Contents
Introduction
Review of the literature
HNF-1β, an endometriosis-specific transcription
factor
The profiles of HNF-1β target genes
CD44v9 as a potential target gene for HNF-1β
Proposed function of CD44v9
Conclusions
Introduction
Endometriosis is an estrogen-dependent condition
associated with chronic pelvic pain and infertility and increases
susceptibility to the development of ovarian cancer. Accumulating
evidence indicates that selective genetic alterations play a role
in the pathogenesis of endometriosis. Upregulation of transcription
factor hepatocyte nuclear factor (HNF)-1β expression occurs in
endometriosis (1–4). However, to what extent HNF-1β
overexpression is involved in its pathogenesis remains to be
determined. This review summarizes recent advances in
HNF-1β-mediated signaling, its target genes and binding molecules
and provides the potential challenges to attempts to identify the
molecular basis of this gene.
Review of the literature
A comprehensive review of the literature was
conducted to investigate the molecular basis of HNF-1β. A Medline
search of the literature was performed using the key words
endometriosis, cancer, target, binding, iron, oxidative stress,
cystine transporter subunit (xCT), osteopontin (OPN), epidermal
growth factor receptor (EGFR), CD44v9 and hyaluronic acid (HA).
English-language publications in PubMed and references from
relevant articles published between 1997 and 2012 were analyzed.
References in the studies identified were also searched and certain
unpublished data were obtained.
HNF-1β, an endometriosis-specific
transcription factor
Several studies demonstrated overexpression of the
HNF-1β gene and protein in endometriosis. HNF-1β is a Pit-1 (POU
class 1 homeobox 1)/Oct-1 (solute carrier family 22)/Unc-86
(POU)/homeodomain-containing transcription factor that regulates
tissue-specific gene expression in the kidney, liver, pancreas and
other epithelial organs. Mutations in this gene produce diabetes
syndrome [maturity-onset diabetes of the young type 5 (MODY5)], as
well as being associated with congenital renal cysts. Expression of
this gene was altered in certain types of cancer. HNF-1β
overexpression increased genomic instability (5). Aberrant expression of HNF-1β in
endometriosis led to the production of detoxification proteins
against persistent inflammation and oxidative stress. This review
enhances our ability to understand the suggested function of this
gene.
The profiles of HNF-1β target genes
Possible genes included in the profiles of HNF-1β
target genes in ovarian cancer are dipeptidyl-peptidase 4 (DPP4,
also known as CD26), osteopontin, angiotensin I converting enzyme 2
(ACE2), FXYD domain containing ion transport regulator 2 (FXYD2),
tissue factor pathway inhibitor 2 (TFPI2), nicotinamide
N-methyltransferase (NNMT), lipopolysaccha-ride-induced TNF factor
(LITAF/PIG7), RNA binding protein with multiple splicing (RBPMS),
annexin A4 (ANXA4) and UDP glucuronosyltransferase 1 family and
polypeptide A1 (UGT1A1) (2). These
genes have been hypothe sized as key drivers of the phenotypic
characteristics. Recent reviews have demonstrated the function of
this molecule in the endometriosis and ovarian cancer (2–4).
In addition, HNF-1β regulates the expression of
several genes in the kidney, including encoding polycystic kidney
and hepatic disease-1 (Pkhd1); encoding polycystic kidney disease-2
(Pkd2) and encoding uromodulin (Umod); kinesin family member 12
(Kif12); encoding crumbs homolog-3 (Crb3); transcription factor
AP-2β (Tcfap2β); encoding transmembrane protein-27 (Tmem27);
encoding bicaudal C homolog 1 (Bicc1); suppressor of cytokine
signaling 3 (SOCS-3); ATPase, Na+/K+
transporting, α 1 polypeptide (ATP1A1); intraflagellar transport 88
homolog (IFT88) and CD44v9 (6, unpublished data). Chromatin
immunoprecipitation experiments confirmed that HNF1β binds to a
number of these genes. Potential target genes are involved in cell
polarity, cystogenesis or both.
The Umod gene acts as an inhibitor of calcium
crystallization and defense against urinary tract infections.
Mutations in this gene induce the autosomal dominant renal
disorders medullary cystic kidney disease-2 (MCKD2) and familial
juvenile hyperuricemic nephropathy (FJHN). These diseases are
characterized by juvenile onset of hyperuricemia, gout and chronic
renal failure.
HNF-1β directly regulates the Pkhd1 promoter.
Mutations in this gene are associated with autosomal recessive
polycystic kidney disease (ARPKD). Thus, the mechanism of cyst
formation in MODY5 patients might involve the downregulation of
Pkhd1 gene transcription.
SOCS-3 encodes a member of the STAT (Signal
transducer and activator of transcription)-induced STAT inhibitor
(SSI) family. SSI family members are cytokine-inducible negative
regulators of cytokine signaling. SOCS-3, induced by various
cytokines including IL-6, IL-10 and interferon (IFN)-γ, has been
proven to inhibit JAK-STAT signal transduction for gp130 cytokines
[leukemia inhibitory factor (IL-6, IL-11, LIF), oncostatin M (OSM),
ciliary neurotrophic factor (CNTF), cardiotropin-1 (CT-1),
cardiotropin-like cytokine (CLC)], as well as insulin, IGF-1,
leptin, prolactin and growth hormone (GH). Overexpression of HNF-1β
results in decreased SOCS-3 expression (7).
CD44v9 as a potential target gene for
HNF-1β
Furthermore, findings of a recent genome-wide
expression analysis have showed specific expression of CD44v9,
cyclin D2, spleen tyrosine kinase (Syk), prolactin, v-myb
myeloblastosis viral oncogene homolog (MyB), heparin-binding
EGF-like growth factor (HB-EGF), Eph-receptor B6 and p16 [also
known as cyclin-dependent kinase inhibitor 2A (CDKN2A)] in the
HNF-1β-transfected cancer cells. CD44 participates in a wide
variety of cell functions, including lymphocyte activation,
recirculation and homing, hematopoiesis, cell-cell interactions,
cell adhesion, proliferation, growth, survival, motility,
migration, angiogenesis, differentiation and tumor metastasis. This
glycoprotein is a receptor for hyaluronic acid (HA) and interacts
with other ligands, such as osteopontin, collagens and matrix
metalloproteinases (MMPs). It is one of the reactive oxygen species
(ROS)-sensitive genes and is upregulated upon tissue injury. HA is
able to serve as a novel therapeutic intervention for organ injury
via the CD44 molecules. CD44 is also one of the cell surface
markers associated with cancer stem cells in several types of tumor
(8). The cell markers CD44, CD133
and aldehyde dehydrogenase (ALDH) are used to identify cancer stem
cells. Although CD44 standard isoform (CD44s) is expressed in
almost all the normal cells (hematopoietic cells and normal
epithelial cell subsets) and cancer cells, variant (CD44v) isoforms
are abundant in epithelial-type carcinomas and the cells that most
often undergo malignant transformation. CD44v9 is the most likely
candidate stem cell marker (9). The
upregulation of CD44v9 may correlate with the malignant potential
of patients with endocervical adenocarcinoma, gastric, esophageal,
colon, prostate and ovarian cancers, multiple myeloma and
hematologic malignancies (10–18).
CD44v9 expression was positively correlated with proliferative
activity, glycogen synthase kinase-3β (GSK-3β) activity, epithelial
mesenchymal transition (EMT) changes and inhibition of Fas-mediated
apoptosis (9,19). CD44 downregulated E-cadherin
expression, upregulated MT1-MMP, which resulted in cell invasion
and migration, suggesting that the cells with CD44v9 overexpression
underwent the EMT process. Increased expression of CD44v9 is likely
to correlate with carcinogenesis, hematogenous and lymph node
metastasis and is predictive of the adverse prognosis for various
carcinomas. However, numerous groups have conducted investigations
evaluating the role of CD44v9 with conflicting data and
conclusions. The downregulation of CD44v9 may correlate with the
poor prognosis of patients with squamous cell carcinoma of the
tongue and uterine cervix, as well as soft tissue sarcomas
(20–24). These data allow us to hypothesize
that assembly of the CD44v9 molecule comprising various subsets of
binding proteins has yielded conflicting findings.
CD44 binds and interacts with several proteins in
regulating signal transduction. These molecules include hyaluronan,
EGFR, leukemia-associated Rho-guanine nucleotide exchange factor
(LARG), also known as Rho guanine nucleotide exchange factor (GEF)
12 (ARHGEF12), IQ motif containing GTPase activating protein 1
(IQGAP1), macrophage migration inhibitory factor (MIF), major
histocompatibility complex, class II invariant chain (CD74), xCT,
Fas and extracellular matrix (ECM) proteins (collagen, laminin and
fibronectin) (25–27).
HA mediates the assembly of complex structures
including CD44 and EGFR and induces activation of Rac1 and RhoA
signaling cascades. LARG associates with CD44 and EGFR to promote
several Ras and RhoA pathway effectors and MMP expression, leading
to cell migration, growth, invasion and tumor survival (25). IQGAP1, an essential scaffolding
protein, forms a complex with CD44 and stimulates HA-induced cell
migration and growth (26). MIF
binds to a CD44-CD74 complex and is involved in the regulation of
macrophage function in host defense and the production of a variety
of host immune modulators (27).
CD74 is a chaperone that regulates antigen presentation and T-cell
activation for immune response and associates with class I/II major
histocompatibility complex.
Furthermore, the expression of CD44 variant forms is
regulated by several molecules including OPN and lipogenic enzymes.
OPN, a glycosylated, secreted multifunctional phosphoprotein, is
involved in cell attachment, chemotaxis, immune cell activation,
ECM remodeling, wound healing, inflammation, as well as tumor
progression. OPN increases the cell surface expression of CD44
variant forms. A high OPN expression level is associated with poor
prognosis and metastasis in several cancer patients.
In addition, a recent study established the
association between expression of key enzymes of de novo
lipogenesis and CD44 (28).
Lipogenic enzymes, fatty acid synthase (FASN) and ATP-citrate
lyase, stimulate the expression of CD44 and subsequently enhances
the activation of PI3K/Akt, met proto-oncogene (hepatocyte growth
factor receptor) (MET) and focal adhesion kinase; also known as
protein tyrosine kinase 2 (FAK) (28).
Proposed function of CD44v9
Endometriotic cells may use inflammatory mechanisms
to promote their growth. The proinflammatory cytokine, tumor
necrosis factor (TNF)-α, is crucial to the endometriosis
progression. TNF-α is highly expressed in the endometriosis
microenvironment. Serum TNF-α levels were higher in patients with
endometriosis compared to controls. The TNF-α receptor signals
through the key regulatory transcription factor nuclear factor
(NF)-κB. TNF-α upregulates the expression of CD44s and CD44 variant
forms via apoptosis-related c-Jun N-terminal kinase (JNK) and p38
mitogen-activated protein kinase (MAPK) pathways (29). Iron overload and oxidative stress
also activate NF-κB. Excessive ROS and oxidative stress are
involved in a stress-related cell cycle regulator and stimulate its
downstream targets, JNK and p38 MAPK (8). ROS abundance in the inflamed tissue
further amplifies several inflammatory reactions.
Endometriosis may induce mechanisms for the
protection against ROS-mediated damage. The mechanisms that operate
to protect genomic DNA from the oxidative damage need to be
identified. HA, a major component of ECM, attenuates DNA damage by
neutralizing the Fe2+-mediated oxidative stress
(30) (Fig. 1). HA has physiological functions,
such as lubrication, water homeostasis and macromolecular
filtering, as well as being a well-documented regulator of cell
behaviors. Of note, high molecular weight forms of HA have
anti-inflammatory properties. It has been established that HA has a
protective effect on cellular genome by neutralizing oxidants
(31). One mechanism involves the
ability of HA to chelate or entrap Fe2+, rendering
Fe2+ unavailable for Fenton’s reaction which produces
oxidative species. Thus, HA attenuates the accumulation of the
by-products of oxidative species and the damage that involves the
formation of DNA double-strand breaks. HA also prevents the
activation of ataxia telangiectasia-mutated (ATM) protein kinase
through the phosphorylation on Ser-1981 (ATM-S1981P) and of the
variants of histone H2A, histone H2AX on Ser-139 that reflect DNA
damage (32).
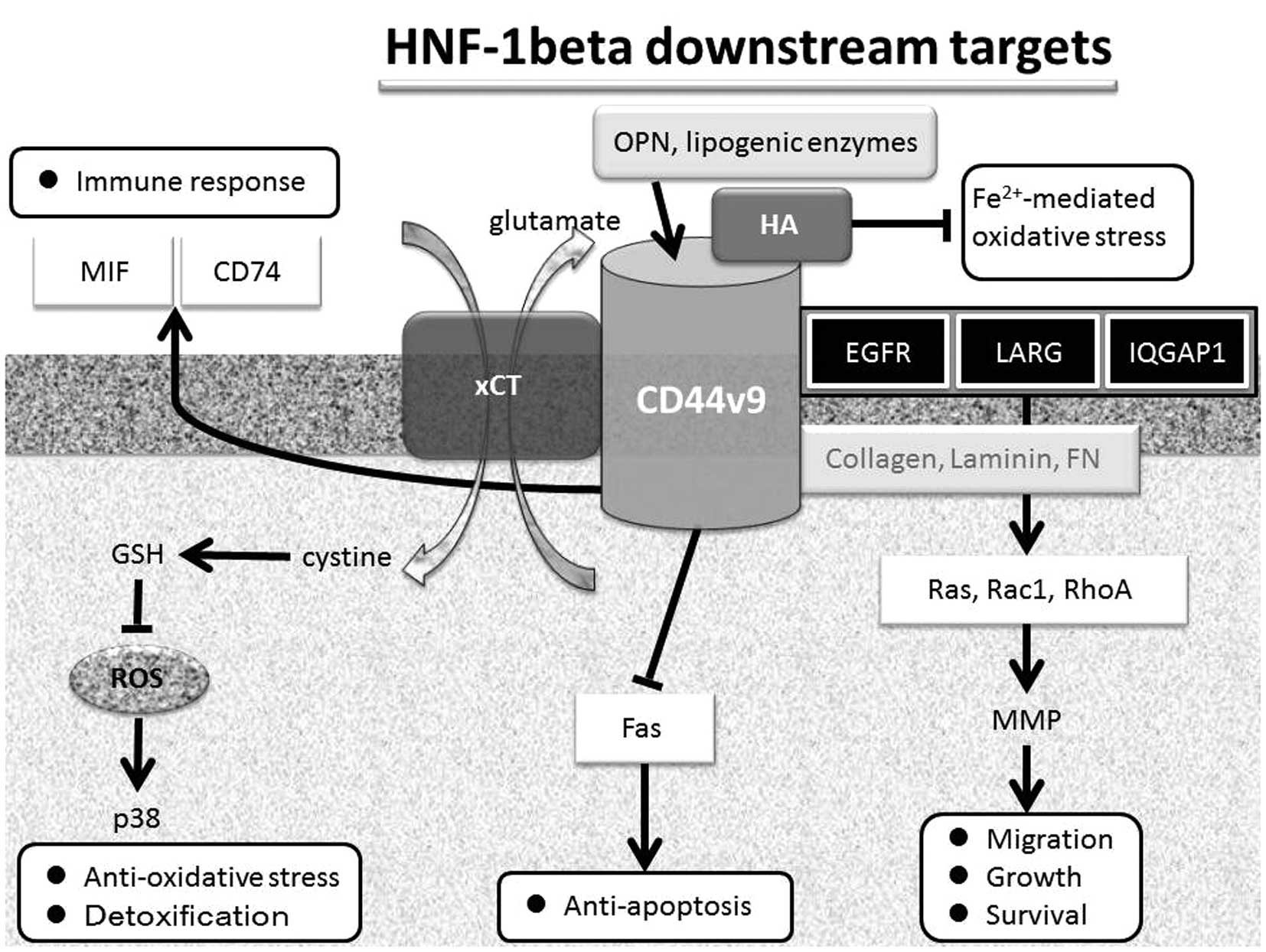 | Figure 1.Proposed function of HNF-1β downstream
targets. HNF-1β regulates the expression of several genes,
including CD44v9, which binds several molecules, including
hyaluronan, EGFR, LARG, IQGAP1, MIF, CD74, xCT, Fas and ECM
proteins. Aberrant expression of HNF-1β is able to promote survival
and also attenuate cell and DNA damage, probably through its
anti-oxidative action and its detoxification process as well as the
potential to minimize the deleterious effects of free iron on
endometriotic tissue. EGRF, epidermal growth factor receptor; LARG,
leukemia-associated Rho-guanine nucleotide exchange factor; IOGAP1,
IQ motif containing GTPase activating protein 1; MIF, macrophage
migration inhibitory factor; CD74, major histocompatibility
complex, class II invariant chain; xCT, cystine transporter
subunit; ECM, extracellular matrix proteins. |
HA binds to its cell receptor CD44. HA-CD44 binding
is important for the initial attachment of endometriotic cells
expressing CD44 molecules to mesothelial-associated HA (33). This interaction is one of the
mechanisms involved in the pathogenesis of endometriosis.
Expression of CD44v9 was detected in the normal endometrial
glandular cell membrane (34).
CD44v9 expression was increased after cell damage (35). Although CD44v9 was not observed in
normal ovarian tissues, ovarian endometriosis shares alterations of
CD44 isoforms, which show the adhesive and aggressive potentials of
endometriotic cells (34,36).
Upregulation of CD44 expression enhances reduced
glutathione (GSH) synthesis (8). A
CD44 variant, CD44v9, specifically regulates redox status (9). CD44v9 interacts with xCT, a
glutamate-cystine transporter (also known as SLC7A11; solute
carrier family 7) and mediates cystine-glutamate exchange (8). xCT controls the intracellular level of
GSH and is thereby an important determinant of intracellular redox
balance. GSH is one of the first lines of defense against ROS
damage. GSH suppresses ROS-mediated p38 MAPK activation, indicating
that xCT is crucially involved in the prevention of such stress
signaling (Fig. 1). Ablation of
CD44 induces loss of xCT and suppresses tumor growth and
metastasis. Such metastasis is dependent on the activity of xCT.
The xCT system is also involved in cisplatin resistance in ovarian
cancer cells through maintaining higher levels of GSH. These
findings establish a function for CD44v in the regulation of ROS
defense and tumor progression (8).
Recent biochemical and immunohistochemical studies
have noted a specific expression of HNF-1β in CCC and genetic
alteration may be involved in oxidative stress (2–4). The
majority of the CCC-specific genes were associated with the
redox-related genes (2). Several
important CCC-related genes overlap with those known to be
regulated by HNF-1β. UGT1A1 and ANXA4, the HNF-β downstream
targets, are also critical enzymes responsible for detoxification
in CCC (2). The HNF-1β-dependent
pathway might be associated with detoxification pathways, possibly
through the upregulation of UGT1A1 and ANXA4 expression as well as
CD44v9-xCT-dependent GSH cascade. GSH is a major cellular
metabolite that protects against oxidative stress and chemical
injury. HNF-1β may attenuate DNA damage and promote cell survival
by upregulating the antioxidant protein expression.
Conclusions
The pathogenesis of endometriosis is closely
associated with iron overload originating from the retrograde flow
of the menstrual blood. This review provides more information
regarding the molecular mechanisms underlying the protection of
oxidative stress afforded by transcription factor HNF-1β. HNF-1β
regulates endometriosis-specific gene expression. Fig. 1 outlines the potential challenges to
understand the suggested function of HNF-1β downstream targets.
HNF-1β regulates the expression of several genes, including CD44v9,
which binds several target molecules. CD44v9 specifically regulates
cell functions, including migration, growth, survival,
anti-apoptosis, immune response and redox status. HA directly
reverses the oxidative stress created by redox-active iron. CD44v9
interacts with xCT, which is responsible for exchanging
intracellular glutamate for extracellular cysteine, and is able to
suppress the ROS-mediated oxidative stress. The HNF-1β-dependent
pathway might be associated with detoxification systems, such as
the CD44v9-xCT-dependent GSH pathway. In conclusion, endometriosis
may induce mechanisms for protecting against the susceptibility to
oxidative stress-induced cell and DNA damage.
Acknowledgements
This study was supported by a
Grant-in-aid for Scientific Research from the Ministry of
Education, Science and Culture of Japan granted to the Department
of Obstetrics and Gynecology, Nara Medical University (Hiroshi
Kobayashi).
References
|
1.
|
Kato N, Sasou S and Motoyama T: Expression
of hepatocyte nuclear factor-1β (HNF-1β) in clear cell tumors and
endometriosis of the ovary. Mod Pathol. 19:83–89. 2006.
|
|
2.
|
Kobayashi H, Yamada Y, Kanayama S,
Furukawa N, Noguchi T, Haruta S, Yoshida S, Sakata M, Sado T and Oi
H: The role of hepatocyte nuclear factor-1beta in the pathogenesis
of clear cell carcinoma of the ovary. Int J Gynecol Cancer.
19:471–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yamada Y, Shigetomi H, Onogi A, Haruta S,
Kawaguchi R, Yoshida S, Furukawa N, Nagai A, Tanase Y, Tsunemi T,
Oi H and Kobayashi H: Redox-active iron-induced oxidative stress in
the pathogenesis of clear cell carcinoma of the ovary. Int J
Gynecol Cancer. 21:1200–1207. 2011.PubMed/NCBI
|
|
4.
|
Shigetomi H, Higashiura Y, Kajihara H and
Kobayashi H: A potential link of oxidative stress and cell cycle
regulation for development of endometriosis. Gynecol Endocrinol.
May 17–2012.(Epub ahead of print).
|
|
5.
|
Yoshioka K, Kunitomo M, Yanai K, Shimizu
H, Nakasono S, Negishi T and Dateki M: Hepatocyte nuclear factor 1β
induced by chemical stress accelerates cell proliferation and
increases genomic instability in mouse liver. J Recept Signal
Transduct Res. 31:132–138. 2011.
|
|
6.
|
Verdeguer F, Le Corre S, Fischer E,
Callens C, Garbay S, Doyen A, Igarashi P, Terzi F and Pontoglio M:
A mitotic transcriptional switch in polycystic kidney disease. Nat
Med. 16:106–110. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ogata K, Shimamura Y, Hamada K, Hisa M,
Bun M, Okada N, Inoue K, Taniguchi Y, Ishihara M, Kagawa T, Horino
T, Fujimoto S and Terada Y: Upregulation of HNF-1β during
experimental acute kidney injury plays a crucial role in renal
tubule regeneration. Am J Physiol Renal Physiol. 303:F689–F699.
2012.
|
|
8.
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T,
Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y,
Ohmura M, Suematsu M, Baba H and Saya H: CD44 variant regulates
redox status in cancer cells by stabilizing the xCT subunit of
system xc(−) and thereby promotes tumor growth. Cancer Cell.
19:387–400. 2011.PubMed/NCBI
|
|
9.
|
Jang Bl, Li Y, Graham DY and Cen P: The
role of CD44 in the pathogenesis, diagnosis, and therapy of gastric
cancer. Gut Liver. 5:397–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lu D, Tawfik O, Pantazis C, Hobart W,
Chapman J and Iczkowski K: Altered expression of CD44 and variant
isoforms in human adenocarcinoma of the endocervix during
progression. Gynecol Oncol. 75:84–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Koyama S, Maruyama T and Adachi S:
Expression of epidermal growth factor receptor and CD44 splicing
variants sharing exons 6 and 9 on gastric and esophageal
carcinomas: a two-color flow-cytometric analysis. J Cancer Res Clin
Oncol. 125:47–54. 1999. View Article : Google Scholar
|
|
12.
|
Seki K, Yamaguchi A, Goi T, Nakagawara G,
Matsukawa S, Urano T and Furukawa K: Inhibition of liver metastasis
formation by anti-CD44 variant exon 9 monoclonal antibody. Int J
Oncol. 11:1257–1261. 1997.PubMed/NCBI
|
|
13.
|
Omara-Opyene AL, Qiu J, Shah GV and
Iczkowski KA: Prostate cancer invasion is influenced more by
expression of a CD44 isoform including variant 9 than by Muc18. Lab
Invest. 84:894–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Goi T, Koneri K, Katayama K, Hirose K and
Yamaguchi A: Evaluation of clinicopathological factors and the
correlation between the adhesion molecule CD44 variant 9 expression
and pulmonary metastases from colorectal cancers. Int Surg.
87:130–136. 2002.PubMed/NCBI
|
|
15.
|
Okano K, Shimoda T and Matsumura Y:
Clinicopathologic and immunohistochemical study of early colorectal
cancer with liver metastases. J Gastroenterol. 34:334–340. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Rodríguez-Rodríguez L, Sancho-Torres I,
Leakey P, Gibbon DG, Comerci JT, Ludlow JW and Mesonero C: CD44
splice variant expression in clear cell carcinoma of the ovary.
Gynecol Oncol. 71:223–229. 1998.PubMed/NCBI
|
|
17.
|
Eisterer W, Bechter O, Hilbe W, van Driel
M, Lokhorst HM, Thaler J, Bloem AC, Günthert U and Stauder R: CD44
isoforms are differentially regulated in plasma cell dyscrasias and
CD44v9 represents a new independent prognostic parameter in
multiple myeloma. Leuk Res. 25:1051–1057. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Akisik E, Bavbek S and Dalay N: CD44
variant exons in leukemia and lymphoma. Pathol Oncol Res. 8:36–40.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Mielgo A, van Driel M, Bloem A, Landmann L
and Günthert U: A novel antiapoptotic mechanism based on
interference of Fas signaling by CD44 variant isoforms. Cell Death
Differ. 13:465–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Echiburú-Chau C, Roy D and Calaf GM:
Metastatic suppressor CD44 is related with oxidative stress in
breast cancer cell lines. Int J Oncol. 39:1481–1489.
2011.PubMed/NCBI
|
|
21.
|
Sato S, Miyauchi M, Takekoshi T, Zhao M,
Kudo Y, Ogawa I, Kitagawa S, Fujita M and Takata T: Reduced
expression of CD44 variant 9 is related to lymph node metastasis
and poor survival in squamous cell carcinoma of tongue. Oral Oncol.
36:545–549. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kahara N, Ozaki T, Doi T, Nishida K, Kawai
A, Shibahara M and Inoue H: CD44 expression in soft tissue
sarcomas. Virchows Arch. 436:574–578. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Imam H, Eriksson B and Oberg K: Expression
of CD44 variant isoforms and association to the benign form of
endocrine pancreatic tumours. Ann Oncol. 11:295–300. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shimabukuro K, Toyama-Sorimachi N, Ozaki
Y, Goi T, Furukawa K, Miyasaka M and Aso T: The expression patterns
of standard and variant CD44 molecules in normal uterine cervix and
cervical cancer. Gynecol Oncol. 64:26–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wang SJ and Bourguignon LY: Role of
hyaluronan-mediated CD44 signaling in head and neck squamous cell
carcinoma progression and chemoresistance. Am J Pathol.
178:956–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kozlova I, Ruusala A, Voytyuk O, Skandalis
SS and Heldin P: IQGAP1 regulates hyaluronan-mediated fibroblast
motility and proliferation. Cell Signal. 24:1856–1862. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ohta S, Misawa A, Fukaya R, Inoue S,
Kanemura Y, Okano H, Kawakami Y and Toda M: Macrophage migration
inhibitory factor (MIF) promotes cell survival and proliferation of
neural stem/progenitor cells. J Cell Sci. 125:3210–3220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Zaytseva YY, Rychahou PG, Gulhati P,
Elliott VA, Mustain WC, O’Connor K, Morris AJ, Sunkara M, Weiss HL,
Lee EY and Evers BM: Inhibition of fatty acid synthase attenuates
CD44-associated signaling and reduces metastasis in colorectal
cancer. Cancer Res. 72:1504–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Li J, Zha XM, Wang R, Li XD, Xu B, Xu YJ
and Yin YM: Regulation of CD44 expression by tumor necrosis
factor-α and its potential role in breast cancer cell migration.
Biomed Pharmacother. 66:144–150. 2012.
|
|
30.
|
Darzynkiewicz Z and Balazs EA: Genome
integrity, stem cells and hyaluronan. Aging (Albany NY). 4:78–88.
2012.PubMed/NCBI
|
|
31.
|
Pauloin T, Dutot M, Warnet JM and Rat P:
In vitro modulation of preservative toxicity: high molecular weight
hyaluronan decreases apoptosis and oxidative stress induced by
benzalkonium chloride. Eur J Pharm Sci. 34:263–273. 2008.
View Article : Google Scholar
|
|
32.
|
Zhao H, Tanaka T, Mitlitski V, Heeter J,
Balazs EA and Darzynkiewicz Z: Protective effect of hyaluronate on
oxidative DNA damage in WI-38 and A549 cells. Int J Oncol.
32:1159–1167. 2008.PubMed/NCBI
|
|
33.
|
Witz CA, Allsup KT, Montoya-Rodriguez IA,
Vaughan SL, Centonze VE and Schenken RS: Pathogenesis of
endometriosis - current research. Hum Fertil (Camb). 6:34–40. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Ketola K, Hilvo M, Hyötyläinen T, Vuoristo
A, Ruskeepää AL, Orešič M, Kallioniemi O and Iljin K: Salinomycin
inhibits prostate cancer growth and migration via induction of
oxidative stress. Br J Cancer. 106:99–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Leir SH, Baker JE, Holgate ST and Lackie
PM: Increased CD44 expression in human bronchial epithelial repair
after damage or plating at low cell densities. Am J Physiol Lung
Cell Mol Physiol. 278:L1129–L1137. 2000.PubMed/NCBI
|
|
36.
|
Daraï E, Leblanc M, Walker-Combrouze F,
Bringuier AF, Madelenat P and Scoazec JY: Expression of cadherins
and CD44 isoforms in ovarian endometrial cysts. Hum Reprod.
13:1346–1352. 1998.PubMed/NCBI
|















