Introduction
Digestive cancer, including gastric, colorectal,
hepatocellular (HC), gallbladder cancer and gastric
mucosa-associated lymphoid tissue (MALT) lymphoma, which has a
higher cancer-related mortality compared to any other system in the
body (1), has become a major public
health issue worldwide. The causes of digestive cancer, including
the interaction between inherited and environmental factors, are
complicated (2). Host genetic
factors may play a vital role in the genesis of digestive cancer
(3). The activation of the immune
system and inflammation, regulated by specific single nucleotide
polymorphisms (SNPs) of common, low-penetrance susceptibility loci
(4), reportedly plays an important
role in cancer susceptibility and progression (5).
Toll-like receptors (TLRs) are material constituents
of the innate immune response by which the host is able to protect
itself from microbial danger and other unsafe agents (6). Inherited polymorphisms in TLR genes
have been demonstrated to directly affect the risk of infectious
diseases, allergy, cardiovascular disease and more significantly
cancer (7). Toll-like receptor 4
(TLR4), which constitutes one of the most active members of TLRs,
promotes the transcription of genes involved in immune activation
(8). Two SNPs, located on
chromosome 9, are reportedly associated with certain types of
digestive cancer (9–20). One is Asp299Gly (299A>G, D299G,
rs4986790), with G instead of the A allele at 896 base pair (bp),
causing glycine to replace aspartic acid at the 299 site of the
amino acid sequence (TLR4_896A/G). The second SNP is Thr399Ile
(399C>T, T399I, rs4986791), with T instead of the C allele at
1,196 bp, causing isoleucine to replace threonine at the 399 site
of the amino acid sequence (TLR4_1196C/T) (21). The common, co-segregating missense
mutations (Asp299Gly and Thr399Ile), which alter the extracellular
structure of this receptor, are correlated with a blunted response
to lipopolysaccharide (LPS) in vivo and in vitro
(21,22). Additionally, these mutations are
correlated with an increased risk of inflammatory diseases
(23,24), due to the fact that these two SNPs
cleave the normal structure of the extracellular domain of the TLR4
and are, therefore, estimated to reduce the reaction to ligands
through alterations in binding (7).
Although numerous studies have investigated the
correlation between the two SNPs and digestive cancer, data are
limited and the results remain controversial. The aim of this
meta-analysis, considering the eligible published studies currently
available, was therefore to review and quantitatively analyze the
results, in order to reach an evidence-based conclusion.
Materials and methods
Search strategy
In order to evaluate the correlation between the
TLR4 gene and digestive cancer susceptibility, several databases,
including PubMed, Web of Science (ISI), the China National
Knowledge Infrastructure (CNKI), the Database of Chinese Scientific
and Technical Periodicals (VIP) and the China Biology Medical
literature database (CBM), were searched on May 25, 2012, using the
following search terms: ‘toll-like receptor 4’, and, ‘cancer’,
‘carcinoma’, ‘tumor’, ‘malignancy’, ‘neoplasm’, and ‘polymorphism’.
In addition, we reviewed the reference lists of the identified
relevant studies and relevant reviews.
Inclusion criteria
The studies included in this meta-analysis were
independently assessed by two investigators using the following
inclusion criteria: i) the original study evaluated the
relationship between the Asp299Gly (299A>G, D299G, rs4986790,
TLR4_896A/G) and Thr399Ile (399C>T, T399I, rs4986791,
TLR4_1196C/T) polymorphisms in the TLR4 gene and digestive cancer
[defined as cancer of the esophagus, stomach, colorectum, pancreas,
gallbladder, liver and gastric MALT lymphoma] risk; ii) in a
case-control or cohort study design; iii) provided the genotype
frequencies or the data could be calculated in order to determine
the odds ratio (OR) with 95% confidence interval (CI). When the
studies were duplicated or overlapped, those with the largest
number of subjects and the most recently published studies were
included in the final analysis.
Data extraction
Two investigators independently extracted and
converted the available data from the retrieved studies into a
standard format for incorporation into a central database. The
information collected from each study was as follows: the name of
the first author, year of publication, country, cancer type,
genotype frequencies for cases and controls, methods of genotyping,
age and gender in cases and controls, and source of control groups
(population- or hospital-based controls). Any discrepancies between
the two reviewers were settled by discussion and consultation with
a third reviewer.
Statistical analysis
The distribution of genotype frequencies in the
control groups was assessed with regard to whether or not they
deviated from the Hardy-Weinberg equilibrium (HWE) by the
Chi-square goodness of fit test. Pooled OR and the corresponding
95% CI were used as measures to estimate the strength of the
correlation between the two polymorphisms (Asp299Gly and Thr399Ile)
and cancer susceptibility. Dominant (GG+AG vs. AA, TT+TC vs. CC),
recessive (GG vs. AA+GA, TT vs. CC+TC) and codominant (G vs. A, T
vs. C) models were calculated separately. The heterogeneity between
the results of collected studies was evaluated with the
I2 index, which calculates the degree of heterogeneity
in the meta-analysis (25). When
the heterogeneity was significant (I2>50%), the
random-effect model (REM) was selected to evaluate the results
using the DerSimonian and Laird method. By contrast
(I2<50%), the fixed-effect model (FEM) was adopted
using the inverse variance method. To explore the potential source
of heterogeneity, we performed a stratified analysis (gastric or
colorectal cancer) and meta-regression analysis to assess the
potentially important covariates across studies. Theoretical
consideration and empirical evidence have suggested that specific
genetic variants causally associated with common diseases could
have small effects (risk ratio mostly <2.0) (26,27),
due to the fact that original studies with a relatively limited
number of participants might be under-powered to detect the effect.
Therefore, for the sensitivity analysis, we excluded the studies
with OR>3.0 as a criterion to control the impact of outlier
values resulting from low cell counts within each single study on
the pooled effect. Influence analysis was conducted, in order to
describe how robust the pooled estimator was in order to exclude
individual studies. Publication bias was estimated by Begg’s funnel
plots (28). The software used was
STATA version 10.1 (StataCorp LP, College Station, TX, USA). The
reported probabilities (P-values) were two-sided and P<0.05 was
considered to indicate a statistically significant difference.
Results
Study characteristics
The general characteristics of the available studies
are shown in Tables I and II. Due to the fact that there was >1
study in an article (14), there
were 12 studies in 10 published articles (9–15,17–19)
with 1,877 cases and 3,181 controls for Asp299Gly polymorphism, and
8 studies in 8 published articles (10,13,15–20)
with 1,062 cases and 1,867 controls for Thr399Ile polymorphism. The
distribution of genotypes in the control groups obeyed the HWE for
the obtained studies, with the exception of one study (17) for the Asp299Gly polymorphism.
 | Table ICharacteristics of TLR4 Asp299Gly
polymorphism genotype distributions for digestive cancer risk in
the studies included in this meta analysis. |
Table I
Characteristics of TLR4 Asp299Gly
polymorphism genotype distributions for digestive cancer risk in
the studies included in this meta analysis.
| Authors | Year | Country | Cancer type | Genotypes
(AA/AG/GG)
| Percentage of male
patients (case/control) | Mean age
(case/control) | Genotyping
method | Control source | P-value of HWC | Refs. |
|---|
| Case | Control |
|---|
| de Oliveira and
Silva | 2012 | Brazil | Gastric | 154/20/0 | 402/31/0 | 77/49 | 62.2/54.7 | PCR-RFLP | PB | 0.440 | (19) |
| Davoodi and
Seow | 2011 | Iran | Colorectal | 58/2/0 | 50/0/0 | Na | Na | PCR-RFLP | PB | 1.000 | (18) |
| Yang, et
ala | 2011 | China | Colorectal | 96/4/2 | 84/2/1 | 63/60 | 52.0/50.0 | PCR-sequencing | HB | 0.035 | (17) |
| Türe-Ozdemir, et
alb | 2008 | Turkey | GML | 38/18/0 | 39/12/0 | 54/59 | 61.0/56.0 | PCR-RFLP | HB | 0.341 | (9) |
| Santini, et
alb | 2008 | Italy | Gastric | 159/11/1 | 140/11/0 | 58/58 | 60.0/56.0 | PCR-RFLP | PB | 0.642 | (10) |
| Garza-Gonzalez,
et al | 2007 | Mexico | Gastric | 72/6/0 | 239/20/0 | 64/Na | 58.6/57.1 | PCR-sequencing | HB | 0.518 | (15) |
| Hold, et
alc | 2007 | Poland | Gastric | 258/51/3 | 387/31/1 | Na | Na | PCR-TaqMan | PB | 0.651 | (14) |
| Hold, et
alc | 2007 | USA | Gastric | 266/38/3 | 194/16/1 | Na | Na | PCR-TaqMan | PB | 0.299 | (14) |
| Hold, et
alc | 2007 | USA | Esophageal | 148/11/0 | 194/16/1 | Na | Na | PCR-TaqMan | PB | 0.299 | (14) |
| Boraska Jelavić,
et al | 2006 | Croatia | Colorectal | 77/10/2 | 84/4/0 | 69/Na | 61.5/Na | PCR-RFLP | PB | 0.827 | (13) |
| Landi, et
al | 2006 | Spain | Colorectal | 251/31/0 | 232/37/0 | Na | Na | PCR-TaqMan | HB | 0.226 | (12) |
| Hellmig, et
alb | 2005 | Germany | GML | 83/4/0 | 837/114/1 | 32/34 | 57.0/56.3 | PCR-TaqMan | HB | 0.151 | (11) |
 | Table IICharacteristics of TLR4 Thr399Ile
polymorphism genotype distributions for digestive cancer risk in
the studies included in this meta analysis. |
Table II
Characteristics of TLR4 Thr399Ile
polymorphism genotype distributions for digestive cancer risk in
the studies included in this meta analysis.
| Authors | Year | Country | Cancer type | Genotypes
(CC/CT/TT)
| Percentage of male
patients (case/control) | Mean age
(case/control) | Genotyping
method | Control source | P-value of HWC | Refs. |
|---|
| Case | Control |
|---|
| de Oliveira and
Silva | 2012 | Brazil | Gastric | 165/9/0 | 421/12/0 | 77/49 | 62.2/54.7 | PCR-RFLP | PB | 0.770 | (19) |
| Agúndez, et
al | 2012 | Spain | HC | 143/12/0 | 472/68/3 | 75/59 | 66.9/44.1 | PCR-TaqMan | HB | 0.746 | (20) |
| Davoodi and
Seow | 2011 | Iran | Colorectal | 58/2/0 | 50/0/0 | Na | Na | PCR-RFLP | PB | 1.000 | (18) |
| Yang, et
al | 2011 | China | Colorectal | 93/8/1 | 86/1/0 | 63/60 | 52.0/50.0 | PCR-sequencing | HB | 0.957 | (17) |
| Srivastava, et
al | 2010 | India | Gallbladder | 195/32/5 | 232/24/1 | 35/36 | 54.1/53.1 | PCR-RFLP | PB | 0.657 | (16) |
| Santini, et
ala | 2008 | Italy | Gastric | 155/15/1 | 147/4/0 | 58/58 | 60.0/56.0 | PCR-RFLP | PB | 0.869 | (10) |
| Garza-Gonzalez,
et al | 2007 | Mexico | Gastric | 77/1/0 | 246/13/0 | 64/NP | 58.6/57.1 | PCR-RFLP | HB | 0.679 | (15) |
| Boraska Jelavić,
et al | 2006 | Croatia | Colorectal | 77/12/0 | 82/5/0 | Na | 61.5/Na | PCR-RFLP | PB | 0.783 | (13) |
Quantitative synthesis
The details of pooled ORs for the correlation
between TLR4 Asp299Gly and Thr399Ile polymorphisms and digestive
cancer risk are summarized in Tables
III and IV.
 | Table IIIPooled measures of the correlation
between TLR4 Asp299Gly polymorphism and digestive cancer. |
Table III
Pooled measures of the correlation
between TLR4 Asp299Gly polymorphism and digestive cancer.
| Disease | Population | Inherited
model | Prior to
sensitivity analysis | Following
sensitivity analysis |
|---|
|
|
|---|
| No. of
cases/controls | Pooled OR (95%
CI) | I2
(%) | No. of
cases/controls | Pooled OR (95%
CI) | I2
(%) |
|---|
|
|
|---|
| FEM | REM | FEM | REM |
|---|
| Overall | All related
studies | Dominant | 1,877/3,181 | 1.373
(1.109–1.700)a | 1.309
(0.923–1.857) | 55.8 | 1,728/3,043 | 1.325
(1.066–1.648)b | 1.217
(0.847–1.748) | 59.3 |
| Recessive | 1877/3181 | 2.355
(0.848–6.641) | 2.355
(0.848–6.641) | 0 | 1389/1730 | 1.562
(0.410–5.948) | 1.562
(0.410–5.948) | 0 |
| Codominant | 1877/3181 | 1.463
(1.193–1.794)a | 1.460
(0.951–2.241) | 73.5 | 1728/3043 | 1.328
(1.079–1.636)a | 1.221
(0.866–1.721) | 58.7 |
| Excluded for
DHWC | Dominant | 1775/3094 | 1.366
(1.100–1.695)a | 1.290
(0.893–1.863) | 59.6 | 1626/2956 | 1.316
(1.056–1.641)b | 1.190
(0.812–1.744) | 63.6 |
| Recessive | 1775/3094 | 2.522
(0.817–7.783) | 2.522
(0.817–7.783) | 0 | 1287/1643 | 1.497
(0.301–7.450) | 1.497
(0.301–7.450) | 0 |
| Codominant | 1775/3094 | 1.456
(1.184–1.790)a | 1.444
(0.917–2.274) | 73.6 | 1626/2956 | 1.318
(1.067–1.628)b | 1.192
(0.828–1.715) | 63.0 |
| Gastric
cancerc | All related
studies | Dominant | 1042/1473 | 1.772
(1.340–2.343)a | 1.685
(1.193–2.382)a | 29.9 | - | - | - | - |
| Recessive | 1042/1473 | 2.852
(0.679–11.978) | 2.852
(0.679–11.978) | 0 | 730/419 | 2.254
(0.353–14.378) | 2.254
(0.353–14.378) | 0 |
| Codominant | 1042/1473 | 1.761
(1.347–2.301)a | 1.696
(1.237–2.326)a | 23.3 | - | - | - | - |
| Colorectal
cancer | All related
studies | Dominant | 533/494 | 1.062
(0.685–1.648) | 1.546
(0.639–3.738) | 52.4 | 384/356 | 0.850
(0.526–1.373) | 0.881
(0.492–1.577) | 11.2 |
| Recessive | 533/494 | 2.607
(0.392–17.343) | 2.607
(0.392–17.343) | 0 | 444/414 | 1.720
(0.153–19.298) | 1.720
(0.153–19.298) | - |
| Codominant | 533/494 | 1.410
(0.932–2.133) | 2.790
(0.561–13.885) | 88.0 | 384/356 | 0.880
(0.557–1.389) | 0.953
(0.491–1.847) | 28.0 |
| Excluded for
DHWC | Dominant | 431/407 | 1.007
(0.634–1.598) | 1.629
(0.480–5.533) | 65.3 | 282/269 | 0.774
(0.465–1.289) | 0.774
(0.465–1.289) | - |
| Recessive | 431/407 | 5.057
(0.239–106.859) | 5.057
(0.239–106.859) | - | - | - | - | - |
| Codominant | 431/407 | 1.372
(0.883–2.131) | 3.420
(0.336–34.857) | 91.9 | 282/269 | 0.788
(0.481–1.289) | 0.788
(0.481–1.289) | - |
 | Table IVPooled measures of the correlation
between TLR4 Thr399Ile polymorphism and digestive cancer. |
Table IV
Pooled measures of the correlation
between TLR4 Thr399Ile polymorphism and digestive cancer.
| Disease | Inherited
model | Prior to
sensitivity analysis | Following
sensitivity analysis |
|---|
|
|
|---|
| No. of
cases/controls | Pooled OR (95%
CI) | I2
(%) | No. of
cases/controls | Pooled OR (95%
CI) | I2
(%) |
|---|
|
|
|---|
| FEM | REM | FEM | REM |
|---|
| Overalla | Dominant | 1062/1867 | 1.454
(1.048–2.016)b | 1.681
(0.888–3.181) | 63.9 | 729/1579 | 1.244
(0.877–1.763) | 1.212
(0.606–2.427) | 68.3 |
| Recessive | 1062/1867 | 2.506
(0.629–9.991) | 2.506
(0.629–9.991) | 0 | 829/1610 | 1.420
(0.234–8.620) | 1.420
(0.234–8.620) | 0 |
| Codominant | 1062/1867 | 1.485
(1.084–2.035)b | 1.706
(0.895–3.251) | 66.4 | 729/1579 | 1.279
(0.915–1.788) | 1.217
(0.601–2.462) | 71.0 |
| Gastric
cancera | Dominant | 423/843 | 1.962
(1.018–3.784)b | 1.611
(0.496–5.236) | 62.1 | 252/692 | 1.388
(0.617–3.123) | 0.852
(0.119–6.084) | 69.2 |
| Recessive | 423/843 | 2.666
(0.108–65.927) | 2.666
(0.108–65.927) | - | - | - | - | - |
| Codominant | 423/843 | 1.982
(1.036–3.791)b | 1.626
(0.500–5.286) | 63.0 | 252/692 | 1.382
(0.619–3.086) | 0.857
(0.124–5.918) | 68.5 |
| Colorectal
cancera | Dominant | 251/224 | 3.372
(1.343–8.466)b | 3.372
(1.343–8.466)b | 0 | 89/87 | 2.556
(0.860–7.591) | 2.556
(0.860–7.591) | - |
| Recessive | 251/224 | 2.586
(0.104–64.299) | 2.586
(0.104–64.299) | - | - | - | - | - |
| Codominant | 251/224 | 3.286
(1.331–8.116)b | 3.286
(1.331–8.116)b | 0 | 89/87 | 2.443
(0.842–7.088) | 2.443
(0.842–7.088) | - |
Asp299Gly polymorphism
This meta-analysis showed no statistically
significant correlation between the G allele and overall digestive
cancer susceptibility in the dominant (REM: OR, 1.309, 95% CI,
0.923–1.857), recessive (FEM: OR, 2.355; 95% CI, 0.848–6.641) and
codominant (REM: OR, 1.460; 95% CI, 0.951–2.241) models. When
studies that deviated from the HWE in the control group were
excluded (17), there were still no
significant effect of G allele on the risk of overall digestive
cancer in the dominant (REM: OR, 1.290; 95% CI, 0.893–1.863),
recessive (FEM: OR, 2.522; 95% CI, 0.817–7.783) and codominant
(REM: OR, 1.444; 95% CI, 0.917–2.274) models.
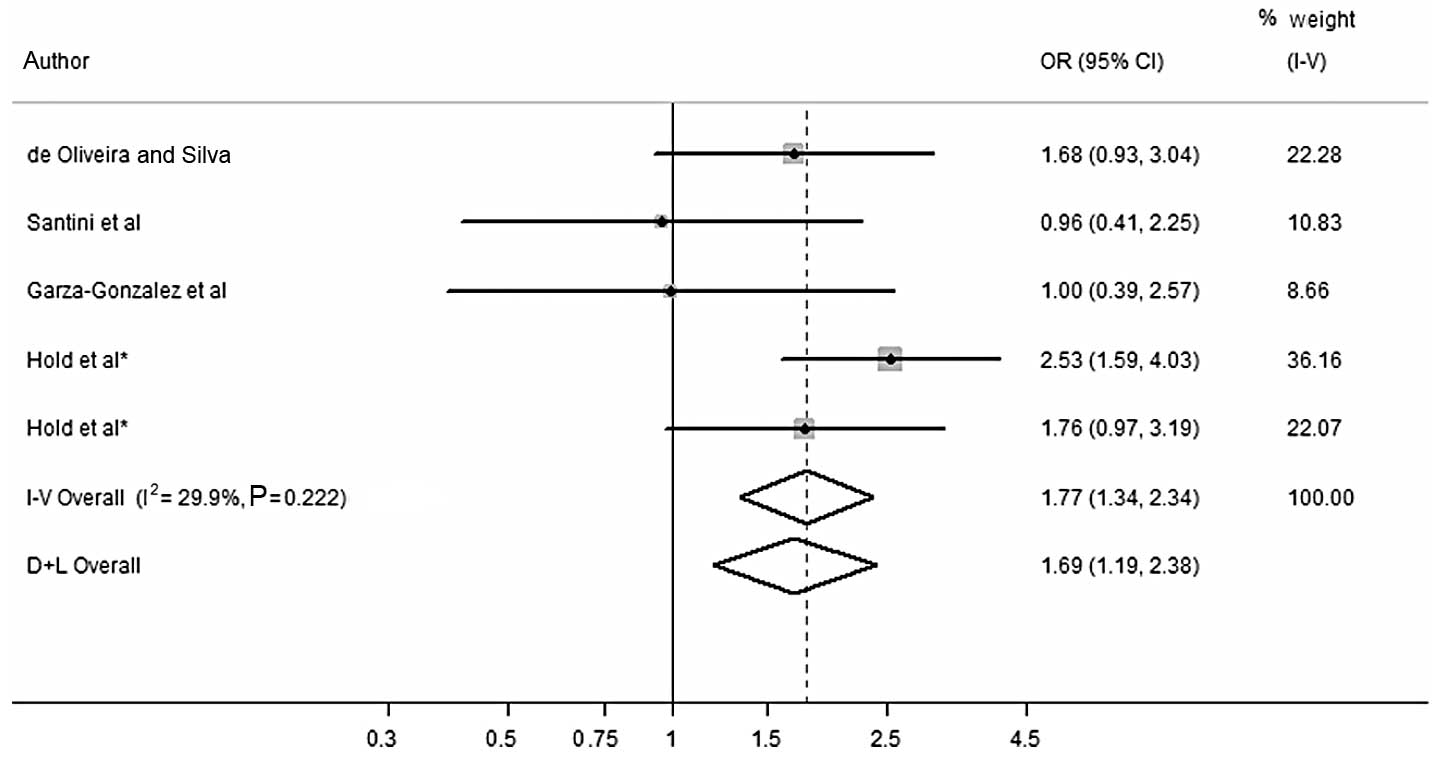
Regarding gastric cancer, a statistically
significant correlation between G allele and an increased risk of
gastric cancer for the dominant (FEM: OR, 1.772; 95% CI,
1.340–2.343) (Fig. 1 and Table III) and codominant (FEM: OR, 1.761;
95% CI, 1.347–2.301) models was detected. However, no statistically
significant correlation was observed between the G allele and
gastric cancer susceptibility in the recessive (FEM: OR, 2.852; 95%
CI, 0.679–11.978) model. No study deviated from the HWE in the
control group for gastric cancer.
Concerning the various types of colorectal cancer,
no statistically significant correlation with the G allele was
found for the dominant (REM: OR, 1.546; 95% CI, 0.639–3.738),
recessive (FEM: OR, 2.607; 95% CI, 0.392–17.343) and codominant
(REM: OR, 2.790; 95% CI, 0.561–13.885) models. Following exclusion
of studies that deviated from the HWE in the control groups
(17), there was still no
statistically significant correlation in the abovementioned
inherited models.
Thr399Ile polymorphism
No statistically significant correlation was found
between the T allele and risk of overall digestive cancer in the
dominant (REM: OR, 1.681; 95% CI, 0.888–3.181), recessive (FEM: OR,
2.506; 95% CI, 0.629–9.991) and codominant (REM: OR, 1.706; 95% CI,
0.895–3.251) models (Table IV). No
study deviated from the HWE in the control group for overall
digestive cancer.
With regard to gastric cancer, this meta-analysis
showed no significant impact of the T allele on the risk of overall
digestive cancer in the dominant (REM: OR, 1.611; 95% CI,
0.496–5.236), recessive (FEM: OR, 2.666; 95% CI, 0.108–65.927) and
codominant (REM: OR, 1.626; 95% CI, 0.500–5.286) models. No study
deviated from the HWE in the control group for gastric cancer.
Regarding colorectal cancer, the correlation with T
allele was significant in the dominant (FEM: OR, 3.372; 95% CI,
1.343–8.466) and codominant (FEM: OR, 3.286; 95% CI, 1.331–8.116)
models. No statistically significant correlation was found in the
recessive (FEM: OR, 2.586; 95% CI, 0.104–64.299) model. No study
deviated from the HWE in the the control group for colorectal
cancer.
Sources of heterogeneity
As shown in Tables
III and IV, prior to
sensitivity analysis, evident heterogeneity among studies in the
dominant and codominant models was demonstrated for Asp299Gly and
Thr399Ile polymorphisms, following the exclusion of studies
deviating from the HWE in the control groups. However, in the
recessive model no significant heterogeneity was found for
Asp299Gly and Thr399Ile polymorphisms, following the exclusion of
studies deviating from the HWE in the control groups.
We performed a univariate meta-regression analysis
with the covariates of publication year, continent (including Asia,
Europe and America), gender [ratio of males (%) in case/control
group), age (ratio of mean age or median age in case/control
group), sample size (the sum of cases and controls) and genotype
method [including polymerase chain reaction-restriction fragment
length polymorphism (PCR-RFLP), PCR-TaqMan probe technique
(PCR-TaqMan) and PCR-sequencing technique (PCR-sequencing)]. No
covariates were demonstrated to have a significant effect on
between-study heterogeneity for Asp299Gly and Thr399Ile
polymorphisms.
Sensitivity analysis
Asp299Gly polymorphism
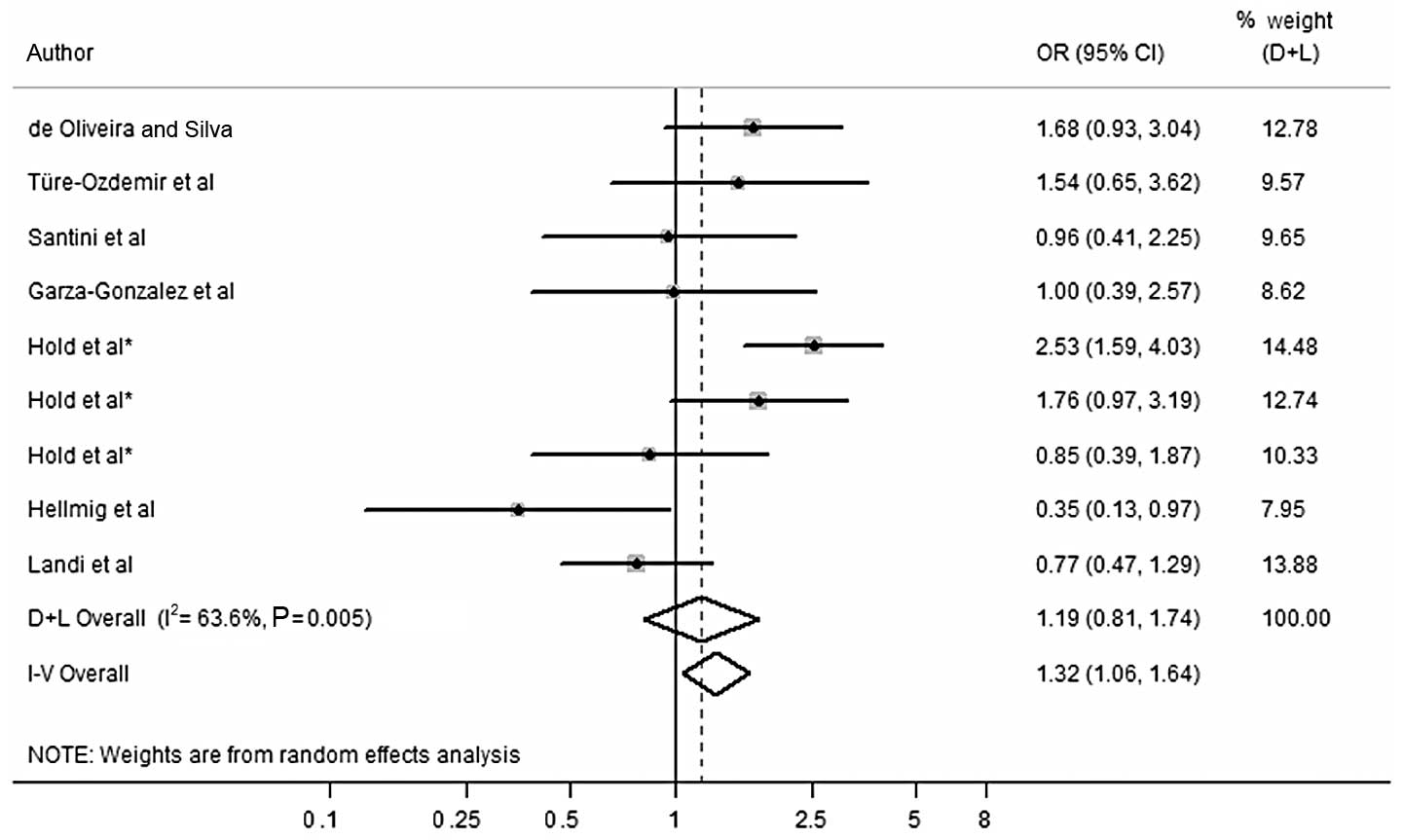
Concerning overall digestive cancer, following the
exclusion of studies with OR>3.0 (13) (OR, 3.273 in the dominant; 5.057 in
the recessive and 4.241 in the codominant model), (18) (OR, 4.316 in the dominant model and
OR, 4.241 in the codominant model), (11) (OR, 3.625 in the recessive model) and
(14) (OR, 4.058 in the recessive
model), the risk effect of G allele in the dominant, recessive and
codominant models was still not significant (Fig. 2). For gastric cancer, following the
exclusion of studies with OR>3.0 (14) (OR, 4.058 in the recessive model), no
significant correlation was found in the G allele in the recessive
model. For colorectal cancer, following the exclusion of studies
with OR>3.0 (13) (OR, 3.273 in
the dominant, 5.057 in the recessive and 4.241 in the codominant
model) and (18) (OR, 4.316 in the
dominant model and OR, 4.241 in the codominant model), the analysis
did not show any significant effect of G allele in the dominant,
recessive and codominant models (Table
III).
Thr399Ile polymorphism
Regarding overall digestive cancer, following the
exclusion of studies with OR>3.0 (18) (OR, 4.316 in the dominant and 4.241
in the codominant model), (10)
(OR, 3.794 in the dominant and 3.897 in the codominant model),
(16) (OR, 5.639 in the recessive
model) and (17) (OR, 8.323 in the
dominant and 8.918 in the codominant model), no significant
correlation was found in the dominant, recessive and codominant
models. Following the exclusion of studies with OR>3.0, for
gastric cancer (10) (OR, 3.794 in
the dominant model and 3.897 in the codominant model) and
colorectal cancer (18) (OR, 4.316
in the dominant model and 4.241 in the codominant model) and
(17) (OR, 8.323 in the dominant
model and 8.918 in the codominant model), no significant
correlation to the T allele was indicated in dominant and
codominant models (Table IV).
Influence analysis
Following the exclusion of studies deviating from
HWE in controls and sensitivity analysis, no individual study was
found to have an impact on the pooled effect in the dominant,
recessive and codominant models for either the Asp299Gly or
Thr399Ile polymorphisms.
Publication bias evaluation
Following the exclusion of studies deviating from
HWE in controls and sensitivity analysis, no significant
publication bias was detected in any of the above-mentioned
inherited models for Asp299Gly and Thr399Ile polymorphisms (data
not shown).
Discussion
In this meta-analysis, we assessed the correlation
between TLR4 gene polymorphisms (Asp299Gly and Thr399Ile) and
digestive cancer susceptibility. As a result, a significant
correlation was found between TLR4 gene and the risk of gastric
cancer.
Epidemiological studies have demonstrated that
chronic inflammation is important in the development of digestive
cancer (29). A variety of chronic
inflammatory statuses, e.g., Barrett’s esophagus, ulcerative
colitis and chronic gastritis induced by Helicobacter pylori
infection, significantly increase the risk of developing digestive
cancer (29,30). Considering the correlation between
inflammation and carcinogenesis, investigators have begun to shed
light on the role of TLRs and innate immune responses in
inflammation-associated carcinogenesis in the
gastrointestinal tract (31–34).
TLR4, which constitutes one of the most active members of the TLRs
family, performs important immune and non-immune functions in the
human intestinal tract (35). From
a theoretical point of view, the activation of TLR4 may irritate
the immune response that protects the organism against tumors,
produce a pro-inflammatory environment and, thus, may promote
carcinogenesis (20). The possible
effect of the two non-synonymous SNPs at rs4986790 (A299G) and
rs4986791 (T399I) in the TLR4 gene on cancer risk has received more
attention (6), as the minor A
allele in Asp299Gly and T allele in Thr399Ile are associated with
reduced activation of nuclear factor-κB (NF-κB) and
pro-inflammatory cytokine expression (36,37).
Recently, studies on the correlation of SNPs
(Asp299Gly and Thr399Ile) in TLR4 gene with the risk of digestive
cancer, including gastric, colorectal, gallbladder, hepatocellular,
esophageal cancer and gastric MALT lymphoma, have conducted
investigations on different ethnicities (9–20).
However, the outcomes were inconclusive and the small sample size
of each study was underpowered to confirm the correlation. Thus, a
larger-scale meta-analysis including all the available studies was
required to evaluate the correlation between TLR4 gene and
digestive cancer susceptibility. This meta-analysis, of 12
published studies (9–20), with 12 studies for Asp299Gly
polymorphism and 8 studies for Thr399Ile polymorphism (10,13,15–20),
constitutes a greater probability to reach an evidence-based
conclusion.
According to a previously published study (38), between-study heterogeneity is common
in the meta-analyses of genetic association studies. In this
meta-analysis, significant between-study heterogeneity in dominant
and codominant models was also demonstrated, regarding overall
digestive and gastric cancer. A series of uncertain factors that
differ among studies may explain between-study heterogeneity, e.g.,
study quality, different sources of population, characteristics of
the sample, non-comparable measures of genotyping, variation of the
covariate and deviation from HWE in certain studies. In order to
investigate the conceivable substantial causes of between-study
heterogeneity, meta-regression was performed. Following the
exclusion of the studies deviating from HWE in controls, this
meta-analysis did not detect any of the above-mentioned covariates
as a substantial contributor to between-study heterogeneity.
In addition, it is noteworthy to consider the
outlier values of OR that could cause significant effects by
chance. Theoretical and empirical evidence has suggested that
specific genetic variants causally associated with common diseases
have limited effects (risk ratios mostly <2.0) (26,27). A
relatively small sample size and possible genotyping errors might
also have an impact on this effect. Besides, large effect estimates
could be induced by unsteady effect estimations due to low cell
counts within each study. Therefore, we conducted a sensitivity
analysis, excluding studies with OR>3.0. However, the
correlation of the G allele of Asp299Gly and Thr399Ile
polymorphisms in the TLR4 gene with digestive, gastric and
colorectal cancer risk, was not significant.
There are significant different incidences of
Asp299Gly and Thr399Ile polymorphisms between the continents
(39). Although we did not find any
significant evidence that different continents were responsible for
the heterogeneity, we could not exclude continent as a potential
contributor to between-study heterogeneity. Moreover, additional
factors that were not analyzed in this study should not be
considered, e.g., cigarette smoking, alcohol intake, amount of
exercise, dietary history and occupation characteristics, since
they potentially affect cancer progression.
In this meta-analysis, no significant publication
bias in the above-mentioned inherited models was identified, a fact
which may result from the limited number of studies included in
this meta-analysis. In summary, this meta-analysis has demonstrated
that the G allele of Asp299Gly polymorphism in the TLR4 gene is
able to increase the risk of gastric cancer. However, due to the
fact that potential biases and confounders could not be completely
excluded, further large-scale, well-designed, comprehensive studies
on various ethnicities, with more detailed individual data, need to
be performed in order for these results to be further
validated.
Acknowledgements
This study was supported by the
Natural Science Foundation of Shandong Province (grant nos.
ZR2009CM111 and ZR2010HM100).
References
|
1
|
Parkin DM, Pisani P and Ferlay J: Global
cancer statistics. CA Cancer J Clin. 49:33–64. 1999. View Article : Google Scholar
|
|
2
|
Pharoah PD, Dunning AM, Ponder BA and
Easton DF: Association studies for finding cancer-susceptibility
genetic variants. Nat Rev Cancer. 4:850–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin BK, Clyne M, Walsh M, et al: Tracking
the epidemiology of human genes in the literature: the HuGE
Published Literature database. Am J Epidemiol. 164:1–4. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le Marchand L: Genome-wide association
studies and colorectal cancer. Surg Oncol Clin N Am. 18:663–668.
2009.PubMed/NCBI
|
|
5
|
Frank B, Hoffmeister M, Klopp N, Illig T,
Chang-Claude J and Brenner H: Polymorphisms in inflammatory pathway
genes and their association with colorectal cancer risk. Int J
Cancer. 127:2822–2830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kutikhin AG: Impact of Toll-like receptor
4 polymorphisms on risk of cancer. Human Immunol. 72:193–206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Omar EM, Ng MT and Hold GL:
Polymorphisms in Toll-like receptor genes and risk of cancer.
Oncogene. 27:244–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seya T, Shime H, Ebihara T, Oshiumi H and
Matsumoto M: Pattern recognition receptors of innate immunity and
their application to tumor immunotherapy. Cancer Sci. 101:313–320.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Türe-Ozdemir F, Gazouli M, Tzivras M, et
al: Association of polymorphisms of NOD2, TLR4 and CD14 genes with
susceptibility to gastric mucosa-associated lymphoid tissue
lymphoma. Anticancer Res. 28:3697–3700. 2008.PubMed/NCBI
|
|
10
|
Santini D, Angeletti S, Ruzzo A, et al:
Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms in
gastric cancer of intestinal and diffuse histotypes. Clin Exp
Immunol. 154:360–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hellmig S, Fischbach W, Goebeler-Kolve
M-E, Fölsch UR, Hampe J and Schreiber S: Association study of a
functional toll-like receptor 4 polymorphism with susceptibility to
gastric mucosa-associated lymphoid tissue lymphoma. Leuk Lymphoma.
46:869–872. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Landi S, Gemignani F, Bottari F, et al:
Polymorphisms within inflammatory genes and colorectal cancer. J
Negat Results Biomed. 5:152006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boraska Jelavić T, Barisić M, Drmic Hofman
I, et al: Microsatellite GT polymorphism in the toll-like receptor
2 is associated with colorectal cancer. Clin Genet. 70:156–160.
2006.PubMed/NCBI
|
|
14
|
Hold GL, Rabkin CS, Chow WH, et al: A
functional polymorphism of toll-like receptor 4 gene increases risk
of gastric carcinoma and its precursors. Gastroenterology.
132:905–912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garza-Gonzalez E, Bosques-Padilla FJ,
Mendoza-Ibarra SI, Flores-Gutierrez JP, Maldonado-Garza HJ and
Perez-Perez GI: Assessment of the toll-like receptor 4 Asp299Gly,
Thr399Ile and interleukin-8 -251 polymorphisms in the risk for the
development of distal gastric cancer. BMC Cancer. 7:702007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srivastava K, Srivastava A, Kumar A and
Mittal B: Significant association between toll-like receptor gene
polymorphisms and gallbladder cancer. Liver Int. 30:1067–1072.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang G, Qiu J, Wang X, Shen Z, Shao S and
Li F: The single nucleotide polymorphisms of toll-like receptor 4
gene and colorectal cancer susceptibility. Chin J Gastrointest
Surg. 14:814–815. 2011.
|
|
18
|
Davoodi H and Seow HF: Variant toll-like
receptor 4 (Asp299Gly and Thr399Ile alleles) and toll-like receptor
2 (Arg753Gln and Arg677Trp alleles) in colorectal cancer. Iran J
Allergy Asthma Immunol. 10:91–99. 2011.PubMed/NCBI
|
|
19
|
de Oliveira JG and Silva AE: Polymorphisms
of the TLR2 and TLR4 genes are associated with risk of gastric
cancer in a Brazilian population. World J Gastroenterol.
18:1235–1242. 2012.PubMed/NCBI
|
|
20
|
Agúndez JA, García-Martín E, Devesa MJ, et
al: Polymorphism of the TLR4 gene reduces the risk of hepatitis C
virus-induced hepatocellular carcinoma. Oncology. 82:35–40.
2012.PubMed/NCBI
|
|
21
|
Arbour NC, Lorenz E, Schutte BC, et al:
TLR4 mutations are associated with endotoxin hyporesponsiveness in
humans. Nat Genet. 25:187–191. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Norata GD, Garlaschelli K, Ongari M, et
al: Effect of the Toll-like receptor 4 (TLR-4) variants on
intima-media thickness and monocyte-derived macrophage response to
LPS. J Intern Med. 258:21–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brand S, Staudinger T, Schnitzler F, et
al: The role of Toll-like receptor 4 Asp299Gly and Thr399Ile
polymorphisms and CARD15/NOD2 mutations in the susceptibility and
phenotype of Crohn’s disease. Inflamm Bowel Dis. 11:645–652.
2005.PubMed/NCBI
|
|
24
|
Gazouli M, Mantzaris G, Kotsinas A, et al:
Association between polymorphisms in the Toll-like receptor 4,
CD14, and CARD15/NOD2 and inflammatory bowel disease in the Greek
population. World J Gastroenterol. 11:681–685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khoury MJ, Little J, Gwinn M and Ioannidis
JP: On the synthesis and interpretation of consistent but weak
gene-disease associations in the era of genome-wide association
studies. Int J Epidemiol. 36:439–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ioannidis JP: Commentary: grading the
credibility of molecular evidence for complex diseases. Int J
Epidemiol. 35:572–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Houghton J and Wang TC: Helicobacter
pylori and gastric cancer: a new paradigm for inflammation
associated epithelial cancers. Gastroenterology. 128:1567–1578.
2005. View Article : Google Scholar
|
|
30
|
Peek RM and Blaser MJ: Helicobacter
pylori and gastrointestinal tract adenocarcinomas. Nat Rev
Cancer. 2:28–37. 2002. View
Article : Google Scholar
|
|
31
|
Huang B, Zhao J, Li H, et al: Toll-like
receptors on tumor cells facilitate evasion of immune surveillance.
Cancer Res. 65:5009–5014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang B, Zhao J, Shen S, et al: Listeria
monocytogenes promotes tumor growth via tumor cell toll-like
receptor 2 signaling. Cancer Res. 67:4346–4352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao H, Gulen MF, Qin J, et al: The
Toll-interleukin-1 receptor member SIGIRR regulates colonic
epithelial homeostasis, inflammation, and tumorigenesis. Immunity.
26:461–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fukata M, Chen A, Vamadevan AS, et al:
Toll-like receptor-4 (TLR4) promotes the development of
colitis-associated colorectal tumors. Gastroenterology.
133:1869–1881. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukata M and Abreu MT: Role of Toll-like
receptors in gastrointestinal malignancies. Oncogene. 27:234–243.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo J, Loke J, Zheng F, et al: Functional
linkage of cirrhosis-predictive single nucleotide polymorphism of
toll-like receptor 4 to hepatic stellate cell responses.
Hepatology. 49:960–968. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo J and Friedman SL: Toll-like receptor
4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis
Tissue Repair. 3:212010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Munafò MR and Flint J: Meta-analysis of
genetic association studies. Trends Genet. 20:439–444.
2004.PubMed/NCBI
|
|
39
|
Ferwerda B, McCall MBB, Alonso S, et al:
TLR4 polymorphisms, infectious diseases, and evolutionary pressure
during migration of modern humans. Proc Natl Acad Sci USA.
104:16645–16650. 2007. View Article : Google Scholar : PubMed/NCBI
|