Introduction
Intrahepatic cholangiocarcinoma (ICC) is a highly
lethal hepatobiliary neoplasm with increasing incidence (1). The established standards for the
treatment of ICC include first-line (gemcitabine and cisplatin),
second-line (FOLFOX) and adjuvant (capecitabine) systemic
chemotherapy (2,3). Due to the high aggressiveness of ICC,
long-term survival is only observed in patients undergoing complete
R0 surgical resection (4). Lymph
node involvement is one of the most important prognostic factors.
Therefore, novel and efficient methods for the diagnosis and
therapy of ICC are urgently needed.
As a serine/threonine kinase, liver kinase B1 (LKB1)
has been shown to be a tumor suppressor that regulates cell growth,
metabolism, survival and polarity (5–7).
LKB1 inactivation can also lead to centromere defects and genome
instability via p53-dependent upregulation of survivin, independent
of AMPK (8). LKB1 regulation of
the tumor immune microenvironment is complex and has received
widespread attention. For example, LKB1 deficiency promotes
neutrophil recruitment and proinflammatory cytokine production to
suppress T-cell activity (9). LKB1
mutations may cause PD-1 inhibitor resistance in KRAS-mutant lung
adenocarcinoma (10). Loss of LKB1
silences the expression of stimulator of interferon genes,
decreasing the sensitivity of KRAS-mutant lung cancer cells to
cytoplasmic double-stranded DNA (11). Certain preclinical therapeutic
methods have been developed to treat malignant tumors with LKB1
loss, such as dual molecular targeted therapy for mTOR (Rapamycin)
and PI3K (BKM-120) (12) as well
as the combination of metformin with cisplatin (13). In addition, LKB1 is involved in
regulating intestinal stem cell fate, skeletal muscle development,
liver regeneration and certain non-neoplastic diseases (14–17).
Exosomes, derived from the endocytic pathway, are
membranous vesicles with a diameter of ~40–200 nm (18,19).
In various diseases, exosomes provide a channel to view the altered
cellular or tissue states, and their detection in biological fluids
potentially offers a multicomponent diagnostic readout (20). Tumor-derived exosomes modulate
intercellular communication between tumor and stromal cells,
influencing malignant phenotypes and the tumor microenvironment
(21–23). Although the regulation of exosomes
has been widely studied in multiple types of cancer, exosomes
derived from and acting on ICC are rarely mentioned. Even so,
exosomal circ-0000284 has been found to be a competitive endogenous
RNA that promotes ICC progression and can be directly transferred
from ICC cells to surrounding normal cells (24). P2RX7 may influence ICC in an
exosome-related manner (25).
Considering the significant role of exosomes in cancer, it was
suggested that exosomes may provide a new window for the diagnosis
and therapy of ICC.
Cholangiocarcinoma represents a diverse group of
epithelial tumors, including intrahepatic, perihilar and distal
types (26). Although the
biological characteristics of the three types of cholangiocarcinoma
are similar, surgical resection is more likely to be performed for
ICC than for the other two types. Therefore, the present study
focused on ICC research. In a recent study, the inhibitory effect
of LKB1 on the transcriptional activity of the immune checkpoint
PD-L1 was uncovered in ICC cells (27). However, the regulatory role of LKB1
in exosomes secreted by ICC cells remains obscure. Exosomes from
cell culture supernatants of ICC cells were extracted and examined
in the present study. Further study uncovered the inhibitory effect
of exosomal LKB1 on PD-L1 and phosphorylated (p)-AKT expression in
cells and exosomes as well as malignant phenotypes and metastasis
of ICC cells. The change in exosomal LKB1 expression in plasma may
be a promising target for the diagnosis and therapy of ICC in the
future.
Materials and methods
Cell culture
The human embryonic kidney fibroblasts 293T (RRID:
CVCL_0063) were purchased from the American Type Culture Collection
(ATCC). The human ICC cells RBE (RRID: CVCL_4896) and HCCC-9810
(RRID: CVCL_6908) were obtained from Wuhan Boster Biological
Technology, Ltd. The culture medium for 293T cells and ICC cells
was composed of DMEM, 10% (v/v) FBS and penicillin-streptomycin
(all from Thermo Fisher Scientific, Inc.). Cells were passaged at
an average of three days and tested routinely to avoid mycoplasma
contamination.
Plasmid construction and lentiviral
infection
The 2nd generation system of lentiviruses was used
in the present study. Plasmids for silencing LKB1 expression were
constructed using pGreenPuro™ small hairpin (sh)RNA
Cloning and Expression Lentivector (System Biosciences, LLC), and
the target sequences for gene silencing are listed below: LT-shLKB1
(CCTGCTGAAAGGGATGCTT) and negative control LT-shControl
(ACTACCGTTGTTATAGGTG). By mixing 8.0 µg gene silencing plasmids,
8.0 µg packaging plasmid psPAX2 and 2.7 µg envelope plasmid pMD2G
with 18 µl transfection reagent Lipofectamine® 2000 in
600 µl Opti-MEM™ (both from Thermo Fisher Scientific,
Inc.) for 20 min at room temperature, plasmids were co-transfected
into 293T cells. After 2 days, cell culture supernatants containing
lentivirus were collected every 8 h and filtered with 0.45-µm
filter membranes. Cells were infected with lentivirus at different
multiplicity of infection (1, 2.5, 5, 10, 20), and silencing
efficiency of LKB1 lentivirus was determined by western blotting 72
h after infection. Puromycin (2 µg/ml; Thermo Fisher Scientific,
Inc.) was used to screen the lentivirus-infected cells, and in
vitro and in vivo experiments were conducted immediately
after the puromycin selection.
Western blot analysis
The cultured cells were sequentially rinsed with
ice-cold PBS, lysed in ice-cold RIPA lysis buffer (Thermo Fisher
Scientific, Inc.) supplemented with proteinase inhibitor cocktail
(Thermo Fisher Scientific, Inc.), and collected into 1.5-ml
microtubes. After incubation on ice for 30 min, cell lysates were
centrifuged at 16,100 × g for 15 min at 4°C. The protein
concentration was determined using a BCA protein quantification kit
(Beijing Dingguo Changsheng Biotechnology Co., Ltd.). An
appropriate amount of BCA working solution was prepared according
to the number of standards and samples and standard BSA protein
solution (1 mg/ml) was dispensed into 96-well plates at 0, 1, 2, 4,
6, 8 and 10 µl and supplemented to 10 µl by adding
ddH2O. At the same time, 10 µl diluted protein samples
were added to each well of 96-well plates. A total of 200 µl of BCA
working solution was added to the sample and protein standard wells
and mixed. The 96-well plates were incubated at 37°C for 30 min and
then cooled to room temperature. The absorbance was measured at 562
nm wavelength on a microplate reader SENERGY HTX (BioTek
Instruments, Inc.). Densitometric analysis was conducted using
software Image Lab (Version 5.2; Bio-Rad Laboratories, Inc.).
Then, 20 µg total protein was electrophoresed in 10%
SDS-PAGE gels and transferred onto PVDF membranes (Merck
Millipore). The PVDF membranes were sequentially blocked with 5%
skim milk for 30 min at room temperature and incubated with the
primary antibodies overnight at 4°C and the secondary antibodies
for 2 h at room temperature. Protein bands on PVDF membranes were
detected by Supersignal West Pico chemiluminescent substrate
(Thermo Fisher Scientific, Inc.).
The commercially obtained antibodies were used
according to the manufacturer's protocol: LKB1 (1 µg/ml; cat. no.
sc-32245, Santa Cruz Biotechnology, Inc.), E-cadherin (1 µg/ml;
cat. no. 3195S), N-cadherin (1 µg/ml; cat. no. 13116S), β-Catenin
(1 µg/ml; cat. no. 8480S), Slug (1 µg/ml; cat. no. 9585S), Snail (1
µg/ml; 3879S; all from Cell Signaling Technology, Inc.), PD-L1
(0.67 µg/ml; cat. no. 17952-1-AP; ProteinTech Group, Inc.), GAPDH
(1 µg/ml; cat. no. MAB374; Merck Millipore), CD9 (1 µg/ml; cat. no.
sc-13118; Santa Cruz Biotechnology, Inc.), TSG101 (1 µg/ml; cat.
no. 102286-T38; Sino Biological), p-AKT (1 µg/ml; cat. no. 4060S),
AKT (1 µg/ml; cat. no. 4691S; both from Cell Signaling Technology,
Inc.), goat anti-mouse IgG (HRP-linked) (1:5,000; cat. no. 401211;
Merck Millipore) and anti-rabbit IgG (HRP-linked) (1:5,000; cat.
no. 7074S; Cell Signaling Technology, Inc.).
Exosome extraction
Exosomes in cell culture supernatants and plasma
specimens of patients with ICC were extracted according to standard
protocols (28,29). The simplified procedures of exosome
extraction are listed below: i) To remove dead cells, cell culture
supernatants and plasma were centrifuged at 2,000 × g for 20 min at
4°C; ii) To remove cell debris, the collected supernatants and
plasma were centrifuged at 10,000 × g for 30 min at 4°C; iii) The
purified supernatants were transferred to clean ultra-tubes and
then ultra-centrifuged at 100,000 × g for 70 min at 4°C; iv) The
precipitates were resuspended with ice-cold PBS and
ultra-centrifuged at 100,000 × g for another 70 min at 4°C; v) The
precipitated exosomes at the bottom of the ultracentrifuge tubes
were resuspended with ice-cold PBS for subsequent research. The
protein markers of exosomes, including CD9, TSG101 and GAPDH, were
examined by western blotting.
Electron microscopy
The morphology of exosomes was observed using a
transmission electron microscope (TEM) JEM-3010 (JEOL, Ltd.), and
the simplified protocol was as follows: i) Exosomes resuspended in
10 µl PBS were mixed with 10 µl of 4% paraformaldehyde for 20 min
at room temperature and then added to the carbon films; ii) After
standing for 20 min, the carbon films were rinsed with PBS twice
for 2 min each time; iii) Exosomes on carbon films were then
stained with 10 µl of 2% uranyl-acetate solution for 1 min at room
temperature; (4) Carbon films were
air-dried for 10 min at room temperature; (5) The morphology of exosomes was
visualized at 100 kV.
Exosome treatment in cell culture
An average of 6×105 ICC cells with
differential expression of LKB1 were seeded into each well of a
six-well culture plate. A total of 24 h later, 2 µg exosomes
resuspended in 10 µl PBS were added to each well of the cell
culture supernatants. After incubation for another 24 h in cell
culture incubator at 37°C with 5% CO2, total protein was
collected from the cells and examined by western blotting.
MTT assay
A total of 500 µl of culture medium containing
10,000 ICC cells with differential expression of LKB1 was added to
each well of a 24-well cell culture plate. A total of 24 h later,
0.50 µg exosomes resuspended in 2.5 µl PBS were added to each well
of the cell culture supernatants. After cultivation for different
times, MTT solution (Sangon Biotech) was added to each well at a
final concentration of 0.50 µg/µl. After 6 h, the culture medium
was carefully discarded and replaced with 150 µl of DMSO (Sangon
Biotech Co., Ltd.). After 20 min of gentle shaking, the OD value at
490 nm was measured and statistically analyzed.
Transwell assay
Transwell experiments were conducted to determine
the migratory ability of ICC cells. Before conducting experiments,
cultured ICC cells in plates were starved with serum-free DMEM for
24 h. Transwell chambers with 8.0-µm PET membrane pores (Corning,
Inc.) were inserted into each well of a 24-well plate. Then, 600 µl
of DMEM supplemented with 10% (v/v) FBS and 0.50 µg exosomes was
added to the lower chamber, and 100 µl of serum-free DMEM
containing 1×105 cells was added to the upper chamber. A
total of 24 h later, the cells were fixed with methyl alcohol for
15 min at room temperature and stained with 0.2% (m/v) crystal
violet (Sigma-Aldrich; Merck KGaA) for 15 min at room temperature.
Then, the remaining cells in the upper chamber were removed with a
medical cotton swab. Images of the migratory cells were captured
under an inverted light microscope, and the number of migratory
cells was counted and statistically analyzed.
Animal experiment
In total, 12 six-week-old male BALB/c nude mice
(weight, ~20 g) were purchased from Hunan SJA Laboratory Animal
Co., Ltd. (Changsha, China) and fed at the animal care facility of
The Central Hospital of Xiangtan. Mice were housed three per cage
with free access to food and sterile water, under 12-h light/dark
cycle at 25°C and 50% humidity. To assess the regulatory effect of
exosomal LKB1 on the metastasis of ICC cells in vivo, mice
were arbitrarily assigned to four groups (n=3 each). Overall,
2.0×106 ICC cells in 200 µl of sterile PBS with
differential expression of LKB1 were injected into mice via the
tail vein. After 7 days, 50 µg of exosomes resuspended in 100 µl of
sterile PBS with differential expression levels of LKB1 were
injected into mice every via the tail vein three days for five
consecutive injections. Fluorescence in live mice was detected
using the IVIS Lumina XR live animal imager (PerkinElmer, Inc.)
according to the expression of green fluorescence protein (GFP) in
lentivirus-infected ICC cells. The mice in the present study were
sacrificed when they experienced a sharp decrease in activity,
water and diet intake. Therefore, 40 days after cell injection,
mice were sacrificed by cervical dislocation. The protocol used for
animal experiments was approved (approval no. 2020073) by the
Animal Care and Experiment Committee of The Central Hospital of
Xiangtan (Xiangtan, China). All applicable international, national,
and/or institutional guidelines for the care and use of animals
were followed in the present study.
Patient information
A total of 42 pairs of cancer and para-cancer tissue
specimens used in the present study were obtained from The Central
Hospital of Xiangtan with informed consent from 2014 to 2019. No
patients received chemotherapy, radiotherapy or immune therapy
before surgery. The age of the patients ranged from 37–74 years
old, with 27 patients being over 60 years old. Among them, 18
patients were male and 24 patients were female. Limited by the
diagnosis and treatment strategies of the hospital for ICC, gene
mutational information for patients is not available. Plasma
specimens of patients with ICC used in the present study were
obtained with written informed consent and approved (approval no.
2019-08-001) by the institutional review boards of The Central
Hospital of Xiangtan and were in accordance with the Declaration of
Helsinki (2000).
Statistical analysis
GraphPad Prism 8 (GraphPad Software, Inc.) was used
to generate statistical graphs, and statistical analyses were
conducted using SPSS 22.0 (IBM Corp.). Student's two-sided unpaired
t-test was used to compare the significance between two groups, and
one-way ANOVA with Tukey-Kramer post hoc test was used to determine
differences among multiple groups. The correlation between the
protein expression of LKB1 and PD-L1 examined by western blotting
was determined by Pearson's correlation analysis (two-tailed). The
Kaplan-Meier method followed by log-rank test was performed to
generate survival curves. Experiments were repeated two or three
times with similar results. Data are represented as the means ± SD
with at least three biological replicates. P<0.05 was considered
to indicate a statistically significant difference.
Results
LKB1 inhibits exosomal PD-L1 in ICC
cells
Our previous research revealed that LKB1 inhibits
ICC by repressing the transcriptional activity of the immune
checkpoint PD-L1 (27). However,
the regulatory effect of exosomal LKB1 on ICC remains unclear. In
the present study, the role of exosomal LKB1 in ICC was
investigated using the RBE and HCCC-9810 cell lines. The high level
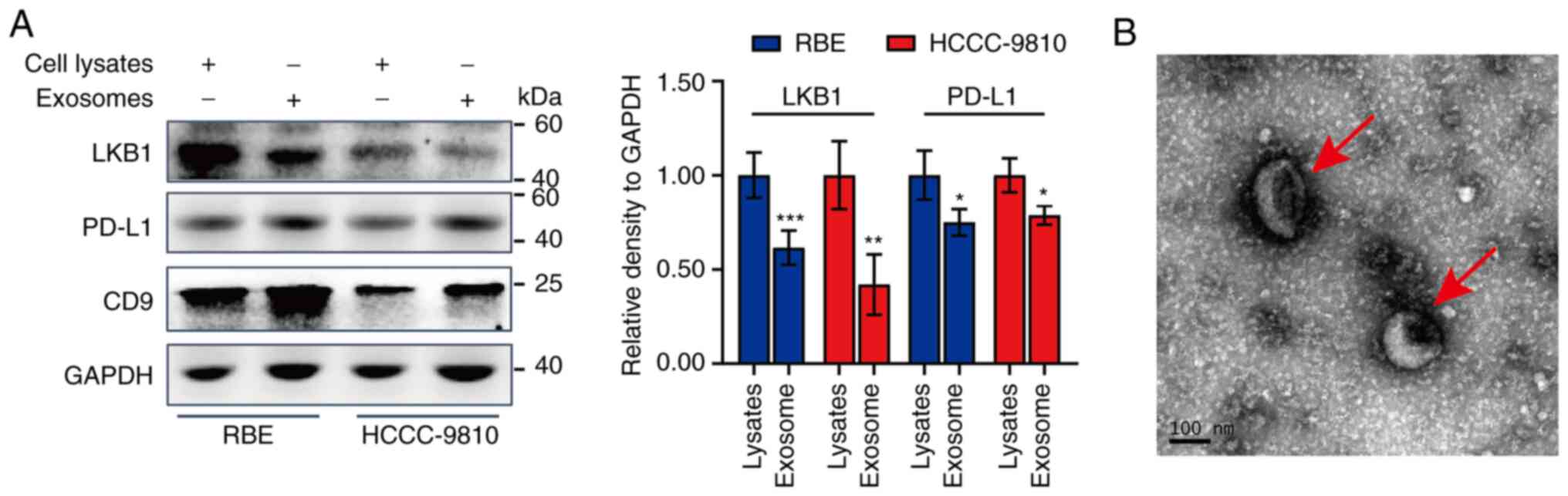
of CD9 protein (Fig. 1A) and the
morphology revealed by TEM (Fig.
1B) suggested the successful extraction of exosomes from cell
culture supernatants of ICC cells. The diameter of the captured
exosomes in the image was ~150 nm (Fig. 1B). Notably, the protein level of
LKB1 in exosomes secreted by RBE and HCCC-9810 cells was lower than
that in cell lysates (Fig. 1A).
Meanwhile, the PD-L1 level in exosomes was higher than that in cell
lysates. These findings indicated that exosomes secreted by ICC
cells may exert a tumor-promoting role and that exosomal LKB1 may
suppress ICC.
To verify this hypothesis, ICC cells stably infected
with lentivirus for silencing LKB1 expression were constructed for
the following research. Silencing LKB1 expression increased the
PD-L1 level in ICC cells as well as that of p-AKT and the
transcription factor Slug (Fig.
2A). Consistent with our previous research, LKB1 did not affect
the protein expression of epithelial-mesenchymal
transition-associated markers, including β-Catenin, E-cadherin, and
N-cadherin (27). Exosomes were
extracted from the cell culture supernatants of ICC cells RBE and
HCCC-9810 with differential expression of LKB1. Exosomal PD-L1 and
LKB1 levels were examined by western blotting. Silencing
intracellular LKB1 downregulated the protein level of exosomal
PD-L1, accompanied by a decrease in the protein level of exosomal
LKB1 (Fig. 2B).
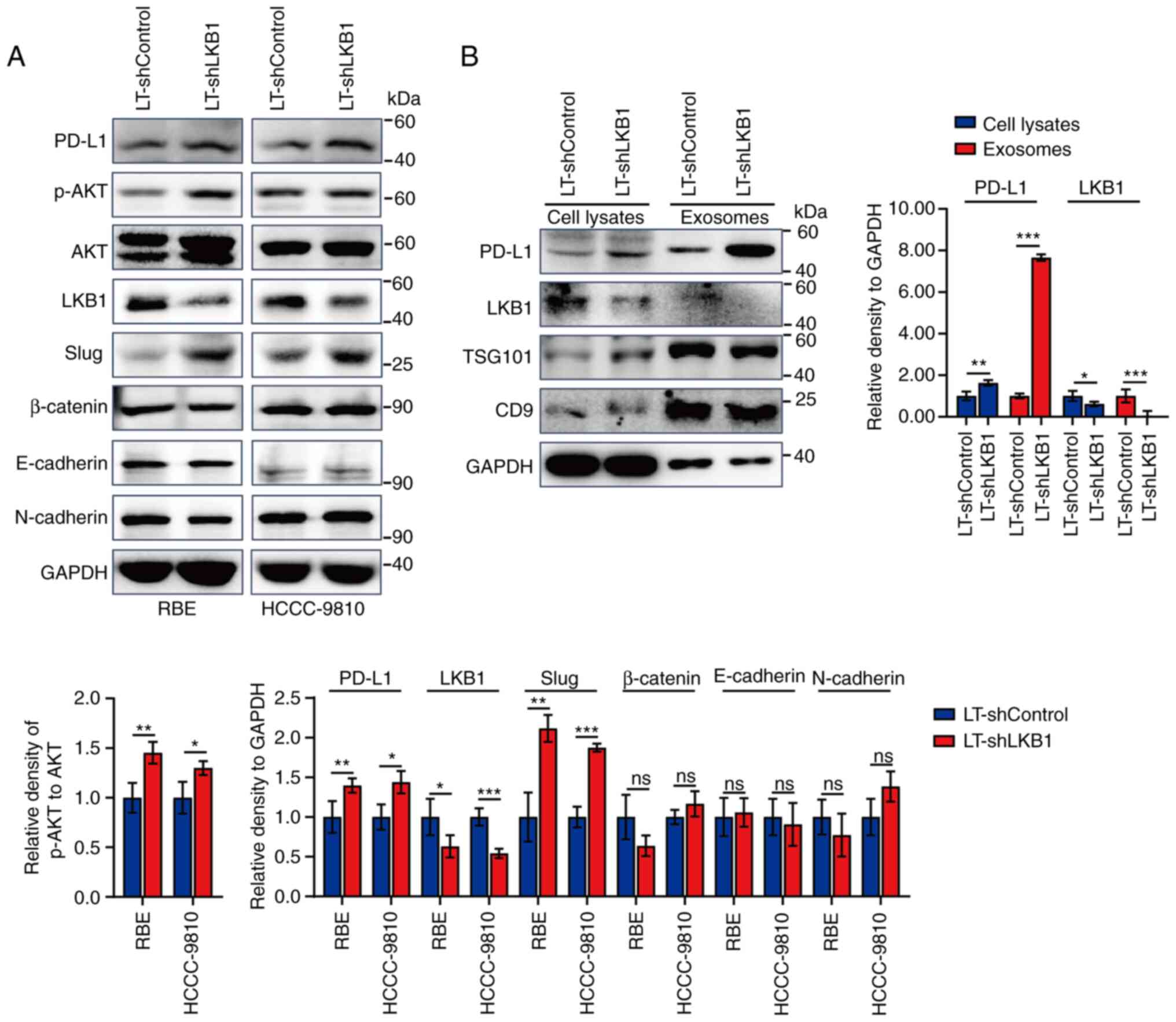 | Figure 2.Negative regulation of LKB1 on
exosomal PD-L1. (A) Western blot analysis for protein expression of
PD-L1, p-AKT, AKT, LKB1 and epithelial-mesenchymal
transition-associated markers (Slug, β-Catenin, E-cadherin, and
N-cadherin) in cell lysates of RBE and HCCC-9810 ICC cells with
LKB1 knockdown. (B) Western blotting for protein expression of
PD-L1, LKB1, TSG101, CD9 and GAPDH in cell lysates and exosomes of
RBE cells with LKB1 knockdown. Data are represented as the means ±
SD and significance was analyzed using Student's two-sided t-test.
n=3 in each group. *P<0.05, **P<0.01 and ***P<0.001. LKB1,
liver kinase B1; PD-L1, programmed death ligand 1; p-,
phosphorylated; ICC, intrahepatic cholangiocarcinoma; sh-, small
hairpin; LT, lentivirus; ns, no significance. |
Exosomal LKB1 inhibits the immune
checkpoint PD-L1 and malignant phenotypes of ICC cells
Next, the inhibitory effect of exosomal LKB1 on ICC
was confirmed in vitro. Notably, compared with exosomes
secreted by LT-shCtrl cells, ICC cells incubated with exosomes
secreted by LT-shLKB1 cells expressed lower levels of LKB1 protein
and higher levels of PD-L1, p-AKT and Slug (Fig. 3A). These findings suggested that
exosomal LKB1 inhibits the immune checkpoint PD-L1 and tumor signal
transduction of ICC cells. Thus, exosomal PD-L1 plays a tumor
suppressor role in ICC.
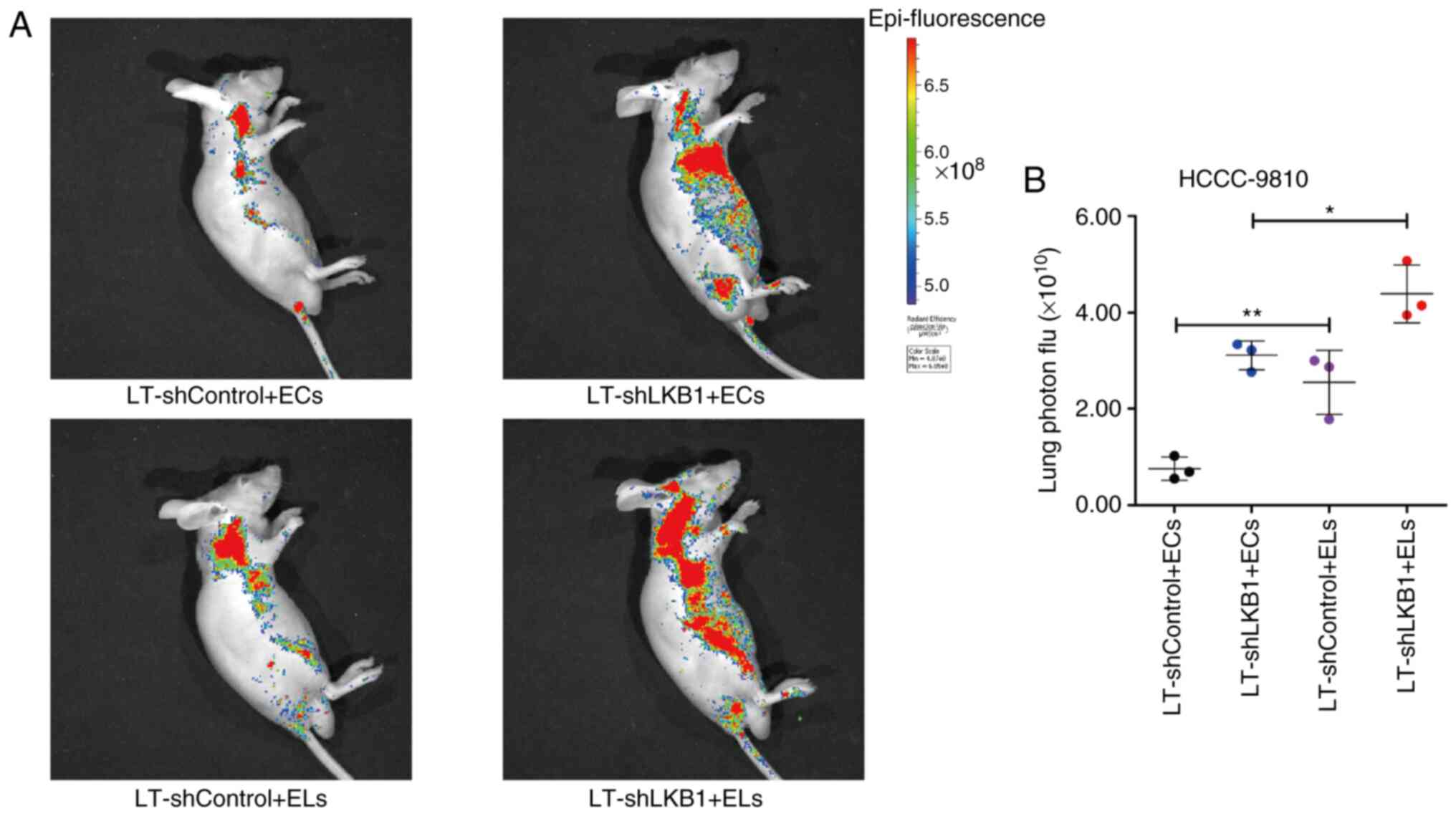
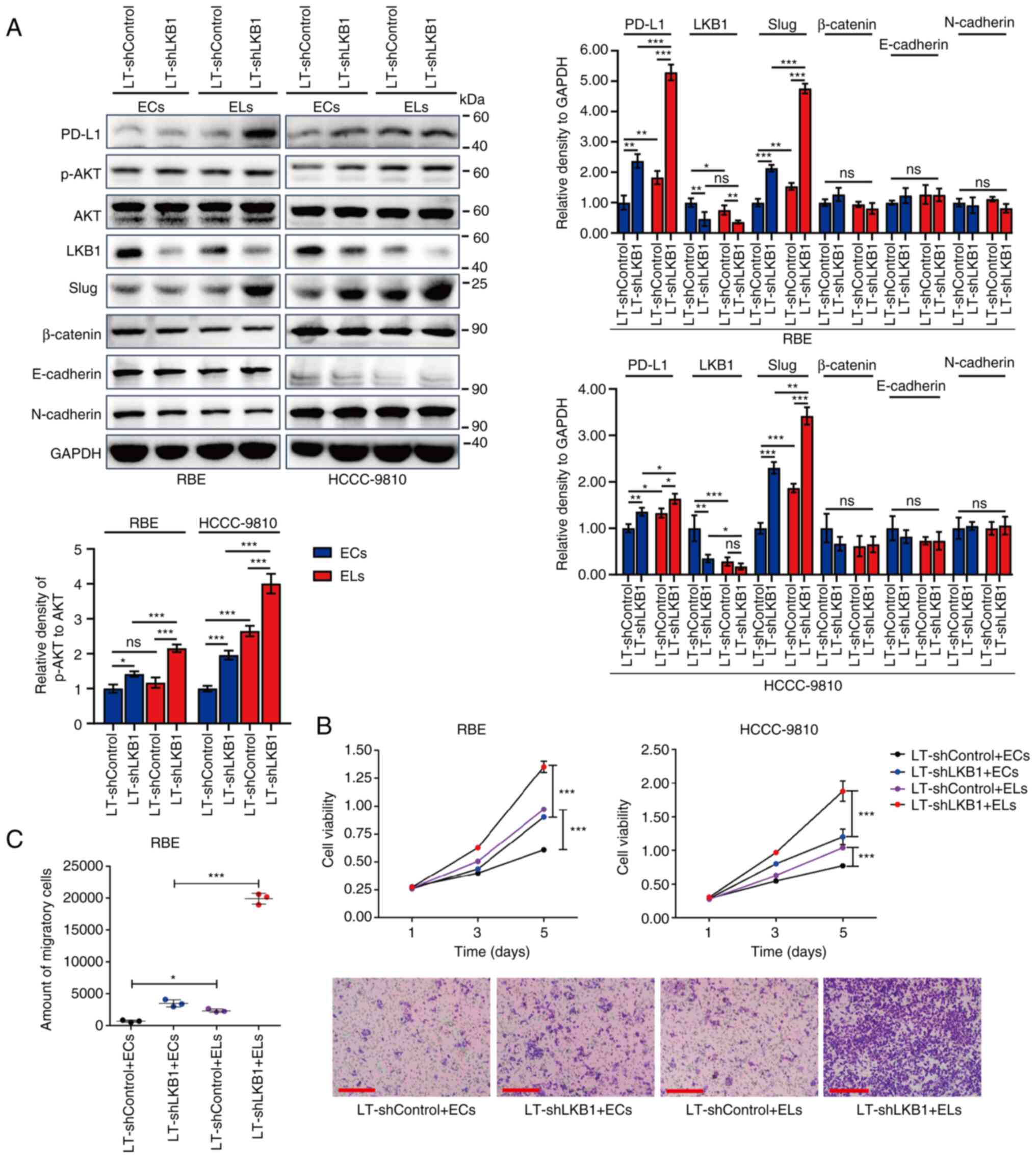 | Figure 3.Regulatory effects of exosomes with
differential expression of LKB1 on malignant phenotypes of ICC
cells. (A) Western blotting for protein expression of PD-L1, p-AKT,
AKT, LKB1 and epithelial-mesenchymal transition-associated markers
(Slug, β-Catenin, E-cadherin and N-cadherin) in lysates of RBE and
HCCC-9810 ICC cells. The adherent ICC cells were incubated with
exosomes extracted from cell culture supernatants of corresponding
ICC cells with differential expression of LKB1 for 24 h. (B)
Proliferation ability of RBE and HCCC-9810 cells as assayed by MTT.
The adherent ICC cells were incubated with exosomes extracted from
cell culture supernatants of corresponding ICC cells with
differential expression of LKB1 for different times. n=4 in each
group. (C) Representative images and statistical analysis of the
migratory RBE and HCCC-9810 ICC cells as examined by Transwell
assays. Cells in the upper chambers of the Transwell were incubated
with exosomes extracted from cell culture supernatants of
corresponding ICC cells with differential expression of LKB1 for 24
h. n=3 in each group. Scale bar, 100 µm. Data are represented as
the means ± SD and significance was analyzed using Student's
two-sided t-test or one-way ANOVA with the Tukey-Kramer post hoc
test. *P<0.05, **P<0.01 and ***P<0.001. LKB1, liver kinase
B1; ICC, intrahepatic cholangiocarcinoma; PD-L1, programmed death
ligand 1; p-, phosphorylated; ECs, exosomes secreted by
LT-shControl cells; ELs, exosomes of LT-shLKB1 cells; sh-, small
hairpin; LT, lentivirus; ns, no significance. |
Next, the malignant phenotypes of ICC cells were
examined after treatment with exosomes with differential expression
of LKB1. Compared with exosomes derived from ICC cells with high
levels of LKB1, exosomes secreted by ICC cells with low levels of
LKB1 significantly promoted the proliferation of ICC cells RBE and
HCCC-9810 (Fig. 3B). Moreover,
exosomes with low levels of LKB1 exhibited a significant effect on
enhancing the migratory ability of ICC cells (Fig. 3C). Therefore, it was identified
that exosomal LKB1 inhibits the malignant phenotypes of ICC
cells.
Exosomal LKB1 suppresses ICC cell
metastasis in vivo
Considering the inhibitory effect of exosomal LKB1
on the migration ability of ICC cells, it was hypothesized that
exosomal LKB1 may influence ICC metastasis. Therefore, animal
experiments were conducted to explore the regulatory effect of
exosomal LKB1 on ICC metastasis. The wild-type ICC cell line
HCCC-9810 was administered to male BALB/c nude mice through tail
vein injection. After 7 days, exosomes of HCCC-9810 cells with
differential expression of LKB1 resuspended in sterile PBS were
administered to mice by tail vein injection. Through tracing the
GFP expression of lentivirus-infected HCCC-9810 cells in
vivo, it was found that mice injected with exosomes with lower
levels of LKB1 exhibited a significantly stronger intensity of
luciferase, while mice injected with exosomes with higher levels of
LKB1 protein exhibited a significantly weaker intensity of
luciferase (Fig. 4A). The
statistical analysis of luciferase intensity showed a significant
difference among the groups (Fig.
4B). These findings suggested that exosomal LKB1 inhibits ICC
metastasis.
Low levels of exosomal LKB1 may
predict poor prognosis of ICC
To further evaluate the clinical importance of
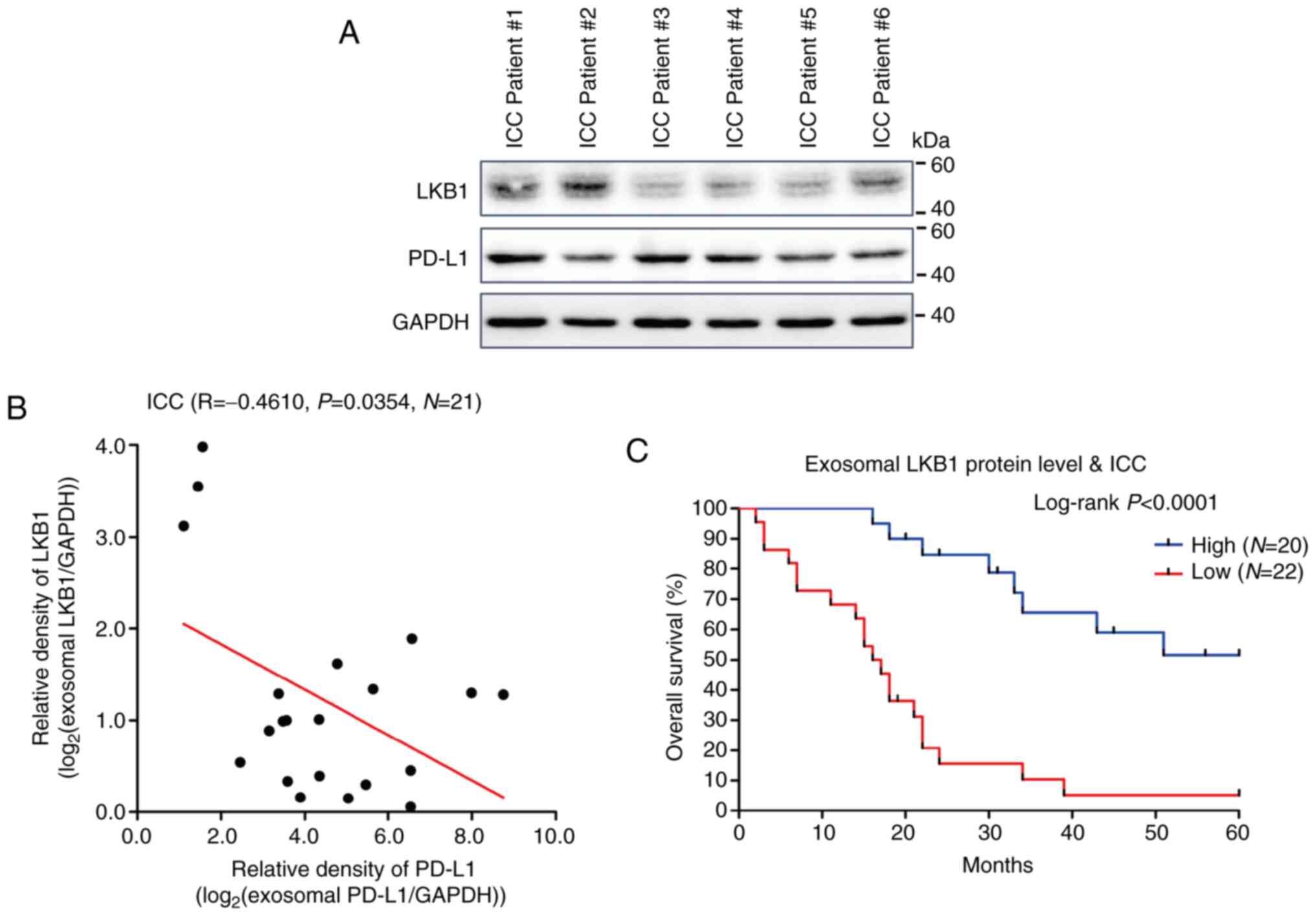
exosomal LKB1 in cancer research, exosomes were extracted from
plasma specimens of patients with ICC. Western blotting revealed
that the expression trends of LKB1 and PD-L1 protein in exosomes in
the plasma of patients with ICC were almost opposite (Fig. 5A). Pearson's correlation (R)
analysis revealed a negative correlation between the protein levels
of LKB1 and PD-L1 in exosomes of plasma specimens (Fig. 5B). These findings are consistent
with the aforementioned observations in cell culture supernatants
of ICC cells. Moreover, the Kaplan-Meier analysis for the overall
survival of ICC indicated that a low level of exosomal LKB1 may
predict poor prognosis of ICC (Fig.
5C). These experimental results from clinical samples suggested
the inhibitory effect of LKB1 on exosomal PD-L1 and demonstrated
the tumor suppressor role of exosomal LKB1 in ICC.
Discussion
Accumulating evidence reflects LKB1 regulation of
the immune checkpoint PD-L1. In KRAS-mutated lung cancer, the LKB1
co-mutation is associated with a reduced level of PD-L1 protein,
resulting in therapeutic resistance to PD-1/PD-L1 inhibitors
(30). Compared with patients with
non-squamous LKB1-mutant non-small-cell lung cancer, patients with
wild-type LKB1 present improved response to anti-PD-L1
immunotherapy. Therefore, loss of LKB1 clearly leads to upregulated
levels of PD-L1 (27,31). In the present study, a low level of
exosomal LKB1 was uncovered in the cell culture supernatants of ICC
cells and the plasma of patients with ICC. At present, no P53
mutations have been reported in ICC cells RBE or HCCC-9810, while
the IDH1 mutation (R132S) exists in RBE cells (32). Both RBE and HCCC9810 cells exhibit
strong migratory and clonogenic abilities (33), while HCCC9810 cells exhibit
stronger resistance to drugs, such as anlotinib and gemcitabine,
than RBE cells (34). HCCC-9810
cells have the ability to form tumors in vivo (35,36),
while RBE cells cannot form tumors in athymic nude mice (37). Based on the aforementioned
findings, it was considered that HCCC-9810 cells may have stronger
aggressive and metastatic ability than RBE cells. These two
differential cell lines, RBE and HCCC-9810, were selected to study
the regulation of exosomal LKB1 on ICC. The downregulation of
intracellular LKB1 led to the downregulation of exosomal LKB1 and
the upregulation of PD-L1, Slug and p-AKT in exosomes. Exosomal
PD-L1 contributes to immunosuppression and is associated with the
anti-PD-1 response (38). Thus,
LKB1 may be involved in immunosurveillance of ICC by suppressing
exosomal PD-L1. Exosomal LKB1 was revealed to downregulate PD-L1
levels in ICC cells, which may provide new insights into the immune
evasion of ICC cells by modulating exosomes.
Moreover, exosomes secreted by ICC cells with low
levels of LKB1 promoted the metastasis of ICC cells. PD-L1 and
p-AKT are both closely related to malignant transformation and poor
prognosis of malignances (39–41).
The crosstalk between the PI3K-AKT-mTOR and LKB1-AMPK signaling
pathways is critical for modulating cancer metastasis, metabolism
and prognosis (42,43), and Slug functions greatly in cancer
development (44). Although the
present study did not show direct evidence of the involvement of
AKT and Slug in the inhibitory effect of LKB1 on ICC metastasis, it
could still be concluded that LKB1 may suppress metastasis via an
exosome-mediated mechanism. These regulatory factors may be the key
points for LKB1 to inhibit immune evasion and metastasis of ICC.
However, the direct mechanism by which exosomal LKB1 regulates ICC
metastasis still needs to be further explored.
Exosomes secreted by cancer cells can be used as
warehouses to transfer biologically active molecules, such as RNA
and proteins, thereby regulating the occurrence and development of
malignances (45,46). Except for CD9, CD63 and TSG101,
both GAPDH and β-actin have been detected in exosomes (47–49),
all of which may be exosomal markers. The abundant level of GAPDH
protein in exosomes secreted by RBE and HCCC-9810 cells suggested
the role of GAPDH as a loading control for exosomes at least.
Limited by experimental conditions and wide spread of COVID-19,
nanoparticle tracking analysis on the extracted exosomes was not
performed, which may need to be further improved in future studies.
Notably, exosomal PD-L1 promotes the immune evasion of cancer cells
and reduces the response efficiency to PD-1 inhibitors (38). The regulatory role of LKB1 in
exosomes remains obscure. Consistent with the inhibition on p-AKT
and Slug, LKB1 was revealed to decrease exosomal PD-L1 in ICC cells
in the present study, providing a new mechanism for understanding
the anticancer effect of LKB1. These findings may provide new
methods for inhibiting the immune evasion of ICC cells by targeting
exosomal LKB1 and PD-L1.
Liquid biopsy has gained momentum in clinical cancer
research. Continuously released by living cells, exosomes contain
DNA, RNA and proteins, providing direction for clinically relevant
diagnosis (50). Owing to their
non-invasive nature and real-time assessment, exosome-based
diagnostics are more readily available to track patients over time
and monitor potential disease progression and therapeutic
intervention in an improved way (51). Through conducting small RNA
sequencing and proteomics evaluation, certain miRNAs and proteins
have been identified with elevated or attenuated expression in
plasma specimens of cancer patients, acting as potential diagnostic
markers for multiple types of cancer (52–54).
In the present study, it was revealed that low expression of
exosomal LKB1 in the plasma of patients with ICC may predict poor
prognosis, further emphasizing the clinical significance of LKB1
research in ICC.
In summary, the significance and innovations of the
present study are mainly reflected in the following aspects. First,
LKB1 was shown to inhibit exosomal PD-L1 in ICC cells. Second,
in vitro and in vivo experiments revealed the
inhibitory effect of exosomal LKB1 on ICC. Third, the low
expression level of exosomal LKB1 was found to be tightly
associated with the metastasis and poor prognosis of ICC. Exosomal
LKB1 exerts a tumor suppressor role in ICC and may be an important
biomarker for the diagnosis and immune therapy of ICC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hunan Natural Science
Foundation (grant no. 2019JJ50626) and the Project of Health and
Family Planning Commission of Hunan Province (grant no.
B20180832).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL, KZ and TM conceived and designed the
experiments. ZL, KZ, JZ, XZ, HL, KP, XL, FL, BJ and MZ performed
the experiments. ZL, KZ, HL, XL and TM managed data. ZL, KZ, JZ, XZ
and TM analyzed the data. KP and TM contributed to reagents,
materials and analysis tools. ZL and TM acquired funding. ZL, KZ
and TM wrote the paper. ZL and KZ confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The protocol used for animal experiments was
approved (approval no. 2020073) by the Animal Care and Experiment
Committee of The Central Hospital of Xiangtan (Xiangtan, China).
All applicable international, national, and/or institutional
guidelines for the care and use of animals were followed in the
present study. Informed consent was obtained from all patients for
collection of plasma specimens. Patient studies were approved
(approval no. 2019-08-001) by the institutional review boards of
The Central Hospital of Xiangtan (Xiangtan, China) and were in
accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LKB1
|
liver kinase B1
|
|
PD-L1
|
programmed death ligand 1
|
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
TEM
|
transmission electron microscopy
|
|
GFP
|
green fluorescence protein
|
References
|
1
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis, and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kelley RK, Bridgewater J, Gores GJ and Zhu
AX: Systemic therapies for intrahepatic cholangiocarcinoma. J
Hepatol. 72:353–363. 2020. View Article : Google Scholar
|
|
3
|
Lunsford KE, Javle M, Heyne K, Shroff RT,
Abdel-Wahab R, Gupta N, Mobley CM, Saharia A, Victor DW, Nguyen DT,
et al: Liver transplantation for locally advanced intrahepatic
cholangiocarcinoma treated with neoadjuvant therapy: A prospective
case-series. Lancet Gastroenterol Hepatol. 3:337–348. 2018.
View Article : Google Scholar
|
|
4
|
El-Diwany R, Pawlik TM and Ejaz A:
Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 28:587–599.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin SG and St Johnston D: A role for
Drosophila LKB1 in anterior-posterior axis formation and epithelial
polarity. Nature. 421:379–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaw RJ, Kosmatka M, Bardeesy N, Hurley
RL, Witters LA, DePinho RA and Cantley LC: The tumor suppressor
LKB1 kinase directly activates AMP-activated kinase and regulates
apoptosis in response to energy stress. Proc Natl Acad Sci USA.
101:3329–3335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li N, Huang D, Lu N and Luo L: Role of the
LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells
(review). Oncol Rep. 34:2821–2826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin LY, Zhao K, Xu LJ, Zhao RX, Werle KD,
Wang Y, Liu XL, Chen Q, Wu ZJ, Zhang K, et al: LKB1 inactivation
leads to centromere defects and genome instability via
p53-dependent upregulation of survivin. Aging (Albany NY).
12:14341–14354. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koyama S, Akbay EA, Li YY, Aref AR,
Skoulidis F, Herter-Sprie GS, Buczkowski KA, Liu Y, Awad MM,
Denning WL, et al: STK11/LKB1 deficiency promotes neutrophil
recruitment and proinflammatory cytokine production to suppress
T-cell activity in the lung tumor microenvironment. Cancer Res.
76:999–1008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Skoulidis F, Goldberg ME, Greenawalt DM,
Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco
SE, Gay L, et al: STK11/LKB1 mutations and PD-1 inhibitor
resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov.
8:822–835. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitajima S, Ivanova E, Guo S, Yoshida R,
Campisi M, Sundararaman SK, Tange S, Mitsuishi Y, Thai TC, Masuda
S, et al: Suppression of STING associated with LKB1 loss in
KRAS-driven lung cancer. Cancer Discov. 9:34–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shukuya T, Yamada T, Koenig MJ, Xu J,
Okimoto T, Li F, Amann JM and Carbone DP: The effect of LKB1
activity on the sensitivity to PI3K/mTOR inhibition in non-small
cell lung cancer. J Thorac Oncol. 14:1061–1076. 2019. View Article : Google Scholar
|
|
13
|
Moro M, Caiola E, Ganzinelli M, Zulato E,
Rulli E, Marabese M, Centonze G, Busico A, Pastorino U, de Braud
FG, et al: Metformin enhances cisplatin-induced apoptosis and
prevents resistance to cisplatin in co-mutated KRAS/LKB1 NSCLC. J
Thorac Oncol. 13:1692–1704. 2018. View Article : Google Scholar
|
|
14
|
Gao Y, Yan Y, Tripathi S, Pentinmikko N,
Amaral A, Päivinen P, Domènech-Moreno E, Andersson S, Wong IPL,
Clevers H, et al: LKB1 represses ATOH1 via PDK4 and energy
metabolism and regulates intestinal stem cell fate.
Gastroenterology. 158:1389–1401.e10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shan T, Xu Z, Liu J, Wu W and Wang Y: Lkb1
regulation of skeletal muscle development, metabolism and muscle
progenitor cell homeostasis. J Cell Physiol. 232:2653–2656. 2017.
View Article : Google Scholar
|
|
16
|
Zhang Y, Meng Q, Sun Q, Xu ZX, Zhou H and
Wang Y: LKB1 deficiency-induced metabolic reprogramming in
tumorigenesis and non-neoplastic diseases. Mol Metab.
44:1011312021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maillet V, Boussetta N, Leclerc J, Fauveau
V, Foretz M, Viollet B, Couty JP, Celton-Morizur S, Perret C and
Desdouets C: LKB1 as a gatekeeper of hepatocyte proliferation and
genomic integrity during liver regeneration. Cell Rep.
22:1994–2005. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang D, Zhang W, Zhang H, Zhang F, Chen L,
Ma L, Larcher LM, Chen S, Liu N, Zhao Q, et al: Progress,
opportunity, and perspective on exosome isolation-efforts for
efficient exosome-based theranostics. Theranostics. 10:3684–3707.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Niel G, D'Angelo G and Raposo G:
Shedding light on the cell biology of extracellular vesicles. Nat
Rev Mol Cell Biol. 19:213–228. 2018. View Article : Google Scholar
|
|
20
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fabris L, Sato K, Alpini G and
Strazzabosco M: The tumor microenvironment in cholangiocarcinoma
progression. Hepatology. 73 (Suppl 1):S75–S85. 2021. View Article : Google Scholar
|
|
22
|
Sinha D, Roy S, Saha P, Chatterjee N and
Bishayee A: Trends in research on exosomes in cancer progression
and anticancer therapy. Cancers (Basel). 13:3262021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang ZJ, Zhang LL, Bi QC, Gan LJ, Wei MJ,
Hong T, Tan RJ, Lan XM, Liu LH, Han XJ and Jiang LP: Exosomal
connexin 43 regulates the resistance of glioma cells to
temozolomide. Oncol Rep. 45:442021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang S, Hu Y, Lv X, Li B, Gu D, Li Y, Sun
Y and Su Y: Circ-0000284 arouses malignant phenotype of
cholangiocarcinoma cells and regulates the biological functions of
peripheral cells through cellular communication. Clin Sci (Lond).
133:1935–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Miao R, Liu T, Xiang X, Gu J, Jia
Y, Li Z, Fu Y, He Y, Zhang Y, et al: IDH1 as a frequently mutated
gene has potential effect on exosomes releasement by epigenetically
regulating P2RX7 in intrahepatic cholangiocarcinoma. Biomed
Pharmacother. 113:1087742019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar
|
|
27
|
Liu Z, Li S, Zeng J, Zhou X, Li H, Liu X,
Li F, Jiang B, Zhao M and Ma T: LKB1 inhibits intrahepatic
cholangiocarcinoma by repressing the transcriptional activity of
the immune checkpoint PD-L1. Life Sci. 257:1180682020. View Article : Google Scholar
|
|
28
|
Théry C, Amigorena S, Raposo G and Clayton
A: Isolation and characterization of exosomes from cell culture
supernatants and biological fluids. Curr Protoc Cell Biol Chapter
3: Unit 3.22. 2006.
|
|
29
|
Shang S, Wang J, Chen S, Tian R, Zeng H,
Wang L, Xia M, Zhu H and Zuo C: Exosomal miRNA-1231 derived from
bone marrow mesenchymal stem cells inhibits the activity of
pancreatic cancer. Cancer Med. 8:7728–7740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Santarpia M, Aguilar A, Chaib I, Cardona
AF, Fancelli S, Laguia F, Bracht JWP, Cao P, Molina-Vila MA,
Karachaliou N and Rosell R: Non-small-cell lung cancer signaling
pathways, metabolism, and PD-1/PD-L1 antibodies. Cancers (Basel).
12:14752020. View Article : Google Scholar
|
|
31
|
Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y,
Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, et al:
Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with
elevated PD-L1 expression. Cancer Cell. 25:590–604. 2014.
View Article : Google Scholar
|
|
32
|
Saha SK, Gordan JD, Kleinstiver BP, Vu P,
Najem MS, Yeo JC, Shi L, Kato Y, Levin RS, Webber JT, et al:
Isocitrate dehydrogenase mutations confer dasatinib
hypersensitivity and SRC dependence in intrahepatic
cholangiocarcinoma. Cancer Discov. 6:727–739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao Q, Zhao YJ, Wang XY, Guo WJ, Gao S,
Wei L, Shi JY, Shi GM, Wang ZC, Zhang YN, et al: Activating
mutations in PTPN3 promote cholangiocarcinoma cell proliferation
and migration and are associated with tumor recurrence in patients.
Gastroenterology. 146:1397–1407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fan S, Ge Y, Liu J, Liu H, Yan R, Gao T,
Fan X, Xiao Z and An G: Combination of anlotinib and gemcitabine
promotes the G0/G1 cell cycle arrest and apoptosis of intrahepatic
cholangiocarcinoma in vitro. J Clin Lab Anal. 35:e239862021.
View Article : Google Scholar
|
|
35
|
Tang L, Wang Y, Wang H, Xu B, Ji H, Xu G,
Ge X, Li Q and Miao L: Long noncoding-RNA component of
mitochondrial RNA processing endoribonuclease is involved in the
progression of cholangiocarcinoma by regulating microRNA-217.
Cancer Sci. 110:2166–2179. 2019. View Article : Google Scholar
|
|
36
|
Yang X, Wang S, Mu Y and Zheng Y:
Schisandrin B inhibits cell proliferation and induces apoptosis in
human cholangiocarcinoma cells. Oncol Rep. 36:1799–1806. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Enjoji M, Sakai H, Nawata H, Kajiyama K
and Tsuneyoshi M: Sarcomatous and adenocarcinoma cell lines from
the same nodule of cholangiocarcinoma. In Vitro Cell Dev Biol Anim.
33:681–683. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng R, Zuo C, Li Y, Xue B, Xun Z, Guo Y,
Wang X, Xu Y, Tian R, Chen S, et al: The innate immune effector
ISG12a promotes cancer immunity by suppressing the canonical
Wnt/β-catenin signaling pathway. Cell Mol Immunol. 17:1163–1179.
2020. View Article : Google Scholar
|
|
40
|
Alzahrani AS: PI3K/Akt/mTOR inhibitors in
cancer: At the bench and bedside. Semin Cancer Biol. 59:125–132.
2019. View Article : Google Scholar
|
|
41
|
He PX, Ma ZL, Han H, Zhang XY, Niu SH, Du
LN, Zheng YC and Liu HM: Expression of programmed death ligand 1
(PD-L1) is associated with metastasis and differentiation in
gastric cancer. Life Sci. 242:1172472020. View Article : Google Scholar
|
|
42
|
Deneka AY, Kopp MC, Nikonova AS, Gaponova
AV, Kiseleva AA, Hensley HH, Flieder DB, Serebriiskii IG and
Golemis EA: Nedd9 restrains autophagy to limit growth of early
stage non-small cell lung cancer. Cancer Res. 81:3717–3726. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chou WC, Rampanelli E, Li X and Ting JP:
Impact of intracellular innate immune receptors on
immunometabolism. Cell Mol Immunol. 19:337–351. 2022. View Article : Google Scholar
|
|
44
|
Gao X, Mi Y, Guo N, Luan J, Xu H, Hu Z,
Wang N, Zhang D, Gou X and Xu L: The mechanism of propofol in
cancer development: An updated review. Asia Pac J Clin Oncol.
16:e3–e11. 2020. View Article : Google Scholar
|
|
45
|
Kanada M, Bachmann MH and Contag CH:
Signaling by extracellular vesicles advances cancer hallmarks.
Trends Cancer. 2:84–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakaoka A, Nakahana M, Inubushi S, Akasaka
H, Salah M, Fujita Y, Kubota H, Hassan M, Nishikawa R, Mukumoto N,
et al: Exosome-mediated radiosensitizing effect on neighboring
cancer cells via increase in intracellular levels of reactive
oxygen species. Oncol Rep. 45:132021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rodrigues G, Hoshino A, Kenific CM, Matei
IR, Steiner L, Freitas D, Kim HS, Oxley PR, Scandariato I,
Casanova-Salas I, et al: Tumour exosomal CEMIP protein promotes
cancer cell colonization in brain metastasis. Nat Cell Biol.
21:1403–1412. 2019. View Article : Google Scholar
|
|
48
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, Garcia-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Skog J, Wurdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008. View Article : Google Scholar
|
|
51
|
Yu W, Hurley J, Roberts D, Chakrabortty
SK, Enderle D, Noerholm M, Breakefield XO and Skog JK:
Exosome-based liquid biopsies in cancer: Opportunities and
challenges. Ann Oncol. 32:466–477. 2021. View Article : Google Scholar
|
|
52
|
Hannafon BN, Trigoso YD, Calloway CL, Zhao
YD, Lum DH, Welm AL, Zhao ZJ, Blick KE, Dooley WC and Ding WQ:
Plasma exosome microRNAs are indicative of breast cancer. Breast
Cancer Res. 18:902016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Choi D, Montermini L, Kim DK, Meehan B,
Roth FP and Rak J: The impact of oncogenic EGFRvIII on the proteome
of extracellular vesicles released from glioblastoma cells. Mol
Cell Proteomics. 17:1948–1964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Matsumoto Y, Kano M, Akutsu Y, Hanari N,
Hoshino I, Murakami K, Usui A, Suito H, Takahashi M, Otsuka R, et
al: Quantification of plasma exosome is a potential prognostic
marker for esophageal squamous cell carcinoma. Oncol Rep.
36:2535–2543. 2016. View Article : Google Scholar : PubMed/NCBI
|