Introduction
Neoplasms in the brain and other parts of the
nervous system are the leading cause of cancer-associated mortality
among men aged <40 years and women aged <20 years (1). These tumor types were estimated to
cause 17,760 deaths in the US in 2019 (1). Gliomas account for >70% of all
primary brain tumors in adults and the majority of patients with
glioma will not survive beyond the first 2 years from diagnosis
even following aggressive chemotherapy or radiotherapy (2). Therefore, it is urgent to identify
novel therapeutic methods to treat this disease. Although the
molecular mechanisms that contribute to tumorigenesis of glioma
have been recently identified (3),
their exact association with the development of this disease has
not been fully clarified.
MicroRNAs (miRs) are small, non-coding (nc),
single-stranded RNAs that are 20–23 nucleotides in length and act
by suppressing gene expression in a variety of eukaryotic organisms
via targeting specific mRNAs (4).
miRs suppress gene expression via complementary binding between
their seed region and the 3′-untranslated region (UTR) of the
target mRNA (4). Through this
regulation, miRs serve a pivotal role in several cellular
processes, including proliferation, cell cycle control, programmed
cell death, differentiation, invasiveness and tissue specific
functions, such as immune response, hormone secretion and
angiogenesis, which are implicated in the development and
progression of hepatocellular carcinoma (5), glioma (6) and lung cancer (7). miRs can be used as diagnostic and
prognostic markers (8) in cancer
as they are regulatory molecules with both oncogene and suppressor
gene functions and constitute major components of intercellular
communication (9). It has been
shown that miRs are involved in several biological processes in
glioma, such as cell proliferation, invasion, angiogenesis and
therapeutic resistance (10).
Significantly decreased expression of miR-49(6)7 has been observed
in numerous types of cancer type, including cutaneous squamous cell
carcinoma (11), breast (12), gastric (13), thyroid (14), colorectal (15), hepatocellular carcinoma (16), pancreatic (17), adrenocortical carcinoma (18), bladder (19), non-small cell lung (20) and cervical cancer (21) as well as other solid tumors.
However, several studies have shown that miR-497 expression is
upregulated in other types of cancer, including chronic lymphocytic
leukemia (22), diffuse large
B-cell lymphoma (23) and renal
cell carcinoma (24). With regards
to glioma, the expression and biological functions of miR-497
remain controversial and require further analysis. miR-497 is
located at the intron of miR-497 host gene (MIR497HG), which has
been demonstrated to be a tumor suppressor gene that suppresses
tumor progression (25,26). However, it remains controversial
whether the long (l)nc RNA MIR497HG is the actual host gene of
miR-497. In the present study, the expression and functions of
miR-497 and its host gene MIR497HG were investigated in glioma
tissue.
Materials and methods
Sample collection and ethics
statement
All human tissue was obtained from patients
undergoing surgery for glioma in the Department of Neurosurgery,
Xijing Hospital, Fourth Military Medical University (Xi'an, China).
A total of 13 male and 17 female glioma patients aged 40–60 years
were recruited between January 2018 and January 2020. The glioma
samples were histologically classified based on the diagnosis of
clinical and pathological grading according to the World Health
Organization guidelines (27).
Among these samples, 22 contained both adjacent and glioma tissues,
while 8 samples only contained glioma tissue. Therefore, the number
of glioma tissues was 30 and the number of adjacent tumor tissues
was 22. The normal brain tissue was collected as negative controls
from patients with cerebral trauma. Written informed consent
conforming to the tenets of the Declaration of Helsinki was
obtained from each participant and the study procedures were
approved by the institutional review board of Xijing Hospital,
Fourth Military Medical University. Expression levels of CCNE1 and
TUSC1 correlated with overall survival time were analyzed using 667
patients derived from The Cancer Genome Atlas (TCGA) database
(28).
Plasmid construction, cell culture and
transfection
The fragments of wild-type (WT) and mutated (Mut)
3′-UTRs of CCNE1 and TUSC1 were amplified via PCR from the human
cDNA library isolated from human peripheral blood and inserted into
the pGL3-promoter vector (Promega Corporation). The PCR
thermocycling conditions were as follows: Initial denaturation at
95°C for 5 min, 35 cycles of denaturation at 95°C for 30 sec,
annealing at 58°C for 30 sec, elongation at 72°C for 2 min sec and
final extension at 72°C for 5 min. The coding regions of CCNE1 were
also generated using PCR amplification and cloned into the
expression vector pCMV-Flag (Invitrogen; Thermo Fisher Scientific,
Inc.). The human cortical neuronal cell line HCN-2 and the glioma
cell lines U251, LN229 and LN18, were cultured for 24 h in DMEM
supplemented with 10% FBS and 2 mM glutamine (Thermo Fisher
Scientific, Inc.). The passaged cells were seeded into 6- or
12-well plates for overnight culture followed by transfection with
plasmids (2 µg) using Lipofectamine® 2000 (5 µl)
(Invitrogen; Thermo Fisher Scientific, Inc.) for 25 min at room
temperature. The subsequent experiments were performed 48 h later.
In the in vitro functional experiments, the oligonucleotides
were chemically synthesized and transfected at a final
concentration of 50 nmol/l according to the manufacturer's
instructions (Guangzhou RiboBio Co., Ltd.). Following transfection
with oligonucleotides, the cells were cultured in complete DMEM and
subsequently harvested for further experiments. All cells were
incubated at 37°C in an atmosphere of 5% CO2.
Intracranial glioma model
Nude mice (male BALB/cA-nu; age, 8 weeks) were
purchased from the Shanghai Experimental Animal Center (Chinese
Academy of Sciences) and maintained under specific pathogen-free
conditions. The animals were housed at 22±2°C, humidity of 55±10%,
12/12-h light/dark cycle and ad libitum access to water and
food. At the start of the experiments, animals weighed 22±2 g. A
total of 15 mice were randomly divided into three groups (Scramble,
miR-497 and MIR497HG; n=5 each). Luciferase modified-glioma cells
were established to overexpress miR-497 or MIR497HG via lentivirus
(Shanhai GeneChem Co., Ltd.) and injected intracranially into each
mouse at a density of 1×106 cells. At 14 days after
inoculation, the tumor-bearing mice were infected with luciferin
(Shanghai Yeasen Biotechnology Co., Ltd.) and the glioma growth was
evaluated via bioluminescence imaging. The mice were sacrificed by
cervical dislocation under pentobarbital sodium intraperitoneal
anesthesia (60 mg/kg) to minimize discomfort. The body weight of
experimental mice was not significantly different compared with
that before tumor cell inoculation. All animal experiments were
approved by the Animal Experiment Administration Committee of The
Fourth Military Medical University. All experiments were performed
in accordance with the recommendations of the Guide for the Care
and Use of Laboratory Animals prepared by the National Academy of
Sciences and published by the National Institutes of Health
(29).
RNA extraction and quantification
assay
Total RNA was extracted from glioma cell line U251,
LN229 and LN18 using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. cDNA was generated using TaqMan MicroRNA Reverse
Transcription (Thermo Fisher Scientific, Inc.) or PrimerScript RT
Reagent kit (Takara Bio, Inc.) according to the manufacturer's
instructions. Reverse transcription-quantitative (RT-q)PCR was
performed using a CFX96™ Real-Time PCR system (Bio-Rad
Laboratories, Inc.) with SYBR-Green reagents (cat. no. DRR041A;
Takara Bio, Inc.) according to the manufacturer's instructions as
previously described (30). The
primers were synthesized by Takara Bio, Inc., as follows: MIR497HG
forward, 5′-GAGATCTCTTGTGGGGGTGC-3′ and reverse,
5′-ACGTAGCAGGGTGTTTCAGG-3′; CCNE1 forward,
5′-AAGGAGCGGGACACCATGA-3′ and reverse, 5′-ACGGTCACGTTTGCCTTCC-3′;
TUSC1 forward, 5′-GCCTCTTCCGTCAGGCTTT-3′ and reverse,
5′-CGGAGTCGGGTTCCTGTAGA-3′ and GAPDH forward,
5′-CTTCAACGACCACTTTGT−3′ and reverse, 5′-TGGTCCAGGGGTCTTACT−3′. To
analyze miR-497 and miR-588 expression levels, Bulge-Loop™ miR
RT-qPCR primer kit (Guangzhou RiboBio Co., Ltd.) was used according
to the manufacturer's instructions. The RNA input was normalized to
the level of human U6 small nuclear RNA (forward,
5′-GTGCTCGCTTCGGCAGCA−3′ and reverse, 5′-CAAAATATGGAACGCTTC−3′).
The quantification was performed via the 2−ΔΔCq method
(31).
Western blot analysis
Western blot analysis was performed following
harvesting of glioma cell LN229 cells and lysis on ice for 30 min
using RIPA buffer supplemented with protease inhibitors (100 mM
Tris-HCl, pH 7.4; 150 mM NaCl; 5 mM EDTA; 1% Triton X-100; 1%
deoxycholate acid; 0.1% SDS, 2 mM phenylmethylsulfonyl fluoride; 1
mM sodium orthovanadate; 2 mM dithiothreitol; 2 mM leupeptin; 2 mM
pepstatin). The cell lysate was centrifuged at 10,000 × g for 15
min (4°C) and the supernatant was collected to extract the total
protein. The concentration levels of the protein samples were
determined using the BCA method (Beyotime Institute of
Biotechnology) and an 20 µl sample was separated via SDS-PAGE (12%)
and transferred onto PVDF membranes. The membranes were blocked at
RT with 5% non-fat dried milk solution for 2 h and incubated with
primary antibodies against CCNE1 (Abcam) and β-actin (1:2,000; cat.
no. EK1002; Boster Biological Technology). Following washing of the
membranes three times with PBS-Tween-20 (0.1%), membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat. no. BA1056; Boster Biological Technology) for 2 h at
room temperature and visualized with an ECL detection system [Multi
Science (Lianke) Biotech Co., Ltd.]. The protein expression was
measured using ImageJ software (version ImageJ 2X; National
Institutes of Health).
Immunofluorescence staining
The slices of normal, adjacent and glioma tissue
derived from patients were examined using in situ
hybridization for miR-497 detection (Wuhan Servicebio Technology
Co., Ltd.) and counterstained with DAPI. The clinical samples were
observed using a laser scanning confocal microscope (FV-1000;
Olympus Corporation). Briefly, the tissues were fixed in DEPC for
12 h at room temperature, followed by dehydration by gradient
alcohol, paraffin embedding and sectioning (8 µm). The slices were
boiled in the retrieval solution for 10–15 min and tissue was
marked with liquid blocker pen. Proteinase K (20 µg/ml; Wuhan
Servicebio Technology Co., Ltd.) working solution was added to
cover objectives and incubated at 37°C for 30 min. Sections were
washed in pure water, then washed three times in PBS (pH 7.4) on a
Rocker device for 5 min each. Then, pre-hybridization solution was
added to each section and incubated for 1 h at 37°C. After removing
the pre-hybridization solution, miR-497 probe hybridization
solution [1 µM Carboxyfluorescein (FAM)]-labelled miR-497 probe
(5′-ACAAACCACAGTGTGCTGCTG −3′) was added overnight at 42°C. Then,
the hybridization solution was removed and the sections were washed
in 2× SSC for 10 min at 37°C, in 1× SSC twice for 5 min each at
37°C and in 0.5× SSC for 10 min at room temperature. The cell
nuclei was stained with DAPI for 8 min in the dark at room
temperature. Finally, microscopic examination was performed to take
photos under a positive fluorescence microscope. DAPI glows blue by
UV excitation wavelength 330–380 nm and emission wavelength 420 nm;
FAM glows green by excitation wavelength 465–495 nm and emission
wavelength 515–555 nm.
Luciferase reporter assay
The target genes of miRNAs were predicted by
bioinformatics analysis via TargetScan (32) and miRanda online tools (33). U251 cells (1×105 cells)
were seeded into a 96-well plate and luciferase reporter plasmids
(Invitrogen; Thermo Fisher Scientific, Inc.) bearing WT or Mut
3′-UTR of CCNE1 were transfected with miR-497 oligonucleotides
(Guangzhou RiboBio Co., Ltd.) and pRL-TK vector by Lipofectamine
(Invitrogen; Thermo Fisher Scientific, Inc.). The 3′-UTRs of TUSC1
were transfected with miR-588 oligonucleotides (Guangzhou RiboBio
Co., Ltd.) and pRL-TK vector. The cells were harvested and lysed
with lysis buffer at 24 h post-transfection (Promega Corporation).
The relative luciferase activity was measured using a Dual
Luciferase Reporter Assay system (Promega Corporation) and
normalized to the relative activity of Renilla. Each
experiment was performed at least five times and the data were
analyzed via paired Student's t-test.
Proliferation assay
The proliferation of glioma cells was analyzed via
MTT assay. The miR-497- or MIR497HG-overexpressing glioma cells
(1×105 cells) were seeded into 96-well plates and cell
proliferation was evaluated at 24, 48, 72 and 96 h using MTT
reagent (5.0 mg/ml in PBS). CCNE1 or miR-588 were overexpressed for
rescue experiments. Following incubation for 4 h at 37°C, the
supernatant was removed and the precipitate was dissolved in DMSO
(Sigma-Aldrich; Merck KGaA). Spectrophotometric absorbance was
measured at 570 nm using a microplate reader (BioTek Instruments
Inc.).
Cell cycle assay
The cell cycle distribution was determined using a
BD Accuri™ C6 Plus Flow Cytometer (BD Biosciences). Briefly, U251
cells were collected and fixed in ice-cold ethanol (70% in PBS)
overnight at 4°C. The cells were treated with 20 g/ml RNase A
(Sigma-Aldrich; Merck KGaA) for 1 h at 37°C to degrade the RNA,
then incubated with 50 µg/ml PI (Sigma-Aldrich; Merck KGaA) in the
dark for 30 min at 4°C. The DNA content was analyzed via flow
cytometry (Accuri™ C6 Plus, BD Biosciences) and all phases of the
cell cycle were analyzed by appropriate gating on the distribution
plot and analyzed by FlowJo (Flowjo, LLC; V10).
Statistical analysis
The data were analyzed using SPSS 12.0 software
(SPSS, Inc.). Comparisons between groups were performed with an
unpaired Student's t-test or one-way ANOVA followed by
Student-Newman-Keuls test using GraphPad Prism software (GraphPad
Software, Inc.; version 5.0). The correlation was evaluated using
Pearson's correlation. Survival analysis was evaluated using the
Kaplan-Meier method and assessed using the log-rank test. Data are
presented as the mean ± SEM. The number of independent experimental
repeats was described in figure legends. P<0.05 was considered
to indicate a statistically significant difference.
Results
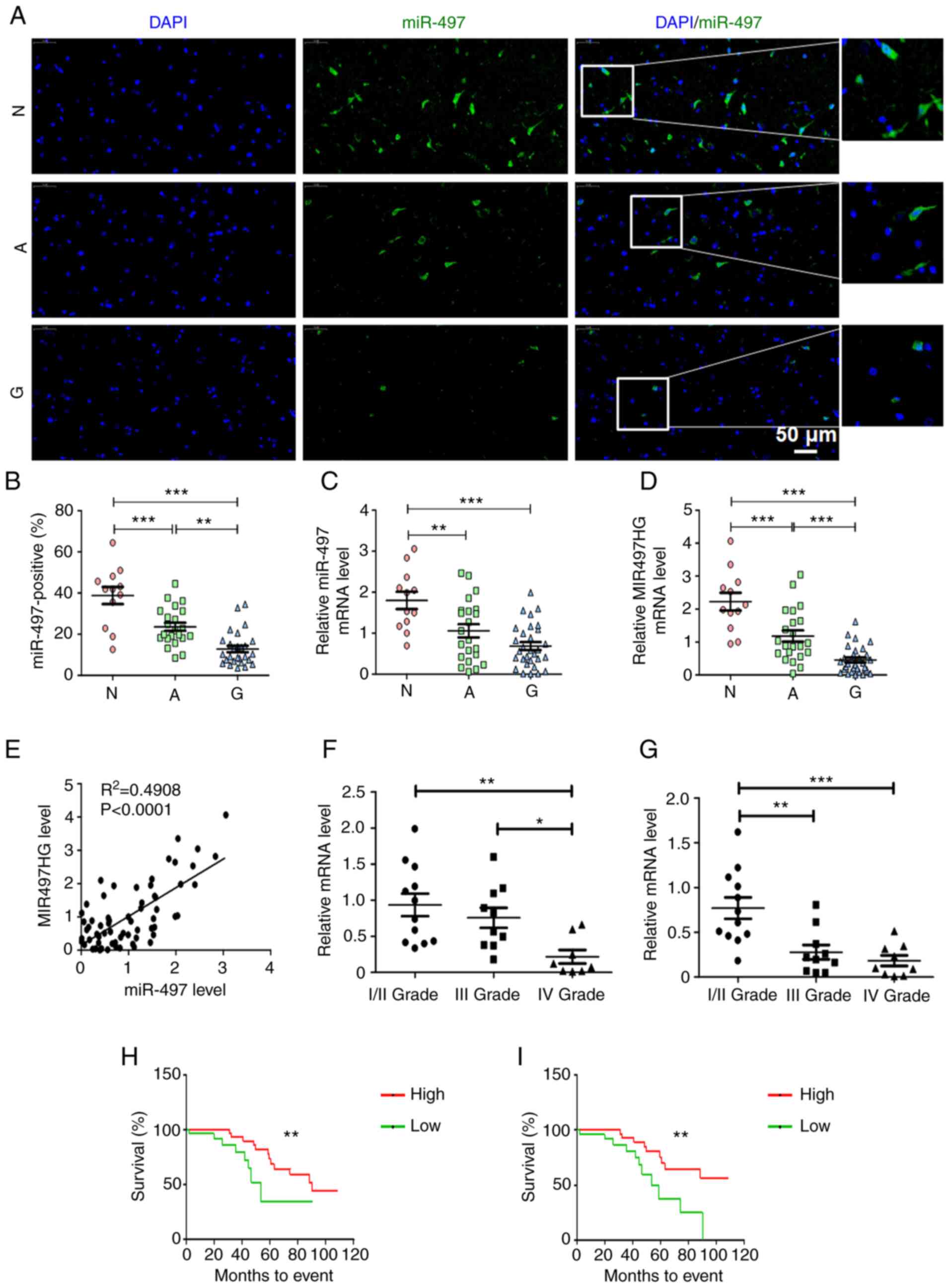
Decreased expression levels of miR-497
and MIR497HG predict poor prognosis of patients with glioma
To investigate the expression profile of miR-497 in
glioma, in situ hybridization staining was used to analyze
the expression levels of this miR in 27 pairs of glioma and
corresponding adjacent paracancerous, as well as normal brain
tissue. The results indicated that the number of miR-497-positive
cells was significantly decreased in glioma compared with adjacent
non-neoplastic and normal brain tissue (Fig. 1A and B). miR-497 is located at the
intron of its host gene, MIR497HG (26). The present study verified the
expression levels of these miRs in patient tissue samples using
RT-qPCR. The data suggested that expression levels of miR-497 and
MIR497HG were lower in glioma than in non-neoplastic and normal
brain tissue (Fig. 1C and D). In
addition, miR-497 expression was highly correlated with that of its
host gene MIR497HG (Fig. 1E).
Expression levels of miR-497 and MIR497HG decreased as the
pathological grade of glioma tumor increased (Fig. 1F and G). Subsequent analysis
indicated that higher expression levels of miR-497 and MIR497HG
were associated with better prognosis and longer survival time of
patients with glioma (Fig. 1H and
I). These data indicated that miR-497 and its host gene
MIR497HG were associated with glioma progression and predicted
better prognosis of patients with glioma.
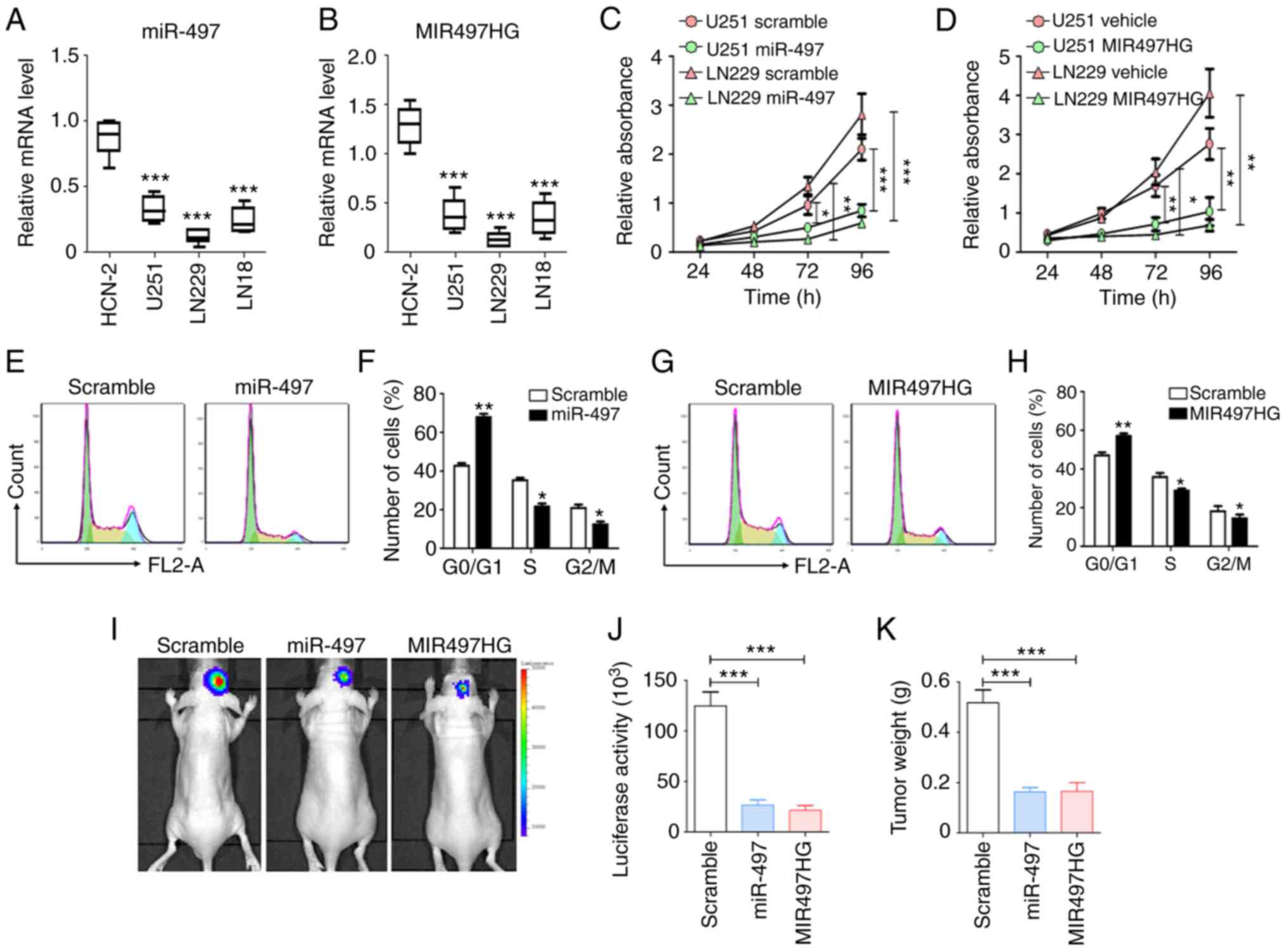
Induced overexpression of miR-497 or
MIR497HG inhibits proliferation and cell cycle progression in
glioma cells
To validate the effects of miR-497 and MIR497HG on
glioma, the expression levels of these two targets were determined
in glioma (U251, LN229 and LN18) and normal human cortical neuron
cell line (HCN-2). The results indicated that the expression levels
of miR-497 and MIR497HG were suppressed in the three glioma cell
lines compared with HCN-2 cells (Fig.
2A and B). To assess the biological functions of miR-497 and
MIR497HG in glioma, these were overexpressed in U251 and LN229
cells. Subsequently, cell proliferation and cell cycle progression
were evaluated using MTT and flow cytometry. The data indicated
that overexpression of either miR-497 or MIR497HG markedly
inhibited proliferation in both cell lines compared with scramble
control groups (Fig. 2C and D).
Similarly, miR-497 and MIR497HG arrested glioma cells at the
G0/G1 phase (Fig. 2E-H). The luciferase-modified glioma
cells were transfected with sequences overexpressing miR-497 or
MIR497HG and injected intracranially into nude mice. After 3 weeks,
glioma growth was evaluated via bioluminescence imaging and tumor
weight measurement, which indicated that miR-497 or MIR497HG
overexpression suppressed glioma progression (Fig. 2I-K). These results indicated that
the miR-497/MIR497HG axis significantly suppressed glioma cell
proliferation both in vitro and in vivo.
CCNE1 is a direct target gene of
miR-497 in glioma cells
The in vitro experiments demonstrated that
miR-497 and MIR497HG blocked glioma cell cycle progression. To
determine the mechanism underlying inhibition of cell proliferation
and cell cycle arrest induced by miR-497, bioinformatics analysis
(TargetScan and miRanda) was performed to predict the potential
targets of miR-497. Although several potential targets that may
participate in the proliferation of tumor cells have been
identified (34–38), CCNE1 directly regulates the cell
cycle of different types of cell and was therefore selected as a
putative target of miR-497 (39–42).
It was predicted that miR-497 binds to the 3′-UTR of CCNE1 based on
a sequence at 485–492 bp (Fig.
3A). To assess whether miR-497 targets CCNE1 by binding to its
3′-UTR region, U251 cells were co-transfected with WT or Mut 3′-UTR
luciferase reporter plasmid and miR-497 mimic or scramble control.
Luciferase activity was decreased in cells co-transfected with WT
3′-UTR reporter plasmid and miR-497 mimic, whereas this effect was
not noted in cells co-transfected with Mut 3′-UTR reporter plasmid
and miR-497 mimic (Fig. 3B).
Moreover, overexpression of miR-497 decreased CCNE1 mRNA and
protein expression levels (Fig. 3C and
D). These data indicated that CCNE1 was a direct target gene of
miR-497 in glioma cells.
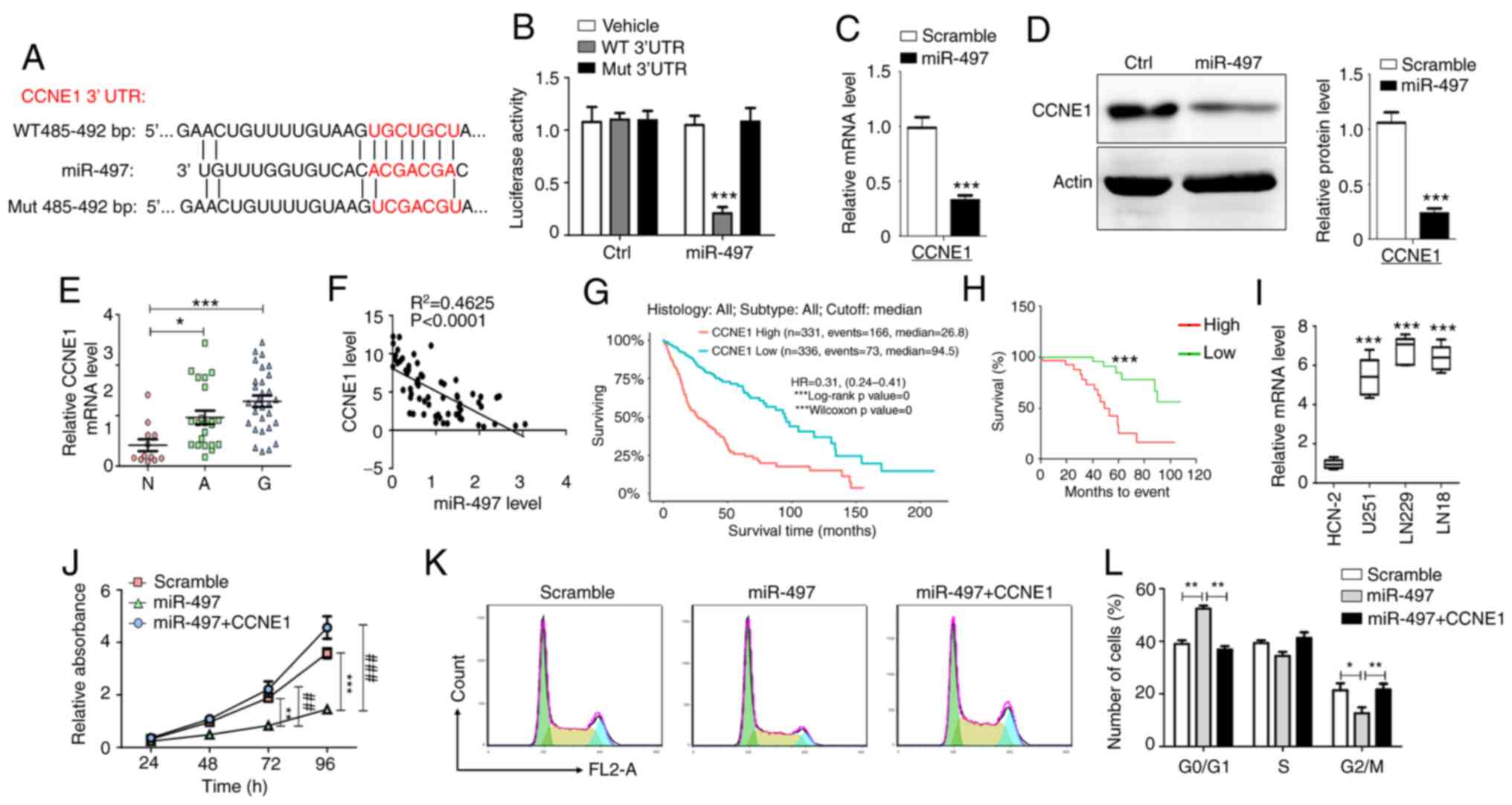 | Figure 3.CCNE1 is a direct functional
downstream target of miR-497. (A) WT and Mut binding sites of
miR-497 in the 3′-UTR of CCNE1. (B) Relative luciferase activity
was determined in U251 cells following co-transfection of miR-497
and luciferase reporter plasmid with WT or Mut CCNE1 3′-UTR (n=5).
Glioma cells were transfected with miR-497 and (C) mRNA and (D)
protein levels of CCNE1 were detected (n=5). (E) mRNA levels of
CCNE1 were determined using RT-qPCR in N (n=12), A (n=22) and G
(n=30). (F) Correlation between CCNE1 and miR-497
(R2=0.4625). (G) Expression levels of CCNE1 correlated
with overall survival time of 667 patients derived from The Cancer
Genome Atlas database. (H) Correlation between expression levels of
CCNE1 and the survival time of patients (n=60). (I) RT-qPCR
analysis was performed to detect CCNE1 mRNA expression in glioma
and HCN-2 cells (n=5). (J) MTT assay demonstrated the effects of
CCNE1 overexpression on inhibition of cell proliferation induced by
miR-497 (n=6). (K and L) Flow cytometry analysis indicated the
effects of CCNE1 overexpression on cell cycle arrest induced by
miR-497 (n=6). Data are presented as the mean ± SEM. *P<0.05, **
or ##P<0.01, *** or ###P<0.001. CCNE1,
cyclin E1; miR, microRNA; WT, wild-type; Mut, mutant; UTR,
untranslated region; RT-q, reverse transcription-quantitative; N,
normal; A, adjacent; G, glioma; Ctrl, control. |
miR-497 targets CCNE1 inhibition of
glioma cell proliferation and CCNE1 predicts poor prognosis of
patients with glioma
CCNE1 mRNA expression levels were measured in glioma
tissue via RT-qPCR analysis. The results indicated that CCNE1 mRNA
expression levels were increased in glioma compared with adjacent
non-neoplastic and normal brain tissue (Fig. 3E). Subsequently, the correlation
between CCNE1 mRNA and miR-497 expression levels was determined in
glioma tissue. The expression levels of CCNE1 mRNA were inversely
correlated with miR-497 expression (Fig. 3F). Patients with high CCNE1
expression demonstrated a shorter overall survival period as
determined by analysis of samples derived from The Cancer Genome
Atlas (TCGA) database and tissue from patients with glioma
(Fig. 3G and H). In addition,
CCNE1 mRNA expression levels were higher in glioma cell lines than
in HCN-2 cells (Fig. 3I).
Restoration of CCNE1 expression reversed the inhibition of
proliferation and induction of cell cycle arrest caused by miR-497
overexpression in U251 cells (Fig.
3J-L). The data suggested that miR-497 targeted CCNE1
inhibition of glioma cell proliferation and CCNE1 predicted poor
prognosis of patients with glioma.
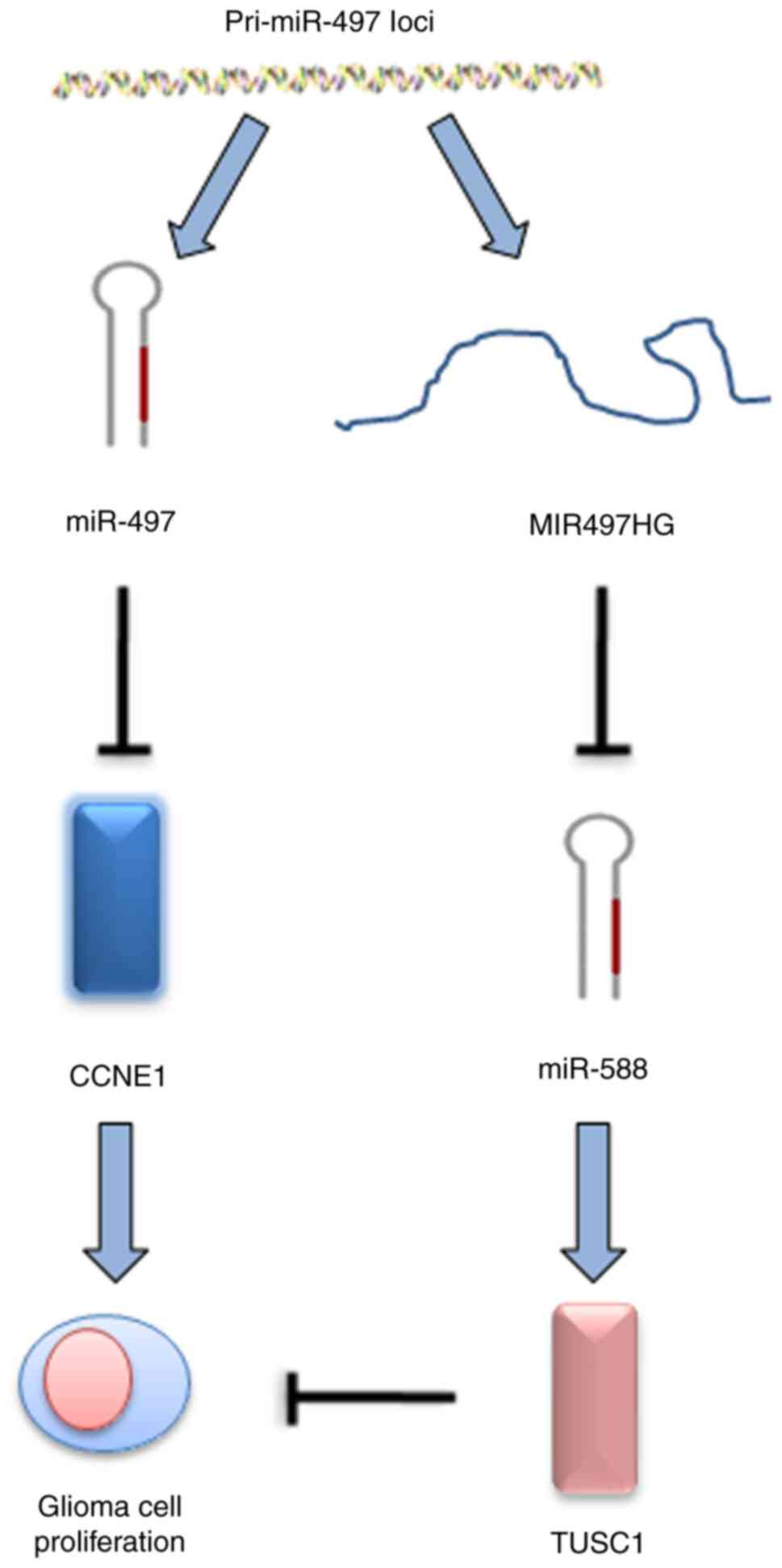
MIR497HG regulates glioma cell
proliferation via the miR-588/TUSC1 axis
Bioinformatics analysis was used to identify target
miRs of MIR497HG. miR-588 was predicted to interact with MIR497HG
and this was verified by the suppression of miR-588 levels in
MIR497HG-overexpressing glioma cells (Fig. 4A and B). The data further indicated
that miR-588 specifically bound to the 3′-UTR of TUSC1 via
complementary interactions, suggesting that TUSC1 expression could
be suppressed by miR-588 (Fig.
4C). This finding was confirmed via RT-qPCR analysis, which
indicated significantly decreased TUSC1 mRNA expression in
miR-588-overexpressing glioma cells (Fig. 4D). In addition, luciferase reporter
assay indicated that miR-588 decreased TUSC1 3′-UTR activity in
glioma cells (Fig. 4E). The
expression levels of miR-588 increased, while those of TUSC1
decreased in glioma compared with paracancerous and normal tissue
(Fig. 4F and G).
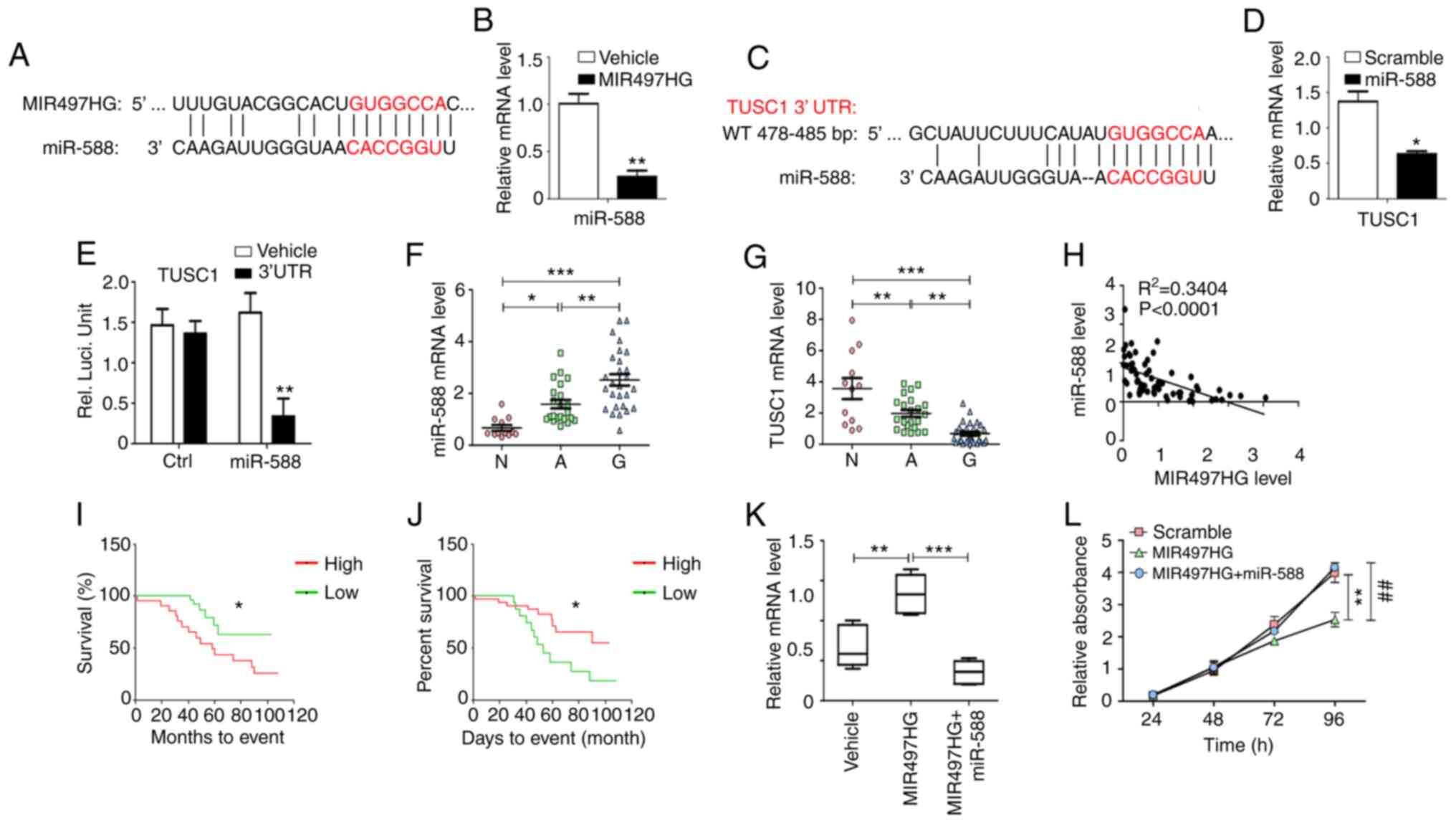 | Figure 4.MIR497HG regulates cell proliferation
and cell cycle via the miR-588/TUSC1 signaling axis. (A)
Bioinformatic analysis indicated that miR-588 may be a specific
downstream miRNA of MIR497HG. (B) Glioma cells overexpressing
MIR497HG were established and mRNA levels of miR-588 were detected
(n=5). (C) Bioinformatics analysis indicated that TUSC1 may be a
specific downstream target of miR-588. (D) Glioma cells
overexpressing miR-588 were established and mRNA expression levels
of TUSC1 were detected (n=5). (E) Relative luciferase activity was
determined in U251 cells following co-transfection of miR-588
plasmid with luciferase reporter plasmid containing TUSC1 3′-UTR.
mRNA expression levels of (F) miR-588 and (G) TUSC1 were detected
in N (n=12), A (n=22) and G (n=30) using reverse
transcription-quantitative PCR analysis. (H) Correlation between
miR-588 and MIR497HG. Correlation between the expression levels of
(I) miR-588 or (J) TUSC1 and survival time of patients (n=60) was
analyzed. (K) Effect of miR-588 was evaluated on the increase in
TUSC1 expression levels induced by MIR497HG. (L) MTT assay
indicated that miR-588 abrogated the inhibition of cell
proliferation induced by MIR497HG. Data are presented as the mean ±
SEM. *P<0.05, ** or ##P<0.01, ***P<0.001.
MIR497HG, miR-497 host gene; miR, microRNA; TUSC1, tumor suppressor
candidate 1; UTR, untranslated region; RT-qPCR, reverse
transcription-quantitative; N, normal; A, adjacent; G, glioma;
Ctrl, control. |
The expression levels of miR-588 exhibited an
inverse correlation with those of MIR497HG in glioma tissue
(Fig. 4H). High levels of miR-588
predicted poor patient prognosis, whereas high levels of TUSC1
indicated longer survival time of patients with primary glioma
(Fig. 4I and J). TCGA database
indicated decreased expression levels of TUSC1 in glioma compared
with non-tumor tissue. However, the expression levels of TUSC1 were
not associated with the prognosis of patients with glioblastoma
(GBM), which did not distinguish between patients with primary and
recurrent glioma (Fig. S1).
U251 cells were transfected with MIR497HG or
MIR497HG + miR-588-expressing plasmids to assess whether TUSC1 was
a downstream target of MIR497HG and miR-588. Elevated MIR497HG
expression induced upregulation of TUSC1 mRNA expression and
inhibited cell proliferation, while overexpression of miR-588
abrogated these effects (Fig. 4K and
L). These results demonstrated a novel signaling pathway that
regulates glioma cell proliferation via the MIR497HG/miR-588/TUSC1
axis.
Discussion
Accumulating evidence has indicated that abnormal
miR-497 expression contributes to carcinogenesis and progression of
multiple types of cancer, including glioma (43–45).
In the present study, expression levels of miR-497 and its host
gene MIR497HG were significantly downregulated in human glioma
tissue, as well as in glioma cell lines. The expression levels of
miR-497 or MIR497HG were inversely correlated with tumor grade.
Lower miR-497 and MIR497HG expression levels were correlated with
poor prognosis of patients with glioma. These results indicated
that miR-497 and MIR497HG expression levels correlated with
pathological features and prognosis of these patients. It has been
previously reported that the lncRNA MIR497HG may be the actual host
gene of miR-497 (26). However,
the present study indicated that miR-497 and lncRNA MIR497HG shared
similar expression profiles in both clinical glioma tissue and
glioma cell lines. In addition, histone H3K27me3 modification is
the hallmark event of promoter methylation. Chromatin
immunoprecipitation-sequencing data derived from glioma cells from
the Cistrome Project (cistrome.org/) exhibit only one unique peak
corresponding to a strong signal of H3K4me3 near the region of
MIR497HG and miR-497 (Gene Expression Omnibus ID: GSM2288202;
CistromeDB ID: 82969) (46). This
indicates that MIR497HG and miR-497 are simultaneously transcribed
in glioma cells. Considering that the histone modifications include
heterogeneity and plasticity, the transcriptional levels of
MIR497HG and miR-497 may be different in different types of cell or
tissue.
The present study is not the first to analyze
miR-497 expression and its correlation with patient prognosis. Lu
et al (45) demonstrated
that miR-497 expression levels are downregulated in glioma tissue
and associated with poor disease progression and decreased overall
survival, suggesting a tumor suppressor function and a potential
therapeutic application of miR-497 in glioma. Regazzo et al
(47) indicated that patients with
GBM, which is considered the most common and malignant subtype of
glioma, are more likely to exhibit downregulated expression levels
of miR-497 compared with patients those with lower tumor grades.
This suggests that serum miR-497 levels may be a novel diagnostic
marker for clinical application in patients with glioma (47). Feng et al (48) revealed that miR-497 expression is
suppressed in human glioma tissue and associated with higher degree
of angiogenesis and poor prognosis. In addition, a genome-wide
miRNA sequence survey was performed and Yang et al (49) reported that the expression levels
of miR-497 are significantly decreased in serum samples of patients
with astrocytoma compared with those of normal control subjects.
The present study verified the aforementioned findings, suggesting
that miR-497 and MIR497HG are tumor suppressors and can be used as
prognostic biomarkers in glioma. The findings of the present study
suggested that the combination of miR-497 and its host gene
MIR497HG can target glioma progression. However, contradictory
findings have also been reported (50,51).
Based on an online dataset analysis, Lan et al (50) demonstrated that miR-497 is
upregulated in glioma tissue and that hypoxia induces expression
levels of miR-497 and its transcriptional levels by binding to the
hypoxia response element and promoting chemoresistance in glioma
cells via programmed cell death. Zhu et al (51) revealed that miR-497 expression
levels are upregulated in glioma cell lines and that this conferred
resistance to temozolomide in these cells by targeting the
mTOR/Bcl-2 pathway. However, the aforementioned study did not
investigate the expression profile of miR-497 in glioma tissue
samples (51). The exact causes of
the different expressions of miR-497 in glioma remain unclear and
require further investigation. Therefore, uniform and globally
standardized experimental protocols have to be developed in
specimen collection, storage, miR extraction, purification,
profiling and analytical methods. Additional multi-center studies
and cohorts are required to achieve consistent and robust results
that can be applied for clinical use.
In cancer progression, miRs function as regulatory
molecules and downregulate the expression levels of downstream
target genes, which are involved in cell differentiation,
proliferation, angiogenesis and other biological processes
(52). Here, induced
overexpression of miR-497 or MIR497HG inhibited cell proliferation
and cell cycle arrest in vitro. CCNE1 is an important cell
cycle regulator that is required for G1/S transition
(53). Bioinformatics analysis and
luciferase assay indicated that CCNE1 was a direct target that was
suppressed by miR-497. Rescue experiments indicated that
overexpression of CCNE1 abrogated inhibition of cell proliferation
and cell cycle arrest caused by miR-497, which was consistent with
a previous study (54). The
expression levels and function of miR-588 have been investigated in
gastric (55) and liver cancer
(56); this miR functions either
as a tumor suppressor or as an oncogenic molecule (54,57–59).
TUSC1 is a putative tumor suppressor gene that has been shown to
serve a role in different types of cancer, including GBM (60,61).
In the present study, miR-588/TUSC1 was identified as the
downstream signaling axis of MIR497HG, which participated in the
regulation of glioma cell proliferation and cell cycle progression.
High levels of TUSC1 indicated longer survival time of patients
with primary glioma. However, the TCGA database indicated that
TUSC1 expression was not associated with the prognosis of patients
with glioblastoma. Considering that the TCGA database did not
distinguish between patients with primary and recurrent glioma,
this inconsistent conclusion may be explained due to the
differences between primary and recurrent gliomas. To the best of
our knowledge, the present study is the first to demonstrate the
importance of the MIR497HG/miR-588/TUSC1 signaling pathway in
glioma progression (Fig. 5).
In conclusion, the present study indicated that
miR-497 and MIR497HG expression levels were downregulated in glioma
tissue and associated with poor disease prognosis. Overexpression
of miR-497 or MIR497HG inhibited proliferation and cell cycle
progression in glioma cells via downregulation of CCNE1 expression
and inhibition of the miR-588/TUSC1 signaling pathway. The present
study may provide new insight into the molecular mechanism of
glioma progression and aid the development of potential therapeutic
targets and prognostic biomarkers for this cancer.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Shuang Wu, Fourth
Military Medical University for providing the space and equipment
for experiments.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (grant no. 81502145)
and National Natural Science Foundation of Shaanxi (grant no.
2016JQ8017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LYJ and MW performed the experiments. YYL performed
data analysis. ZLD and SZL designed and directed the study and
wrote the manuscript. MW and YYL confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xijing Hospital of Fourth Military Medical University
(approval no. 20190238) and was performed in accordance with the
Declaration of Helsinki. Written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diamandis P and Aldape KD: Insights from
molecular profiling of adult glioma. J Clin Oncol. 35:2386–2393.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laug D, Glasgow SM and Deneen B: A glial
blueprint for gliomagenesis. Nat Rev Neurosci. 19:393–403. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao J, Li H, Zhao S, Wang E, Zhu J, Feng
D, Zhu Y, Dou W, Fan Q, Hu J, et al: Epigenetic silencing of
miR-144/451a cluster contributes to HCC progression via paracrine
HGF/MIF-mediated TAM remodeling. Mol Cancer. 20:462021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li SZ, Ren KX, Zhao J, Wu S, Li J, Zang J,
Fei Z and Zhao JL: miR-139/PDE2A-Notch1 feedback circuit represses
stemness of gliomas by inhibiting Wnt/β-catenin signaling. Int J
Biol Sci. 17:3508–3521. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Q, Luo X, Terp MG, Li Q, Li Y, Shen L,
Chen Y, Jacobsen K, Bivona TG, Chen H, et al: DDX56 modulates
post-transcriptional Wnt signaling through miRNAs and is associated
with early recurrence in squamous cell lung carcinoma. Mol Cancer.
20:1082021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Godlewski J, Krichevsky AM, Johnson MD,
Chiocca EA and Bronisz A: Belonging to a network-microRNAs,
extracellular vesicles, and the glioblastoma microenvironment.
Neuro Oncol. 17:652–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Liang H, Zhang J, Zen K and Zhang
CY: Secreted microRNAs: A new form of intercellular communication.
Trends Cell Biol. 22:125–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hassan A, Mosley J, Singh S and Zinn PO: A
comprehensive review of genomics and noncoding RNA in gliomas. Top
Magn Reson Imaging. 26:3–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng Z, Zhu Y, Zhang Y, Wilhelmsen K, Jia
C, Jin J, Xue Q, Feng X, Zhang F and Yu B: Effects of ghrelin on
pulmonary NOD2 mRNA expression and NF-κB activation when protects
against acute lung injury in rats challenged with cecal ligation
and puncture. Int Immunopharmacol. 13:440–445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan Q, Zhao P, Li J, Xie X, Xu M, Zhang Y,
Mu D, Li W, Sun R, Liu W, et al: 17β-Estradiol administration
attenuates seawater aspiration-induced acute lung injury in rats.
Pulm Pharmacol Ther. 24:673–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu YB, Wan BB, Liu G, Wu YX, Chen D, Lu
MD, Chen JL, Yu RQ, Chen DZ and Pang QF: Nrf2 protects against
seawater drowning-induced acute lung injury via inhibiting
ferroptosis. Respir Res. 21:2322020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buntwal L, Sassi M, Morgan AH, Andrews ZB
and Davies JS: Ghrelin-mediated hippocampal neurogenesis:
Implications for health and disease. Trends Endocrinol Metab.
30:844–859. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Menigatti M, Staiano T, Manser CN,
Bauerfeind P, Komljenovic A, Robinson M, Jiricny J, Buffoli F and
Marra G: Epigenetic silencing of monoallelically methylated miRNA
loci in precancerous colorectal lesions. Oncogenesis. 2:e562013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Y, Wei RR, Huang GL, Zhang MY, Yuan YF
and Wang HY: Checkpoint kinase 1 is negatively regulated by miR-497
in hepatocellular carcinoma. Med Oncolo. 31:8442014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Ye Z, Mei D, Gu H and Zhang J:
Long noncoding RNA DLX6-AS1 promotes tumorigenesis by modulating
miR-497-5p/FZD4/FZD6/Wnt/β-catenin pathway in pancreatic cancer.
Cancer Manag Res. 11:4209–4221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Özata DM, Caramuta S, Velázquez-Fernández
D, Akçakaya P, Xie H, Höög A, Zedenius J, Bäckdahl M, Larsson C and
Lui WO: The role of microRNA deregulation in the pathogenesis of
adrenocortical carcinoma. Endocr Relat Cancer. 18:643–655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei Z, Hu X, Liu J, Zhu W, Zhan X and Sun
S: MicroRNA-497 upregulation inhibits cell invasion and metastasis
in T24 and BIU-87 bladder cancer cells. Mol Med Rep. 16:2055–2060.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sen LS, Karakoyun B, Yeğen C, Akkiprik M,
Yüksel M, Ercan F, Özer A and Yeğen BÇ: Treatment with either
obestatin or ghrelin attenuates mesenteric
ischemia-reperfusion-induced oxidative injury of the ileum and the
remote organ lung. Peptides. 71:8–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo M, Shen D, Zhou X, Chen X and Wang W:
MicroRNA-497 is a potential prognostic marker in human cervical
cancer and functions as a tumor suppressor by targeting the
insulin-like growth factor 1 receptor. Surgery. 153:836–847. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maura F, Cutrona G, Mosca L, Matis S,
Lionetti M, Fabris S, Agnelli L, Colombo M, Massucco C, Ferracin M,
et al: Association between gene and miRNA expression profiles and
stereotyped subset #4 B-cell receptor in chronic lymphocytic
leukemia. Leuk Lymphoma. 56:3150–3158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Troppan K, Wenzl K, Pichler M, Pursche B,
Schwarzenbacher D, Feichtinger J, Thallinger GG, Beham-Schmid C,
Neumeister P and Deutsch A: miR-199a and miR-497 are associated
with better overall survival due to increased chemosensitivity in
diffuse large B-cell lymphoma patients. Int J Mol Sci.
16:18077–18095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matthay MA, McAuley DF and Ware LB:
Clinical trials in acute respiratory distress syndrome: Challenges
and opportunities. Lancet Respir Med. 5:524–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Yang FJ, Wang YG, Su GF and Miao
X: LncRNA MIR497HG inhibits proliferation and migration of retinal
endothelial cells under high-level glucose treatment via
miRNA-128-3p/SIRT1 axis. Eur Rev Med Pharmacol Sci. 24:5871–5877.
2020.PubMed/NCBI
|
|
26
|
Zhuang C, Liu Y, Fu S, Yuan C, Luo J,
Huang X, Yang W, Xie W and Zhuang C: Silencing of lncRNA MIR497HG
via CRISPR/Cas13d induces bladder cancer progression through
promoting the crosstalk between Hippo/Yap and TGF-β/Smad signaling.
Front Mol Biosci. 7:6167682020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ganini C, Amelio I, Bertolo R, Bove P,
Buonomo OC, Candi E, Cipriani C, Di Daniele N, Juhl H, Mauriello A,
et al: Global mapping of cancers: The cancer genome atlas and
beyond. Mol Oncol. Jul 10–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American Physiological
Society. Physiologist. 39:199, 208–211. 1996.PubMed/NCBI
|
|
30
|
Wang H, Yan X, Ji LY, Ji XT, Wang P, Guo
SW and Li SZ: miR-139 Functions as an antioncomir to repress glioma
progression through targeting IGF-1 R, AMY-1, and PGC-1β. Technol
Cancer Res Treat. 16:497–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peterson SM, Thompson JA, Ufkin ML,
Sathyanarayana P, Liaw L and Congdon CB: Common features of
microRNA target prediction tools. Front Genet. 5:232014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riffo-Campos AL, Riquelme I and
Brebi-Mieville P: Tools for sequence-based miRNA Target Prediction:
What to Choose? Int J Mol Sci. 17:19872016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chae DK, Park J, Cho M, Ban E, Jang M, Yoo
YS, Kim EE, Baik JH and Song EJ: MiR-195 and miR-497 suppress
tumorigenesis in lung cancer by inhibiting SMURF2-induced TGF-β
receptor I ubiquitination. Mol Oncol. 13:2663–2678. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng L, Cheng K, Zang R, Wang Q and Wang
J: miR-497-5p inhibits gastric cancer cell proliferation and growth
through targeting PDK3. Biosci Rep. 39:BSR201906542019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hassan N, Zhao JT, Glover A, Robinson BG
and Sidhu SB: Reciprocal interplay of miR-497 and MALAT1 promotes
tumourigenesis of adrenocortical cancer. Endocr Relat Cancer.
26:677–688. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia Y, Hu C, Lian L, Hui K, Wang L, Qiao
Y, Liu L, Liang L and Jiang X: miR497 suppresses malignant
phenotype in nonsmall cell lung cancer via targeting KDR. Oncol
Rep. 42:443–452. 2019.PubMed/NCBI
|
|
38
|
Yang L, Cai Y, Zhang D, Sun J, Xu C, Zhao
W, Jiang W and Pan C: miR-195/miR-497 Regulate CD274 expression of
immune regulatory ligands in triple-negative breast cancer. J
Breast Cancer. 21:371–381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Li X, Zhang J and Mao L: E6
hijacks KDM5C/lnc_000231/miR-497-5p/CCNE1 axis to promote cervical
cancer progression. J Cell Mol Med. 24:11422–11433. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han Z, Zhang Y, Yang Q, Liu B, Wu J, Zhang
Y, Yang C and Jiang Y: miR-497 and miR-34a retard lung cancer
growth by co-inhibiting cyclin E1 (CCNE1). Oncotarget.
6:13149–13163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei W, Zhang WY, Bai JB, Zhang HX, Zhao
YY, Li XY and Zhao SH: The NF-κB-modulated microRNAs miR-195 and
miR-497 inhibit myoblast proliferation by targeting Igf1r, Insr and
cyclin genes. J Cell Sci. 129:39–50. 2016.PubMed/NCBI
|
|
42
|
Furuta M, Kozaki K, Tanimoto K, Tanaka S,
Arii S, Shimamura T, Niida A, Miyano S and Inazawa J: The
tumor-suppressive miR-497-195 cluster targets multiple cell-cycle
regulators in hepatocellular carcinoma. PLoS One. 8:e601552013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luo G, He K, Xia Z, Liu S, Liu H and Xiang
G: Regulation of microRNA-497 expression in human cancer. Oncol
Lett. 21:232021.PubMed/NCBI
|
|
44
|
Boldrin E, Gaffo E, Niedermayer A, Boer
JM, Zimmermann M, Weichenhan D, Claus R, Münch V, Sun Q,
Enzenmüller S, et al: MicroRNA-497/195 is tumor-suppressive and
cooperates with CDKN2A/B in pediatric acute lymphoblastic leukemia.
Blood. Jun 7–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu F, Ye Y, Zhang H, He X, Sun X, Yao C,
Mao H, He X, Qian C, Wang B, et al: miR-497/Wnt3a/c-jun feedback
loop regulates growth and epithelial-to-mesenchymal transition
phenotype in glioma cells. Int J Biol Macromol. 120:985–991. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Turcan S, Makarov V, Taranda J, Wang Y,
Fabius AWM, Wu W, Zheng Y, El-Amine N, Haddock S, Nanjangud G, et
al: Mutant-IDH1-dependent chromatin state reprogramming,
reversibility, and persistence. Nat Genet. 50:62–72. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Regazzo G, Terrenato I, Spagnuolo M,
Carosi M, Cognetti G, Cicchillitti L, Sperati F, Villani V,
Carapella C, Piaggio G, et al: A restricted signature of serum
miRNAs distinguishes glioblastoma from lower grade gliomas. J Exp
Clin Cancer Res. 35:1242016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feng F, Kuai D, Wang H, Li T, Miao W, Liu
Y and Fan Y: Reduced expression of microRNA-497 is associated with
greater angiogenesis and poor prognosis in human gliomas. Hum
Pathol. 58:47–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang C, Wang C, Chen X, Chen S, Zhang Y,
Zhi F, Wang J, Li L, Zhou X, Li N, et al: Identification of seven
serum microRNAs from a genome-wide serum microRNA expression
profile as potential noninvasive biomarkers for malignant
astrocytomas. Int J Cancer. 132:116–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lan J, Xue Y, Chen H, Zhao S, Wu Z, Fang
J, Han C and Lou M: Hypoxia-induced miR-497 decreases glioma cell
sensitivity to TMZ by inhibiting apoptosis. FEBS Lett.
588:3333–3339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhu D, Tu M, Zeng B, Cai L, Zheng W, Su Z
and Yu Z: Up-regulation of miR-497 confers resistance to
temozolomide in human glioma cells by targeting mTOR/Bcl-2. Cancer
Med. 6:452–462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang R, Xing L, Zheng X, Sun Y, Wang X and
Chen J: The circRNA circAGFG1 acts as a sponge of miR-195-5p to
promote triple-negative breast cancer progression through
regulating CCNE1 expression. Mol Cancer. 18:42019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shen L, Orillion A and Pili R: Histone
deacetylase inhibitors as immunomodulators in cancer therapeutics.
Epigenomics. 8:415–428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen Y, Zhang J, Gong W, Dai W, Xu X and
Xu S: miR-588 is a prognostic marker in gastric cancer. Aging.
13:2101–2117. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Z, Mo H, Sun L, Wang L, Chen T, Yao B,
Liu R, Niu Y, Tu K, Xu Q and Yang N: Long noncoding RNA
PICSAR/miR-588/EIF6 axis regulates tumorigenesis of hepatocellular
carcinoma by activating PI3K/AKT/mTOR signaling pathway. Cancer
Sci. 111:4118–4128. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu M, Zhang X, Li H, Zhang P and Dong W:
MicroRNA-588 is downregulated and may have prognostic and
functional roles in human breast cancer. Med Sci Monit.
23:5690–5696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao N, Lin T, Zhao C, Zhao S, Zhou S and
Li Y: MicroRNA-588 is upregulated in human prostate cancer with
prognostic and functional implications. J Cell Biochem. Oct
5–2017.(Epub ahead of print).
|
|
59
|
Zhou X and Xu M, Guo Y, Ye L, Long L, Wang
H, Tan P and Xu M: MicroRNA-588 regulates invasion, migration and
epithelial-mesenchymal transition via targeting EIF5A2 pathway in
gastric cancer. Cancer Manag Res. 10:5187–5197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shan Z, Shakoori A, Bodaghi S, Goldsmith
P, Jin J and Wiest JS: TUSC1, a putative tumor suppressor gene,
reduces tumor cell growth in vitro and tumor growth in vivo. PLoS
One. 8:e661142013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang R, Yu W, Liang G, Jia Z, Chen Z,
Zhao L, Yuan Y, Zhou X, Li D, Shen S, et al: Tumor suppressor
candidate 1 suppresses cell growth and predicts better survival in
glioblastoma. Cell Mol Neurobiol. 37:37–42. 2017. View Article : Google Scholar : PubMed/NCBI
|