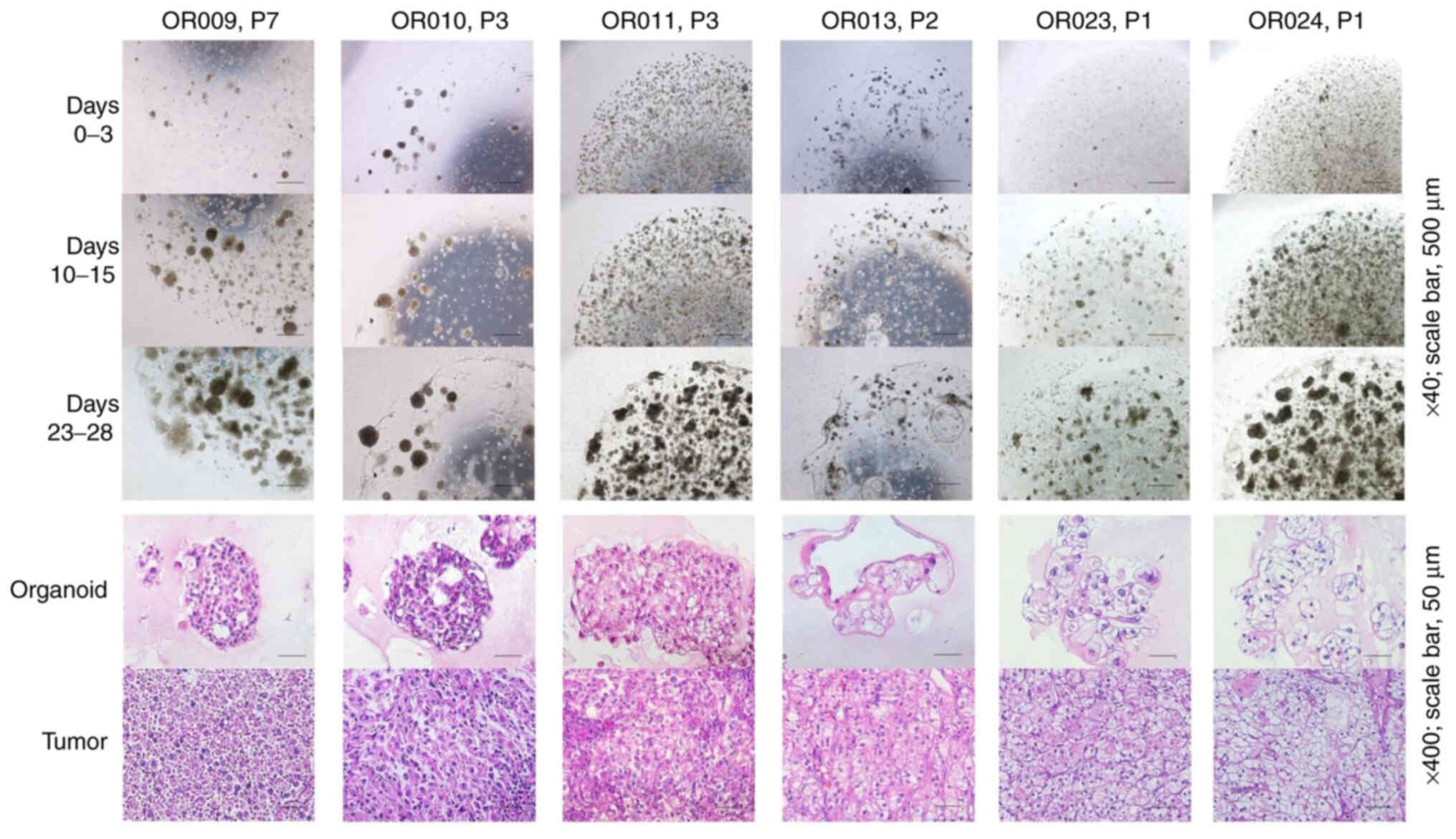
The TOs grew in dense clusters of cells, forming aggregates. A high
degree of association between the morphological structure of RCC
tumors and their corresponding TOs was observed (Fig. 2). The present study not only confirmed
the histology of clear cells, a common histological feature of RCC
in TOs, but also the features of chromophobe RCC and renal
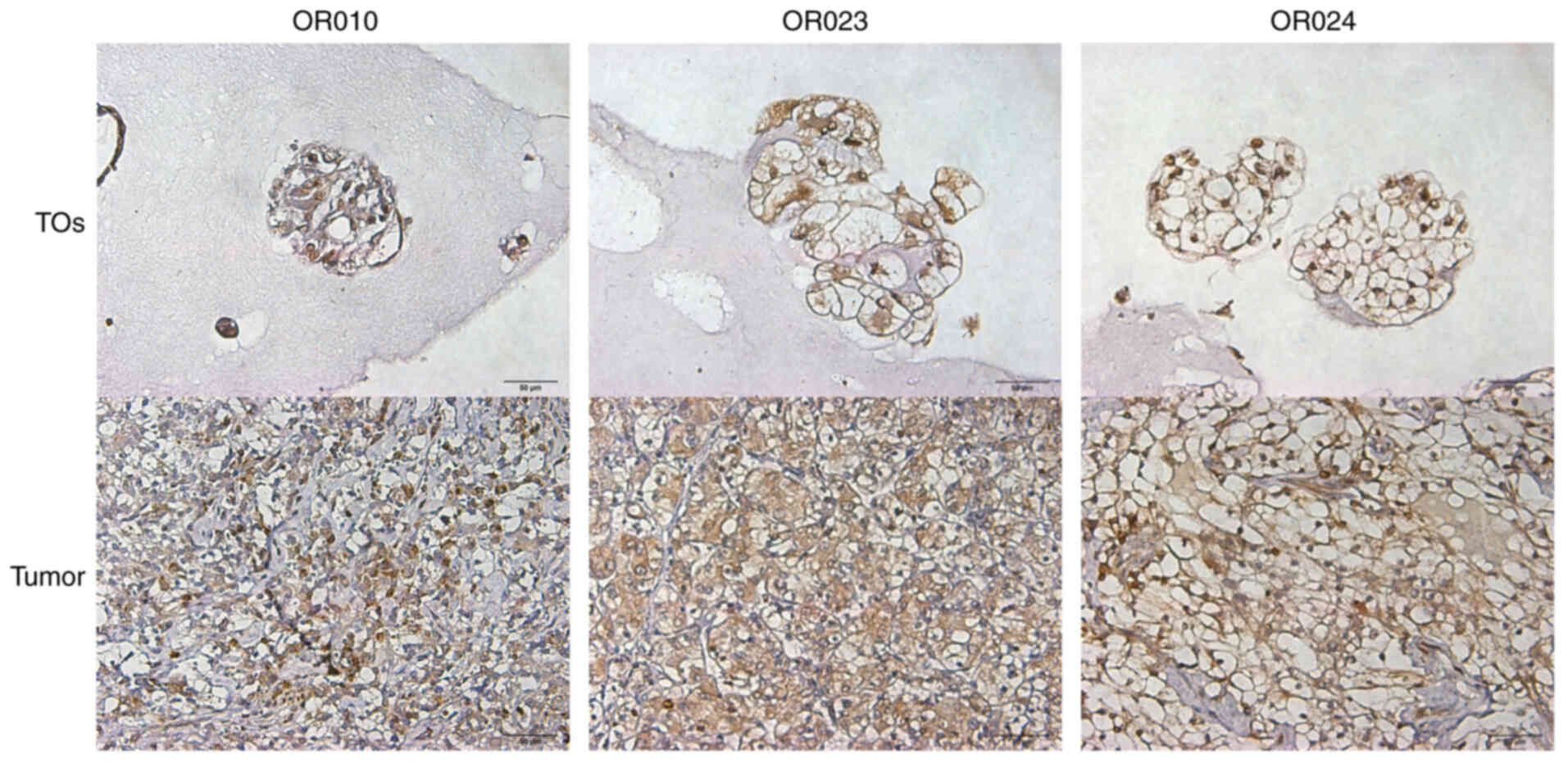
carcinoma with sarcomatoid variants. Using IHC staining, it was
found that Bcl-2 was highly expressed in cancer cells from both RCC
tissues and the corresponding TOs (Fig.
3).
WES of renal tumors and tumor-derived
TOs
To investigate whether the TOs preserved the genetic
characteristics of their corresponding RCC tumors, WES of the TOs
and their corresponding primary tumor tissues was performed using a
NovaSeq 6000 system. The allele frequencies of the variant genes
represented the multiclonal capacity of a TO culture compared to
parental cancer tissue. Although 6–39% of the allele settings
differed between the tumor tissues and TOs, the analysis of the WES
data revealed concordance in numerous gene mutations in the
parental RCC tissue and corresponding TOs. Common RCC genetic
alterations, such as von Hippel-Lindau (VHL) and polybromo 1
(PBRM1) mutations, were detected in the RCC tumors and
corresponding TOs (Fig. 4). The
results suggested that patient-derived TOs resembled clinical
tumors and that TOs could serve as a potential ex vivo model
of RCC.
 | Figure 4.Summary of the genetic alterations
identified in parental RCC and corresponding tumor organoids by
whole-exome sequencing (cases OR009, OR011, OR024 and OR026).
Representative genes known to be mutated in kidney cancer are shown
in bold font. RCC, renal cell carcinoma; m, multi-hit; in, frame
del; sp, splice site; f, frameshift; n, nonsense; miss, missence;
up, upstream; down, downstream; st, structural interaction. RCC,
renal cell carcinoma. |
Patient-derived TOs respond
differently to treatment with TKIs
To explore the utility of the TO cultures as
potential tumor models for the evaluation of drug efficacy, the RCC
SOC TKIs, sunitinib, axitinib, pazopanib, sorafenib and
cabozantinib, were examined in four patient-derived RCC TO cultures
(Fig. 5). To avoid the divergence of
the parent tumor and TO lines due to potential clonal selection at
late TO passages, each TO line was examined at an early passage. It
was found that the GI50 values for the TKIs ranged from
2 to 50 µM (Fig. 5). The TOs were
defined as TKI-resistant when the GI50 of the drug was
higher than the clinically relevant concentration (CRDC) of the
same drug. The CRDC is defined as the peak plasma concentration of
a drug. Although all four TO cultures were resistant to sunitinib
(CRDC, 200 nM), axitinib (CRDC, 160 nM) and cabozantinib (CRDC, 4
µM), it was found that three of the four TO cultures were sensitive
to pazopanib (CRDC, 130 µM) and all TOs were sensitive to sorafenib
(CRDC, 20 µM) (Fig. 5B) (15). The pazopanib GI50 was
8–10-fold lower than its CRDC in the OR009, OR011 and OR013 cases,
whereas the sorafenib GI50 was 2–5-fold lower than its
CRDC in the same cases (Fig. 5B).
These findings support the selection of pazopanib as the most
effective drug for the OR009, OR011 and OR013 RCC cases, and
sorafenib for case OR024.
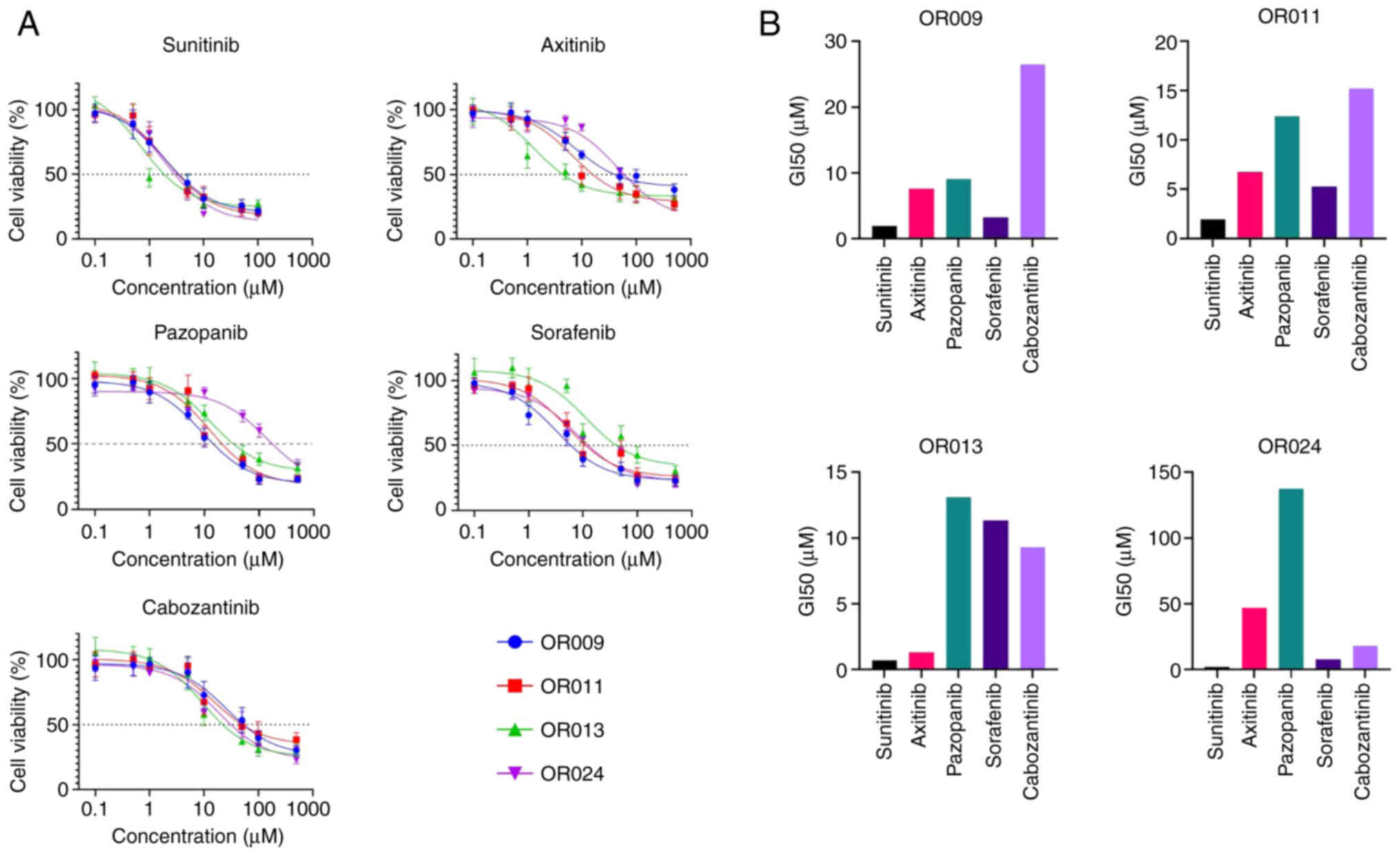 | Figure 5.Analysis of the antitumor effects of
TKIs in RCC tumor organoids. (A) Analysis of cancer cell viability
using colorimetric CellTiter assay in OR009, OR011, OR013 and OR024
tumor organoids treated with the TKIs, sunitinib, axitinib,
pazopanib, sorafenib and cabozantinib, for 72 h. (B)
GI50 for each TKI in RCC TO models using GraphPrism 8.0.
TKI, tyrosine kinase inhibitor; RCC, renal cell carcinoma; TO,
tumor organoid; GI50, drug concentration that inhibited
the growth of cancer cells by 50%. |
Discussion
Oncologists aim to ensure that cancer patients are
offered the optimal treatment options from the start in order to
avoid delayed treatment, potential side-effects of ineffective
treatments, and unnecessary expenses of therapies that do not
benefit them.
The main objective of the present study was to
develop and test patient-derived 3D TOs as a new personalized ex
vivo tumor model to determine the most effective treatment for
individual patients with RCC. The development of this approach
includes the generation of TO culture from a patient's tumor biopsy
specimen, development and execution of a functional personalized
test of chemotherapeutic and targeted therapeutics in the
laboratory, followed by the confirmation of the effectiveness of
the predicted drug therapy in the clinic.
Usually, treatment with SOC drugs is initiated 30–90
days following the surgical resection of the tumor and the
successful post-operative recovery of the patient. It provides a
treatment-free period (30–90 days) to establish TOs, test SOC drugs
in TOs, and identify the most effective therapeutic agent to begin
the treatment of the patient. In metastatic cases, tumor core
biopsy may be another source of tumor material to establish TOs.
Tumor core biopsy can be performed at any time point during the
patient's treatment, and it does not affect previous or current
therapies administered to the patient. The method presented herein
may prove to be useful for the selection of an effective therapy
for patients who develop recurrence following radical nephrectomy,
and for deciding on the second-line treatment for patients with RCC
with disease progression during primary systemic therapy. This
novel approach facilitates the efficient and rapid development of
tumor-derived TOs with subsequent testing and identification of the
optimal personalized treatment options for cancer patients.
Tumor-derived organoids have been developed in
various cancer types, including lung (16), gastrointestinal (17), colon (18), prostate (8) and bladder (12) cancers. An initial report on the
development of TOs from clear cell RCC (four cases) was published
in 2019 (19). The present study
selected the development of 3D TO models as the generation of 2D
cell lines from primary tumors is inefficient, as it involves
extensive adaptation and clonal selection for in vitro 2D
culture conditions. As only rare clones are able to expand and can
be maintained over several passages in 2D culture, the derived 2D
cell lines may have undergone substantial genetic alterations and
may no longer recapitulate the genetic heterogeneity of the
original tumors. In the present study, a novel method for the
efficient development of 3D TO cultures from freshly resected RCC
tumor samples was developed. Importantly, this method allows the
growth of RCC TOs using a simple culture medium without costly
supplements (e.g., Noggin, gastrin, R-spondin, or Wnt3A) (4,11,20,21). While
previous TO studies have used Dulbecco's modified Eagle
medium/nutrient mixture F-12 (DMEM/F12) medium with multiple
supplements, the present study established TO cultures in 15 (75%)
of 20 RCC cases using hepatocyte culture medium. The quality of the
tumor specimen is critical for TO development; however, further
investigations are required in order to understand other factors
affecting the successful establishment of TO culture.
Clinical tumors and patient-derived TO cultures
share a number of features, including morphology, cell-cell
interaction, signal transduction, gene and protein expression,
hypoxia, differential zones of proliferation, pH, drug response and
resistance (10,22). Consistent with previously published
studies on other types of cancer (8,12,15–17), the
present study found that patient-derived RCC TO cultures possessed
in vivo features of RCC clinical tumors, such as morphology,
genetic alterations and the expression of the Bcl-2 anti-apoptotic
protein, suggesting the potential use of the patient-derived RCC TO
culture as a model for personalized medicine.
The evaluation of the therapeutic response of
patient-derived TOs to antitumor drugs may be a potential approach
for the development of personalized treatment. To the best of our
knowledge, the present study is first to demonstrate the
development of a method for selecting the optimal treatment options
for patients with RCC by testing the SOC TKIs in patient-derived TO
models. Although ICIs are becoming the mainstay of first-line
treatment for metastatic RCC (23–25), the
development of a TO assay for the testing of ICIs remains
challenging due to the indirect effect of immunotherapeutic agents
on cancer cells mediated by host immunity, which is difficult to
recapitulate ex vivo. The development of TO-based assays for
the testing of immuno-oncology therapeutics is a subject of future
research studies. TKIs remain key targeted therapeutics in
second-line RCC therapy (26). The
selection of patients with RCC who are most likely to respond to
TKI therapy is crucial for improving patient survival. The findings
of the present study demonstrated a difference in response to TKI
treatment in different ex vivo TO cultures established from
RCC tumors. The present findings are consistent with those of
previous studies that observed variations in drug sensitivity in
different TO cultures (27–30). Herein, the ex vivo therapeutic
response was defined as that with a GI50 lower than the
CRDC (peak plasma concentration). This approach can be applied to
evaluate the therapeutic response of targeted or chemotherapeutic
drugs in various TO models. The ex vivo testing method used
herein for the selection of the most effective TKI in
patient-derived RCC TO models requires validation in clinical
settings.
In conclusion, the present study demonstrated the
development of a patient-derived TO culture and presented a novel
approach for the ex vivo evaluation of the therapeutic
response as a potential testing model for the selection of
personalized therapy for patients with RCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by a research grant
from the Department of Urology, Division of Molecular Oncology,
Niigata University Graduate School of Medical and Dental Sciences,
Niigata, Japan (grant no. CH29017; August, 2018).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the figshare repository, https://doi.org/10.6084/m9.figshare.14993409.v1.
Authors' contributions
AK, VB and YT conceived and designed the study. AK,
TA, HK, YS, MM, VB, AU, KS and YT developed the study methodology.
AK, TA, HK, YS and MM performed the experiments. AK, VB and YT
analyzed and interpreted the data. AK, VB, AU and YT wrote,
reviewed and revised the manuscript. YT provided administrative,
technical, or material support. VB, AU, KS and YT supervised the
study. AK and VB confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Niigata University (approval no. 2018-0254).
Written informed consent was obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kabaria R, Klaassen Z and Terris MK: Renal
cell carcinoma: Links and risks. Int J Nephrol Renovasc Dis.
9:45–52. 2016.PubMed/NCBI
|
|
2
|
Escudier B, Porta C, Schmidinger M,
Rioux-Leclercq N, Bex A, Khoo V, Grünwald V, Gillessen S and
Horwich A; ESMO Guidelines Committee. Electronic address, :
clinicalguidelines@esmo.org: Renal cell carcinoma: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 30:706–720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malone ER, Oliva M, Sabatini PJ, Stockley
TL and Siu LL: Molecular profiling for precision cancer therapies.
Genome Med. 12:82020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fatehullah A, Tan SH and Barker N:
Organoids as an in vitro model of human development and disease.
Nat Cell Biol. 18:246–254. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clevers H: Modeling development and
disease with organoids. Cell. 165:1586–1597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rookmaaker MB, Schutgens F, Verhaar MC and
Clevers H: Development and application of human adult stem or
progenitor cell organoids. Nat Rev Nephrol. 11:546–554. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kapałczyńska M, Kolenda T, Przybyła W,
Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski
Ł and Lamperska K: 2D and 3D cell cultures-a comparison of
different types of cancer cell cultures. Arch Med Sci. 4:910–919.
2018.PubMed/NCBI
|
|
8
|
Broutier L, Mastrogiovanni G, Verstegen
MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R,
Sidorova O, Gaspersz MP, et al: Human primary liver cancer-derived
organoid cultures for disease modeling and drug screening. Nat Med.
12:1424–1435. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao D, Vela I, Sboner A, Iaquinta PJ,
Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora
VK, et al: Organoid cultures derived from patients with advanced
prostate cancer. Cell. 159:176–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ganesh K, Wu C, O'Rourke KP, Szeglin BC,
Zheng Y, Sauvé CG, Adileh M, Wasserman I, Marco MR, Kim AS, et al:
A rectal cancer organoid platform to study individual responses to
chemoradiation. Nat Med. 25:1607–1614. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romero-Calvo I, Weber CR, Ray M, Brown M,
Kirby K, Nandi RK, Long TM, Sparrow SM, Ugolkov A, Qiang W, et al:
Human organoids share structural and genetic features with primary
pancreatic adenocarcinoma tumors. Mol Cancer Res. 17:70–83. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito Y, Muramatsu T, Kanai Y, Ojima H,
Sukeda A, Hiraoka N, Arai E, Sugiyama Y, Matsuzaki J, Uchida R, et
al: Establishment of patient-derived organoids and drug screening
for biliary tract carcinoma. Cell Rep. 27:1265–1276.e4. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SH, Hu W, Matulay JT, Silva MV,
Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB, et
al: Tumor evolution and drug response in patient-derived organoid
models of bladder cancer. Cell. 173:515–528.e17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bilim V, Yuuki K, Itoi T, Muto A, Kato T,
Nagaoka A, Motoyama T and Tomita Y: Double inhibition of XIAP and
Bcl-2 axis is beneficial for retrieving sensitivity of renal cell
cancer to apoptosis. Br J Cancer. 98:941–949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liston DR and Davis M: Clinically relevant
concentrations of anticancer drugs: A guide for nonclinical
studies. Clin Cancer Res. 23:3489–3498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ,
Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, et al: Patient-derived
lung cancer organoids as in vitro cancer models for therapeutic
screening. Nat Commun. 10:39912019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aberle MR, Burkhart RA, Tiriac H, Olde
Damink SW, Dejong CH, Tuveson DA and van Dam RM: Patient-derived
organoid models help define personalized management of
gastrointestinal cancer. Br J Surg. 105:e48–e60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van de Wetering M, Francies HE, Francis
JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J,
Taylor-Weiner A, Kester L, et al: Prospective derivation of a
living organoid biobank of colorectal cancer patients. Cell.
161:933–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grassi L, Alfonsi R, Francescangeli F,
Signore M, De Angelis ML, Addario A, Costantini M, Flex E, Ciolfi
A, Pizzi S, et al: Organoids as a new model for improving
regenerative medicine and cancer personalized therapy in renal
diseases. Cell Death Dis. 10:2012019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neal JT, Li X, Zhu J, Giangarra V,
Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, et
al: Organoid modeling of the tumor immune microenvironment. Cell.
175:1972–1988.e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vlachogiannis G, Hedayat S, Vatsiou A,
Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford
I, Burke R, et al: Patient-derived organoids model treatment
response of metastatic gastrointestinal cancers. Science.
359:920–926. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bian S, Repic M, Guo Z, Kavirayani A,
Burkard T, Bagley JA, Krauditsch C and Knoblich JA: Author
correction: Genetically engineered cerebral organoids model brain
tumor formation. Nat Methods. 15:7482018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Motzer RJ, Penkov K, Haanen J, Rini B,
Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S,
Uemura M, et al: Avelumab plus Axitinib versus Sunitinib for
advanced renal-cell carcinoma. N Engl J Med. 380:1103–1115. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: KEYNOTE-426 investigators. Pembrolizumab plus Axitinib
versus Sunitinib for advanced renal-cell carcinoma. N Engl J Med.
380:1116–1127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: CheckMate 214 investigators. Nivolumab
plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma.
N Engl J Med. 378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zerdes I, Tolia M, Tsoukalas N, Mitsis M,
Kardamakis D, Pistevou-Gombaki K, Tsekeris P and Kyrgias G:
Systemic therapy of metastatic renal cell carcinoma: Review of the
current literature. Urologia. 86:3–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Qin T, Huang Y, Li Y, Chen G and
Sun C: Drug screening model meets cancer organoid technology.
Transl Oncol. 13:1008402020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M and Izpisua Belmonte JC:
Organoids-preclinical models of human disease. N Engl J Med.
380:569–579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bleijs M, van de Wetering M, Clevers H and
Drost J: Xenograft and organoid model systems in cancer research.
EMBO J. 38:e1016542019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Drost J and Clevers H: Organoids in cancer
research. Nat Rev Cancer. 18:407–418. 2018. View Article : Google Scholar : PubMed/NCBI
|