Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
burgeoning health problem that affects 1/3 of the adult population
and an increasing number of children in developed countries
(1,2). NAFLD includes a wide spectrum of
histologic abnormalities ranging from hepatic steatosis (3) to non-alcoholic steatohepatitis (NASH)
that may progress to cirrhosis, and subsequent end-stage liver
disease and hepatocellular carcinoma (4,5). NAFLD
is characterized by fat accumulation in the liver in patients with
no statistically significant alcohol consumption, and is
particularly associated with metabolic syndrome comprising
hypertension, insulin resistance (IR), obesity and dyslipidaemia
(6). NAFLD has been shown to be
strongly and independently associated with an increased risk of
type 2 diabetes and cardiovascular disease (7,8).
The pathogenesis of NAFLD is complex and not fully
elucidated. The classical supporting theory is the ‘two-hit’
hypothesis (9,10), whereby IR leads to hepatic steatosis
(first hit), while the steatosis subsequently sensitizes the liver
to a variety of metabolic injuries (second hit) leading to
necroinflammation and fibrosis. Since IR is crucial in the
pathogenesis of NAFLD, insulin-sensitizing drugs, such as metformin
may offer a therapeutic benefit. Metformin probably interrupts
mitochondrial oxidative processes, resulting in a reduced ATP/AMP
ratio and subsequently in the activation of AMP-activated protein
kinase, a major cell regulator of lipid and glucose metabolism
(11,12). The activation of AMP-activated
protein kinase in the liver stimulates β-oxidation of fatty acids
and inhibits de novo synthesis, thus potentially leading to
reduced liver steatosis (13).
At present, the ideal treatment for NAFLD has yet to
be identified. Current treatments, such as lifestyle modification
and weight loss, are often difficult to achieve and hard to
maintain (14). Therefore, new
therapeutic approaches for the management of NAFLD are required.
Metformin is non-hepatotoxic and cost-effective, therefore, it is
more suitable for long-term treatment (15). Thus, a number of clinical trials
have evaluated the use of metformin in the treatment of NAFLD
(16,17). However, the mixed results,
heterogeneous therapeutic approaches and small number of patients
have limited its widespread use in clinical practice. A recent
meta-analysis of randomized control trials (RCT) including
sub-analysis of the efficacy of metformin on histological and
biochemical outcomes in biopsy-proven NASH, suggested that
metformin is not an effective treatment for NASH (17). The authors explained the strict
inclusion criteria with the histological definition of NAFLD or
NASH, while only three RCTs were included as barriers to the
meta-analysis with regards to the effect of metformin on NAFLD.
However, several studies, including more recent RCT data, and an
additional review have shown that metformin may improve metabolic
variables in NAFLD patients, especially in patients meeting the
diagnostic criteria of metabolic syndrome (18). Therefore, the aim of this systematic
review and meta-analysis was to further assess the beneficial and
harmful effects of metformin on NAFLD.
Materials and methods
Search strategy
A systematic computer-assisted search was conducted
by two independent investigators (Y.L. and L.L.) with disagreements
resolved by mutual discussion. Databases searched during April 2012
included Medline, Cochrane the Central Register of Controlled
Trials, the Cochrane Database of Systematic Reviews, Embase,
Science Citation Index Expanded and The Chinese Biomedical
Database. The meeting proceedings (American Gastroenterological
Association/American Association for the Study of Liver
Diseases/Digestive Disease Week meeting abstracts/European
Association for the Study of the Liver) and reference lists of
reviews were searched manually for additional relevant studies. The
authors of locally published and unpublished studies were contacted
for thoroughness. Search terms included ‘Metformin’, ‘Biguanides’,
‘Glucophag’, ‘NAFLD’, ‘NASH’, ‘nonalcoholic fatty liver disease’,
‘nonalcoholic steatohepatitis’, ‘liver fat’, ‘fatty liver’,
‘steatosis’, ‘AST’, ‘ALT’, ‘aminotransferase’, ‘liver enzymes’ and
‘trial’.
Inclusion and exclusion criteria
Two investigators (Y.L. and D.C.) determined the
inclusion and exclusion criteria, and reviewed the titles and
abstracts of the studies identified. Inclusion criteria were: i)
RCTs using metformin in patients with NAFLD or NASH; ii) NAFLD or
NASH diagnosed by histology or suggestive imaging findings
(ultrasound, computed tomography, magnetic resonance imaging) with
abnormal aminotransferase; iii) comparators could be placebo, no
intervention or other intervention and iv) adult patients of any
gender or ethnic origin (age, ≥18 years). Exclusion criteria were:
i) non-human studies or non-randomized trials; ii) participants
with addressed alcoholic, drug-induced, total parenteral
nutrition-induced, viral or genetic causes of liver injury; iii)
combination of metformin and other therapeutic approaches (e.g.,
thiazolidinediones and antioxidants) and iv) letters/case reports
or studies enrolling <10 subjects, or manuscripts without
adequate data or reviews. There was no restriction on
languages.
Outcome measures
The primary outcome was a change in histological
response quantified by needle biopsy and histological assessment,
proton magnetic resonance spectroscopy (1H MRS), or inferred by
ultrasonography (US). The secondary outcomes included alanine
aminotransferase (ALT), aspartate aminotransferase (AST), insulin
sensitivity (measured by homeostasis model assessment of IR
‘HOMA-IR’) and body mass index (BMI). Incident adverse events were
also evaluated.
Data extraction
Two investigators (Y.L. and L.L.) independently
assessed the selected studies. Y.L. abstracted data and then L.L.
checked the data extraction. The agreement in data extraction
measured by a κ statistic and discrepancies were resolved by
consensus. The data extracted included: i) study: date, location
and funding of the trial, length of follow-up, use of
intention-to-treat analyses and the publication status; ii)
patients: number, inclusion and exclusion criteria, mean (or
median) age and gender ratio; iii) treatment: dose, duration and
mode of administration of various metformin and/or of additional
comparators and v) outcome: histological response, ALT, AST, BMI,
HOMA-IR and adverse effects.
Missing data
In the cases where the information of interest was
not presented in the published studies, the investigators contacted
the corresponding authors to obtain additional information. For
incomplete or missing dichotomous data, sensitivity analysis was
performed by excluding the studies that appeared as outliers in the
forest plots. For missing standard deviations (SD) of the mean
change in continuous data, and where the P-value was provided for
the two groups, SD was calculated by converting the P-value into a
t-value with appropriate degrees of freedom (19). Studies in which participants dropped
out, thereby presenting these percentages, were not excluded.
Methodological quality assessment
Methodological quality was assessed by two
investigators (Y.L. and J.W.) using the Cochrane Risk of Bias Tool
with a potential risk of bias of high, low or unclear (19). Quality assessment was based on the
following domains: i) sequence generation; ii) allocation
concealment; iii) blinding of participants, personnel and outcome
assessors; iv) completeness of outcome data; v) unbiased outcome
reporting and vi) source of funding. If all six domains were
well-described, the studies were categorized as high quality,
otherwise as low quality.
Data analysis
The analyses were conducted using the Review Manager
(RevMan Version 5.1.6 Copenhagen: The Cochrane Collaboration 2012).
The primary outcome (histological response) was assessed as a
dichotomous variable (presented as OR with 95% CI). The secondary
outcomes (ALT, AST, HOMA-IR and BMI) were presented as continuous
variables (presented as MD and 95% CI). Five analyses were carried
out to compare the effect of i) metformin vs. control on liver
histological response, ii) metformin vs. control on ALT change and
iii) metformin vs. control on AST change, iv) metformin vs. control
on BMI change and v) metformin vs. control on HOMA-IR change.
Random effect models were used in most meta-analyses, while
statistical heterogeneity was evaluated using Cochran’s Q statistic
(P<0.10) and the I2 statistic. Where Cochran’s Q
statistic (P>0.10) or an I2 statistic was >50%,
the summary meta-analysis was abandoned and potential sources were
examined using stratified analyses. Subgroup analysis was carried
out for two groups: i) diabetics and non-diabetics; ii) NAFLD and
NASH population. Sensitivity analysis was performed by excluding
lower quality studies from the meta-analysis and assessing the
effect on the summary estimate.
Results
Literature search
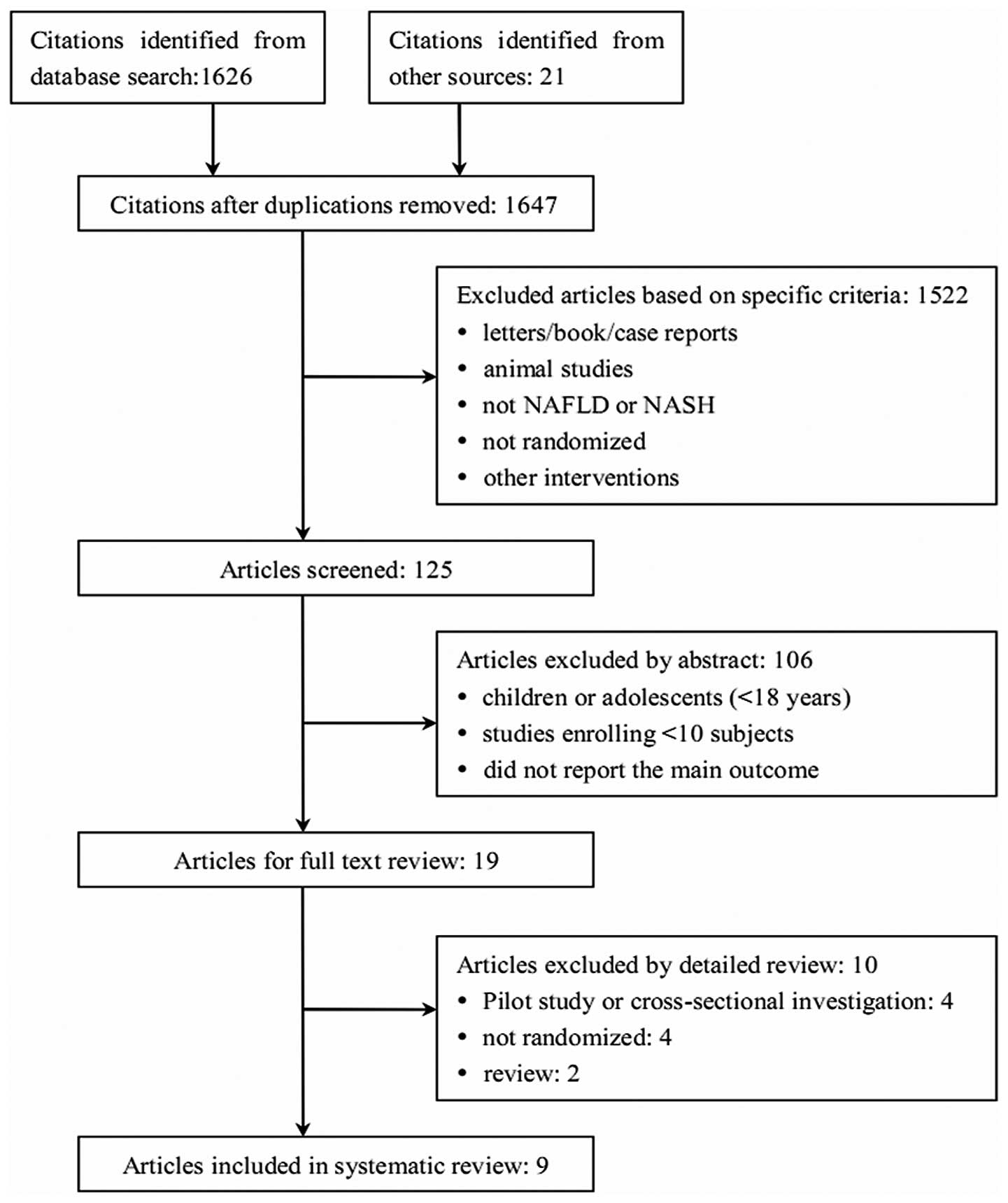
A total of 1,647 non-duplicated studies were
retrieved from the broad search terms used. After review of the
titles and abstracts of these studies, 1,628 were excluded.
Nineteen studies underwent full text review and 10 were
subsequently excluded for the following reasons: 4 were pilot
studies or cross-sectional investigations, 4 were not randomized
and 2 were reviews. Therefore, 9 studies were included in this
systematic review and meta-analysis (Fig. 1).
Trial characteristics
The trials were published in English between 2004
and 2011 and reported similar inclusion and exclusion criteria
(Table I). The nine trials included
comprised a total study population of 417 individuals. The dose of
metformin ranged from 0.5 to 3 g/day, while the duration of
treatment was 4 months in a sole study, 6 months in four studies
and 12 months in the remaining four studies. Three trials were
placebo-controlled (n=130) (20–22),
three used diet as the control (n=168) (23–25),
while the remaining three trials used diet and exercise as the
control (n=119) (26–28). The mean age of the participants
ranged from 41 to 54 years.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| Study author | Year of
publication | N | Dropout, n (%) | Mean age
(years) | Country | Intervention (dose)
(g/day) | Comparators (dose)
(kcal/d) | Duration
(months) | Diabetes or IGT
(%) | Refs. |
|---|
| Bugianesi et
al | 2005 | 82a | 0 | 43 | Italy | Metformin (2) | Diet | 12 | 7 | 23 |
| Akyüz et
al | 2007 | 36a | 0 | 45 | Turkey | Metformin
(0.85) | Diet +
exercise | 12 | 19 | 26 |
| Garinis et
al | 2010 | 50 | 5 (10) | 44 | Italy | Metformin (1) | Diet (1300) | 6 | 0 | 24 |
| Haukeland et
al | 2009 | 48 | 4 (8.3) | 47 | Norway | Metformin
(2.5–3) | Placebo | 6 | 27 | 20 |
| Idilman et
al | 2008 | 49a | 0 | 47 | Turkey | Metformin
(1.7) | Diet +
exercise | 12 | 0 | 27 |
| Nar and Gedik | 2009 | 34 | 0 | 47 | Turkey | Metformin
(1.7) | Diet +
exercise | 6 | 100 | 28 |
| Shields et
al | 2009 | 19 | 3 (15.8) | 47 | USA | Metformin
(0.5–1) | Placebo + diet +
exercise | 12 | 0 | 21 |
| Sofer et
al | 2011 | 63 | 11 (17.5) | 54 | Israel | Metformin
(0.85–1.7) | Placebo | 4 | 13 | 22 |
| Uygun et
al | 2004 | 36 | 2 (5.9) | 41 | Turkey | Metformin
(1.7) | Diet
(1600–1800) | 6 | 0 | 25 |
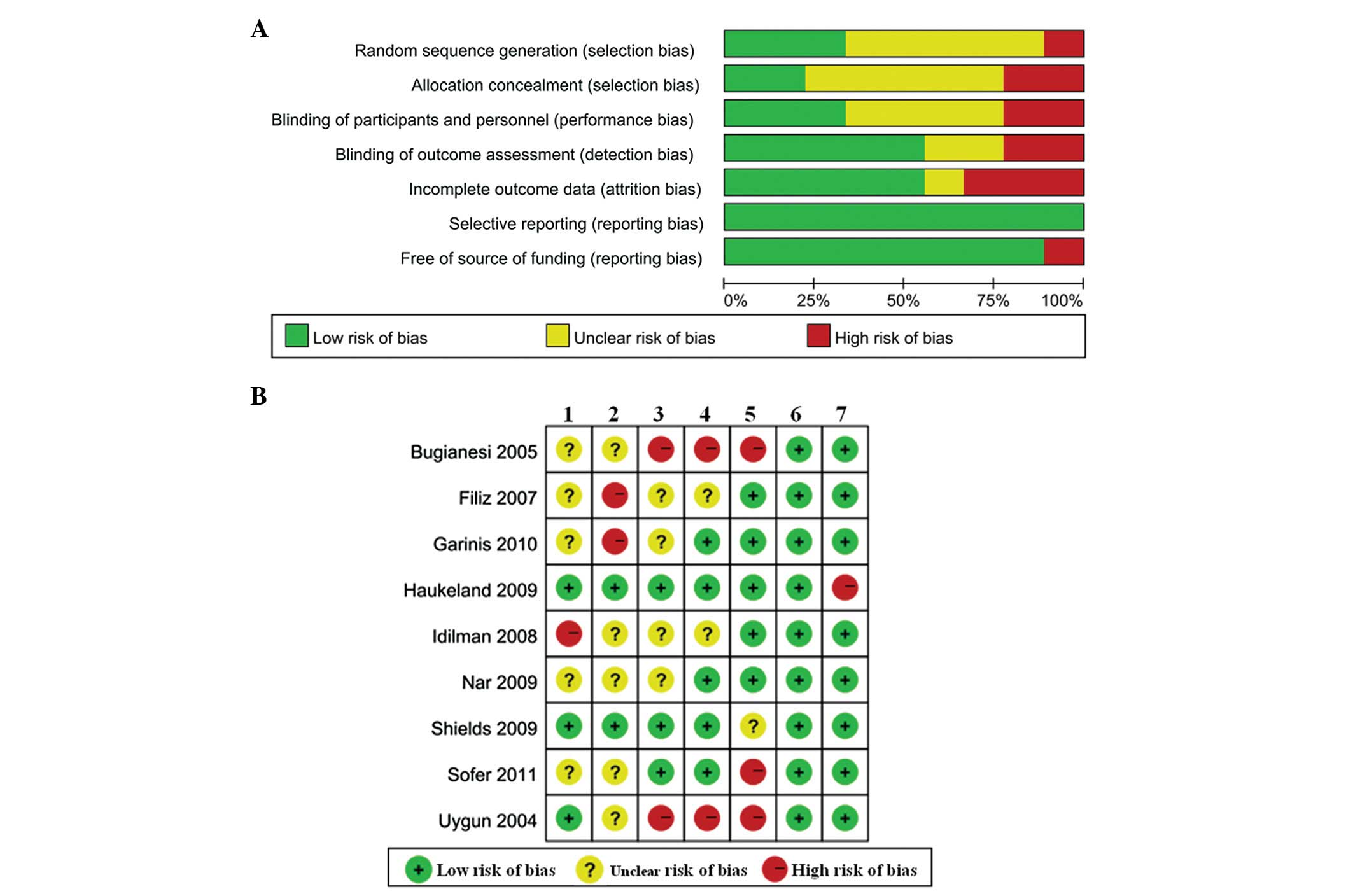
Methodological quality and risk of
bias
The consensus between two investigators regarding
the study selection and the quality assessment of trials was 0.81
and 0.92, respectively. The methodological quality and risk of bias
of the nine included studies are shown in Fig. 2. Overall, three studies (20,21,25)
had a low risk of bias in random sequence generation and two
studies (20,21) had a low risk of bias in allocation
concealment. Blinding of outcome assessment was performed in five
studies (20–22,24,28)
and only one study was funded by a Health Authority (20).
Outcomes reporting
The primary outcome was reported separately
(Table II). Two studies (24,28)
provided the data of the liver fatty changes diagnosed by US. Four
studies (20,21,25,27)
reported the number of patients that improved following diagnosis
by liver biopsy. The secondary outcomes were integrated, especially
with respect to ALT and AST. Partial data of BMI and HOMA-IR were
obtained from the figures provided, only a small proportion of data
was not reported.
 | Table II.Primary and secondary outcomes of the
included studies. |
Table II.
Primary and secondary outcomes of the
included studies.
| Study author | Year of
publication | Primary outcome
| Secondary outcomes
|
|---|
| Population
(diagnosis) | Histology | ALT | AST | BMI | HOMA-IR | Refs. |
|---|
| Bugianesi et
al | 2005 | NAFLD (biopsy) | Incomplete
dataa | Fig. 4 | NR | Yes | Fig. 4 | 23 |
| Akyüz et
al | 2007 | NAFLD (US and
biopsy) | Incomplete
dataa | Yes | Yes | Yes | Yes | 26 |
| Garinis et
al | 2010 | NAFLD (US) | Fatty changes
datab | Yes | Yes | Yes | Yes | 24 |
| Haukeland et
al | 2009 | NAFLD (biopsy) | Number
improved | Fig. 4 | Fig. 4 | Yes | Yes | 20 |
| Idilman et
al | 2008 | NASH (biopsy) | Number
improved | Yes | Yes | Yes | Yes | 27 |
| Nar and Gedik | 2009 | NAFLD (US) | Fatty changes
datab | Yes | Yes | Yes | Yes | 28 |
| Shields et
al | 2009 | NASH (biopsy) | Number
improved | Fig. 4 | Fig. 4 | Fig. 4 | Fig. 4 | 21 |
| Sofer et
al | 2011 | NAFLD (US) | NR | Yes | Yes | NR | Yes | 22 |
| Uygun et
al | 2004 | NASH (US and
biopsy) | Number
improved | Yes | Yes | Yes | NR | 25 |
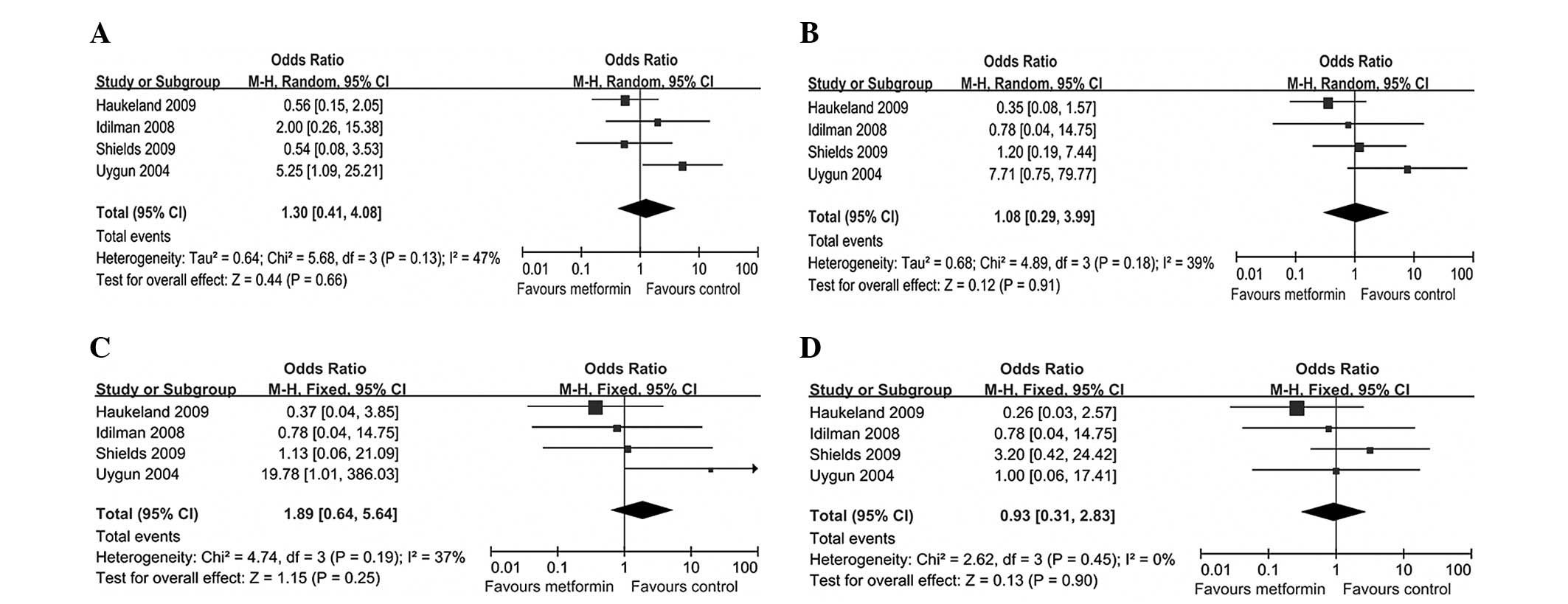
Primary outcome
Histological response
Histological response was assessed only in four
studies (20,21,25,27),
evaluating the scores of steatosis, inflammation, hepatocellular
ballooning and fibrosis prior and subsequent to treatment. Since
these scores were not continuous variables, we calculated the
number of patients with improvement in each histological variable.
Participants treated with metformin showed no improvement in
histological variables compared to those treated with placebo or
diet and exercise. OR was 1.30 (95% CI, 0.41–4.08;
I2=47%; P=0.66) for steatosis, 1.08 (95% CI, 0.29–3.99;
I2=39%; P=0.91) for inflammation, 1.89 (95% CI,
0.64–5.64; I2=37%; P=0.25) for hepatocellular
ballooning, and 0.93 (95% CI, 0.31–2.83; I2= 0%; P=
0.90) for fibrosis (Fig. 3). When
liver fat [diagnosed by US (24,28)]
data alone were analyzed, metformin did not affect any liver fat
(OR=0.93; 95% CI, 0.32–2.74; I2=0%; P=0.89). No
improvement was observed in the histological variables in the
subgroup analyses of diabetic or non-diabetic patients.
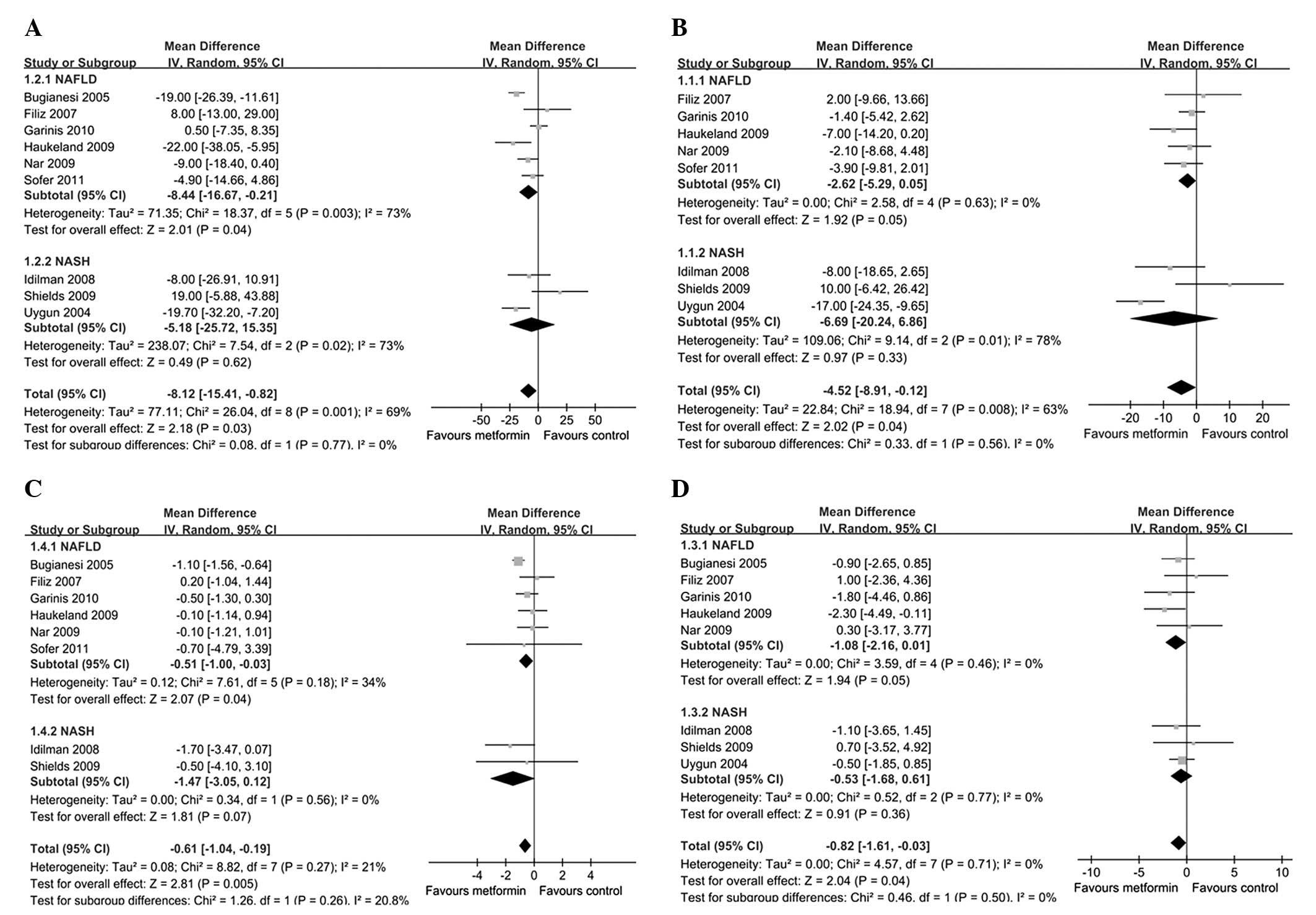
Secondary outcomes
ALT
ALT activity was evaluated in the included studies.
A statistically significant reduction was observed in ALT in
metformin-treated patients (MD, −8.12 U/l; 95% CI, −15.41 to −0.82
U/l; P=0.03) (Fig. 4A).
Heterogeneity was high (I2=69%). Subgroup analysis
showed improvement in ALT in NAFLD (MD, −8.44 U/l;
I2=73%; P=0.04), but not in NASH patients (MD, −5.18
U/l; P=0.62), although the analysis of the two subgroups
demonstrated statistically significant heterogeneity
(I2=73%).
AST
AST was reported in 8/9 studies. Overall,
metformin-treated patients showed only a modest, but statistically
significant (P=0.04) decrease in AST (MD, −4.52 U/l; 95% CI, −8.91
to −0.12 U/l; I2=63%) compared to placebo or diet and
exercise (Fig. 4B). In the subgroup
analysis, significant differences were observed in the metformin
and control groups in NAFLD (MD, −2.62 U/l; I2=0%;
P=0.05), but not in NASH patients (MD, −6.69 U/l; P=0.33), although
analysis of the latter subgroup showed a marked heterogeneity
(I2=78%).
HOMA-IR
HOMA-IR activity was reported in eight studies,
showing a modest reduction in metformin-treated patients (MD,
−0.61; 95% CI, −1.04 to −0.19; I2=21%; P=0.005)
(Fig. 4C). However, in the subgroup
analysis, statistically significant differences were only found in
NAFLD (MD, −0.51; I2=34%; P=0.04) but not in NASH
patients (MD, −1.47; I2=0%; P=0.07).
BMI
A noteworthy benefit of metformin therapy vs. the
control was observed on BMI (MD, −0.82 kg/m2; 95% CI,
−1.61 to −0.03 kg/m2; I2=0%; P=0.04)
(Fig. 4D). In the subgroup analysis
of NASH, the mean reduction in BMI was modest and statistically not
significant (MD, −0.53 kg/m2, I2=0%;
P=0.36).
Adverse events
Of the nine studies, only four provided detailed
information on gastrointestinal side-effects, such as gas, bloating
and mild abdominal pain (n=17, 8.5%) in the metformin group
(20,22,25,28).
In their study, Haukeland et al(20) reported that only one patient had
developed exanthema. However, none of the patients discontinued
metformin due to intolerance during treatment.
Discussion
In this systematic review, nine randomized clinical
trials were included, six of which were on NAFLD and three on NASH.
The nine trials had a small sample size and a high variability of
group size (ranging from 19 to 82 participants). A total of 417
participants were enrolled. A number of patients were overweight or
had diabetes. Two trials were considered to be of high
methodological quality (20,21),
in terms of allocation sequence generation, allocation concealment,
blinding and outcome data. The remaining seven trials (22–28)
were inadequate or unclear in at least one of the five components
used to assess methodological quality. Considerable heterogeneity,
such as inclusion criteria, sample sizes, duration of treatment and
methods of outcome assessment were present in these trials. Thus,
the adequacy of this evidence should be considered carefully.
The effect of metformin on liver histology remains
unclear. Our findings demonstrate that metformin does not improve
the condition of NAFLD or NASH patients with the histological
spectrum of steatosis, inflammation, hepatocellular ballooning and
fibrosis, consistent with the findings of previously published
systematic studies (17,29,30).
The current data should be interpreted objectively and
dialectically. First, although the nine studies assessed the effect
of metformin on histological response, five studies could not be
included in the histological response analyses, due to the fact
that three of them provided insufficient data and two employed US
as the means of diagnosis. Furthermore, the histopathologic grading
system was different in the four included studies, inevitably
resulting in heterogeneity. The two studies assessing hepatic
steatosis by US also suggested that metformin had no significant
effect on hepatic steatosis. Although perfect evidence is absent,
the studies included in this review demonstrate that metformin has
no effect on histological response in NAFLD.
Regarding the liver enzymes, there were eight and
nine studies assessing the effect of metformin on ALT and AST,
respectively. Overall, they showed a statistically significant
reduction in ALT and AST levels in the metformin group. Certain
reviews (31,32) have shown improvements in liver
enzymes only in the single-arm trails. However, this has not yet
been confirmed by data obtained from RCTs. Therefore, our findings
may be more objective and reliable. In the NASH subgroup analysis,
the metformin group had a tendency to exhibit lower ALT and AST
levels compared to the control group, although this tendency was
not statistically significant (P=0.62 and 0.33). A number of the
included studies reported ALT to be a marker for hepatic
inflammation. We found an average ALT reduction of 8.12 U/l.
However, ALT should not be considered to be a marker for liver
improvement, since improvement in liver tests is not correlated
with histological findings (33,34).
IR was assessed using HOMA-IR (20–24,26–28)
and serum insulin levels, in eight and one study, respectively
(25). As expected, insulin
sensitivity markedly improved in the metformin, compared to the
control group. In their study, Rakoski et al(17) reported that non-diabetic patients
may be particularly susceptible to the insulin-sensitizing
properties of glitazone and that early intervention may prevent
worsening of IR (35). However, our
subgroup analysis detected no statistically significant differences
between diabetic and non-diabetic patients, suggesting only that
the improving trend in HOMA-IR was more obvious in NAFLD compared
to NASH patients.
Due to the incomplete data on body weight, we
calculated BMI instead and found a favourable response to metformin
treatment in patients with NAFLD. This improvement in BMI was
associated with an improvement in IR, as HOMA-IR scores were
markedly lower at the end of the included studies in the two
groups. However, three included studies showed no improvement in
BMI (21,26,28).
The reason for this result is unclear, but may be associated with
the relatively low dose in the three studies (mean dose of 0.76
g/day). Therefore, the dose or the duration of therapy should be
more carefully determined in metformin treatment.
Our study had several limitations. First, the
methodological limitations: the small number of patients, the lack
of randomization and blinded measures and the incomplete
histological outcomes. Second, although publication bias is a
significant factor (assessed using funnel plots), it is
uninformative due to the small number of studies available for our
analysis. However, the included studies yielded negative results
for an improvement in histological response. Third, meta-analysis
of weighting studies draws conclusions resulting in the largest
variance for the pooled effect size. Thus, 95% CIs for the OR and
MD are likely to be wider and more conservative than expected. We
performed sensitivity analysis after excluding the studies that
appeared as outliers in the forest plots, which did not have a
significant impact on the results. Finally, the histopathologic
grading system was inconsistent in the included studies, likely to
have affected the accuracy of the data.
In the included studies, the duration of treatment
ranged from 4 to 12 months (median duration, 8.5 months), and the
dose of metformin ranged from 0.85 to 3 g/day (median dose, 1.5
g/day). Due to the limited number of the included studies, the
optimal dose and the duration of therapy were difficult to
determine. In addition, IR is widely considered to be a pivotal
feature of NAFLD, which is strongly and independently associated
with the increased risk of type 2 diabetes (36,37).
Although metformin has been used for the treatment of diabetes for
>50 years, it has currently become one of the first-line
treatment options in the management of diabetes, and is recommended
by a number of international guidelines and consensus (38). Metformin may therefore be used to
treat NAFLD and diabetes simultaneously. Therefore, application of
the optimal dose of metformin in order to decrease NAFLD-associated
risk of diabetes is a promising treatment approach. Further
randomized clinical trails are required to confirm his hypothesis.
This review shows that metformin has mild side-effects, thus can be
used safely for long periods of time, and that no patient
discontinued metformin due to intolerance or side-effects. However,
we should be cautious about this conclusion, since evidence
pointing towards important renal toxicity was reported in previous
studies (39). The unexpected
adverse events should be monitored carefully in future studies.
Although we cannot determine the optimal dose and duration of
therapy, the studies included in this review provide current
available evidence on the beneficial as well as harmful effects of
metformin in NAFLD.
Due to the heterogeneity of the inclusion criteria
for patient enrollment, patients aged <18 years of age were
excluded. However, several studies have evaluated the potential
involvement of metformin in the therapy of pediatric patients with
NAFLD (40–42). Findings regarding pediatric patient
population were similar to those regarding adults, supporting the
beneficial effects of metformin on biochemical and metabolic, but
not on histological response. Despite the fact that this systematic
review shows no histological benefit and a modest biochemical and
metabolic benefit of metformin, whether or not metformin should be
widely used in clinical trials remains to be determined.
Investigators and clinicians should be aware of this issue, since
the changes in histology in NAFLD have been observed in larger
studies with long follow-up periods. In this systematic review, the
follow-up period of the included trials on average was 8.5 months.
Since the sample size was small, RCTs with large sample sizes and a
long follow-up need to be conducted. The use of metformin might be
limited to NAFLD to improve biochemical and metabolic features and
might not be used for NASH. Furthermore, as metformin is less
expensive compared to most other treatment modalities for NAFLD, it
is considered affordable for the affected population.
In summary, this systematic review has shown that
metformin may not improve histological response, but it can improve
biochemical and metabolic features in NAFLD. Therefore, metformin
remains a promising drug for the treatment of NAFLD due to its
metabolic effects and safety profile. However, in the treatment of
NASH, our subgroup analyses do not support this finding. RCTs with
a low risk of bias need to be conducted in order to assess the
beneficial or harmful effects of metformin on NAFLD or NASH.
Moreover, RCTs with a large sample size and long follow-up period
should be designed. Furthermore, future studies should develop a
uniform assessment method of liver biopsy and investigate the
optimal dose and duration of metformin therapy to achieve the
maximal sustainable effect.
Abbreviations:
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
BMI
|
body mass index
|
|
CI
|
confidence intervals
|
|
HOMA-IR
|
homeostasis model assessment of
insulin resistance
|
|
IR
|
insulin resistance
|
|
MD
|
mean difference
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
RCT
|
randomized clinical trial
|
|
SD
|
standard deviation
|
|
OR
|
odds ratio
|
|
US
|
ultrasonography
|
Acknowledgements
The authors would like to thank Ms.
Xie-Wan Chen and Ms. Xiao-Qing Zhan (Medical English Department,
Third Military Medical University, Chongqing, China) for the
critical reading of the manuscript and their valuable advice. This
study was supported by the Natural Science Foundation of China
(grant no. 81072362 and 81200297).
References
|
1.
|
Cohen JC, Horton JD and Hobbs HH: Human
fatty liver disease: old questions and new insights. Science.
332:1519–1523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Chalasani N, Younossi Z, Lavine JE, et al:
The diagnosis and management of non-alcoholic fatty liver disease:
practice guideline by the American Gastroenterological Association,
American Association for the Study of Liver Diseases, and American
College of Gastroenterology. Gastroenterology. 142:1592–1609. 2012.
View Article : Google Scholar
|
|
3.
|
Wieckowska A, McCullough AJ and Feldstein
AE: Noninvasive diagnosis and monitoring of nonalcoholic
steatohepatitis: present and future. Hepatology. 46:582–589. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Liou I and Kowdley KV: Natural history of
nonalcoholic steatohepatitis. J Clin Gastroenterol. 40:S11–S16.
2006.
|
|
5.
|
Farrell GC and Larter CZ: Nonalcoholic
fatty liver disease: from steatosis to cirrhosis. Hepatology.
43:S99–S112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Alberti KG, Zimmet P and Shaw J; IDF
Epidemiology Task Force Consensus Group: The metabolic syndrome - a
new worldwide definition. Lancet. 366:1059–1062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Targher G, Day CP and Bonora E: Risk of
cardiovascular disease in patients with nonalcoholic fatty liver
disease. N Engl J Med. 363:1341–1350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dunn W, Xu R, Wingard DL, et al: Suspected
nonalcoholic fatty liver disease and mortality risk in a
population-based cohort study. Am J Gastroenterol. 103:2263–2271.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Angulo P: Nonalcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
McCullough AJ: Update on nonalcoholic
fatty liver disease. J Clin Gastroenterol. 34:255–262. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Owen MR, Doran E and Halestrap AP:
Evidence that metformin exerts its anti-diabetic effects through
inhibition of complex 1 of the mitochondrial respiratory chain.
Biochem J. 348:607–614. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhou G, Myers R, Li Y, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Browning JD and Horton JD: Molecular
mediators of hepatic steatosis and liver injury. J Clin Invest.
114:147–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Moseley RH: Therapy for nonalcoholic fatty
liver disease. J Clin Gastroenterol. 42:332–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Loomba R, Lutchman G, Kleiner DE, et al:
Clinical trial: pilot study of metformin for the treatment of
non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 29:172–182.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lam B and Younossi ZM: Treatment options
for nonalcoholic fatty liver disease. Therap Adv Gastroenterol.
3:121–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Rakoski MO, Singal AG, Rogers MA and
Conjeevaram H: Meta-analysis: insulin sensitizers for the treatment
of non-alcoholic steatohepatitis. Aliment Pharmacol Ther.
32:1211–1221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mazza A, Fruci B, Garinis GA, et al: The
role of metformin in the management of NAFLD. Exp Diabetes Res.
2012:7164042012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Higgins PTJ and Green S: Cochrane Handbook
for Systematic Reviews of Interventions. Version 5.1.6. The
Cochrane Collaboration, 2011 (Available at: https://www.cochrane-handbook.org).
Accessed April 2, 2012.
|
|
20.
|
Haukeland JW, Konopski Z, Eggesbo HB, et
al: Metformin in patients with non-alcoholic fatty liver disease: a
randomized, controlled trial. Scand J Gastroenterol. 44:853–860.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Shields WW, Thompson KE, Grice GA, et al:
The effect of metformin and standard therapy versus standard
therapy alone in nondiabetic patients with insulin resistance and
nonalcoholic steatohepatitis (NASH): a pilot trial. Therap Adv
Gastroenterol. 2:157–163. 2009. View Article : Google Scholar
|
|
22.
|
Sofer E, Boaz M, Matas Z, et al: Treatment
with insulin sensitizer metformin improves arterial properties,
metabolic parameters, and liver function in patients with
nonalcoholic fatty liver disease: a randomized, placebo-controlled
trial. Metabolism. 60:1278–1284. 2011. View Article : Google Scholar
|
|
23.
|
Bugianesi E, Gentilcore E, Manini R, et
al: A randomized controlled trial of metformin versus vitamin E or
prescriptive diet in nonalcoholic fatty liver disease. Am J
Gastroenterol. 100:1082–1090. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Garinis GA, Fruci B, Mazza A, et al:
Metformin versus dietary treatment in nonalcoholic hepatic
steatosis: a randomized study. Int J Obes (Lond). 34:1255–1264.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Uygun A, Kadayifci A, Isik AT, et al:
Metformin in the treatment of patients with non-alcoholic
steatohepatitis. Aliment Pharmacol Ther. 19:537–544. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Akyüz F, Demir K, Ozdil S, et al: The
effects of rosiglitazone, metformin, and diet with exercise in
nonalcoholic fatty liver disease. Dig Dis Sci. 52:2359–2367.
2007.PubMed/NCBI
|
|
27.
|
Idilman R, Mizrak D, Corapcioglu D, et al:
Clinical trial: insulin-sensitizing agents may reduce consequences
of insulin resistance in individuals with non-alcoholic
steatohepatitis. Aliment Pharmacol Ther. 28:200–208. 2008.
View Article : Google Scholar
|
|
28.
|
Nar A and Gedik O: The effect of metformin
on leptin in obese patients with type 2 diabetes mellitus and
nonalcoholic fatty liver disease. Acta Diabetol. 46:113–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Musso G, Gambino R, Cassader M, et al: A
meta-analysis of randomized trials for the treatment of
nonalcoholic fatty liver disease. Hepatology. 52:79–104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Musso G, Cassader M, Rosina F and Gambino
R: Impact of current treatments on liver disease, glucose
metabolism and cardiovascular risk in non-alcoholic fatty liver
disease (NAFLD): a systematic review and meta-analysis of
randomised trials. Diabetologia. 55:885–904. 2012. View Article : Google Scholar
|
|
31.
|
Chavez-Tapia NC, Barrientos-Gutierrez T,
Tellez-Avila FI, et al: Insulin sensitizers in treatment of
nonalcoholic fatty liver disease: Systematic review. World J
Gastroenterol. 12:7826–7831. 2006.PubMed/NCBI
|
|
32.
|
Adams LA and Angulo P: Treatment of
non-alcoholic fatty liver disease. Postgrad Med J. 82:315–322.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Mofrad P, Contos MJ, Haque M, et al:
Clinical and histologic spectrum of nonalcoholic fatty liver
disease associated with normal ALT values. Hepatology.
37:1286–1292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Adams LA, Sanderson S, Lindor KD, et al:
The histological course of nonalcoholic fatty liver disease: a
longitudinal study of 103 patients with sequential liver biopsies.
J Hepatol. 42:132–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Orlando R, Azzalini L, Orando S, et al:
Bile acids for non-alcoholic fatty liver disease and/or
steatohepatitis. Cochrane Database Syst Rev. 1:CD0051602007.
|
|
36.
|
Filik L: Role of type 2 diabetes mellitus
in nonalcoholic fatty liver disease. Eur J Clin Invest.
41:1367–1368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Bonapace S, Perseghin G, Molon G, et al:
Nonalcoholic fatty liver disease is associated with left
ventricular diastolic dysfunction in patients with type 2 diabetes.
Diabetes Care. 35:389–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Stumvoll M, Nurjhan N, Perriello G, et al:
Metabolic effects of metformin in non-insulin-dependent diabetes
mellitus. N Engl J Med. 333:550–554. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Hemmingsen B, Christensen LL, Wetterslev
J, et al: Comparison of metformin and insulin versus insulin alone
for type 2 diabetes: systematic review of randomised clinical
trials with meta-analyses and trial sequential analyses. BMJ.
344:1771–1779. 2012. View Article : Google Scholar
|
|
40.
|
Nadeau KJ, Ehlers LB, Zeitler PS, et al:
Treatment of non-alcoholic fatty liver disease with metformin
versus lifestyle intervention in insulin-resistant adolescents.
Pediatr Diabetes. 10:5–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Lavine JE, Schwimmer JB, Van Natta ML, et
al: Effect of vitamin E or metformin for treatment of nonalcoholic
fatty liver disease in children and adolescents: the TONIC
randomized controlled trial. JAMA. 305:1659–1668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Lavine JE, Schwimmer JB, Molleston JP, et
al: Treatment of nonalcoholic fatty liver disease in children:
TONIC trial design. Contemp Clin Trials. 31:62–70. 2010. View Article : Google Scholar : PubMed/NCBI
|