Introduction
Lung cancer is a major cause of mortality from
malignant diseases, due to its high incidence, malignant behavior
and lack of major advancements in treatment strategy (1). Lung cancer was the leading indication
for respiratory surgery (47.5%) in 2009 in Japan (2), with >30,000 patients undergoing
surgery due to lung cancer at Japanese institutions during the same
year (2). The clinical behavior of
non-small cell lung cancer (NSCLC) is largely associated with its
stage. Treatment of the disease by surgery is only achieved in
cases at an early stage of NSCLC (3).
An imbalance in immune regulation affects
tumor-specific T-cell immunity in the cancer microenvironment and
reshapes tumor progression and metastasis (4). The lack of immunostimulatory
activation may be harmful if it impairs immune responses against
cancer (5). Several receptor-ligand
interactions are known to trigger anti-apoptotic pathways that
prevent activation-induced T-cell death (6,7).
Programmed death 1 (PD-1) protein, a T-cell co-inhibitory receptor,
and one of its ligands, programmed cell death 1 ligand 1 (PD-L1),
are involved in the ability of tumor cells to escape the host’s
immune system. PD-L1 is selectively expressed in a number of tumors
(8–10). The blockade of interactions between
PD-1 and PD-L1 enhances the immune function in vitro and
mediates antitumor activity in preclinical models (8,9).
Recent studies have suggested that antibody-mediated blockade of
PD-L1 (10) and PD-1 (11) induced durable tumor regression and
prolonged stabilization of the disease in certain patients with
advanced cancers, including NSCLC. In their study, Topalian et
al(12) demonstrated that
immunohistochemical (IHC) analysis detected no objective response
in PD-L1-negative patients. However, 36% of the patients with
PD-L1-positive tumors had an objective response, although the
sample number for IHC was small (n=42). Thus, PD-L1 might be a
critical factor in cancer immunotherapy.
In this study, we examined PD-L1 mRNA
expression in Japanese NSCLC and adjacent normal lung tissues, by
real-time quantitative polymerase chain reaction (qPCR) using
LightCycler (Roche Molecular Biochemicals, Mannheim, Germany)
(13) in surgically treated cases.
The findings were compared to the clinicopathological parameters of
the NSCLC and PD-L1 gene status.
Patients and methods
Patients
The study group comprised NSCLC patients who had
undergone surgery at the Department of Surgery, Nagoya City
University Hospital (Nagoya, Japan) between 2006 and 2009. The
tumor samples were immediately frozen and stored at −80°C until
they were assayed. Patient consent was obtained from the patients.
The study was approved by the ethics committee of the university.
The clinical and pathological characteristics of the 123 NSCLC
patients for PD-L1 mRNA gene analyses were as follows: 80
(65.0%) were male and 43 were female, 95 (77.2%) were diagnosed
with adenocarcinomas, 79 (64.2%) were smoker and 44 (35.8%) were
non-smoker, and 81 (65.9%) were pathological stage I (Table I).
 | Table I.Clinicopathological parameters of 123
lung cancer patients. |
Table I.
Clinicopathological parameters of 123
lung cancer patients.
| PD-L1
|
|---|
| Factors | No. of patients
(n=123) (%) | T/N ratio of
PD-L1/β-actin mRNA levels | P-value |
|---|
| Stage | | | |
| I | 81 (65.9) | 5.523±13.780 | III–IV vs. II |
| II | 20 (16.3) | 2.213±4.422 | 0.0345 |
| III–IV | 22 (17.9) | 13.359±29.768 | |
| Tumor status | | | |
| pT1 | 56 (45.5) | 3.492±8.494 | T4 vs. T1 |
| pT2 | 49 (39.8) | 6.670±15.718 | 0.0235 |
| pT3 | 6 (4.9) | 12.231±24.958 | |
| pT4 | 12 (9.8) | 15.811±36.883 | |
| Lymph node
metastasis | | | |
| Negative | 90 (73.2) | 5.502±13.588 | 0.3456 |
| Positive | 33 (26.8) | 8.798±24.337 | |
| Age (years) | | | |
| ≤65 | 59 (48.0) | 5.382±10.094 | 0.5359 |
| >65 | 64 (52.0) | 7.295±21.597 | |
| EGFR mutation | | | |
| Positive | 28 (22.8) | 7.412±21.261 | 0.3976 |
| Negative | 95 (73.6) | 6.084±15.780 | |
| Smoking | | | |
| BI=0 | 44 (35.8) | 8.268±21.856 | 0.3644 |
| BI>0 | 79 (64.2) | 5.339±13.806 | |
| Pathological
subtypes | | | |
| Adeno | 95 (77.2) | 7.344±19.206 | 0.2543 |
| Non-adeno | 28 (22.8) | 3.139±4.683 | |
| Gender | | | |
| Male | 80 (65.0) | 5.536±16.039 | 0.4539 |
| Female | 43 (35.0) | 7.969±19.000 | |
PCR assay for PD-L1 gene
Total RNA was extracted from NSCLC and adjacent
normal lung tissues using the Isogen kit (Nippon Gene, Tokyo,
Japan), according to the manufacturer’s instructions. RNA
concentration was determined by NanoDrop ND-1000 Spectrophotometer
(Nano Drop Technologies Inc., Rockland, DE, USA). Approximately 10
cases were excluded for each assay since tumor cells were
insufficient in number to extract tumor RNA. RNA (1 μg) was reverse
transcribed by the first strand cDNA synthesis kit with 0.5 μg
oligo(dT)16 (Roche Diagnostics GmbH, Mannheim, Germany),
according to the manufacturer’s instructions. The reaction mixture
was incubated at 25°C for 15 min, 42°C for 60 min, 99°C for 5 min
and at 4°C for 5 min. The cDNA concentration was determined by a
NanoDrop ND-1000 Spectrophotometer. Approximately 200 ng of each
cDNA was used for PCR analysis. To ensure the fidelity of mRNA
extraction and reverse transcription, the samples were subjected to
qPCR amplification with the β-actin primers (Nihon Gene
Laboratory, Miyagi, Japan) using LightCycler-FastStart DNA Master
HybProbe Kit (Roche Diagnostics GmbH). The PD-L1 qPCR assay
reactions were performed using the LightCycler FastStart DNA Master
SYBR-Green I kit (Roche Diagnostics GmbH) in a 20 μl reaction
volume. The primer sequences for PD-L1 gene were: forward:
5′-CAAAGAATTTTGGTTGTGGA-3′ and reverse: 5′-AGCTTCTCCTCTCTCTTGGA-3′
(155 base pairs). The cycling conditions were as follows: initial
denaturation at 95°C for 10 min, followed by 40 cycles at 95°C for
10 sec, annealing at 54°C for 10 sec and extension at 72°C for 7
sec.
Statistical analysis
Statistical analysis was carried out using the
Student’s t-test for unpaired samples and Wilcoxon’s signed
rank-sum test for paired samples. Correlation coefficients were
determined using the Chi-square test. Fisher’s PLSD test was used
to adjust multiple comparisons. The overall survival of lung cancer
patients was examined by the Kaplan-Meier method, while differences
were examined by the log-rank test. The analysis was carried out
using the StatView software package (Abacus Concepts, Inc.,
Berkeley, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
PD-L1 mRNA status in Japanese lung cancer
patients
The PD-L1 gene status was quantified for 123
NSCLC samples and adjacent normal lung tissues. The
PD-L1/β-actin mRNA levels showed no statistically
significant difference in lung cancer (131.398±421.596) and
adjacent normal lung tissues (78.182±254.092, P=0.1482). The
tumor/normal (T/N) ratio of PD-L1/β-actin mRNA levels
was >2 in 49 cases and >1 in 63 cases. The T/N ratio of
PD-L1/β actin mRNA levels did not correlate with
gender (male vs. female, P=0.4539), age (age ≤65 vs. >65,
P=0.5359), smoking status (smoker vs. non-smoker, P=0.3644) and
EGFR mutations status (wild type vs. mutant patients, P=0.3976).
The T/N ratio of PD-L1/β-actin mRNA level did not
correlate with pathological subtypes (adeno-carcinoma vs. others,
P=0.2543) and lymph node metastasis (P=0.3456). The T/N ratio of
PD-L1/β-actin mRNA level showed a gradual increase in
pathological T stages, and was markedly higher in pathological T4
cases (15.811±35.883) when compared to the T1 cases (3.492±8.494,
P=0.0235). The T/N ratio of PD-L1/β-actin mRNA levels
was markedly higher in pathological stage III–IV (13.359±29.768)
compared to stage II cases (2.213±4.422, P=0.0345), likely the
effect of advanced T statuses.
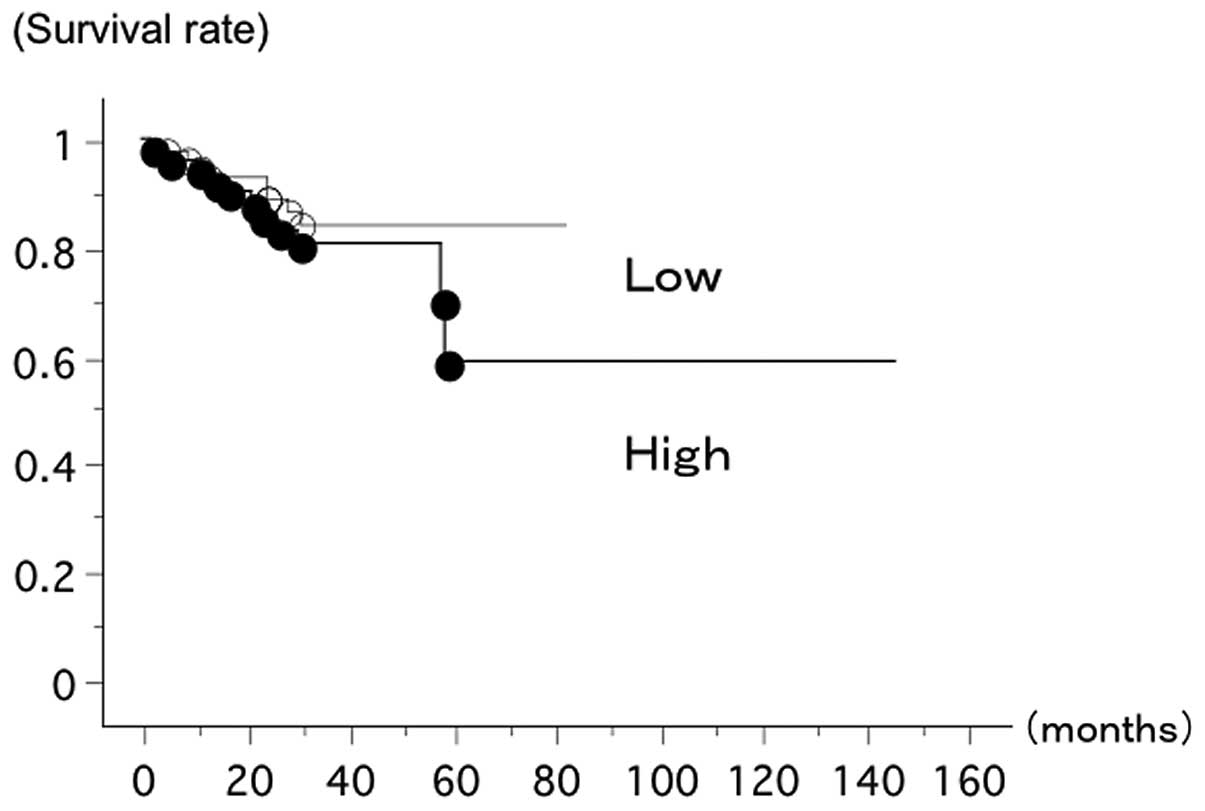
The overall survival of 123 lung cancer patients
from Nagoya City University (Nagoya, Japan), with follow-up through
July 31, 2012, was studied in reference to the PD-L1 gene
status. The survival of the patients with a T/N ratio of
PD-L1/β-actin mRNA level ≥1 (n=64, 8 deceased) and
those with a T/N ratio of PD-L1/β-actin mRNA level
<1 (n=59, 11 deceased) showed no statistically significant
difference (log-rank test, P=0.2336) (Fig. 1).
Discussion
In this study, we focused on one of the PD-1
ligands, PD-L1, to establish whether or not it might be a new
molecular target for NSCLC. The results showed that PD-L1
mRNA expression was correlated with tumor invasion in surgically
resected NSCLC using LightCycler.
Human cancers harbor numerous genetic and epigenetic
changes, generating neoantigens that are potentially recognizable
by the immune system (14). Tumors
develop multistep resistance systems, including local
immuno-suppression, induction of tolerance and systemic dysfunction
in T-cell signaling (15–18). In addition, tumors utilize several
pathways to escape immune destruction. These observations generated
intensive efforts to develop immunotherapeutic approaches for
cancer, including immune-checkpoint-pathway inhibitors, such as
anti-CTLA-4 antibody (19,20) and anti-PD-L1 therapy (11,12).
PD-1 is a key immune-checkpoint receptor expressed
by activated T cells that mediates immuno-suppressions. PD1 ligands
PD-L1 (B7-H1) and PD-L2 (B7-DC) are expressed by tumor and stromal
cells (8,21–23).
Thus PD-L1 may also act as a molecule target for tumor
progression in various types of cancer. In vitro, inhibition
of the interaction between PD-1 and PD-L1 may enhance T-cell
responses and mediate preclinical antitumor activity (8,9).
Investigations into the role of anti-PD-1 antibody in advanced
solid tumors are currently ongoing (24). Recent studies by Brahmer et
al(11) and Topalian et
al(12) have reported the
safety and activity of anti-PD1 or PD-L1 immunotherapy in cancers
including NSCLC. In NSCLC, 10% of patients exhibited a response to
anti-PD-L1 antibody (11), while
18% of NSCLC patients exhibited a response to anti-PD-1 antibody
(12). Notably, in the latter
report (12), PD-L1 expression
correlated with response. Of the limited number (n=42) of
pretreatment tumor samples (12),
none of the patients with PD-L1-negative tumors had an objective
response. However, 36% with PD-L1-positive tumors had an objective
response.
In our analysis, PD-L1 expression correlated
with tumor invasion. Tumor cells expressing PD-L1 might exhibit a
high progression potential in NSCLC. However, only half of the
tumors had >1 T/N ratio of PD-L1 mRNA levels, while only
one third of the tumors had >2 T/N ratio of PD-L1 mRNA
levels. Thus, potential of basing patient selection for the
suppression of PD-L1 signaling on PD-L1 expression in tumors
requires prospective assessment. In addition, the development and
validation of strategies to improve effective identification of the
high-responder patient population with anti-PD-L1 strategies are
important and likely to play a role in clinical practice.
In conclusion, PD-L1 might drive the tumor invasion
of NSCLC in certain patient populations, while providing a
candidate for blockade of its function as a strategy to antagonize
the progression process.
Acknowledgements
The authors would like to thank Mrs.
Yuka Toda for her excellent technical assistance. This study was
funded by Grants-in-Aid for Scientific Research, Japan Society for
the Promotion of Science (JSPS) (nos. 24592097 and 23659674) and a
grant for cancer research of the Program for developing the
supporting system for upgrading the education and research (2009)
of the Ministry of Education, Culture, Sports, Science and
Technology of Japan.
References
|
1.
|
Ginsberg RJ, Kris MK and Armstrong G:
Cancer of the lung. Principles and Practice of Oncology. 4th
edition. Lippincott; Philadelphia: pp. 673–682. 1993
|
|
2.
|
Sakata R, Fujii Y and Kuwano H: Thoracic
and cardiovascular surgery in Japan during 2009: annual report by
the Japanese Association for Thoracic Surgery. Gen Thorac
Cardiovasc Surg. 59:636–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Postmus PE: Chemotherapy for non-small
cell lung cancer: the experience of the Lung Cancer Cooperative
Group of the European Organization for Research and Treatment of
Cancer. Chest. 113:28S–31S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zou W: Immunosuppressive networks in the
tumour environment and their therapeutic relevance. Nature Rev
Cancer. 5:263–274. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chen L, Linsley PS and Hellstrom KE:
Costimulation of T cells for tumor immunity. Immunol Today.
14:483–486. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Boise LH, Noel PJ and Thompson CB: CD28
and apoptosis. Curr Opin Immunol. 7:620–625. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Watts TH and DeBenedette MA: T cell
co-stimulatory molecules other than CD28. Curr Opin Immunol.
11:286–293. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dong H, Strome SE, Salomao DR, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: a potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Iwai Y, Ishida M, Tanaka Y, et al:
Involvement of PD-L1 on tumor cells in the escape from host immune
system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad
Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Brahmer JR, Tykodi SS, Chow LQ, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Topalian SL, Hodi FS, Brahmer JR, et al:
Safety, activity, and immune correlates of anti-PD-1 antibody in
cancer. N Engl J Med. 366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wittwer CT, Ririe KM, Andrew RV, et al:
The LightCycler: a microvolume multi sample fluorimeter with rapid
temperature control. Biotechniques. 22:176–181. 1997.PubMed/NCBI
|
|
14.
|
Sjoblom T, Jones S, Wood LD, et al: The
consensus coding sequences of human breast and colorectal cancers.
Science. 314:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Topalian SL, Weiner GJ and Pardoll DM:
Cancer immunotherapy comes of age. J Clin Oncol. 29:4828–4836.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Drake CG, Jaffee E and Pardoll DM:
Mechanisms of immune evasion by tumors. Adv Immunol. 90:51–81.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mizoguchi H, O’Shea JJ, Longo DL, et al:
Alterations in signal transduction molecules in T lymphocytes from
tumor-bearing mice. Science. 258:1795–1598. 1792. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hodi FS, O’Day SJ, McDermott DF, et al:
Improves survival with ipilimumab in patients with metastatic
melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar
|
|
20.
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Freeman GJ, Long AJ, Iwai Y, et al:
Engagement of the PD-1 immunoinhibitory receptor by a novel B7
family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Brahmer JR, Drake CG, Wollner I, et al:
Phase I study of single-agent anti-programmed death-1 (MDX-1106) in
refractory solid tumors: safety, clinical activity,
pharmacodynamics, and immunologic correlates. J Clin Oncol.
28:3167–3175. 2010. View Article : Google Scholar
|